The Interaction between Psychological Stress and Iron Status on Early-Life Neurodevelopmental Outcomes
Abstract
1. Introduction
2. Psychological Stress and Iron Status
2.1. Psychological Stress—Definitions and Prevalence
2.2. Studies on Stress and Iron Status
2.2.1. Prenatal Stress and Offspring Iron Status
2.2.2. Postnatal Stress and Iron Status
3. Shared Neurodevelopmental Outcomes of Pre-Natal and Early-Life Stress and Iron Deficiency
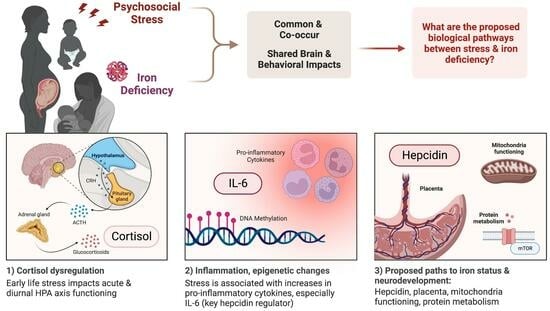
4. Hypothesized Biological Pathways between Psychological Stress and Iron Status
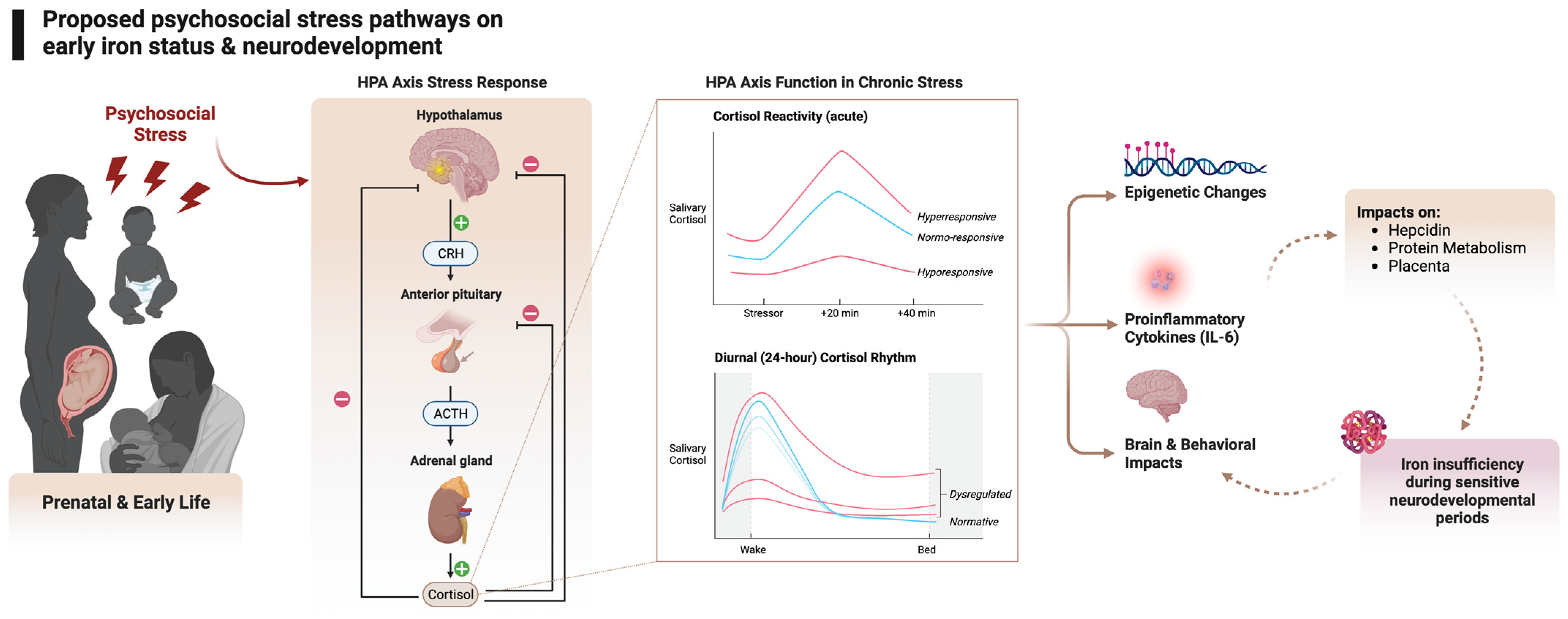
4.1. Stress and the HPA Axis
4.2. Stress and Inflammation
4.3. Stress, Inflammation, and Hepcidin
4.4. Other Mechanisms
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K. Iron assessment to protect the developing brain. Am. J. Clin. Nutr. 2017, 106, 1588S–1593S. [Google Scholar] [CrossRef]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Pena-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M.; Nutrition Impact Model Study Group. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef]
- Cusick, S.; Georgieff, M.; Rao, R. Approaches for Reducing the Risk of Early-Life Iron Deficiency-Induced Brain Dysfunction in Children. Nutrients 2018, 10, 227. [Google Scholar] [CrossRef]
- Georgieff, M.K. Iron deficiency in pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef]
- Lopresti, A.L. The Effects of Psychological and Environmental Stress on Micronutrient Concentrations in the Body: A Review of the Evidence. Adv. Nutr. 2020, 11, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Hantsoo, L.; Kornfield, S.; Anguera, M.C.; Epperson, C.N. Inflammation: A Proposed Intermediary Between Maternal Stress and Offspring Neuropsychiatric Risk. Biol. Psychiatry 2019, 85, 97–106. [Google Scholar] [CrossRef]
- Slavich, G.M. Social Safety Theory: A Biologically Based Evolutionary Perspective on Life Stress, Health, and Behavior. Annu. Rev. Clin. Psychol. 2020, 16, 265–295. [Google Scholar] [CrossRef]
- McEwen, B.S. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology 2000, 22, 108–124. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Herrick, H.W.B. The Effect of Stressful Life Events on Postpartum Depression: Results from the 1997–1998 North Carolina Pregnancy Risk Assessment Monitoring System (PRAMS); North Carolina State Center for Health Statistics: Raleigh, NC, USA, 2000. [Google Scholar]
- Whitehead, N.; Hill, H.A.; Brogan, D.J.; Blackmore-Prince, C. Exploration of Threshold Analysis in the Relation between Stressful Life Events and Preterm Delivery. Am. J. Epidemiol. 2002, 155, 117–124. [Google Scholar] [CrossRef][Green Version]
- Lu, M.C.; Chen, B. Racial and ethnic disparities in preterm birth: The role of stressful life events. Am. J. Obstet. Gynecol. 2004, 191, 691–699. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Preventing Violence against Children; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- U.S. Department of Health & Human Services; Administration on Children, Youth and Families; Children’s Bureau. Child Maltreatment 2021; U.S. Department of Health & Human Services: Washington, DC, USA, 2021. [Google Scholar]
- United Nations Children’s Fund. A Familiar Face: Violence in the Lives of Children and Adolescents; UNICEF: New York, NY, USA, 2017. [Google Scholar]
- Brannon, P.M.; Taylor, C.L. Iron Supplementation during Pregnancy and Infancy: Uncertainties and Implications for Research and Policy. Nutrients 2017, 9, 1327. [Google Scholar] [CrossRef]
- Rahman, M.M.; Abe, S.K.; Rahman, M.S.; Kanda, M.; Narita, S.; Bilano, V.; Ota, E.; Gilmour, S.; Shibuya, K. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: Systematic review and meta-analysis1,2. Am. J. Clin. Nutr. 2016, 103, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M.; Quigg, A.M.; Hurley, K.M.; Pepper, M.R. Iron deficiency and iron-deficiency anemia in the first two years of life: Strategies to prevent loss of developmental potential. Nutr. Rev. 2011, 69 (Suppl. S1), S64–S70. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J.; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Armony-Sivan, R.; Aviner, S.; Cojocaru, L.; Fytlovitch, S.; Ben-Alon, D.; Eliassy, A.; Babkoff, H.; Lozoff, B.; Anteby, E. Prenatal maternal stress predicts cord-blood ferritin concentration. J. Perinat. Med. 2013, 41, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.K.; Tamayo-Ortiz, M.; Cantoral, A.; Schnaas, L.; Osorio-Valencia, E.; Wright, R.J.; Tellez-Rojo, M.M.; Wright, R.O. Maternal Prenatal Psychological Stress and Prepregnancy BMI Associations with Fetal Iron Status. Curr. Dev. Nutr. 2020, 4, nzaa018. [Google Scholar] [CrossRef]
- Rendina, D.N.; Blohowiak, S.E.; Coe, C.L.; Kling, P.J. Maternal Perceived Stress during Pregnancy Increases Risk for Low Neonatal Iron at Delivery and Depletion of Storage Iron at One Year. J. Pediatr. 2018, 200, 166–173.e2. [Google Scholar] [CrossRef]
- Coe, C.L.; Lubach, G.R.; Shirtcliff, E.A. Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatr. Res. 2007, 61, 520–524. [Google Scholar] [CrossRef]
- Teng, W.F.; Sun, W.M.; Shi, L.F.; Hou, D.D.; Liu, H. Effects of restraint stress on iron, zinc, calcium, and magnesium whole blood levels in mice. Biol. Trace Elem. Res. 2008, 121, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhou, J.; Huang, X.; Li, M. Effects of psychological stress on serum iron and erythropoiesis. Int. J. Hematol. 2008, 88, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, J.B.; Wang, W.Y.; Wang, L.; Ma, L.; Shen, H.; Li, M. Psychological stress induces hypoferremia through the IL-6-hepcidin axis in rats. Biochem. Biophys. Res. Commun. 2008, 373, 90–93. [Google Scholar] [CrossRef]
- Li, Y.J.; Zheng, Y.Y.; Qian, J.X.; Chen, X.M.; Shen, Z.L.; Tao, L.P.; Li, H.X.; Qin, H.H.; Li, M.; Shen, H. Preventive Effects of Zinc Against Psychological Stress-Induced Iron Dyshomeostasis, Erythropoiesis Inhibition, and Oxidative Stress Status in Rats. Biol. Trace Elem. Res. 2012, 147, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.B.; Shen, H.; Chen, C.J.; Wang, W.Y.; Yu, S.Y.; Zhao, M.; Li, M. The effect of psychological stress on iron absorption in rats. BMC Gastroenterol. 2009, 9, 83. [Google Scholar] [CrossRef]
- Li, H.F.; Jiang, S.X.; Yang, C.; Yang, S.; He, B.; Ma, W.Q.; Zhao, R.Q. Long-Term Dexamethasone Exposure Down-Regulates Hepatic TFR1 and Reduces Liver Iron Concentration in Rats. Nutrients 2017, 9, 617. [Google Scholar] [CrossRef]
- Guo, S.H.; Yang, C.; Jiang, S.X.; Ni, Y.D.; Zhao, R.Q.; Ma, W.Q. Repeated Restraint Stress Enhances Hepatic TFR2 Expression and Induces Hepatic Iron Accumulation in Rats. Biol. Trace Elem. Res. 2020, 196, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Smoak, B.L.; Patterson, K.Y.; LeMay, L.G.; Veillon, C.; Deuster, P.A. Biochemical indices of selected trace minerals in men: Effect of stress. Am. J. Clin. Nutr. 1991, 53, 126–131. [Google Scholar] [CrossRef]
- Reid, B.M.; East, P.; Blanco, E.; Doom, J.R.; Burrows, R.A.; Correa-Burrows, P.; Lozoff, B.; Gahagan, S. Early-life adversity is associated with poor iron status in infancy. Dev. Psychopathol. 2022, 1–12. [Google Scholar] [CrossRef]
- Lozoff, B.; Georgieff, M.K. Iron deficiency and brain development. Semin. Pediatr. Neurol. 2006, 13, 158–165. [Google Scholar] [CrossRef]
- Beard, J.L.; Connor, J.R. Iron status and neural functioning. Annu. Rev. Nutr. 2003, 23, 41–58. [Google Scholar] [CrossRef]
- Beard, J.L.; Wiesinger, J.A.; Connor, J.R. Pre- and postweaning iron deficiency alters myelination in Sprague-Dawley rats. Dev. Neurosci. 2003, 25, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Kwik-Uribe, C.L.; Gietzen, D.; German, J.B.; Golub, M.S.; Keen, C.L. Chronic marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition. J. Nutr. 2000, 130, 2821–2830. [Google Scholar] [CrossRef] [PubMed]
- Oloyede, O.B.; Folayan, A.T.; Odutuga, A.A. Effects of Low-Iron Status and Deficiency of Essential Fatty-Acids on Some Biochemical-Constituents of Rat-Brain. Biochem. Int. 1992, 27, 913–922. [Google Scholar] [PubMed]
- Yu, G.S.; Steinkirchner, T.M.; Rao, G.A.; Larkin, E.C. Effect of prenatal iron deficiency on myelination in rat pups. Am. J. Pathol. 1986, 125, 620–624. [Google Scholar]
- Lozoff, B.; Beard, J.; Connor, J.; Felt, B.; Georgieff, M.; Schallert, T. Long-Lasting Neural and Behavioral Effects of Iron Deficiency in Infancy. Nutr. Rev. 2006, 64 (Suppl. S2), S34–S43. [Google Scholar] [CrossRef] [PubMed]
- de Deungria, M.; Rao, R.; Wobken, J.D.; Luciana, M.; Nelson, C.A.; Georgieff, M.K. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr. Res. 2000, 48, 169–176. [Google Scholar] [CrossRef]
- Rao, R.; Tkac, I.; Townsend, E.L.; Gruetter, R.; Georgieff, M.K. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J. Nutr. 2003, 133, 3215–3221. [Google Scholar]
- Jorgenson, L.A.; Wobken, J.D.; Georgieff, M.K. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev. Neurosci. 2003, 25, 412–420. [Google Scholar] [CrossRef]
- Siddappa, A.M.; Georgieff, M.K.; Wewerka, S.; Worwa, C.; Nelson, C.A.; DeRegnier, R.A. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr. Res. 2004, 55, 1034–1041. [Google Scholar] [CrossRef]
- Geng, F.; Mai, X.; Zhan, J.; Xu, L.; Zhao, Z.; Georgieff, M.; Shao, J.; Lozoff, B. Impact of Fetal-Neonatal Iron Deficiency on Recognition Memory at 2 Months of Age. J. Pediatr. 2015, 167, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.B.; Orlando, M.; Eddins, A.; MacDonald, M.; Monczynski, C.; Wang, H. In Utero Iron Status and Auditory Neural Maturation in Premature Infants as Evaluated by Auditory Brainstem Response. J. Pediatr. 2010, 156, 377–381. [Google Scholar] [CrossRef]
- Wachs, T.D.; Pollitt, E.; Cueto, S.; Jacoby, E. Relation of neonatal iron status to individual variability in neonatal temperament. Dev. Psychobiol. 2005, 46, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Tancredi, D.J.; Krakowiak, P.; Hansen, R.L.; Ozonoff, S. Maternal Intake of Supplemental Iron and Risk of Autism Spectrum Disorder. Am. J. Epidemiol. 2014, 180, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Insel, B.J.; Schaefer, C.A.; McKeague, I.W.; Susser, E.S.; Brown, A.S. Maternal iron deficiency and the risk of schizophrenia in offspring. Arch. Gen. Psychiatry 2008, 65, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Riggins, T.; Miller, N.C.; Bauer, P.J.; Georgieff, M.K.; Nelson, C.A. Consequences of Low Neonatal Iron Status Due to Maternal Diabetes Mellitus on Explicit Memory Performance in Childhood. Dev. Neuropsychol. 2009, 34, 762–779. [Google Scholar] [CrossRef] [PubMed]
- Jabès, A.; Thomas, K.M.; Langworthy, S.; Georgieff, M.K.; Nelson, C.A. Functional and Anatomic Consequences of Diabetic Pregnancy on Memory in Ten-Year-Old Children. J. Dev. Behav. Pediatr. 2015, 36, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Clark, K.M.; Jing, Y.; Armony-Sivan, R.; Angelilli, M.L.; Jacobson, S.W. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J. Pediatr. 2008, 152, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, A.F.; Koss, M.; Burden, M.J.; Jonides, J.; Nelson, C.A.; Kaciroti, N.; Jimenez, E.; Lozoff, B. Iron deficiency in infancy and neurocognitive functioning at 19 years: Evidence of long-term deficits in executive function and recognition memory. Nutr. Neurosci. 2010, 13, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Jorgenson, L.A.; Sun, M.; O’Connor, M.; Georgieff, M.K. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus 2005, 15, 1094–1102. [Google Scholar] [CrossRef]
- Vallée, M.; Maccari, S.; Dellu, F.; Simon, H.; Le Moal, M.; Mayo, W. Long-term effects of prenatal stress and postnatal handling on age-related glucocorticoid secretion and cognitive performance: A longitudinal study in the rat. Eur. J. Neurosci. 1999, 11, 2906–2916. [Google Scholar] [CrossRef] [PubMed]
- Vallée, M.; Mayo, W.; Dellu, F.; Le Moal, M.; Simon, H.; Maccari, S. Prenatal Stress Induces High Anxiety and Postnatal Handling Induces Low Anxiety in Adult Offspring: Correlation with Stress-Induced Corticosterone Secretion. J. Neurosci. 1997, 17, 2626–2636. [Google Scholar] [CrossRef] [PubMed]
- Piazza, P.V.; Le Moal, M. Pathophysiological Basis of Vulnerability to Drug Abuse: Role of an Interaction Between Stress, Glucocorticoids, and Dopaminergic Neurons. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, V.; Koehl, M.; Le Moal, M.; Abrous, D.N. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc. Natl. Acad. Sci. USA 2000, 97, 11032–11037. [Google Scholar] [CrossRef]
- Sandman, C.A.; Curran, M.M.; Davis, E.P.; Glynn, L.M.; Head, K.; Baram, T.Z. Cortical Thinning and Neuropsychiatric Outcomes in Children Exposed to Prenatal Adversity: A Role for Placental CRH? Am. J. Psychiatry 2018, 175, 471–479. [Google Scholar] [CrossRef]
- Swales, D.A.; Stout-Oswald, S.A.; Glynn, L.M.; Sandman, C.; Wing, D.A.; Davis, E.P. Exposure to traumatic events in childhood predicts cortisol production among high risk pregnant women. Biol. Psychol. 2018, 139, 186–192. [Google Scholar] [CrossRef]
- Chan, J.C.; Nugent, B.M.; Bale, T.L. Parental Advisory: Maternal and Paternal Stress Can Impact Offspring Neurodevelopment. Biol. Psychiatry 2018, 83, 886–894. [Google Scholar] [CrossRef]
- O’Donnell, K.J.; Glover, V.; Barker, E.D.; O’Connor, T.G. The persisting effect of maternal mood in pregnancy on childhood psychopathology. Dev. Psychopathol. 2014, 26, 393–403. [Google Scholar] [CrossRef]
- Van den Bergh, B.R.H.; van den Heuvel, M.I.; Lahti, M.; Braeken, M.; de Rooij, S.R.; Entringer, S.; Hoyer, D.; Roseboom, T.; Raikkonen, K.; King, S.; et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci. Biobehav. Rev. 2020, 117, 26–64. [Google Scholar] [CrossRef]
- Gunnar, M.; Reid, B.M. Early Deprivation Revisited: Contemporary Studies of the Impact on Young Children of Institutional Care. Annu. Rev. Dev. Psychol. 2019, 1, 93–118. [Google Scholar]
- Pollak, S.D.; Nelson, C.A.; Schlaak, M.; Roeber, B.; Wewerka, S.; Wiik, K.L.; Frenn, K.; Loman, M.M.; Gunnar, M.R. Neurodevelopmental effects of early deprivation in post-institutionalized children. Child Dev. 2010, 81, 224–236. [Google Scholar] [PubMed]
- Roy, P.; Rutter, M.; Pickles, A. Institutional care: Associations between overactivity and lack of selectivity in social relationships. J. Psychol. Psychiatry 2004, 45, 866–873. [Google Scholar]
- Burgess, P.W.; Stuss, D.T. Fifty Years of Prefrontal Cortex Research: Impact on Assessment. J. Int. Neuropsychol. Soc. 2017, 23, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.E.; Pollak, S.D. Early life stress and neural development: Implications for understanding the developmental effects of COVID-19. Cogn. Affect. Behav. Neurosci. 2022, 22, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Turecki, G.; Meaney, M.J. Effects of the Social Environment and Stress on Glucocorticoid Receptor Gene Methylation: A Systematic Review. Biol. Psychiatry 2016, 79, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Brunson, K.L.; Kramar, E.; Lin, B.; Chen, Y.; Colgin, L.L.; Yanagihara, T.K.; Lynch, G.; Baram, T.Z. Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 2005, 25, 9328–9338. [Google Scholar] [CrossRef] [PubMed]
- Danielewicz, J.; Hess, G. Early life stress alters synaptic modification range in the rat lateral amygdala. Behav. Brain Res. 2014, 265, 32–37. [Google Scholar] [CrossRef]
- Ishikawa, J.; Nishimura, R.; Ishikawa, A. Early-life stress induces anxiety-like behaviors and activity imbalances in the medial prefrontal cortex and amygdala in adult rats. Eur. J. Neurosci. 2015, 41, 442–453. [Google Scholar] [CrossRef]
- Wei, L.; David, A.; Duman, R.S.; Anisman, H.; Kaffman, A. Early life stress increases anxiety-like behavior in Balbc mice despite a compensatory increase in levels of postnatal maternal care. Horm. Behav. 2010, 57, 396–404. [Google Scholar] [CrossRef]
- Bolton, J.L.; Molet, J.; Regev, L.; Chen, Y.C.; Rismanchi, N.; Haddad, E.; Yang, D.Z.; Obenaus, A.; Baram, T.Z. Anhedonia Following Early-Life Adversity Involves Aberrant Interaction of Reward and Anxiety Circuits and Is Reversed by Partial Silencing of Amygdala Corticotropin-Releasing Hormone Gene. Biol. Psychiatry 2018, 83, 137–147. [Google Scholar] [CrossRef]
- Berman, A.K.; Lott, R.B.; Donaldson, S.T. Periodic maternal deprivation may modulate offspring anxiety-like behavior through mechanisms involving neuroplasticity in the amygdala. Brain Res. Bull. 2014, 101, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Monroy, E.; Hernandez-Torres, E.; Flores, G. Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. J. Chem. Neuroanat. 2010, 40, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Bagot, R.C.; van Hasselt, F.N.; Champagne, D.L.; Meaney, M.J.; Krugers, H.J.; Joels, M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol. Learn. Mem. 2009, 92, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Ivy, A.S.; Rex, C.S.; Chen, Y.C.; Dube, C.; Maras, P.M.; Grigoriadis, D.E.; Gall, C.M.; Lynch, G.; Baram, T.Z. Hippocampal Dysfunction and Cognitive Impairments Provoked by Chronic Early-Life Stress Involve Excessive Activation of CRH Receptors. J. Neurosci. 2010, 30, 13005–13015. [Google Scholar] [CrossRef]
- Oomen, C.A.; Soeters, H.; Audureau, N.; Vermunt, L.; van Hasselt, F.N.; Manders, E.M.M.; Joels, M.; Lucassen, P.J.; Krugers, H. Severe Early Life Stress Hampers Spatial Learning and Neurogenesis, but Improves Hippocampal Synaptic Plasticity and Emotional Learning under High-Stress Conditions in Adulthood. J. Neurosci. 2010, 30, 6635–6645. [Google Scholar] [CrossRef]
- Swales, D.A.; Winiarski, D.A.; Smith, A.K.; Stowe, Z.N.; Newport, D.J.; Brennan, P.A. Maternal depression and cortisol in pregnancy predict offspring emotional reactivity in the preschool period. Dev. Psychobiol. 2018, 60, 557–566. [Google Scholar] [CrossRef]
- Van den Bergh, B.R.; Van Calster, B.; Smits, T.; Van Huffel, S.; Lagae, L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: A prospective study on the fetal origins of depressed mood. Neuropsychopharmacol. 2008, 33, 536–545. [Google Scholar] [CrossRef]
- Grantham-McGregor, S.; Ani, C. A review of studies on the effect of iron deficiency on cognitive development in children. J. Nutr. 2001, 131, 649S–666S. [Google Scholar]
- Skalicky, A.; Meyers, A.F.; Adams, W.G.; Yang, Z.; Cook, J.T.; Frank, D.A. Child food insecurity and iron deficiency anemia in low-income infants and toddlers in the United States. Matern. Child Health J. 2006, 10, 177–185. [Google Scholar] [CrossRef]
- Auerbach, M.; Abernathy, J.; Juul, S.; Short, V.; Derman, R. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J. Matern.-Fetal Neonatal Med. 2021, 34, 1002–1005. [Google Scholar] [CrossRef]
- Baskin, R.; Hill, B.; Jacka, F.N.; O’Neil, A.; Skouteris, H. The association between diet quality and mental health during the perinatal period. A systematic review. Appetite 2015, 91, 41–47. [Google Scholar] [CrossRef]
- Monk, C.; Lugo-Candelas, C.; Trumpff, C. Prenatal Developmental Origins of Future Psychopathology: Mechanisms and Pathways. Annu. Rev. Clin. Psychol. 2019, 15, 317–344. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C. Adapted from “Hypothalamic-Pituitary-Adrenal Axis” by BioRender.com. Available online: https://app.biorender.com/biorender-templates (accessed on 21 August 2023).
- Herman, J.P.; Cullinan, W.E. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997, 20, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.L.; Gunnar, M.R. The development of stress reactivity and regulation during human development. Int. Rev. Neurobiol. 2020, 150, 41–76. [Google Scholar] [PubMed]
- Adam, E.K.; Quinn, M.E.; Tavernier, R.; McQuillan, M.T.; Dahlke, K.A.; Gilbert, K.E. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 83, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Hellhammer, D.H. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 1994, 19, 313–333. [Google Scholar] [CrossRef]
- Levine, A.; Zagoory-Sharon, O.; Feldman, R.; Lewis, J.G.; Weller, A. Measuring cortisol in human psychobiological studies. Physiol. Behav. 2007, 90, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Stalder, T.; Jarczok, M.; Almeida, D.M.; Badrick, E.; Bartels, M.; Boomsma, D.I.; Coe, C.L.; Dekker, M.C.; Donzella, B.; et al. The CIRCORT database: Reference ranges and seasonal changes in diurnal salivary cortisol derived from a meta-dataset comprised of 15 field studies. Psychoneuroendocrinology 2016, 73, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.M.; Parker, K.J. Stress inoculation-induced indications of resilience in monkeys. J. Trauma. Stress 2007, 20, 423–433. [Google Scholar] [CrossRef]
- Boyce, W.T.; Ellis, B.J. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 2005, 17, 271–301. [Google Scholar] [CrossRef]
- Lyons, D.M.; Parker, K.J.; Schatzberg, A.F. Animal models of early life stress: Implications for understanding resilience. Dev. Psychobiol. 2010, 52, 402–410. [Google Scholar] [CrossRef]
- Tronick, E. The Inherent Stress of Normal Daily Life and Social Interaction Leads to the Development of Coping and Resilience, and Variation in Resilience in Infants and Young Children: Comments on the Papers of Suomi and Klebanov & Brooks-Gunn. Ann. NY Acad. Sci. 2006, 1094, 83–104. [Google Scholar] [CrossRef]
- Leneman, K.B.; Gunnar, M.R. Developmental Timing of Stress Effects on the Brain. In The Oxford Handbook of Stress and Mental Health; Harkness, K.L., Hayden, E.P., Eds.; Oxford University Press: Oxford, UK, 2020. [Google Scholar] [CrossRef]
- Thayer, Z.M.; Wilson, M.A.; Kim, A.W.; Jaeggi, A.V. Impact of prenatal stress on offspring glucocorticoid levels: A phylogenetic meta-analysis across 14 vertebrate species. Sci. Rep. 2018, 8, 4942. [Google Scholar] [CrossRef]
- Gunnar, M.R.; Davis, E.P. The effects of stress on early brain and behavioral development. In Cognitive Development; Rakic, P., Rubenstein, J., Tager-Flusberg, H.B., Eds.; Elsevier: New York, NY, USA, 2013; Volume 2, pp. 447–466. [Google Scholar]
- Gitau, R.; Cameron, A.; Fisk, N.M.; Glover, V. Fetal exposure to maternal cortisol. Lancet 1998, 352, 707–708. [Google Scholar] [CrossRef]
- Howland, M.A.; Sandman, C.A.; Glynn, L.M. Developmental origins of the human hypothalamic-pituitary-adrenal axis. Expert Rev. Endocrinol. Metab. 2017, 12, 321–339. [Google Scholar] [CrossRef]
- Gunnar, M.R.; Vazquez, D. Stress Neurobiology and Developmental Psychopathology. In Developmental Psychopathology: Vol. 2. Developmental Neuroscience; Cicchetti, D., Cohen, D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; Volume 2. [Google Scholar]
- Gunnar, M.R. Reactivity of the hypothalamic-pituitary-adrenocortical system to stressors in normal infants and children. Pediatrics 1992, 90, 491–497. [Google Scholar]
- Wang, L.; Wang, H.; Li, L.; Li, W.; Dong, X.; Li, M.; Lv, L. Corticosterone induces dysregulation of iron metabolism in hippocampal neurons in vitro. Biol. Trace Elem. Res. 2010, 137, 88–95. [Google Scholar] [CrossRef]
- Reid, B.M.G.; Keenan, K. Association between Cortisol Stress Response and Iron Status in Pregnancy: Insights from a Study of Pregnant Black Women with Medicaid Insurance. In Proceedings of the International Society of Psychoneuroendocrinology Annual Conference, London, UK, 30 August–1 September 2023. [Google Scholar]
- Segerstrom, S.C.; Miller, G.E. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004, 130, 601–630. [Google Scholar] [CrossRef]
- Bower, J.E.; Kuhlman, K.R. Psychoneuroimmunology: An Introduction to Immune-to-Brain Communication and Its Implications for Clinical Psychology. Annu. Rev. Clin. Psychol. 2023, 19, 331–359. [Google Scholar] [CrossRef]
- Glaser, R.; Kiecolt-Glaser, J.K. Science and society—Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 2005, 5, 243–251. [Google Scholar] [CrossRef]
- Weber, M.D.; Godbout, J.P.; Sheridan, J.F. Repeated Social Defeat, Neuroinflammation, and Behavior: Monocytes Carry the Signal. Neuropsychopharmacology 2017, 42, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Reader, B.F.; Jarrett, B.L.; McKim, D.B.; Wohleb, E.S.; Godbout, J.P.; Sheridan, J.F. Peripheral and central effects of repeated social defeat stress: Monocyte trafficking, microglial activation, and anxiety. Neuroscience 2015, 289, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Marsland, A.L.; Walsh, C.; Lockwood, K.; John-Henderson, N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav. Immun. 2017, 64, 208–219. [Google Scholar] [CrossRef] [PubMed]
- MacCormack, J.K.; Gaudier-Diaz, M.M.; Armstrong-Carter, E.L.; Arevalo, J.M.G.; Meltzer-Brody, S.; Sloan, E.K.; Cole, S.W.; Muscatell, K.A. Beta-adrenergic blockade blunts inflammatory and antiviral/antibody gene expression responses to acute psychological stress. Neuropsychopharmacology 2021, 46, 756–762. [Google Scholar] [CrossRef]
- Cole, S.W. The Conserved Transcriptional Response to Adversity. Curr. Opin. Behav. Sci. 2019, 28, 31–37. [Google Scholar] [CrossRef]
- Cole, S.W.; Conti, G.; Arevalo, J.M.; Ruggiero, A.M.; Heckman, J.J.; Suomi, S.J. Transcriptional modulation of the developing immune system by early life social adversity. Proc. Natl. Acad. Sci. USA 2012, 109, 20578–20583. [Google Scholar] [CrossRef]
- Marie-Mitchell, A.; Cole, S.W. Adverse childhood experiences and transcriptional response in school-age children. Dev. Psychopathol. 2022, 34, 875–881. [Google Scholar] [CrossRef]
- Bower, J.E.; Kuhlman, K.R.; Ganz, P.A.; Irwin, M.R.; Crespi, C.M.; Cole, S.W. Childhood maltreatment and monocyte gene expression among women with breast cancer. Brain Behav. Immun. 2020, 88, 396–402. [Google Scholar] [CrossRef]
- Miller, G.E.; Chen, E.; Sze, J.; Marin, T.; Arevalo, J.M.; Doll, R.; Ma, R.; Cole, S.W. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-kappa B signaling. Biol. Psychiatry 2008, 64, 266–272. [Google Scholar] [CrossRef]
- Kuhlman, K.R.; Horn, S.R.; Chiang, J.J.; Bower, J.E. Early life adversity exposure and circulating markers of inflammation in children and adolescents: A systematic review and meta-analysis. Brain Behav. Immun. 2020, 86, 30–42. [Google Scholar] [CrossRef]
- Baumeister, D.; Akhtar, R.; Ciufolini, S.; Pariante, C.M.; Mondelli, V. Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol. Psychiatry 2016, 21, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, K.R.; Cole, S.W.; Craske, M.G.; Fuligni, A.J.; Irwin, M.R.; Bower, J.E. Enhanced Immune Activation Following Acute Social Stress among Adolescents with Early-Life Adversity. Biol. Psychiatry Glob. Open Sci. 2023, 3, 213–221. [Google Scholar] [CrossRef] [PubMed]
- McGowan, P.O.; Sasaki, A.; D’Alessio, A.C.; Dymov, S.; Labonte, B.; Szyf, M.; Turecki, G.; Meaney, M.J. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009, 12, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Crisan, T.O.; Netea, M.G.; Joosten, L.A.B. Innate immune memory: Implications for host responses to damage-associated molecular patterns. Eur. J. Immunol. 2016, 46, 817–828. [Google Scholar] [CrossRef]
- Hanke, M.L.; Powell, N.D.; Stiner, L.M.; Bailey, M.T.; Sheridan, J.F. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav. Immun. 2012, 26, 1150–1159. [Google Scholar] [CrossRef]
- Coussons-Read, M.E.; Okun, M.L.; Nettles, C.D. Psychological stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav. Immun. 2007, 21, 343–350. [Google Scholar] [CrossRef]
- Reid, B.; Danese, A. Challenges in researching the immune pathways between early life adversity and psychopathology. Dev. Psychopathol. 2020, 32, 1597–1624. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Iron Sequestration and Anemia of Inflammation. Semin. Hematol. 2009, 46, 387–393. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Augustine, L.F.; Nair, K.M.; Rao, S.F.; Rao, M.V.; Ravinder, P.; Balakrishna, N.; Laxmaiah, A.; Vazir, S. Adolescent Life-Event Stress in Boys Is Associated with Elevated IL-6 and Hepcidin but Not Hypoferremia. J. Am. Coll. Nutr. 2014, 33, 354–362. [Google Scholar] [CrossRef]
- Gambling, L.; Charania, Z.; Hannah, L.; Antipatis, C.; Lea, R.G.; McArdle, H.J. Effect of iron deficiency on placental cytokine expression and fetal growth in the pregnant rat. Biol. Reprod. 2002, 66, 516–523. [Google Scholar] [CrossRef]
- Cao, C.; Prado, M.A.; Sun, L.; Rockowitz, S.; Sliz, P.; Paulo, J.A.; Finley, D.; Fleming, M.D. Maternal Iron Deficiency Modulates Placental Transcriptome and Proteome in Mid-Gestation of Mouse Pregnancy. J. Nutr. 2021, 151, 1073–1083. [Google Scholar] [CrossRef]
- Manji, H.; Kato, T.; Di Prospero, N.A.; Ness, S.; Beal, M.F.; Krams, M.; Chen, G. Impaired mitochondrial function in psychiatric disorders. Nat. Rev. Neurosci. 2012, 13, 293–307. [Google Scholar] [CrossRef]
- Bastian, T.W. Potential Mechanisms Driving Mitochondrial Motility Impairments in Developing Iron-Deficient Neurons. J. Exp. Neurosci. 2019, 13, 117906951985835. [Google Scholar] [CrossRef] [PubMed]
- Bastian, T.W.; Von Hohenberg, W.C.; Georgieff, M.K.; Lanier, L.M. Chronic Energy Depletion due to Iron Deficiency Impairs Dendritic Mitochondrial Motility during Hippocampal Neuron Development. J. Neurosci. 2019, 39, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Bastian, T.W.; Rao, R.; Tran, P.V.; Georgieff, M.K. The Effects of Early-Life Iron Deficiency on Brain Energy Metabolism. Neurosci. Insights 2020, 15, 263310552093510. [Google Scholar] [CrossRef] [PubMed]
- Glombik, K.; Stachowicz, A.; Slusarczyk, J.; Trojan, E.; Budziszewska, B.; Suski, M.; Kubera, M.; Lason, W.; Wedzony, K.; Olszanecki, R.; et al. Maternal stress predicts altered biogenesis and the profile of mitochondrial proteins in the frontal cortex and hippocampus of adult offspring rats. Psychoneuroendocrinology 2015, 60, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, L.; Chen, J.; Nomura, Y. Mitochondrial Gene Expression Profiles Are Associated with Maternal Psychological Stress in Pregnancy and Infant Temperament. PLoS ONE 2015, 10, e0138929. [Google Scholar] [CrossRef]
- Guo, S.; Dong, Y.; Cheng, X.; Chen, Z.; Ni, Y.; Zhao, R.; Ma, W. Chronic Psychological Stress Disrupts Iron Metabolism and Enhances Hepatic Mitochondrial Function in Mice. Biol. Trace Elem. Res. 2023, 201, 1761–1771. [Google Scholar] [CrossRef]
- Prendergast, A.J.; Humphrey, J.H. The stunting syndrome in developing countries. Paediatr. Int. Child Health 2014, 34, 250–265. [Google Scholar] [CrossRef]
- Belfort, M.B.; Ramel, S.E. NICU Diet, Physical Growth and Nutrient Accretion, and Preterm Infant Brain Development. Neoreviews 2019, 20, e385–e396. [Google Scholar] [CrossRef]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR Signaling in Growth and Metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef]
- Monk, C.; Georgieff, M.K.; Osterholm, E.A. Research review: Maternal prenatal distress and poor nutrition—Mutually influencing risk factors affecting infant neurocognitive development. J. Child Psychol. Psychiatry 2013, 54, 115–130. [Google Scholar] [CrossRef]
- Suchdev, P.S.; Boivin, M.J.; Forsyth, B.W.; Georgieff, M.K.; Guerrant, R.L.; Nelson, C.A. Assessment of Neurodevelopment, Nutrition, and Inflammation From Fetal Life to Adolescence in Low-Resource Settings. Pediatrics 2017, 139, S23–S37. [Google Scholar] [CrossRef]
- Lubach, G.R.; Coe, C.L. Preconception maternal iron status is a risk factor for iron deficiency in infant rhesus monkeys (Macaca mulatta). J. Nutr. 2006, 136, 2345–2349. [Google Scholar]
| Insufficient Iron during Gestation and in Infancy | Maternal Stress Exposure and HPA Activation in Pregnancy, Infancy, Toddlerhood |
|---|---|
| Abnormal acute brain function | |
| Poor recognition memory * [44,45] Slower speed of neural processing * [46] Poor bonding and maternal interaction * [47] Impacts on hippocampus [41,43,54]; metabolic [42] and dendritic structure changes *† [43] Altered brain metabolism, myelination [35,36,37,38,39], neurotransmitter function *† [40] | ↑ cortical thinning and decrements in cognitive functioning * [59] Alterations in synaptic signaling and epigenetics in the hippocampus and amygdala, linked to ↑ anxiety and depressive behaviors † [69,70,71,72,73,74,75] ↓ Dendritic arborization in PFC, hippocampus † [76,77] Changes in hippocampal synaptic plasticity, ↓ spatial memory learning † [68,78,79] |
| Acute and long-term neurobehavioral abnormalities | |
| Motor Dysfunction † [52] Socio-Affective † [52] Neurocognitive, including ↓ memory performance (3rd trimester ID) * [50,51] | Impaired learning * [55] Impaired attention regulation and EF † [64,65,66] ↑ Anxiety and depressive behaviors * [56] ↑ Emotional reactivity in preschool-age offspring * [80] |
| Long-term mental health abnormalities | |
| ↑ Risk of ASD (1st trimester ID) * [48] ↑ Risk of schizophrenia (2nd trimester ID) * [49] | ↑ Risk for ASD schizophrenia [61] ↑ Risk of anxiety, *† depression, *† ADHD * [53,62,63,81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reid, B.M.; Georgieff, M.K. The Interaction between Psychological Stress and Iron Status on Early-Life Neurodevelopmental Outcomes. Nutrients 2023, 15, 3798. https://doi.org/10.3390/nu15173798
Reid BM, Georgieff MK. The Interaction between Psychological Stress and Iron Status on Early-Life Neurodevelopmental Outcomes. Nutrients. 2023; 15(17):3798. https://doi.org/10.3390/nu15173798
Chicago/Turabian StyleReid, Brie M., and Michael K. Georgieff. 2023. "The Interaction between Psychological Stress and Iron Status on Early-Life Neurodevelopmental Outcomes" Nutrients 15, no. 17: 3798. https://doi.org/10.3390/nu15173798
APA StyleReid, B. M., & Georgieff, M. K. (2023). The Interaction between Psychological Stress and Iron Status on Early-Life Neurodevelopmental Outcomes. Nutrients, 15(17), 3798. https://doi.org/10.3390/nu15173798