Anti-Diabetic Potential of Polyphenol-Rich Fruits from the Maleae Tribe—A Review of In Vitro and In Vivo Animal and Human Trials
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. In Vitro Studies
| Species | Sample Type, Composition | Model, Study Design | Tested Parameters, Observed Effects * | Ref. |
|---|---|---|---|---|
| Amelanchier alnifolia Nutt. | crude 80% EtOH extract, water fraction, and EtOAc fraction; detected compounds (HPLC-MS): phenolic acids (chlorogenic, caffeic, hydroxybenzoic acids), anthocyanins (cyanidin monoglycosides, cyanidin 3,5-diglucoside), proanthocyanidins (oligomers, catechin/epicatechin, epicatechin gallate) | cellular studies (L6rat skeletal muscle cells) and in vitro enzyme inhibition; control: 3,3-tetramethylene-glutaric acid (ALR activity), cells without an extract (glycogen accumulation) | ALR inhibition: strong activity (82 ± 0.73% inhibition at 5µg/mL) for EtOAc, other extracts not active; glycogen accumulation in non-insulin-stimulated cells (glucose uptake): 76%, 92%, and 23% changes for 80% EtOH extract, water, and EtOAc fractions, respectively, compared to controls; phenolic acids, anthocyanins, and proanthocyanidins responsible for hypoglycaemic activity (based on literature studies and extracts’ composition) | [40] |
| 70% acetone extract of whole fruits, flesh, and peels from different cultivars; polyphenols 1.11–2.27 g GAE/100 g (fruits), 0.45–0.96 (flesh), 1.11–2.86 (peel) (spectrophotometry), the contents of monophosphate nucleotides and free amino acids were also determined (LC-MS) | in vitro enzyme inhibition; control: - | α-amylase inhibition: IC50 = 18.33–31.70 mg/mL (fruits), 22.53–42.15 (flesh), 11.05–18.41 (peels); α-glucosidase inhibition: IC50 = 27.83–42.23 mg/mL (fruits), 28.86–43.79 (flesh), 23.60–37.06 (peels); free amino acids responsible for observed effects (PCA analysis) | [55] | |
| Amelanchier Medik. sp. | 70% acetone extract, polyphenols 13.8–15.2 mg/g of dry fruits (depending on clone), including hydroxycinnamic acids, anthocyanins, flavonols, and hydroxybenzoic acids (HPLC) | in vitro enzyme inhibition and cellular studies (on βTC3 pancreatic β-cells); control: sodium orthovanadate (for PTP1B) | α-amylase inhibition: IC50 = 4.3–5.3 mg/mL depending on clone (better than, i.a., mulberry fruits); α-glucosidase inhibition: IC50 = 116.7–134.3 mg/mL depending on clone (very low activity); PTP1B inhibition: IC50 = 1.2 mg/mL (better than, i.a., mulberry fruits); cytoprotection of βTC3 pancreatic β-cells: very low effect on cell viability and no effect on cell proliferation | [36] |
| Aronia melanocarpa (Michx.) Elliott | acidified EtOH extract; anthocyanins 128.36 μg/mL (HPLC-MS: cyanidin monoglycosides) | cellular studies (HepG2 human hepatoma cell line and C2C12 mouse myoblast cell line with palmitic acid induced insulin resistance (tested) or normal cells (control) | glucose uptake: ↑, optimal extract concentration 40 μg/mL; glycogen level: ↑; protein expression: ↑ GLUT-4, ↑ IRS-1, ↑ p-GSK-3β, ↓ SOCS3, ↓ p-IRS-1, ↓ GSK-3β compared to insulin-resistant cells (effects on glucose transport and insulin sensitivity); anthocyanins responsible for observed effects (based on literature studies and extracts’ composition) | [28] |
| standardised Aronia berry extract powder (Fort Wayne, IN, USA); polyphenols 40%, anthocyanins 15% | cellular studies (RAW 264.7 and mouse bone-marrow-derived macrophages (BMDMs)); normal or LPS-stimulated cells (control), LPS +50, 100 mg/mL extract (tested) | mRNA expression levels: ↓ GLUT-1, HK1, and G6PD in comparison to LPS-stimulated controls (to the level comparable to normal controls); anthocyanins responsible for observed effects (based on literature studies and extract composition) | [35] | |
| EtOH and 50% EtOH extracts; detected compounds (HPLC-MS): cyanidin monoglycosides; epicatechin; procyanidins B2, B5, and C1 | in vitro enzyme inhibition; control: acarbose IC50 = 130 ± 20 μg/mL | α-glucosidase inhibition: EtOH inactive; 50% EtOH IC50 = 3.5 ± 0.1 μg/mL; cyanidin glycosides IC50 = 0.37–5.5 μg/mL (the highest activity for cyanidin arabinoside); procyanidins IC50 = 3.8–5.5 μg/mL (the highest activity for procyanidin C1) | [12] | |
| 80% EtOH and acidified MeOH extracts; polyphenols 98–148 mg GAE/g of EtOH extract (1079–1921 mg GAE/100 g of fresh fruits), anthocyanins 249–447 mg/100 g fruits, proanthocyanidins 2.46–3.74 mg PB2E/100 g fruits (HPLC/spectrophotometry) | in vitro enzyme inhibition; control: acarbose IC50 = 130 ± 20 μg/mL | α-glucosidase inhibition: 80% EtOH IC50 = 0.70–0.88 μg/mL; acidified MeOH IC50 = 0.030–0.049 μg/mL (depending on the cultivars); anthocyanins responsible for observed effects (based on previous studies and extract composition) | [13] | |
| lyophilised wine samples prepared with or without additional sugar and enzyme; detected compounds (HPLC-MS): catechin; epicatechin; protocatechuic, gallic, chlorogenic, caffeic, p-coumaric, and ellagic acids | in vitro enzyme inhibition; control: acarbose IC50 = 77.8 ± 5.7 μg/mL | α-glucosidase inhibition: IC50 = 49–50 μg/mL (without additional sugar); IC50 = 28–30 μg/mL (with additional sugar); chlorogenic and caffeic acids’ contributions to the IC50 values: 16.4–19.5% and 4.78–6.15%, respectively (based on inhibitory curves of pure compounds and statistical analysis) | [14] | |
| MeOH and water dry extracts | in vitro enzyme inhibition; control: acarbose IC50 = 1.31 ± 0.12 μg/mL | α-amylase inhibition: MeOH (2.5 mg of extract/mL) inhibition = 58.6 ± 1.9%; water extract (2.5 mg of extract/mL) inhibition = 49.7 ± 3.8% | [56] | |
| 60% EtOH and water extracts | in vitro enzyme inhibition; control: acarbose IC50 = 2.4 ± 0.4 μg/mL | α-amylase inhibition: water extract IC50 = 2632 ± 208.5 μg/mL, 60% EtOH IC50 = 1130 ± 91.19 μg/mL | [57] | |
| water, MeOH, and acetic acid extracts; composition (mg/100 mg) of acetic acid extract (HPLC-MS): neochlorogenic acid 4.22 mg, chlorogenic acid 1.26 mg, cyanidin monoglycosides 8.94 mg | in vitro enzyme inhibition; control: - | α-amylase inhibition: MeOH IC50 = 10.31 ± 0.04 mg/mL; water 13.55 ± 0.04 mg/mL; acetic acid 14.85 ± 0.06 mg/mL; the activity of pure compounds: the strongest for chlorogenic acid (IC50 = 0.57 ± 0.16 mg/mL) and cyanidin-3-glucoside (IC50 = 1.74 ± 0.04 mg/mL) | [58] | |
| Aronia prunifolia (Marshall) Rehder | 80% EtOH and acidified MeOH extracts; polyphenols 175 mg GAE/g of EtOH extract (2996 mg GAE/100 g of fresh fruits), anthocyanins 737 mg/100 g fruits, proanthocyanidins 4.79 mg PB2E/100 g fruits (HPLC/spectrophotometry) | in vitro enzyme inhibition; control: acarbose IC50 = 130 ± 20 μg/mL | α-glucosidase inhibition: 80% EtOH IC50 = 0.88 ± 0.08 μg/mL; acidified MeOH IC50 = 0.030 ± 0.005 μg/mL; anthocyanins responsible for observed effects (based on previous studies and extract composition) | [13] |
| Chaenomeles japonica (Thunb.) Lindl. ex Spach | 60% EtOH and water extracts before in vitro digestion, water extract after digestion; detected compounds (HPLC-MS): epicatechin, procyanidin B2, procyanidin oligomers, quercetin-O-hexoside, epigallocatechin-3-gallate, catechin/epicatechin gallate | in vitro enzyme inhibition; control: acarbose IC50 = 2.4 ± 0.4 μg/mL | α-amylase inhibition (before digestion): water extract IC50 = 53.61 ± 5.074 μg/mL, 60% EtOH IC50 = 48.69 ± 4.993 μg/mL; α-amylase inhibition (after digestion): 55.41–58.48% inhibition at 50 μg/mL after gastric condition and about 50% after intestinal condition; catechin/epicatechin derivatives responsible for observed effects (based on literature studies and extract composition) | [57] |
| 70% acetone extract; detected compounds (HPLC): epigallocatechin (23.17 mg/g), (+) catechin, procyanidin B1, procyanidin C1, (−) epicatechin, epigallocatechin gallate, epicatechin gallate, hydroxybenzoic acids, hydroxycinnamic acids, and flavonols | in vitro enzyme inhibition and cellular studies (on βTC3 pancreatic β-cells); control: sodium orthovanadate (for PTP1B) | α-amylase inhibition: IC50 = 1.68–2.41 mg/mL depending on assay (better than, i.a., mulberry fruits); α-glucosidase inhibition: unable to detect; PTP1B inhibition: IC50 = 1.22 mg/mL (better than, i.a., mulberry fruits); cytoprotection of βTC3 pancreatic β-cells: highly positive effects on cell viability and proliferation; flavan-3-ols responsible for observed effects (based on literature studies and extract composition) | [36] | |
| 70% acetone extract; polyphenols 489.85 mg/g of dry preparation, including (+)-catechin, (−)-epicatechin, epigallocatechingallate, procyanidins B1 and C1, hydroxybenzoic acids, and flavonols (HPLC) | cellular studies on the human hepatoma cell line HepG2; control: - | ↑ level of p-AMPK compered to cells under normal or hyperglycaemic conditions; genes’ expression (5 mg/mL extract vs. cells under normal conditions): ↑ GLUT-4, IRS-2; ↓ PEPCK, PGC-1α, FOXO1, PTP1B; unchanged GLUT-2, G6Pase, GYS2, IRS-1, GSK-3α; genes’ expression (hyperglycaemic conditions): ↑ GLUT-2; ↓ PEPCK; unchanged GLUT-4, G6Pase, GYS2, IRS-1, IRS-2, GSK-3α, PTP1B, PGC-1α, FOXO1; glycogen content: ↑ at 5 mg/mL to a higher level than with metformin (at 5 mM); glucose production: ↑ at 5 mg/mL (weaker activity then metformin); glucose uptake: ↑ at 5 mg/mL to a higher level than with metformin (at 5 mM) | [29] | |
| Chaenomeles speciosa (Sweet) Nakai | 20, 40, 60, 80, and 100% MeOH; water; 20, 40, 60, 80, and 100% EtOH extracts; and crude polysaccharide fraction; polyphenols 2–318 mg GAE/g (HPLC: catechin; epicatechin; chlorogenic, gallic, caffeic, protocatechuic, p-coumaric, syringic, and vanillic acids), triterpenes 23–62 mg OAE/g (oleanolic and ursolic acids), polysaccharides 8–64 mg glucose/g | in vitro enzyme inhibition; control: - | α-glucosidase-inhibitory activity: IC50 about 0.2–6.2 mg/mL, the highest activity for 60% EtOH, 20% EtOH, and 60% MeOH extracts; α-glucosidase-inhibitory activity (pure compounds detected in the samples: about 10–100% inhibition at 0.5 mg/mL, the highest activity for the polysaccharide fraction and oleanolic acid); polysaccharides, oleanolic acid, and different polyphenols responsible for observed effects (based on activity studies of pure compounds and PCA analysis) | [59] |
| water extract before and after gastric and/or intestinal digestion; polyphenols 7.87–12.7 mg GAE/g (HPLC: phenolic acids, catechin, epicatechin), triterpenes 39–48 mg OAE/g (ursolic and oleanolic acids), anthocyanins 0.6–1.6 mg CyE/g | in vitro enzyme inhibition and influence on starch digestion; control: - | α-glucosidase inhibition: IC50 about 0.35–0.7 mg/mL, with the lowest activity after intestinal digestion; effects on the glucose release during in vitro digestion (from corn starch): 98% inhibition, higher then observed for pure native compounds (25–65% inhibition) | [60] | |
| 80% EtOH extracts (different origin of fruits); polyphenols about 19–29 mg GAE/g of plant material, flavonoids about 22–47 mg QE/g, polysaccharides about 20–26 mg glucose/g | in vitro enzyme inhibition; control: quercetin | α-glucosidase inhibition: IC50 about 60–210 mg QE/g of plant material (depending on fruit origin); flavonoids and polysaccharides responsible for observed activity (based on correlation studies) | [61] | |
| Chaenomeles speciosa (Sweet) Nakai, Chaenomeles sinensis (Thouin) Koehne | 60% MeOH extracts from peels, flesh, and endocarps of two species (C. speciosa, CSP; C. sinensis, CSS); polyphenols 356–405 (CSP) and 345–590 (CSS) mg GAE/g, triterpenes 29–43 (CSP) and 16–40 (CSS) mg OAE/g, detected compounds (HPLC-MS): five phenolic acids, two triterpenes, and three flavonoids | in vitro enzyme inhibition; control: - | α-glucosidase inhibition (C. speciosa): IC50 = 1.32–2.66 mg/mL, with the highest activity for the extract from flesh; α-glucosidase inhibition (C. sinensis): IC50 = 0.44–2.28 mg/mL, with the highest activity for the extract from the peel; α-glucosidase inhibition ratio after in vitro digestion: ↓ about 2–20-fold depending on the sample ferulic acid and triterpenes responsible for observed effects (based on correlation studies) | [62] |
| 60% MeOH extracts from peels and flesh of C. speciosa (CSP, 12 varieties) and C. sinensis (CSS, 1 variety); polyphenols about 100–270 (CSP, flesh), 170–360 (CSP, peel), 200 (CSS, flesh), and 350 mg GAE/g (CSS, peel); detected compounds (HPLC-MS): catechin; epicatechin; rutin; hyperoside; myricetin; quercetin; kaempferol; chlorogenic, gallic, caffeic, protocatechuic, syringic, oleanolic, and ursolic acids | in vitro enzyme inhibition; control: - | α-glucosidase inhibition (C. speciosa): IC50 about 0.06–0.35 mg/mL (peel extracts) and about 0.04–0.42 mg/mL (flesh extracts), with the highest activity for the extracts from flesh or peel, depending on the variety; α-glucosidase inhibition (C. sinensis): IC50 about 0.05 mg/mL (peel extract) and about 0.07 mg/mL (flesh extract); α-glucosidase inhibition of pure compounds detected in the samples: IC50 about 0.05–1.8 mg/mL (with the highest activity for hyperoside, quercetin, mirycetin, catechin, epicatechin, protocatechuic acid, chlorogenic acid, and oleanolic acid) | [63] | |
| Chaenomeles japonica (Thunb.) Lindl. ex Spach, Chaenomeles speciosa (Sweet) Nakai, Chaenomeles × superba (Frahm) Rehder. | 80% MeOH (1% HCl) extracts of C. japonica (5 cultivars), C. speciosa (3 cultivars), and C. superba (11 cultivars); polyphenols 56–170 mg/g, (HPLC-MS: (+)-catechin; (−)-epicatechin; procyanidins B2, B3, and C1, procyanidin oligomers and polymers; chlorogenic acids); contents of pectins, sugars, and organic acids also determined | in vitro enzyme inhibition; control: - | α-amylase inhibition: 16.11–17.45 mg/mL (C. japonica), 16.88–18.48 mg/mL (C. speciosa), 13.88–18.25 mg/mL (C. superba); α-glucosidase inhibition: 6.09–15.19 mg/mL (C. japonica), 5.74–12.48 mg/mL (C. speciosa), 2.67–8.54 mg/mL (C. superba); sugars, L-ascorbic acid, and flavan-3-ols responsible for α-amylase- and α-glucosidase-inhibitory activity (based on hierarchical clustering analysis) | [64] |
| Cotoneaster bullatus Bois, Cotoneaster zabelii C.K.Schneid., Cotoneaster integerrimus Medik. | 70% MeOH extract; polyphenols 62.13–81.26 mg GAE/g (HPLC: chlorogenic acids, (−)-epicatechin, procyanidins B2 and C1, quercetin 3-(2”-xylosyl)galactoside, rutin, hyperoside, isoquercitrin, quercitrin) | in vitro enzyme inhibition and effects on AGE formation; Control: acarbose (IC50 = 169.52 and 5.78 µg/mL for α-glucosidase and α-amylase, respectively), aminoguanidin (IC50 = 71.09 µg/mL for AGEs) | α-glucosidase inhibition: IC50 = 48.89, 57.73, 80.12 µg/mL for C. integerrimus, C. zabelii, and C. bullatus (IC50 = 232–416 µg/mL for (−)-epicatechin, hyperoside, quercetin 3-(2”-xylosyl)galactoside, and procyanidin B2); α-amylase inhibition: IC50 = 941–1083 µg/mL; inhibition of AGE formation: IC50 = 106.36, 118.94, 166.62 µg/mL for C. integerrimus, C. zabelii, and C. bullatus (IC50 = 2.20–15.78 µg/mL for procyanidin B2, (−)-epicatechin, hyperoside, and quercetin 3-(2”-xylosyl)galactoside) | [15] |
| Crataegus azarolus var. aronia L. | water extract | in vitro enzymatic starch digestion and glucose movement; control: acarbose 0.1 mg/mL (97.6% reduction in starch digestion); guar gum 50 mg/mL (glucose movement assay, iAUC ↓ by 30.8%) | enzymatic starch digestion: dose-dependent ↓ in glucose level, significant effect at 0.5–10 mg/mL (22.0–70.7% reduction, IC50 = 3.5 mg/mL); glucose movement (postprandial glucose level in vitro): lack of effects | [65] |
| Crataegus laevigata (Poir.) DC. | MeOH and water extracts | in vitro enzyme inhibition; control: acarbose IC50 = 1.31 ± 0.12 μg/mL | α-amylase inhibition: MeOH (2.5 mg of extract/mL), 35.4 ± 4.7% inhibition; water extract (2.5 mg of extract/mL), 41.1 ± 15.3% inhibition | [56] |
| 80% EtOH extract and fractions (EtOAc, n-butanol); polyphenols 136.54–697.23 mg GAE/g, flavonoids 21.46–154.51 mg QE/g, flavonols 19.21–68.83 mg QE/g | in vitro enzyme inhibition; control: acarbose IC50 = 275.43 ± 1.59 μg/mL | α-glucosidase inhibition: IC50 = 16.12–30.80 μg/mL, with the highest activity for the n-butanol fraction; caffeic acid, epicatechin, naringenin and quercetin responsible for observed effects (based on literature studies and extract composition) | [16] | |
| Crataegus microphylla K. Koch | EtOH, acidified (0.5% HCl) EtOH, 50% EtOH, MeOH, acidified MeOH, 50% MeOH, water, acidified water extracts; polyphenols 5.00–57.28 mg GAE/g | in vitro enzyme inhibition; control: acarbose IC50 = 31.92 ± 0.08 μg/mL | α-glucosidase inhibition: IC50 = 250.94–731.81 μg/mL, with the highest activity for the acidified MeOH extract | [66] |
| Crataegus pinnatifida Bunge | MeOH extract and fractions (methylene chloride, EtOAc, n-butanol, water fractions); isolated compounds: hyperoside, chlorogenic acid, 3-epicorosolic acid, ursolic acid, oleanolic acid, β-sitosterol, β-sitosterol glucoside | in vitro enzyme inhibition and effects on the formation of AGEs; control: acarbose IC50 = 81.65 ± 4.07 μg/mL (α-glucosidase), ursolic acid IC50 = 1.00 ± 0.09 μg/mL (PTP1B), quercetin IC50 = 0.75 ± 0.07 μg/mL (ALR), aminoguanidine IC50 = 127.06 ± 7.10 μg/mL (AGEs) | α-glucosidase inhibition: IC50 = 22.70–122.11 μg/mL; PTP1B inhibition: IC50 = 1.41–18.75 μg/mL; Rat lens ALR inhibition: IC50 = 9.09–160.54 μg/mL; inhibition of AGEs formation: IC50 = 65.83–88.90 μg/mL; the highest activity observed for EtOAc (α-glucosidase, PTP1B, ALR inhibition) or MeOH extract (AGE formation); inhibitory potential of 3-epicorosolic acid IC50 = 4.08 and 30.18 μg/mL (PTP1B and α-glucosidase tests); different compounds responsible for observed effects (based on extract composition and literature studies) | [17] |
| MeOH extract; polyphenols 101.56 mg tannic acid equivalents/g, flavonoids 44.52 mg CE/g | in vitro enzyme inhibition; control: acarbose IC50 = 2.15 ± 0.86 μg/mL | α-glucosidase inhibition: IC50 = 766.22 ± 8.14 μg/mL | [67] | |
| water extract before and after gastric and/or intestinal digestion; polyphenols 3.07–11.7 mg GAE/g (HPLC: five phenolic acids, catechin, epicatechin), triterpenes 28.4–47.4 mg OAE/g (ursolic and oleanolic acids), anthocyanins 0.77–1.8 mg CyE/g | in vitro enzyme inhibition and influence on starch digestion; control: - | α-glucosidase inhibition: IC50 about 0.3–0.75 mg/mL, with the lowest activity after intestinal digestion; effects on glucose release during in vitro digestion (from corn starch): 83% inhibition, higher then observed for pure native compounds (25–65% inhibition) | [60] | |
| MeOH, EtOH, EtOAc, acetone, dichloromethane, chloroform, n-hexane extracts; polyphenols 12.1–63.5 mg GAE/g (HPLC: chlorogenic acid, hyperoside, epicatechin, procyanidin B2) | in vitro enzyme inhibition and molecular docking studies; control: acarbose IC50 = 317.80 ± 16.36 μg/mL | α-glucosidase inhibition: IC50 = 42.35–207.46 μg/mL, with the highest activity for acetone, MeOH, and EtOH extracts (IC50 = 42.35–58.69 μg/mL); activity of isolated compounds: IC50 = 34.98–170.37 μg/mL, with the highest activity for hyperoside (also high affinity for enzyme/molecular docking studies) | [68] | |
| Cydonia oblonga Mill. | 70% EtOH macerate | in vitro enzyme inhibition; control: acarbose IC50 = 275.98 ± 1.57 μg/mL | α-glucosidase inhibition: IC50 = 326.48 ± 18.56 μg/mL | [69] |
| 70% EtOH extract from pulp fruit callus; polyphenols 10.98 mg/100 g (pulp) and 91.58 mg/100 g (callus), mainly chlorogenic acid, 5-p-coumaroylquinic acid, neochlorogenic acid, and (−)-epicatechin (LC-MS, GC-MS) | in vitro enzyme inhibition; control: acarbose about 75% and 50% inhibition at 32 μg (α-amylase and α-glucosidase, respectively) | α-amylase inhibition: about 25% inhibition at 250–1000 μg; α-glucosidase inhibition: about 40% inhibition at 250 μg and about 75% inhibition at 500 μg | [70] | |
| Cydonia oblonga Mill.; Malus domestica (Suckow) Borkh. | fruit puree; flavan-3-ols 355.3 and 34.1 mg/kg (Cydonia and Malus, respectively), flavonols 1269 and 245.1 mg/kg, hydroxycinnamic acid derivatives 306.6 and 140.6 mg/kg, anthocyanins 62.2 and 67.6 mg/kg (HPLC) | in vitro enzyme inhibition; control: acarbose | α-amylase inhibition: IC50 = 164 and 161 mg/mL (Cydonia and Malus, respectively); α-glucosidase inhibition: IC50 = 177 and 166 mg/mL (Cydonia and Malus, respectively) | [71] |
| Malus domestica (Suckow) Borkh. | 80% EtOH extract; 15 phenolics detected (HPLC), with phlorizin and chlorogenic acid as the dominant compounds | cellular studies on the human hepatoma cell line HepG2 with insulin resistance induced by high glucose levels (tested) or normal cells (control) | glucose uptake and glycogen content: ↑ in comparison to insulin-resistant cells (phlorizin and chlorogenic acid activity: comparable); protein levels: ↑ p-IRS2/IRS2, ↑ p-AKT/AKT, ↑ p-GSK3β/GSK3β, ↑ p-FOXO1/FOXO1, p-IRS1/IRS1 unchanged (insulin-resistant cells); phlorizin activity (10 μg/mL): comparable; chlorogenic acid: not active | [37] |
| Phytonutriance® polyphenolic extract Appl’In™; polyphenols min. 80%, phlorizin > 5% | cellular studies on Ishikawa Var 1 endometrial cells; control: genistein; about 30% inhibition at 2.7 μg/mL | glucose uptake: > 60% inhibition at 50 μg/mL (phlorizin equivalent 2.5 μg/mL); phlorizin responsible for observed effects (based on literature studies and extract composition) | [72] | |
| standardised commercial extract (Appl’In by DIANA Food SAS); polyphenols 67%, including 40% flavonoid monomers and phenolic acids (HPLC: flavan-3-ols, dihydrochalcones, flavonols, hydroxycinnamic acids) | cellular studies on Caco-2 cells and Xenopus oocytes injected to express SGLT1; control: cells without extract | total and sodium-independent (GLUT-mediated) glucose uptake (Caco-2 cells): 51% and 46% ↓ at 0.3 mg polyphenols/mL (corresponding to the physiological dose that may be reached after consumption of 600 mg of polyphenols (900 mg of apple extract) in the human study); glucose uptake in oocytes (SGLT1 mediate): ↓ at 0.125–4.0 mg apple polyphenols/mL (dose-independent), for phloretin and phlorizin 59% and 85% ↓ at 0.5 mM, respectively; different compounds responsible for observed effects (based on literature studies and extract composition) | [32] | |
| standardised commercial extract (Appl’In, Diana Naturals, France); polyphenols min. 80%, phlorizin > 5% | cellular studies on Caco-2 cells; control: dapagliflozin IC50 for SGLT1 inhibition ~0.5 µM = 0.2 mg/L | glucose uptake: 90% ↓ of SGLT1 at 0.2 mg/mL; ↓ GLUT-2 IC50 = 0.45 mg/mL (in comparison: phlorizin IC50 for SGLT1 = 0.13 mg/L, phloretin IC50 for GLUT-2 = 22 mg/L) | [34] | |
| standardised commercial extract (Appl’In, Diana Naturals, France); polyphenols min. 80%, phlorizin > 5%; 14 compounds detected (HPLC), including quercetin glycosides, dihydrochalcones, phenolic acids, and procyanidin oligomers | cellular studies on Caco-2 cells (TC7 subclone); control: cells without extract | glucose uptake: ↓ at 1.12 µg/mL (IC50 = 1.19 ± 0.35 mg/mL.); different compounds responsible for observed effects (based on literature studies and extract composition) | [73] | |
| 80% MeOH extract (+0.1% formic acid); polyphenols 3.61 mg/g of fresh fruits, including flavanols, flavones, flavonols, dihydrochalcones, hydroxycinnamic acids, and anthocyanins (18 compounds, HPLC) | cellular studies on Caco-2 cells; control: cells with high glucose (HG) content and normal cells (NC) | formation of AGEs: ↓ compared to HG, the level at 0.8 mmol/L comparable to NC; glycolaldehyde-modified proteins: ↓ compared to HG, the levels at 0.4 and 0.8 mmol/L were comparable to NC; glyoxalase I activity: ↑ in the HG cells; at similar levels in the NC and tested cells (0.4–0.8 mmol/L extract); glyoxalase II activity: unchanged | [74] | |
| MeOH extract from apple juice; 10 phenolics detected (chlorogenic acid 0.33 mM, procyanidins dimer B type 0.05 mM, (−)-epicatechin 0.06 mM, p-coumaroylquinic acid 0.085 mM, phloretin glycosides 0.09 mM, quercetin and kaempferol glycosides 0.09 mM) (HPLC-MS) | cellular studies on Caco-2 cells; control: cells without extract or phenolics | glucose transport: ↓, with greater inhibition under sodium-free conditions (apical GLUT-2, IC50 = 136 mg) than under sodium-dependent conditions (SGLT1 and GLUT-2, IC50 = 300 mg); contribution of phenolics to observed effect: quercetin-3-O-rhamnoside 26% (IC50 = 31 μM), phlorizin 52% (IC50 = 146 μM), chlorogenic acid 12% (IC50 = 2570 μM) | [33] | |
| commercial extract (BioActive Food GmbH); polyphenols 44% (catechin equivalents), phlorizin 16%, quercetin 12.43%, chlorogenic acid 5.57% (HPLC/spectrophotometry) | cellular studies on Xenopus oocytes injected to express SGLT1; control: cells without extract | glucose uptake: ↓, extract IC50 = 2.0 ± 0.24 µg/mL; phlorizin IC50 = 0.46 ± 0.19 µM, quercetin IC50 = 0.62 ± 0.05 mM, chlorogenic acid not active | [75] | |
| juice: raw, after thermal or ultrasound pasteurisation and before or after in vitro digestion; polyphenols 5.33 mg/L (chlorogenic and p-coumaroylquinic acids, phlorizin and phloretin xyloqlucosides, epigallocatechin gallate, quercetin monoglycosides; HPLC-MS) | in vitro enzyme inhibition; control: acarbose IC50 = 0.09 μg/mL | α-glucosidase inhibition: IC50 = 6.24 mg/mL (raw sample), 0.92 mg/mL (sample after digestion); ↓ after thermal pasteurisation (not digested sample, 18.41 mg/mL) or ↑ (thermal pasteurisation, digested sample, 0.44 mg/mL); effects with acarbose: synergy at combinatory concentration of 2 mg/mL (i.e., up to 40% inhibition), antagonism at 2–9 mg/mL, and additive activity at >9 mg/mL; different compounds responsible for observed effects (based on literature studies and extract composition) | [25,76] | |
| DMSO extract; 5% quercetin, 30% phlorizin | in vitro receptor binding; control: rosiglitasone IC50 = 0.043 ± 0.004 µg/mL (binding affinity test) | binding affinity to PPARγ receptor: moderate (IC50 = 0.49 µg/mL); effects on rosiglitazone-mediated DRIP205/TRAP220 coactivator: strong antagonism (IC50 = 0.15 ± 0.03 µg/mL); phlorizin, phloretin, epicatechin and catechin responsible for observed effects (based on literature studies and apple composition) | [38] | |
| water and 12% EtOH extracts from peel and pulp, with or without different elicitor treatments; polyphenols 0.35–1.44 mg GAE/g of fresh fruits for peel, 0.05–0.32 mg GAE/g for pulp; detected compounds (HPLC): chlorogenic acid, p-coumaric acid, gallic acid, quercetin | in vitro enzyme inhibition; control: - | α-glucosidase inhibition: higher activity found for extracts from peel, as well as 12% EtOH extracts; the activity of extracts from peel (without elicitors) ↓ after fruit storage, and the activity of extracts from pulp ↑ and then ↓ depending on the storage time (0–3 months); α-amylase inhibition: higher activity found for extracts from pulp, as well as water extracts; the activity of extracts from peel (without elicitors) ↑ after storage, while the activity of extracts from pulp was similar after storage; chlorogenic acid and quercetin responsible for observed effects (based on literature studies and extract composition) | [77] | |
| 12% EtOH and water extracts from peel and pulp of different cultivars; polyphenols 266–781 μg GAE/g fresh material for peel extracts and 30–143 μg GAE/g for pulp extracts; detected compounds (HPLC): catechin, chlorogenic, and p-coumaric acids; quercetin | in vitro enzyme inhibition; control: - | α-amylase inhibition: the highest activity for pulp (about 0–60% at 100 μL) or peel water extracts (about 0–55%); α-glucosidase inhibition: higher activity for pulp water extract (69–83% at 50 μL) than pulp 12% EtOH extract (50–70% at 50 μL); higher or similar activity for peel 12% EtOH extract (54–98% at 50 μL) than peel water extract (52–93% at 50 μL); polyphenols responsible for observed effects (based on correlation studies) | [78] | |
| acetone–ethanol extract (1:3), purified with 95% MeOH (polyphenols 390.8 μg CE/mg,) and fractionated with acetonitrile (II: polyphenols 61.3 μg CE/mg), EtOAc (III: polyphenols 459.3 μg CE/mg), MeOH (IV: polyphenols 620.6 μg CE/mg), and fraction I residue; 14 compounds detected (HPLC-MS) | in vitro enzyme inhibition; control: acarbose IC50 = 840 ± 100 μg/mL, quercetin 661 ± 7 μg/mL | α-glucosidase inhibition: IC50 = 19–67 μg/mL (purified 95% MeOH extract and fractions III-IV); chlorogenic acid isomers, flavan-3-ols monomers and oligomers, quercetin and phloretin glycosides responsible for observed effects (based on literature studies and extract composition) | [18] | |
| 70% EtOH extract; polyphenols 534.39 mg GAE/g (HPLC: phlorizin; (−)-epigallocatechin; (−)-epicatechin; (+)-catechin; procyanidins B1 and B2; quercetin glycosides; chlorogenic, p-coumaroylquinic, and caffeic acids | in vitro enzyme inhibition and molecular docking studies; control: acarbose IC50 = 0.76 μg/mL | α-glucosidase inhibition: IC50 = 15 μg/mL; phlorizin, (−)-epicatechin, and tannins responsible for observed effects (based on activity study of pure compounds); inhibition mechanism: competitive or mixed type, potential conformational change of enzyme was suggested | [79] | |
| apple extract | effects on the formation of AGEs in plasma in vitro; control: - | concentration-dependent (at 100–2000 mmol) inhibition of AGE formation (up to fourfold ↓), depending on the glucose concentration (5.5–50 mmol) and time of study | [80] | |
| water extract from the juice; | in vitro enzyme inhibition; control: quercetin IC50 = 153.85 ± 5.38 μg/mL (ALR), 67.5 ± 0.8 μg/mL (SDH) | ALR inhibition: IC50 = 171.63 ± 5.42 μg/mL; SDH inhibition: IC50 = 56.52 ± 4.95 μg/mL | [41] | |
| juice (fermented and unfermented); compounds: 19 polyphenols (HPLC: mainly chlorogenic acid at about 95–145 mg/L, epicatechin 70–105 mg/L, procyanidin B2 70–95 mg/L), sugars, and organic acids | in vitro enzyme inhibition; control: acarbose, with about 60–80% inhibition at 0.025–0.1 mg/mL | α-glucosidase inhibition: about 43% inhibition at 0.1 mL/mL (fermented and unfermented samples); at 0.5 mL/mL, about 67% (unfermented) and 78% (fermented) | [81,82] | |
| Malus pumila Mill. | 80% MeOH extracts (0.5% formic acid) from the peel and flesh of different cultivars; polyphenols 25.12–281.73 mg/100 g, phlorizin 1.10–68.54 mg/100 g (peel > flesh), procyanidins 6.03–78.76 mg/100 g (flesh > peel) (HPLC) | cellular studies on HepG2 human hepatocellular liver carcinoma cells; control: cells without extracts | glucose uptake: ↑, with the highest activity for peel extract from the Red Delicious cultivar, with the highest total polyphenols and phlorizin content; phlorizin responsible for observed effect (based on correlation and literature studies) | [83] |
| commercial extract (Exxentia®); polyphenols 57.5%, including phlorizin (9.9%), chlorogenic acid (15.8%), and quercetin (0.4%) | cellular studies on L6.C11 rat skeletal muscle myoblast cells (insulin sensitivity mechanisms) and ex vivo α-glucosidase inhibition on isolated rat intestinal mucosa; control: cells not treated with extract or rosiglitazone (Rosi, 10 μmol/L) | glucose uptake: maximal ↑ by 63.6%, EC50 = 4.2 ± 0.7 μg/mL; insulin-stimulated glucose uptake: increasing synergistic effects at 5–25 μg/mL of extract and 50 nM of insulin; GLUT-4 levels: not changed in total membranes, ↑ in a plasma membrane fraction (GLUT-4 translocation, at 25 μg/mL); ↑ p-Akt/Akt by about 50% (unchanged for Rosi); PPARγ level and PPARγ-mediated transcription ↑ (comparable to Rosi), ERK1/2 not affected (for extract at 25 μg/mL); α-glucosidase inhibition: IC50 12.54–18.05 μg/mL (three models) | [30] | |
| Malus Mill. sp. | fruit water extract | in vitro enzyme inhibition; control: acarbose | α-glucosidase inhibition: 8 mg acarbose equivalents/g | [84] |
| apple juice; polyphenols 0.78 mg GAE/mL | effects on AGE formation in vitro control: - | weak inhibition (about 5%) at 10 µL of juice/mL compared to pomegranate juice (about 95% inhibition) | [85] | |
| MeOH extract from red or yellow apples; polyphenols about 4 mg GAE/g dry fruits | in vitro enzyme inhibition; control: - | α-amylase inhibition: not active; α-glucosidase inhibition: 49.5% (yellow apple) or 95.4% (red apple) inhibition at 10 mg/mL concentration; | [86] | |
| Malus Mill. sp. (276 Malus species/cultivars including Malus sieversii and Malus domestica) | 80% MeOH extract + 0.1% HCl + sonification; polyphenols 0.49–2.61 mg/g fresh fruits (HPLC-MS: phenolic acids, flavan-3-ols, proanthocyanidins, flavonols, dihydrochalcones) | in vitro enzyme inhibition and effects on AGE formation; control: acarbose (IC50 = 0.21 mg/mL and 0.58 µg/mL for α-glucosidase and α-amylase), sitagliptin (IC50 = 0.044 µg/mL for DPP IV), aminoguanidin (IC50 = 24 µg/mL for AGEs) | α-glucosidase inhibition: IC50 = 7.1–256 mg/mL (0.01 mg/mL for phlorizin, 0.028–0.073 mg/mL for chlorogenic acid, epicatechin, procyanidin B2, and quercetin 3-O-galactoside); α-amylase inhibition: IC50 = 5.3–21.5 mg/mL for 10 cultivars with the highest total polyphenols (485 and 749 µg/mL for quercetin 3-O-galactoside and epicatechin); DPP IV inhibition: 10.3 mg/mL to inactive for 10 selected cultivars (75 and 90 µg/mL for chlorogenic acid and quercetin 3-O-galactoside); inhibition of AGE formation: IC50 = 5.2 mg/mL to inactive for 10 selected cultivars (17.1 and 23 µg/mL for quercetin 3-O-galactoside and epicatechin) | [42] |
| Mespilus germanica L. | acidified 80% EtOH extract, along with its water and 80% MeOH fractions; polyphenols 32.05–74.93 mg/g (HPLC: phenolic acids, procyanidin B2, catechin, and epicatechin) | in vitro enzyme inhibition; control: acarbose 100% inhibition at 50 mg/mL | α-amylase inhibition: about 47%, 27%, and 52% inhibition at 5 mg/mL for EtOH, MeOH, and water fractions, respectively; phenolic acids (e.g., gallic, chlorogenic, and ferulic acids) responsible for observed effects (based on literature studies and extract composition) | [87] |
| EtOH and water extracts; polyphenols 6.93 mg GAE/g dry fruits | in vitro enzyme inhibition; control: - | α-amylase inhibition: not active; α-glucosidase inhibition: 71.5% at 20 mg/mL for EtOH, 44.9% for water extract (comparable to onion extract) | [88] | |
| EtOH extract; polyphenols 16.5 mg GAE/g, flavonoids 1.99 mg QE/g | in vitro enzyme inhibition; control: acarbose, with about 85% inhibition at 10 μg/mL (α-amylase), and about 85% inhibition at 25 μg/mL (α-glucosidase) | α-amylase inhibition: about 35% at 10 and 100 μg/mL; α-glucosidase inhibition: about 99% at 25 μg/mL | [89] | |
| Pyracantha fortuneana (Maxim.) H.L.Li | proanthocyanidin fraction from 70% acetone extract; compounds (HPLC): epicatechin, catechin, A-type and B-type procyanidins, procyanidin glucosides | in vitro enzyme inhibition and molecular docking studies; control: acarbose IC50 = 307 ± 1 μg/mL | α-glucosidase inhibition: IC50 = 0.15 ± 0.01 μg/mL; reversible, non-competitive inhibition; alteration of the catalytic configuration of the enzyme’s active site; procyanidins responsible for observed effects (based on extract composition and molecular docking studies) | [20] |
| 50–90% MeOH, EtOH and acetone extracts; polyphenols 9.67–17.33 mg GAE/g, 25 compounds detected (HPLC-MS: flavonoids and phenolic acids), flavonoids 0.34–1.03 mg QE/g, polysaccharides 72.87–103.65 mg glucose/g | in vitro enzyme inhibition; control: acarbose IC50 = 1071 ± 29 μg/mL | α-glucosidase inhibition: IC50 = 350–1870 μg/mL, with the highest activity observed for 50% and 70% acetone extracts; polyphenols responsible for observed effects (correlation studies) | [19] | |
| Pyrus bretschneideri Rehder | 60% MeOH from peel and pulp; polyphenols about 2.9 and 8.1 mg GAE/g in pulp and peel, respectively (HPLC-MS: catechin; epicatechin; rutin; chlorogenic, p-coumaric, vanillic, gallic, and ferulic acids), flavonoids about 1.5 and 6.3 mg RE/g, terpenes about 0.9 and 4.3 mg OAE/g (oleanolic and ursolic acids) | in vitro enzyme inhibition; control: compounds detected in the tested extracts, 2–89% inhibition at 40 μg/mL | α-glucosidase inhibition: IC50 190 μg/mL for peel and 1220 μg/mL for pulp extract; activity of model compounds: the highest activity found for ferulic acid, rutin, and vanillic acid (about 80–89% inhibition at 40 μg/mL) | [90] |
| Pyrus communis L. | MeOH extracts from peel, flesh, or peel + flesh of different Pyrus cultivars | in vitro enzyme inhibition; control: acarbose 79.75 ± 1.86% inhibition at 0.5 mg/mL (α-amylase), 70.16 ± 1.60% inhibition at 0.5 mg/mL (α-glucosidase) | α-amylase inhibition: 1.20–18.49% for peel at 6 mg/mL, flesh or flesh + peel not active; α-glucosidase inhibition: highly dependent on the cultivar, with the highest activity for “Takiša”, i.e., 76.50–99.64% inhibition at 0.5 mg/mL; for wild Pyrus communis, 13.59% inhibition at 0.5 mg/mL and 63.36% at 1.0 mg/mL (peel) | [91] |
| juice before or after fermentation; polyphenols about 0.25–0.6 mg GAE/g fresh fruits (HPLC: catechin; rutin; chlorogenic, p-coumaric, protocatechuic, benzoic, and gallic acids) | in vitro enzyme inhibition; control: - | α-amylase inhibition: ↑ after fermentation; α-glucosidase inhibition: ↑, ↑ or unchanged after fermentation (depending on the length, pH, and cultivar) | [92] | |
| juice from different cultivars before or after fermentation; proteins 3.8–7.8 mg/mL, phenolics about 0.4 mg/mL (HPLC: epicatechin, p-coumaric and caffeic acids, quercetin derivatives) | in vitro enzyme inhibition; control: - | α-amylase inhibition: not active; α-glucosidase inhibition: ↑ or ↑ after fermentation (depending on the length, bacteria, and pH), about 5–80% inhibition at 10–50 μL | [93] | |
| 12% EtOH and water extracts from peel and pulp of different cultivars; polyphenols 270–1300 μg GAE/g fresh material for peel and 27–150 μg GAE/g for pulp (HPLC: chlorogenic, caffeic, protocatechuic, p-coumaric, and gallic acids; catechin; quercetin derivatives) | in vitro enzyme inhibition; control: - | α-amylase inhibition: the highest activity for pulp water extract (about 20–50% at 100 μL); α-glucosidase inhibition: depending on the study and cultivar (about 10–60% at 10 μL); polyphenols responsible for observed effects (based on correlation studies or extract composition) | [94,95] | |
| Pyrus pashia Buch.-Ham ex D. Don | EtOAc fraction of 70% EtOH extract before or after NaOH hydrolysis; tannins 780 mg CE/100 g of fresh fruits, sugars 15.93 g/100 g | in vitro enzyme inhibition; control: acarbose IC50 = 55 and 440 μg/mL (α-amylase and α-glucosidase) | α-amylase inhibition: IC50 = 72–100 μg/mL; α-glucosidase inhibition: IC50 = 85–330 μg/mL | [21] |
| Pyrus pyrifolia (Burm.f.) Nakai | 80% EtOH + 70% acetone extract from two cultivars fractionated on Sephadex; polyphenols 20.9–28.5 mg CE/g | in vitro enzyme inhibition; control: - | α-glucosidase inhibition: IC50 = 21.3–66.4 μg/mL; oligomeric and polymeric polyphenols responsible for observed effects (based on activity testing of phenolic fractions) | [96] |
| water extract | in vitro enzyme activity; control: acarbose about 10–40% inhibition at 0.05–1 mg/mL | GK activity: ↑ at 5 and 10 mg/mL; α-glucosidase inhibition: about 10–30% at 0.25–1 mg/mL | [39] | |
| 50% EtOH extract from pear pomace; | cellular studies on 3T3-L1 mouse cells; control: cells not treated with extract (glucose uptake), rosiglitazone 1 μM (Rosi, for protein expression) | glucose uptake: ↑ at 100 and 250 μg/mL; protein expression: ↑ p-IRS-1 (Tyr632) and p-Akt at 100 μg/mL (better than Rosi), ↑ GLUT-4 (comparable to Rosi) | [31] | |
| Pyrus pyrifolia (Burm.f.) Nakai, P. ussuriensis Maxim. Ex Rupr., P. betulifolia Bunge, P. bretschneideri Rehder | juices before and after in vitro digestion; polyphenols about 0.18–0.4 mg/mL and 0.18–0.4 mg/mL, polysaccharides about 4.5–8 mg/mL and 4.5–12 mg/mL (before and after digestion, respectively) | in vitro enzyme inhibition; control: acarbose | α-amylase inhibition: about 1.2–1.6 mg acarbose/mL before digestion, 1.2–2.2 mg acarbose/mL after digestion; α-glucosidase inhibition: about 4.5–6.5 mg acarbose/mL before digestion, 5–12.5 mg acarbose/mL after digestion; polyphenols and polysaccharides responsible for observed effects (based on correlation studies or extract composition) | [97] |
| Sorbus aucuparia L. | 80% acetonitrile extract (whole) and fractions (Sephadex); compounds in the whole extract: hydroxycinnamic acids (chlorogenic acids), flavonols, proanthocyanidins, anthocyanins (LC-MS) | in vitro enzyme inhibition; control: acarbose IC50 = 0.08 μg/mL | α-amylase inhibition: whole-extract IC50 = 4.5 μg GAE/mL; the activity of the proanthocyanidin-rich fraction was comparable to that of the whole extract; synergy with acarbose; proanthocyanidins responsible for observed effects (based on activity testing of phenolic fractions) | [27] |
| 80% acetonitrile extract (whole) and fractions (Sephadex); compounds in the whole extract: 12 phenolics, including chlorogenic acid (65%), dicaffeoylquinic and coumaroylquinic acids, quercetin-3-O-glucoside, and caffeoyl glucose (LC-MS) | in vitro enzyme inhibition; control: acarbose IC50 = about 40 μg/mL | α-glucosidase inhibition: whole-extract IC50 = about 30 μg GAE/mL, synergy with acarbose, no synergy with other berries (blackcurrant); proanthocyanidin-rich fraction IC50 > 100 μg GAE/mL; chlorogenic acids responsible for observed effects (based on extract composition and literature studies) | [26] | |
| water extract; polyphenols 19.13 mg GAE/g, flavonoids 9.62 mg CE/g | in vitro enzyme inhibition; control: acarbose IC50 = 6 ± 0.2 μg/mL (α-amylase), IC50 = 86 ± 2.7 μg/mL (α-glucosidase) | α-amylase inhibition: IC50 > 800 μg/mL; α-glucosidase inhibition: IC50 = 50 μg/mL; polyphenols responsible for observed effects (based on extract composition and literature studies) | [23] | |
| 50% MeOH and 50% acetone extracts and fractions (Et2O, EtOAc, n-butanol, water); polyphenols 1.31–274.79 mg/g (51 compounds (HPLC-MS), including caffeic and ferulic acids pseudodepsides, flavonols, and proanthocyanidins) | effects on the formation of AGEs in vitro; control: aminoguanidine (AG) | inhibition of AGE formation: IC50 = 22.37–55.33 µmol AG/mg of extract (2–4-fold higher activity than AG); IC50 for chlorogenic acid, quercetin 3-O-β-sophoroside, and procyanidin B2: 152, 254, and 486 µmol AG/mg, respectively | [43] | |
| 60% EtOH and water extracts | in vitro enzyme inhibition; control: acarbose IC50 = 2.4 ± 0.4 μg/mL | α-amylase inhibition: water extract IC50 = 1236 ± 177.0 μg/mL, 60% EtOH IC50 = 973.9 ± 61.60 μg/mL | [57] | |
| Sorbus decora (Sarg.) CK Schneid. | 80% EtOH extract | effects on the formation of AGEs in vitro; control: quercetin IC50 = 6.1 ± 1.8 μM (about 1.84 μg/mL) | inhibition of AGE formation: IC50 = 192.7 ± 37.1 μg/mL | [98] |
| Sorbus domestica L. | water extract | in vitro enzyme inhibition; control: acarbose IC50 = 120 ± 23 μg/mL (α-amylase), IC50 = 548 ± 21 μg/mL (α-glucosidase) | α-amylase inhibition: IC50 = 8768 μg/mL; α-glucosidase inhibition: IC50 = 417 μg/mL | [99] |
| MeOH extract and fractions (dichloromethane, Et2O, EtOAc, butanol, water) of fruits at different stages of maturity; polyphenols 2.27–341 mg GAE/g (47 compounds (LC-MS), including flavonoids, benzoic and cinnamic acid derivatives, and biphenyls) | in vitro enzyme inhibition; control: sorbinil, 45% inhibition at 0.25 μM | ALR inhibition: 72–93% for Et2O and EtOAc fractions at 50 μg/mL, >50% inhibition for dichloromethane fraction, <40% inhibition for butanol and water fractions; flavonoids and hydroxycinnamoyl esters responsible for observed effects (based on extract composition and statistical analysis) | [44] | |
| Sorbus torminalis (L.) Crantz | water extract; polyphenols 24.21 mg GAE/g, flavonoids 15.69 mg CE/g | in vitro enzyme inhibition; control: acarbose IC50 = 6 ± 0.2 μg/mL (α-amylase), IC50 = 86 ± 2.7 μg/mL (α-glucosidase) | α-amylase inhibition: IC50 = 307 μg/mL; α-glucosidase inhibition: IC50 = 27 μg/mL; polyphenols responsible for observed effects (based on extract composition and literature studies) | [23] |
| Sorbus species from subgenus Aria ** and Sorbus *** | 80% acetone extracts and fractions (carbohydrates and phenolics, only from S. norvegica); detected compounds (NMR): chlorogenic and neochlorogenic acids, carbohydrates | in vitro enzyme inhibition; control: acarbose IC50 = 0.047 ± 0.006 μg/mL (α-amylase), IC50 = 742 ± 147 μg/mL (α-glucosidase), | α-amylase inhibition: IC50 = 2.5–12.3 μg/mL (Aria), the highest activity—S. norvegica; IC50 = 30.3–2540 μg/mL (Sorbus), the highest activity—S. hybrid, S. aucuparia, S. meinichii; α-glucosidase inhibition: IC50 = 0.9–3.7 μg/mL (Aria), the highest activity—S. alnifolia, S. minima, S. norvegica; IC50 = 4.6–300 μg/mL (Sorbus), the highest activity—S. hybrida, S. aucuparia, S. meinichii; carbohydrates and polyphenols contributed to the observed effects (based on correlation studies and activity testing of fractions from S. norvegica) | [22] |
| Vauquelinia corymbose Bonpl. | water extract; detected compounds (HPLC): prunasin, (−)-epicatechin, hyperoside, isoquercetin, quercitrin, quercetin-3-O-(6″-benzoyl)-β-galactoside, picein, methylarbutin | in vitro enzyme inhibition and molecular docking studies; control: acarbose IC50 = 500 μM (yeast α-glucosidase), IC50 = 100 μM (rat small intestinal α-glucosidase) | α-glucosidase inhibition: IC50 = 28.6 μg/mL (yeast α-glucosidase); the most active compound: quercetin-3-O-(6″-benzoyl)-β-galactoside (IC50 = 30 μM for yeast α-glucosidase and 430 μM for rat small-intestinal α-glucosidase; mixed-type inhibitor) | [24] |
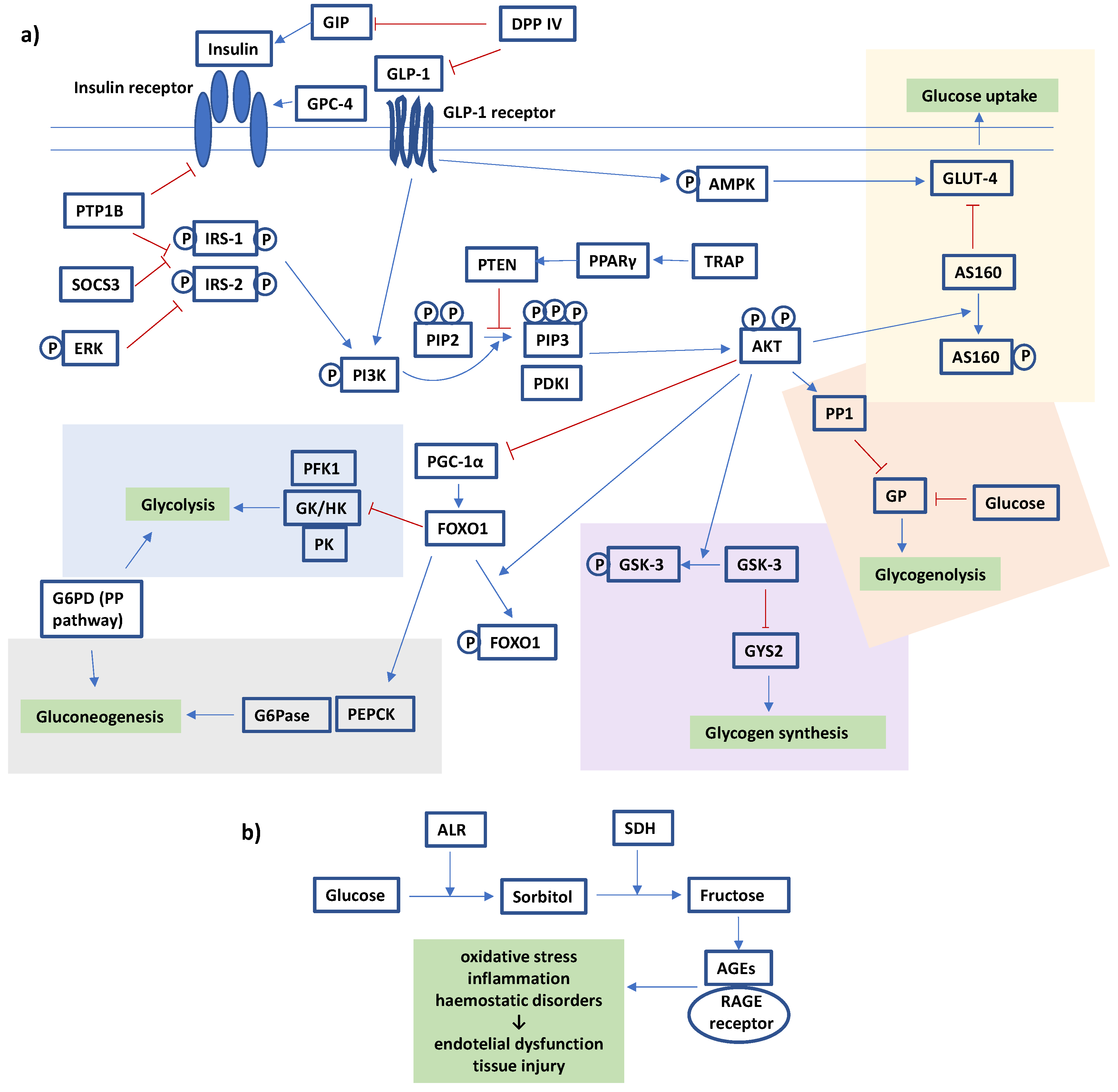
3.2. In Vivo Animal Studies
- (1)
- The effects on intestinal absorption of glucose;
- (2)
- The effects on skeletal, hepatic, or adipose transport of glucose;
- (3)
- The changes in the expression of proteins involved in the insulin signalling pathway;
- (4)
- The modulation of the activity of enzymes involved in glucose metabolism;
- (5)
- The inhibition of glucose-derived protein damage.
| Species | Sample Type, Composition | Model, Study Design | Tested Parameter, Observed Effects * | Ref. |
|---|---|---|---|---|
| Amelanchier alnifolia (Nutt.) Nutt. ex M. Roem. | berry powder; anthocyanins 5011 mg/kg of dry weight (HPLC-MS: cyanidin-3-O-galactoside 74%, cyanidin-3-O-glucoside 18%, cyanidin-3-O-arabinoside, cyanidin-3-O-xyloside) | male diabetic or C57BL/J wild-type mice (n = 5–8/group); duration: 4–5 weeks; tested: diabetic or wild-type mice supplemented with berry powder (0.2, 1, 5, 20%); control: non-supplemented mice (diabetic or wild-type) | blood glucose: ↓ in diabetic mice supplemented with berry powder compared to diabetic non-supplemented mice by 17–41%, depending on the dose (the highest changes for 5% berry powder, i.e., ~8.0 g/kg/day) | [124,125,126] |
| berry powder; anthocyanins 5011 mg/kg of dry weight (HPLC-MS: cyanidin-3-O-galactoside 74%, cyanidin-3-O-glucoside 18%, cyanidin-3-O-arabinoside, cyanidin-3-O-xyloside) | C57BL/6J male mice (n = 8/group); duration: 10–15 weeks; tested: mice fed a high-fat, high-sucrose diet supplemented with berry powder (1, 2.5, 5%, HFHS + B1%, HFHS + B2.5%, HFHS + B5%) or cyanidin 3-O-glucoside (7.2 mg/kg/day), or non-supplemented mice (HFHS); control: low-fat-diet mice | fasting plasma glucose: ↓ in supplemented mice compared to HFHS, the reduction in HFHS + B2.5% (level comparable to control) was significantly lower than in the HFHS + B1% group; plasma insulin and HOMA-IR: ↓ in supplemented mice compared to HFHS (no variation by dose); effects on gut microbiota: multiple changes, i.a., altered α-diversity and β-diversity of gut microbiota (dose ≥ 2.5%), reduced ratio of Firmicutes/Bacteroidetes compared to HFHS; cyanidin 3-O-glucoside activity: similar to Amelanchier berry powder containing an equal amount of cyanidin 3-O-glucoside | [104,105,106,107] | |
| berry powder; flavonoid glycosides 211.79 mg/100 g, quercetin 82.34 mg/100 g, anthocyanins 281 mg/100 g, phenolic acids 108 mg/100 g (HPLC) | male Wistar rats (n = 12/group); duration: 16 weeks; tested: corn starch or high-carbohydrate, high-fat diet + berry powder (26.83 g/kg of food = cyanidin glucoside 8 mg/kg/day) (CS + B or HFHC + B); control: corn starch (CS) or high-carbohydrate, high-fat diet (HFHC) | blood glucose: higher in HFHC and HFHC + B groups compared to CS and CS + B (dependence only on diet; no effect of fruit supplementation); OGTT: ↓ iAUC (0–120 min) of blood glucose in CS + B compared to CS, ↓iAUC in HFHC + B compared to HFHC, higher in both HFHC and HFHC + B compared to CS and CS + B (dependence on both diet and supplementation); gene expression: normalisation of HK1, GP (to a level comparable to controls), and ↑G6Pase | [121] | |
| Aronia melanocarpa (Michx.) Elliott | juice; anthocyanins 5.986 nmol/mL (HPLC: cyanidin 3,5-diglucoside, cyanidin 3-O-arabinoside, cyanidin 3-O-galactoside, cyanidin 3-O-glucoside) | KKAy male mice (n = 5/group); duration: 49 days; tested: Aronia juice (A) or cyanidin 3,5-diglucoside (10 μg/mL solution); control: water-drinking mice | blood glucose: ↓ by about 61% (21 days) and by about 42% (49 days); HbA1c: ↓ by about 33% (21 days) and by about 44% (49 days); DPP IV activity in the serum: ↓ by 62% (49 days); serum active GLP-1 level: about 10-fold ↑ (49 days); cyanidin 3,5-diglucoside: similar but weaker effects compared to Aronia juice | [119] |
| juice; proteins, carbohydrates, fats, minerals, fibres and energy density determined | C57BL/6JmsSlc or KKAy male mice (n = 5/group); duration: 28 days; tested: Aronia-drinking mice; control: water-drinking mice | blood glucose: ↓ by about 55% (only KKAy mice); serum insulin: ↓ (only KKAy mice); DPP IV activity in the serum and liver: ↑ (only C57BL/6JmsSlc mice); DPP IV activity in the intestine: ↓ by about 35% in the ‘upper small intestine’ and about 46% in the ‘lower small intestine’ (KKAy mice); α-glucosidase activity in the upper region of the small intestine: ↓ by about 58% (KKAy mice); expression level: ↓ GIP in the “upper small intestine” and ↑ GLP-1 expression in the “lower small intestine” (KKAy mice) | [115] | |
| juice; anthocyanins 9.572 mg/g, procyanidins 5.328 mg/g, flavonols 3.089 mg/g, hydroxycinnamic acids 2.71 mg/g (HPLC) | C57BL/6J male mice (n = 10/group); duration: 12 weeks; tested: low-fat, high-sucrose, and high-fat diet supplemented with Aronia juice concentrate (1.44 g anthocyanins/kg of diet); control: non-supplemented mice | plasma glucose and insulin: no differences between groups | [127] | |
| standardised Aronia berry extract powder (Fort Wayne, IN, USA); polyphenols 40%, anthocyanins 15% | C57BL/6J male mice (n = 10/group); duration: 14 weeks; tested: high-fat, high-sucrose diet + 0.2% (w/w) Aronia extract; control: low-fat (NC) or high-fat, high-sucrose diet (HFHS) | fasting blood glucose: ↓ compared to HFHS group; PPARγ and GLUT-4 mRNA expression levels in adipocyte fraction: ↑ compared to HFHS group (not statistically significant); anthocyanins responsible for observed effects (based on literature studies and extract composition) | [35] | |
| anthocyanin purified powder extract (80% EtOH + 0.1% HCl + purification); anthocyanins 986.48 mg/g (cyanidin monoglycosides) | C57BL/6J male mice (n = 10/group); STZ-induced diabetes; duration: 5 weeks; tested: high-fat diet, diabetic + 150/300 mg/kg Aronia extract (D/A150 and D/A300) or 200 mg/kg metformin (M); control: low-fat (NC) or high-fat diet, diabetic (DC) | blood glucose: dose-dependent ↓ compared to DC; serum insulin and HbA1c: dose-dependent ↓ compared to DC; liver glycogen: ↑ compared to DC (D/A300 and M); hepatic protein expression: ↑ p-GSK-3β (all groups), ↑ GLUT-4 (D/A300 and M), ↓ SOCS3, (all groups), unchanged GSK-3β and IRS-1 compared to DC; anthocyanins responsible for observed effects (testing of pure anthocyanin fraction activity) | [100] | |
| 70% EtOH extract; detected compounds (HPLC-MS): gallic acid, chlorogenic acid, quercetin, kaempferol | male ICR mice (n = 8/group); STZ-induced diabetes; duration: 31 days; tested: Aronia-extract-treated diabetic mice (10 or 100 mg/kg, D/A10 or D/A100); control: normal (NC) or diabetic (DC) mice | serum glucose: ↓ compared to DC (D/A100 = 208.60 ± 31.05 mg/dL, DC mice = 486.60 ± 81.94 mg/dL, NC mice = 175.67 ± 10.60 mg/dL); serum insulin: ↑ compared to DC (D/A100 = 2.50 ± 0.39 ng/mL, DC mice = 1.34 ± 0.54 ng/mL, NC mice = 2.66 ± 0.36 ng/mL); effect on tissue injury: Aronia extract treatment attenuated histological changes induced by STZ in the liver and pancreatic tissues | [111] | |
| juice; anthocyanins 240 mg/100 mL (HPLC) | male Wistar rats (n = 12/group); duration: 16 weeks; tested: maize starch + Aronia juice (9.4 mg anthocyanins/kg/day) or high-carbohydrate, high-fat diet + Aronia juice (7.8 mg anthocyanins/kg/day); control: maize starch + water (C), high-carbohydrate, high-fat diet + water (HFHC) | OGTT: iAUC (0–120 min) of blood glucose ↑ in HFHC (786 mmol/L min), ↓ in Aronia groups (658 or 648 mmol/L min) compared to C rats (591 mmol/L min); plasma insulin: ↑ in HFHC (4.1 μmol/L), ↓ in Aronia groups (2.3 or 1.1 μmol/L) compared to C rats (1.4 μmol/L); anthocyanins responsible for observed effects (based on juice composition and literature studies) | [128] | |
| 60% EtOH commercial extract containing at least 10% anthocyanins | male Wistar rats (n = 6/group); duration: 6 weeks; tested: fructose-rich diet + 100 or 200 mg/kg Aronia extract dissolved in water; control: fructose-rich diet + water | blood glucose and insulin: ↓ by about 10% (glucose) and 30% (insulin) regardless of dosage; expression of proteins: ↑ mRNA levels of IRS-1, IRS-2, PI3KR1, GLUT-1, GLUT-4, and GYS (by 1.5–2.3-fold at 200 mg/kg dosage), and ↓ mRNA levels of PTEN and GSK-3β (by 0.61–0.62-fold at 200 mg/kg) | [117] | |
| juice; ascorbic acid 29 g/L, anthocyanins 1.3 mg/mL, carotenes 97.8 µg/L, polyphenols 31.84 g/L | male Wistar albino rats (n = 10/group); alloxan-induced diabetes; duration: 6 weeks; tested: normal Aronia-drinking rats (N/A, 10 mL/kg), diabetic Aronia-drinking rats (D/A); control: diabetic water-drinking rats (DC), normal water-drinking rats (NC) | blood glucose: ↓ for D/A rats compared to DC rats (D/A = 136.8 ± 14.6 mg/dL, DC = 220.4 ± 23.5 mg/dL, NC mice = 103.5 ± 11.2 mg/dL) | [129] | |
| juice; polyphenols 709.3 mg/100 mL; flavonoids 189.4 mg/100 mL; anthocyanins 106.8 mg/100 mL, L-ascorbic acid 3.0 mg/100 ml | male Wistar rats (n = 6/group); STZ-induced diabetes; duration: 6 weeks; tested groups: normal (N/A10 and N/A20) or diabetic (D/A10 and D/A20) rats drinking Aronia juice (10 or 20 mL/kg); control: normal (NC) or diabetic (DC) rats drinking water (10 mL/kg) | plasma glucose: DC rats = 17.5 ± 2.9 mmol/l; NC rats = 7.2 ± 0.6 mmol/l; N/A10 and N/A20 rats—no significant changes compared to NC rats; D/A10 and D/A20 rats—↓ to levels comparable to NC rats (by 44% and 42% compared to DC rats, respectively); anthocyanins and flavonoids responsible for observed effects (based on juice composition and literature studies) | [130] | |
| commercial extract; polyphenols 714.1 mg/g, including anthocyanins 56.6% (cyanidin monoglycosides), flavanols 21.6% (procyanidins, epicatechin), phenolic acids 14.7% (chlorogenic and neochlorogenic acids), flavonols 7.1% (quercetin glycosides) (HPLC) | male Wistar rats (n = 8/group); STZ-induced diabetes; duration: 4 weeks; tested: diabetic rats with diet modified by 8% lard and 65% fructose + Aronia extract (0.2%); control: diabetic (DC) or normal rats (NC) fed a standard casein diet enriched with 0.5% cholesterol | activity of microbial enzymes in the caecal digesta: β-glucuronidase activity ↑ in the DC group compared to NC, and ↓ in the Aronia group to a level no different vs. NC; the activity of α- and β-glucosidase and α- and β-galactosidase did not differ; mucosal disaccharidase activities: sucrase and maltase activity comparable between the NC and Aronia groups and ↑ in DC; in the case of lactase the trend was the opposite; serum glucose: no differences between the Aronia and NC groups, remarkably ↑ in the DC group | [114] | |
| 60% EtOH: 0.1% HCl (1:20) extract, purified and concentrated; anthocyanins 254.72 mg/g (cyanidin monoglycosides), flavonoids 11.66 mg/g (quercetin glycosides), phenolics acids 75.97 mg/g (caffeic, chlorogenic, and neochlorogenic acids) (HPLC-MS) | male Wistar rats (n = 6/group); STZ-induced diabetes; duration: 8 weeks; tested: diabetic rats fed a high-fat diet and 100 or 400 mg/kg Aronia extract (D/A100 or D/A400); control: normal rats, standard diet (NC) and diabetic rats, high-fat diet (DC) | OGTT: iAUC (0–120 min) of blood glucose ↓ by 24.40% in the D/A400 rats compared to DC rats; blood glucose: ↓ by 2.76 and 4.25 mmol/L compared to DC rats; serum insulin: ↓ compared to DC rats (statistically insignificant); HOMA-IR: 3.26 ± 0.56 (NC), 15.42 ± 4.17 (DC), 11.24 ± 3.10 (D/A100), 8.12 ± 1.94 (D/A400); hepatic glycogen: ↑ compared to DC rats; liver enzyme activity: GK and PK ↑ compared to DC rats, PEPCK and G6Pase ↓ compared to DC rats; hepatic protein expression: ↑ p-IRS-2, p-PI3K, p-Akt, p-GSK-3β, and GLUT-2 (by 2.03–4.02-fold for D/A400), and ↓ IRS-2 and GSK-3β (by 1.53–2.76-fold for D/A400), compared to DC | [116] | |
| water extract; anthocyanins 579.1 mmol/g (cyanidin monoglycosides), (+)-catechin 10.7 mmol/g, chlorogenic acid 32.7 mmol/g, caffeic acid 13.9 mmol/g (HPLC) | male Sprague-Dawley rats (n = 6/group); duration: 4 weeks; tested: high-fat diet + Aronia extract (17.4 g extract/kg of diet); control: non-supplemented rats | serum glucose: ↓ (8.20 ± 0.31 mM/dL) compared to controls (9.17 ± 0.38 mM/dL); OGTT: iAUC (0–120 min) of blood glucose ↓ by 20.27%; anthocyanins, proanthocyanidins, and chlorogenic acid responsible for observed effects (based on extract composition and literature studies) | [131] | |
| pomace | Polish Merino health lambs (n = 8/group); duration: 90 days; tested: 150 g or 300 g (A150 or A300) of Aronia pomace/kg of the feed mixture (twice a day, intake monitored and adjusted to the lambs’ growing period); control: non-supplemented lambs | serum glucose: A150 = 2.42 ± 0.31 mmol/L, A300 = 1.55 ± 0.66 mmol/L, control = 3.38 ± 0.31 mmol/L; total phenolic contents in the liver or serum: A150 = 7.49 mg GAE/g of liver and 2.41 mg GAE/mL of serum; A300 = 7.46 mg GAE/g of liver and 3.93 mg GAE/mL of serum; control = 4.42 mg GAE/g of liver and 1.77 mg GAE/mL of serum | [132] | |
| Chaenomeles sinensis (Thouin) Koehne | EtOH extract with removed sugars; polyphenols 350 mg GAE/g, including procyanidins (>90%) | KKAy male mice (n = 4–5/group); duration: 4 weeks; tested: diabetic mice fed a high-fat diet + extract (14.3 g/kg diet); control: diabetic mice fed a high-fat diet (DC) | OGTT: ↓ blood glucose after 15 min, iAUC (0–120 min) unchanged; blood glucose: ↓ after 1–4 weeks; HbA1c: unchanged (↓, but not significantly); intermediate products of glycation: 3-DG level not affected, GO and MG levels ↓ about twofold; procyanidins responsible for observed effects (based on extract composition and literature studies) | [123] |
| Crataegus azarolus var. aronia L. | water decoction of unripe fruits | female Sprague-Dawley rats (n = 10/group); STZ-induced diabetes; duration: 24 days; tested: diabetic (D) or normal (N) rats + Crataegus decoction of 50 or 350 mg fruits/mL (D50, D350, N50, N350); controls: normal rats (NC) and diabetic rats (DC) | blood glucose: ↓ in N350 compared to NC rats, ↓ in D50 and D350 compared to DC rats | [133] |
| Crataegus laevigata (Poir.) DC. | 70% EtOH extract | Sprague-Dawley rats (n = 12/group); STZ-induced diabetes; duration: 6 h; tested: diabetic rats + 200, 400, 600, 800, 1000, 1200 mg/kg Crataegus extract; controls: normal rats (NC), diabetic rats (DC), and diabetic rats + glipizide (10 mg/kg) | blood glucose: dose-dependent ↓ compared to DC, hypoglycaemic effect of Crataegus extract at 1200 mg/mL comparable to glipizide; OGTT: effective ↓ in glucose levels after 30, 60, and 90 min (dosage 800 and 1200 mg/mL) | [101] |
| Crataegus meyeri Pojark. | water extract from fresh pulp | male Wistar rats (n = 10/group); STZ-induced diabetes; duration: 10 weeks; tested: diabetic rats + 300 mg/kg Crataegus extract; controls: normal rats (NC) and diabetic rats (DC) | insulin level and QUICKI: unchanged; glucose: ↓ compared to DC (NC 118 ± 32 mg/dL, DC 538 ± 47 mg/dL, Crataegus 348 ± 41 mg/dL); HOMA-IR: ↓ (NC 130 ± 19, DC 177 ± 16, Crataegus 119 ± 21) | [134] |
| Crataegus monogyna Jacq. | 70% MeOH extract | male Wistar rats (n = 8/group); STZ-induced diabetes; duration: 3 weeks; tested: diabetic rats + 100, 200 or 400 mg/kg Crataegus extract; controls: normal rats (NC) and diabetic rats (DC) | serum glucose: ↓ compared to DC rats (to levels comparable to NC; all dosages); effects on pancreatic tissue: Crataegus extract (at 200 and 400 mg/kg) ameliorated the degeneration of pancreatic acinar cells; damage to lobules, acini, and oedema observed in the tissue of DC rats | [135] |
| Crataegus orientalis subsp. presliana K.I.Chr. | 90% EtOH extract | Kunming mice (n = 8/group); STZ-induced diabetes; duration: 4 weeks; tested: diabetic mice + extract 1.8 g/kg; controls: normal (NC) or diabetic mice (DC) | blood glucose: ↓ compared to DC (statistically insignificant); ALR activity: ↓ compared to DC (statistically insignificant) | [112] |
| Crataegus pinnatifida Bunge | proanthocyanidin fraction from 70% EtOH extract; procyanidins 81.85 mg/100 mg (LC-MS: epicatechin 36.12%; procyanidins B2, B5, and C1) | male Wistar rats (n = 12/group); duration: 8 weeks; tested: high-fat diet + 50, 100, or 200 mg/kg Crataegus preparation; controls: standard diet (NC) or high-fat diet (HFD) | fasting blood glucose: lack of differences (tested and control groups); OGTT: glucose level ↓, iAUC (0–120 min) ↓ compared to HFD; insulin: dose-dependent ↓ compared to HFD; alleviation of liver histopathological changes compared to HFD; procyanidins responsible for observed effects (based on activity testing of extract fraction) | [110] |
| MeOH extract; total phenolics 66.94 mg GAE/g | male Sprague-Dawley rats (n = 9/group); STZ-induced diabetes; duration: 2 weeks; tested: diabetic rats fed a high-fat diet + Crataegus extract at 50, 100, or 200 mg/kg; controls: normal rats, standard diet (NC); diabetic rats, high-fat diet (DC); diabetic rats, high-fat diet + orlistat (40 mg/kg) | blood glucose: dose-dependent ↓ compared to DC; 5.62 ± 0.39 mmol/L (NC), 20.25 ± 1.9 mmol/L (DC), 10.5—17.9 mmol/L (Crataegus), about 10 mmol/L (orlistat); serum insulin: dose-dependent ↓ compared to DC; 147.74 ± 15.61 pg/mL (NC), 238.59 ± 21.01 pg/mL (DC), 176.82—206.76 (Crataegus), about 300 pg/mL (orlistat) | [136] | |
| Crataegus pinnatifida var. major N.E.Br. | 70% acidic EtOH (0.1% HCl) extract; polyphenols 594.15 mg/g, including chlorogenic acid 15.2%, procyanidin B2 20.3%, epicatechin, proanthocyanidin B-type oligomers, cyanidin 3-galactoside, and quercetin glycosides (HPLC-MS) | male Wistar rats (n = 8/group); STZ-induced diabetes; duration: 12 weeks; tested: diabetic rats fed a high-fat diet + 300 mg/kg Crataegus extract; controls: normal rats, standard diet (NC); diabetic rats, high-fat diet (DC); diabetic rats, high-fat diet + metformin (150 mg/kg) | blood glucose: 44.2% ↓ compared to DC rats (likewise for metformin); OGTT: glucose level ↓ after 30 min (17.32 mmol/L) compared to DC rats (25.80 mmol/L), comparable to metformin (15.73 mmol/L); serum insulin: 34.1% ↓ compared to DC rats (about 50% reduction for metformin); improved histology of skeletal muscle, liver, and aortic vessels; phosphorylation of proteins: ↑ p-GLUT-4, p-IRS-1, p-Akt, and p-PI3K in the liver; ↑ p-IRS-1 and p-Akt but no effects on p-PI3K in the skeletal muscle (comparable to metformin) | [102] |
| n-butanol fraction of 80% MeOH extract | C57BL/6J male mice (n = 5–9/group); duration: 8 weeks; tested: high-fat diet + Crataegus extract at 200, 500, or 1000 mg/kg (CE200–1000); controls: low-fat diet (NC), high-fat diet (HF), and high-fat diet + rosiglitazone (10 mg/kg, Rosi10) | plasma glucose and insulin: ↓ compered to HF, comparable to NC in the CE1000 and Rosi10 groups; insulin resistance test: ↓ compared to HF (significantly in the CE500, CE1000, and Rosi10 groups); GLUT-4 expression (skeletal muscle): the mRNA level ↓ in HF and ↑ in CE200–1000 and Rosi10 compared to NC; protein expression in the liver: ↓ PEPCK (CE500, -1000, and Rosi10) compared to HF, ↑ p-AMPK (all groups) compared to NC and HF; OGTT: ↓ in glucose levels after 30 min in low-fat diet mice supplemented with 200 mg/kg extract compared to NC, and after 30, 60, 90, 120, and 180 min in mice supplemented with 1000 and 2000 mg/kg | [118] | |
| Crataegus L. sp. | water extract from dried pulp; polyphenols 20.18 mg GAE/g, flavonoids 8.50 mg QE/g | male Wistar rats (n = 6–8/group); STZ-induced diabetes; duration: 14 days; tested: diabetic rats + extract at 100, 300, or 1000 mg/kg; controls: normal rats (NC) and diabetic rats (DC) | blood glucose: ↓ compared to DC at 100 and 300 mg/kg; unchanged at 1000 mg/kg | [137] |
| water extract from dried pulp | male Wistar rats (n = 10/group); STZ-induced diabetes; duration: 10 weeks; tested: diabetic rats + extract at 100 mg/kg ± resistance training; controls: normal rats (NC) and diabetic rats (DC) | blood glucose: unchanged compared to DC; fastening serum insulin: ↑ compared to DC (NC 25.0 μIU/mL; DC 6.93 μIU/mL, extract 8.12 μIU/mL) | [138] | |
| 80% EtOH extract; detected compounds (HPLC): chlorogenic acid, catechin, epigallocatechin gallate, quercetin, kaempferol and apigenin derivatives (including hyperoside and vitexin) | male Wistar rats (n = 8/group); STZ-induced diabetes; duration: 12 weeks; tested: diabetic rats + extract at 100 mg/kg ± resistance training; controls: normal rats (NC) and diabetic rats (DC) | blood glucose: ↓ compared to DC (NC 6.78 mmol/L; DC 22.98 mmol/L, extract 17.96 mmol/L); serum insulin: ↑ compared to DC (NC 10.25 μU/mL; DC 4.98 μU/mL, extract 7.67 μU/mL); GPLD1 serum level: ↓ compared to DC; GPC-4 serum level: ↑ compared to DC (statistically insignificant); HOMA-IS: ↑ compared to DC (statistically insignificant) | [120] | |
| Cydonia oblonga Mill. | 30% EtOH extract; chlorogenic acid 0.75 mg/g (HPLC) | C57BL/6N mice (n = 10/group); duration: 8 weeks; tested: high-fat diet + extract at 50, 100, or 200 mg/kg; controls: normal diet (NC) and high-fat diet (HFD), | serum glucose: unchanged; serum insulin: ↓ compared to HFD (NC 2.62 ng/mL, HFD 9.12 ng/mL, Cydonia 5.67–6.51 ng/mL); HOMA-IR: ↓ compared to HFD (all dosages); QUICKI: ↑ compared to HFD at 200 mg/kg; p-AMPK/AMPK ratio ↑ about twofold compared to HFD (200 mg/kg), PPARγ mRNA expression ↓ about twofold (all dosages) chlorogenic acid responsible for observed effects (based on extract composition and literature studies) | [108] |
| Malus domestica (Suckow.) Borkh. | commercial extract (Biosearch S.A.); polyphenols 80%, phlorizin min. 5% (HPLC) | male Wistar rats (n = 12/group); duration: 56 days; tested: rats fed a high-fat, high-sucrose diet + extract at 700 mg/kg (HFS + M); controls: standard diet (NC) and high-fat, high-sucrose diet (HFS) | serum glucose: ↓ compared to HFS (HFS + M 5.50 ± 0.22 mmol/L, HFS 6.36 ± 0.28 mmol/L, NC 5.45 ± 0.23 mmol/L); serum insulin: ↓ compared to HFS (HFS + M 14.6 µU/mL, HFS 22.9 µU/mL, NC 9.12 µU/mL); HOMA-IR: ↓ compared to HFS (HFS + M 3.45, HFS 6.65, NC 2.18) | [109] |
| juice and peel water extracts; polyphenols 69.68 ± 2.17 mg GAE/100 mL (juice), 673.46 ± 6.90 mg GAE/ 100 g dry extract | male Wistar rats (n = 8/group); STZ-induced diabetes; duration: 21 days; tested: diabetic rats + apple juice (15 mL/kg) or peel extract (1 g/kg); controls: normal rats (NC) and diabetic rats (DC) | blood glucose: ↓ compared to DC after the first, second, and third weeks, to levels comparable to NC (juice: 87.87–134.83 mg/dL, extract: 88.00–105.82 mg/dL, DC: 374.67–432.14 mg/dL, NC: 81.53–89.57 mg/dL) | [139] | |
| extract dissolved in water | Wistar rats (n = 5/group); fructose-induced diabetes; duration: 28 days; tested: diabetic rats + apple extract (500 mg/kg); controls: normal rats (NC) and diabetic rats (DC) | blood glucose: ↓ compared to DC, to levels comparable to NC (apple: 81.00 ± 3.61 mg/dL, DC: 116.70 ± 9.13 mg/dL, NC: 76.33 ± 3.84 mg/dL) | [140] | |
| commercial extract (BioActive Food GmbH); polyphenols 44% (catechin equivalents), phlorizin 16%, quercetin 12.43%, chlorogenic acid 5.57% (HPLC/spectrophotometry) | male C57BL/6N mice (n = 10/group); duration: acute consumption; tested: mice fed a high-fat diet + 12.24 mg of extract or 1.96 mg of phlorizin; controls: normal diet (NC) and high-fat diet (HFC) | OGTT: iAUC (0–15, 0–30, 0–60 min) ↓ compared to HFC (to levels comparable to NC), with the highest reduction after 15 min (likewise for phlorizin); glucose uptake in everted jejunal rings: inhibited (EC50 = 8.9 ± 2.2 µg/mL for extract and 4.2 ± 0.6 µM for phlorizin); glucose uptake (everted jejunal sacs): SGLT1 inhibited (reversible manner) | [75] | |
| peel extract, purified, sugar-free; polyphenols 606 mg GAE/g (HPLC: chlorogenic acid, epicatechin, quercetin glycosides), anthocyanins 7.17 mg/g | C57BL/6J male mice (n = 8/group); duration: 10 weeks; tested: high-fat diet + 0.2% (w/w) extract or quercetin; controls: low-fat diet (NC) and high-fat diet (HFC) | fasting blood glucose: ↓ compared to HFC (comparable to NC); IPGTT: ↓ iAUC (0–2 h) of blood glucose compared to HFC; serum insulin: ↓ compered to HFC; activity of quercetin: comparable to apple extract | [141] | |
| juice (fermented or not); compounds: 19 polyphenols (HPLC: mainly chlorogenic acid about 95–145 mg/L, epicatechin 70–105 mg/L, procyanidin B2 70–95 mg/L), sugars, and organic acids | C57BL/6J mice; STZ-induced diabetes; duration: 4 weeks; tested: high-fat diet + juice (fermented or unfermented); controls: normal mice, normal diet (NC); diabetic mice, high-fat diet (DC); diabetic mice, acarbose (A) | fasting blood glucose: ↓ compared to DC (both fermented and unfermented juice), for fermented juice and acarbose to levels comparable to NC; insulin and HOMA-IR: similar trend as for glucose levels; QUICKI: ↑ compared to DC (fermented and unfermented juice), for fermented juice and acarbose to levels comparable to NC | [82] | |
| Malus Mill. sp. | 70% MeOH macerate | albino mice; duration: 22 days; tested: plant extract 1 g/kg, 2 g/kg, or 3 g/kg; control: non-supplemented mice | G6Pase activity: dose-dependent inhibition (activity at 1 g/kg = 20.7–22.0, at 2 g/kg = 10.4–13.4, at 3 g/kg = 1.3–2.4, compared to control = 70.35), higher than observed for, e.g., mulberry fruit extract | [122] |
| Malus pumila Mill. | procyanidin fraction from juice (without chlorogenic acid and phlorizin) | male B6.Cg-Lepob/J mice (C57BL/6J background) (n = 6/group); obese, insulin-resistant, moderate hyperglycaemia; duration: 4 weeks; tested: 0.5% extract dissolved in water, ad libitum, control: non-supplemented obese mice | OGTT (0–120 min): blood glucose ↓ at 15 and 30 min, serum insulin unchanged; insulin TT (0–120 min): blood glucose ↓ at 15, 30, 45, and 60 min; HOMA-IR: ↓ (27.3 ± 7.9) compared to control mice (76.0 ± 13.3); pancreatic islet size: β-cell area ↓ by about 21%, number of islets unchanged (↓ in pancreatic cells’ hypertrophy); pyruvate TT: glucose ↓ at 15–30 min (↓ in hepatic gluconeogenesis); protein phosphorylation: ↑ p-Akt; suppression of hepatic inflammation resulting in insulin signalling improvement | [113] |
| commercial extract (Exxentia®); polyphenols 57.5%, including phlorizin (9.9%), chlorogenic acid (15.8%), quercetin (0.4%) | obese male Zucker fatty rats—insulin-resistant model (n = 10/group); duration: acute consumption (for TT) and 4 weeks; tested: (TT) for first meal maltodextrin + 150 mg extract/kg, for second meal maltodextrin only, (chronic) standard diet + 128 mg extract/kg; control: (TT) maltodextrin, (chronic) standard diet | acute meal TT: iAUC (0–120 min) ↓ for glucose and unchanged for insulin; second acute meal TT: iAUC (0–240 min) for glucose ↓, glucose levels higher compared to tested rats after first meal; chronic effect on insulin sensitivity: iAUC (0–180) ↓ for both glucose and insulin levels, insulin sensitivity ↑ (glucose infusion rate required to establish euglycaemia ↑ by 45%) | [30] | |
| Pyrus bretschneideri Rehder | 60% MeOH extract from peel and pulp; polyphenols about 2.9/8.1 mg GAE/g pulp/peel (HPLC-MS: catechin; epicatechin; rutin; chlorogenic, p-coumaric, vanillic, gallic, and ferulic acids), flavonoids about 1.5/6.3 mg RE/g, terpenes (oleanolic and ursolic acids) about 0.9/4.3 mg OAE/g | male Kunming mice (n = 10/group); STZ-induced diabetes; duration: 3 weeks; tested: diabetic mice, high-fat diet + 500 mg/kg peel or pulp extract; controls: normal mice, normal diet (NC); diabetic mice, high-fat diet (DC) | blood glucose: ↓ (8.2–8.6 mmol/L) in the peel group after 2–3 weeks compared to DC (14.7–16.0 mmol/L); OGTT: ↓ blood glucose in the peel group compared to DC, ↓ iAUC (0–3 h) | [90] |
| Pyrus communis L. | EtOAc and 80% EtOH extracts; phytochemical screening: carbohydrates, phenolics, tannins, and flavonoids | Wistar rats (n = 6/group); dexamethasone-induced diabetes; duration: 11 days; tested: diabetic rats + 200 mg/kg EtOAc or 80% EtOH; controls: normal rats (NC), diabetic rats (DC), and diabetic rats + glibenclamide (5 mg/kg) | OGTT: ↓ blood glucose after 60 min compared to DC (comparable to glibenclamide); urine sugar: significant levels in DC rats, trace amounts in tested groups and glibenclamide controls; blood glucose: ↓ from 3rd to 11th day compared to DC (comparable to glibenclamide) | [103] |
| Pyrus pyrifolia (Burm.f.) Nakai | 80% EtOH + 70% acetone extract from different cultivars; polyphenols 20.9–28.5 mg CE/g | male DDY mice (n = 8/group); duration: acute consumption; tested: 250 or 500 mg/kg Pyrus extract; control: non-supplemented mice | oral starch TT: ↓ blood glucose after 30 min at 250 mg/kg and after 30, 60, and 120 min at 500 mg/g; ↓ iAUC (2 h) at 500 mg/kg | [96] |
| 50% EtOH extract from pomace | C57BL/6J male mice (n = 10/group); duration: 8 weeks; tested: high-fat diet + 200 or 400 mg/kg extract; control: high-fat diet + water | blood glucose: unchanged; insulin: ↓ at 400 mg/kg (not significantly) HOMA-IR: ↓ at 400 mg/kg; protein expression: ↑ p-IRS-1 (Tyr632), ↑ GLUT-4, ↓ p-IRS-1 (Ser307) | [31] | |
| Sorbus aucuparia L. | EtOH extract | Kunming mice (n = 8/group); STZ-induced diabetes; duration: 4 weeks; tested: high-glucose, high-fat diet mice + extract at 10, 50, or 100 mg/L; controls: normal-diet mice (NC) and high-glucose, high-fat-diet mice (HGFD) | blood glucose: ↓ in the extract-fed mice compared to HGFD (dose-dependent, about twofold ↓ at 100 mg/L); IPGTT: ↓ serum glucose in the extract-fed mice compared to HGFD (dose-dependent, at 50 mg/L significant ↓ after 15 min, at 100 mg/L significant ↓ after 5–120 min) | [142] |
| Sorbus norvegica Hedl. | 80% acetone extract; detected compounds (NMR): chlorogenic and neochlorogenic acids, carbohydrates | C57BL/6J male mice (n = 6–10/group); STZ-induced diabetes; duration: acute consumption (3.5 h); tested: mice fed starch (2 g/kg) and berry extract (600, 900, or 1250 mg/kg) or mice fed glucose (2 g/kg) and berry extract (1250 mg/kg); controls: mice fed starch and acarbose (25 mg/kg, positive control) or mice fed starch/glucose (negative control) | oral starch TT: ↓ maximal blood glucose compared to negative controls; the activity of berry extract at 900–1250 mg/kg was comparable to that of acarbose; for extract at 1250 mg/kg, ↓ iAUC; OGTT: for extract at 1250 mg/kg, ↓ in blood glucose after 30 min compared to the negative control | [22] |
3.3. Human Studies
| Species | Sample Type, Composition | Model, Study Design | Tested Parameter, Observed Effects * | Ref. |
|---|---|---|---|---|
| Aronia melanocarpa (Michx.) Elliott | juice; proteins, carbohydrates, fats, minerals, fibres, and energy density determined | open-label, randomised, two-period, one-way crossover study; 37 healthy Japanese patients (women and men, >30 years old); 100 mL of Aronia juice (tested) or 100 mL of water (control) + 200 g of rice; duration: acute consumption | postprandial blood glucose: ↓ iAUC (0–150 min) by > 50% | [146] |
| juice; low-calorie, sugar-free | (1) 41 diabetic patients (16 insulin-dependent, 25 non-insulin-dependent, women and men, 3–62 years old); glucose at baseline (control) vs. 60 min after ingestion of 200 mL of juice or 200 mL of juice and standard meal (tested); duration: acute consumption; (2) 21 diabetic patients (non-insulin-dependent, women and men, 42–62 years old) drinking 200 mL of juice daily (tested) and 23 diabetic patients (non-insulin-dependent, women and men, 48–67 years) without supplementation (controls); duration: 3 months | fasting blood glucose (1): ↓ from 14.23 ± 1.32 mmol/L at baseline to 11.4 ± 0.89 mmol/L after 60 min; postprandial blood glucose (1): ↓ (statistically insignificant); blood glucose (2): ↓ from 13.28 ± 4.55 mmol/L at baseline to 9.10 ±3.05 mmol/L after 3 months; HbA1c (2): ↓ from 9.39 ± 2.16% at baseline to 7.49 ± 1.33% after 3 months | [143] | |
| juice; total phenolics 413.0 ± 5.1 mg GAE/100 g of fresh fruits, total anthocyanins 172.7 ± 4.4 mg/100 g | 35 diabetic patients (women and men, 35–65 years old, diabetes type 2); 3 × 50 mL per day of Aronia juice (tested), and the same patients not supplemented with Aronia juice (control); duration: 3 months for supplementation, next 3 months for self-control | fasting blood glucose: ↓ (statistically insignificant); HbA1c: ↓ from 59.1–59.4 mmol/mol (baseline and control) to 55.1 ± 14.7 mmol/mol | [144] | |
| juice | 11 overweight and 11 normal-weight patients (women and men, 51.9 ± 3.9 years old); 3 × 50 mL per day of Aronia juice (tested) and baseline parameters (control); duration: 3 months | fasting blood glucose and HbA1c: not changed | [151] | |
| standardised commercial extract (Alixir 400 PROTECT®, polyphenols (431 mg/30 mL), anthocyanins (120 mg/30 mL), potassium sorbate (35.1 mg/30 mL)); detected compounds (HPLC): cyanidin and quercetin glycosides | prospective, open-label, clinical case-series study; 143 patients with metabolic syndrome, with or without diabetes type 2 (women and men, 50–60 years old); 30 mL of Aronia extract per day, control: baseline parameters; duration: 28 days | blood glucose: ↓, especially in diabetic groups (6.40–6.82 mmol/L) compared to baseline levels (7.97–8.41 mmol/L) | [145] | |
| Malus domestica (Suckow) Borkh. | standardised commercial extract (Appl’In by DIANA Food SAS); total polyphenols 67%, including 40% flavonoid monomers and phenolic acids (HPLC: flavan-3-ols, dihydrochalcones, flavonols, hydroxycinnamic acids) | randomised, controlled, double-blind crossover study; 20 healthy men and 5 postmenopausal women (20–60 years old); 200 mL drink containing 1200 mg of apple phenolics (tested) or <5 mg of phenolics (control) + a high-carbohydrate meal; duration: acute consumption | postprandial plasma glucose: ↓ iAUC (0–30 min), ↓ Cmax, ↑ Tmax; postprandial insulin: ↓ iAUC (0–30 min and 0–120 min), ↓ at 10, 20, 30, and 45 min, ↑ Tmax; C-peptide: ↓ iAUC (0–30 min), ↓ at 10, 20, 30, and 45 min, ↑ Tmax; GIP: ↓ iAUC (0–30 min and 0–120 min), ↓at 10, 20, 30, 45, 60, and 75 min, ↓ Cmax decreased, ↑ Tmax; | [32] |
| standardised commercial extract (Appl’In, Diana Naturals, France); min. 80% polyphenols, >5% phlorizin | randomised controlled trial, balanced incomplete block design; 65 healthy adults (women and men, average 20–50 years old), apple extract group: 33 adults; 2 g of plant extract (tested) or not supplemented (control) + rice porridge (~50 g available carbohydrate); duration: acute consumption | postprandial glucose: ↓ iAUC (0–2 h) by about 25.4%, effect comparable to those of mulberry fruit and leaf extracts; postprandial insulin: ↓ iAUC (0–2 h) by about 22.3% urine glucose: no glucosuria observed | [34] | |
| standardised commercial extract (Appl’In, Diana Naturals, France); polyphenols min. 80%, phlorizin > 5%; 14 compounds detected (HPLC), including quercetin glycosides, dihydrochalcones, phenolic acids, and procyanidin oligomers | randomised, controlled, double-blind crossover study; 30 healthy adults (women and men, 18–68 years old); drink without (control) or with 1.8, 1.35, or 0.9 g of apple extract (tested) + a 75 g starch/sucrose meal; duration: acute consumption | postprandial blood glucose: ↓ iAUC (0–30 min) by about 8.99–15.6 mmol/L per minute (all doses), iAUC (0–120 min and 0–240 min) unchanged, ↑Tmax, Cmax unchanged; postprandial insulin and C-peptide: similar trends as for glucose concentration; GIP: ↓ at 30 and 60 min (1.8 and 1.35 g), Tmax and Cmax unchanged; urinary glucose: unchanged different compounds responsible for observed effects (based on literature studies, extract composition, and serum metabolite studies) | [73] | |
| apple powder from unripe apples; total sugars 153.44 g/kg, water-soluble pectins 27.73 g/kg, phlorizin 12.61 g/kg, chlorogenic acid 18.90 g/kg, catechin, epicatechin, quercetin glycosides, phloretin-2-O-D-xyloglucoside (HPLC) | open-label, randomised crossover study; six healthy females with increased risk of cardiovascular disease and diabetes; 25 g of apple preparation (tested) or not supplemented (control) + glucose; duration: acute consumption | OGTT: glucose at 15 to 30 min reduced ↓ by about twofold, urinary glucose excretion after 2–4 h ↑ by about fivefold; phlorizin responsible for observed effects (based on extract composition and urine metabolite studies) | [147] | |
| commercial extract (BioActive Food GmbH); total polyphenols 44% (catechin equivalents), phlorizin 16%, quercetin 12.43%, chlorogenic acid 5.57% (HPLC/spectrophotometry) | randomised crossover study; 10 healthy men (23.5 ± 3.1 years old); 2.8 g of capsuled apple extract (tested) or not supplemented (control) + glucose; duration: acute consumption | OGTT: ↓ iAUC (0–15, 0–30, and 0–45 min), glucose level at 15, 30, and 45 min timepoints not significantly changed insulin: ↓ iAUC (0–30, 0–45, 0–60, and 0–90 min), with the highest reduction after 30 min; urinary glucose: 4.9-fold higher in the first 3 h | [75] | |
| Malus pumila Mill. | polyphenolic extract; 48.9% procyanidins (dimers, trimers, tetramers, pentamers, hexamers, and polymers), 14.1% flavan-3-ols (monomers) and 10.5% phloretin glucosides, including phlorizin (HPLC) | double-blinded, placebo-controlled study; 65 patients (30–60 years old) with normal (<100 mg/dL), high–normal (100–109 mg/dL), and borderline (110–125 mg/dL) glucose; 600 mg of apple polyphenols/day (tested) or placebo (control); duration: 12 weeks | OGTT: glucose ↓ after 30 min in the high–normal and borderline groups (164.0 ± 7.4 mg/dL) compared to controls (194.7 ± 10.4 mg/dL), ↓ iAUC (0–2 h), normal group—lack of changes; fasting plasma insulin, HOMA-I andR, HbA1c: unchanged; procyanidins responsible for observed effects (based on extract composition and literature studies) | [148] |
| Malus sylvestris (L.) Mill. | fruits | randomised pre-/post-test; 22 diabetes mellitus type II patients (40–55 years old); 300 g of apple/day (tested) or patients not given fruits (control); duration: 14 days | fasting blood glucose: ↓ by 40.27 ± 23.018 mg/dL in the apple group compared to baseline parameters; in the control group ↓, but not statistically | [150] |
| Malus Mill. sp. | clear or cloudy commercial juice; phlorizin 67/148 µM, chlorogenic acid 378/1304 µM, phloretin xyloglucoside 16/105 µM, D-fructose 6.28/59.3 g/L, D-glucose 22.6/17.9 g/L, sucrose 26.6/27.8 g/L (clear/cloudy juice) (HPLC) | three-way, single-blind, randomised crossover study; nine healthy adults (women and men, 24 ± 3.2 years old); 400 mL of clear or cloudy apple juice (tested) or drink without apple juice (control); duration: acute consumption | plasma glucose: ↓ at 15 and 30 min for clear juice, ↓ at 15 min and ↑ at 45–60 min for cloudy juice, ↓ iAUC (0–30 min), glucose absorption delayed; plasma insulin: ↓ over the first 90 min; GIP: ↓ over the first 90 min, ↓ iAUC (0–30 min); GLP-1: ↑ over the first 90 min (cloudy juice); chlorogenic acid and phlorizin responsible for observed effects (based on extract composition and literature studies) | [152] |
| Malus pumila Mill.; Pyrus pyrifolia (Burm.f.) Nakai | fruits; Malus: 58% fructose, 30.7% glucose, 11.3% sucrose; Pyrus: 57.3% fructose, 36.7% glucose, 6% sucrose; | randomised, eight-period crossover trial; 14 healthy women (18–25 years old); tested: Malus/Pyrus + iso-carbohydrate test meals (M + IC or P + IC) or hyper-carbohydrate test meals (M + HC or P + HC); IC, 50 g of available carbohydrate; HC, 65 g of available carbohydrate; 845–1342 kcal; control: water + iso-carbohydrate or hyper-carbohydrate test meals (W + IC or W + HC); duration: acute consumption | Malus: ↓ iAUC (0–180 min) by 45.7% and 19.0% for M + IC and M + HC, respectively; ↓ Cmax by 51.3% and 27.9% for M + IC and M + HC, respectively; maximum amplitude of glycaemic excursion ↓ by about 30–46%; Pyrus: ↓ iAUC (0–180 min) by 30.5% and 18.3% for M + IC and M + HC, respectively; ↓ Cmax by about 40%; maximum amplitude of glycaemic excursion ↓ by about 35–40% | [149] |
3.4. Polyphenols and Other Chemical Contributors to the Anti-Diabetic Activity of Maleae Fruits
3.4.1. Anthocyanins’ Contribution to the Amelanchier and Aronia Fruits’ Activity
3.4.2. Dihydrochalcone’s Contribution to the Malus Fruits’ Activity
3.4.3. Flavonols’ Contribution to the Chaenomeles, Cotoneaster, Malus, Pyrus, Sorbus, and Vauquelinia Fruits’ Activity
3.4.4. Proanthocyanidins’ Contribution to the Aronia, Crataegus, Chaenomeles, Cotoneaster, Malus, Pyracantha, Pyrus, and Sorbus Fruits’ Activity
3.4.5. Phenolic Acids’ Contribution to the Aronia, Chaenomeles, Malus, Mespilus, Pyrus, and Sorbus Fruits’ Activity
3.4.6. Contribution of Non-Phenolic Compounds to the Crataegus, Chaenomeles, and Sorbus Fruits’ Activity, and Their Synergy with Polyphenols
3.4.7. Impact of Monosaccharides on Fruits’ Anti-Diabetic Potential
3.5. The Anti-Diabetic Potential of the Most Promising Maleae Fruits—Concluding Thoughts
3.5.1. The Anti-Diabetic Potential of Aronia melanocarpa Fruits
3.5.2. The Anti-Diabetic Potential of Malus domestica Fruits
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; ISBN 978-2-930229-98-0. [Google Scholar]
- WHO Library Cataloguing. Global Report on Diabetes; WHO: Geneve, Switzerland, 2016; Volume 978, ISBN 9789241565257. [Google Scholar]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron. Physician 2016, 8, 1832–1842. [Google Scholar] [CrossRef]
- Tan, S.Y.; Mei Wong, J.L.; Sim, Y.J.; Wong, S.S.; Mohamed Elhassan, S.A.; Tan, S.H.; Ling Lim, G.P.; Rong Tay, N.W.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 364–372. [Google Scholar] [CrossRef]
- Reed, J.; Bain, S.; Kanamarlapudi, V. A review of current trends with type 2 diabetes epidemiology, aetiology, pathogenesis, treatments and future perspectives. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 3567–3602. [Google Scholar] [CrossRef]
- Fereidoon, S.; Cesarettin, A. (Eds.) Handbook of Functional Beverages and Human Health; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781466596429. [Google Scholar]
- Sun, C.; Liu, Y.; Zhan, L.; Rayat, G.R.; Xiao, J.; Jiang, H.; Li, X.; Chen, K. Anti-diabetic effects of natural antioxidants from fruits. Trends Food Sci. Technol. 2021, 117, 3–14. [Google Scholar] [CrossRef]
- Sun, J.; Shi, S.; Li, J.; Yu, J.; Wang, L.; Yang, X.; Guo, L.; Zhou, S. Phylogeny of Maleae (Rosaceae) based on multiple chloroplast regions: Implications to genera circumscription. Biomed. Res. Int. 2018, 2018, 6–9. [Google Scholar] [CrossRef]
- Mandal, D.; Wermund, U.; Phavaphutanon, L.; Cronje, R. (Eds.) Temperate Fruits; Series statement: Innovations in horticultural science; Apple Academic Press: Palm Bay, FL, USA, 2020; ISBN 9781003045861. [Google Scholar]
- United States Department of Agriculture. Fresh apples, grapes, and pears: World markets and trade. In Foreign Agricultural Service; United States Department of Agriculture: Washington, DC, USA, 2019; pp. 1–10. [Google Scholar]
- WFO. The World Flora Online. Available online: http://www.worldfloraonline.org/taxon/wfo-0000550491#synonyms (accessed on 20 August 2023).
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef]
- Wangensteen, H.; Bräunlich, M.; Nikolic, V.; Malterud, K.E.; Slimestad, R.; Barsett, H. Anthocyanins, proanthocyanidins and total phenolics in four cultivars of aronia: Antioxidant and enzyme inhibitory effects. J. Funct. Foods 2014, 7, 746–752. [Google Scholar] [CrossRef]
- Čakar, U.; Grozdanić, N.; Pejin, B.; Vasić, V.; Čakar, M.; Petrović, A.; Djordjević, B. Impact of vinification procedure on fruit wine inhibitory activity against α-glucosidase. Food Biosci. 2018, 25, 1–7. [Google Scholar] [CrossRef]
- Kicel, A.; Magiera, A.; Skrzywanek, M.; Malczuk, M.; Olszewska, M.A. The inhibition of α-glucosidase, α-amylase and protein glycation by phenolic extracts of Cotoneaster bullatus, Cotoneaster zabelii, and Cotoneaster integerrimus leaves and fruits: Focus on anti-hyperglycemic activity and kinetic parameters. Molecules 2022, 27, 7081. [Google Scholar] [CrossRef]
- Mecheri, A.; Amrani, A.; Benabderrahmane, W.; Bensouici, C.; Boubekri, N.; Benaissa, O.; Zama, D.; Benayache, F.; Benayache, S. In Vitro Pharmacological Screening of Antioxidant, Photoprotective, Cholinesterase, and α-Glucosidase Inhibitory Activities of Algerian Crataegus oxyacantha Fruits and Leaves Extracts. Pharm. Chem. J. 2021, 54, 1150–1156. [Google Scholar] [CrossRef]
- Chowdhury, S.S.; Islam, M.N.; Jung, H.A.; Choi, J.S. In vitro antidiabetic potential of the fruits of Crataegus pinnatifida. Res. Pharm. Sci. 2014, 9, 11–22. [Google Scholar] [PubMed]
- de Oliveira Raphaelli, C.; dos Santos Pereira, E.; Camargo, T.M.; Vinholes, J.; Rombaldi, C.V.; Vizzotto, M.; Nora, L. Apple Phenolic Extracts Strongly Inhibit α-Glucosidase Activity. Plant Foods Hum. Nutr. 2019, 74, 430–435. [Google Scholar] [CrossRef]
- Wang, H.; Ye, Y.H.; Wang, H.H.; Liu, J.; Liu, Y.J.; Jiang, B.W. HPLC-QTOF-MS/MS profiling, antioxidant, and α-glucosidase inhibitory activities of Pyracantha fortuneana fruit extracts. J. Food Biochem. 2019, 43, e12821. [Google Scholar] [CrossRef]
- Wei, M.; Chai, W.M.; Yang, Q.; Wang, R.; Peng, Y. Novel Insights into the Inhibitory Effect and Mechanism of Proanthocyanidins from Pyracantha fortuneana Fruit on α-Glucosidase. J. Food Sci. 2017, 82, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Bettadiah, B.K.; Kudachikar, V.B. Food Bioscience Chemical composition and in vitro antihyperglycemic potential of Kainth fruit (Pyrus pashia Buch.-Ham ex D.Don). Food Biosci. 2021, 42, 101119. [Google Scholar] [CrossRef]
- Broholm, S.L.; Gramsbergen, S.M.; Nyberg, N.T.; Jäger, A.K.; Staerk, D. Potential of Sorbus berry extracts for management of type 2 diabetes: Metabolomics investigation of 1H NMR spectra, α-amylase and α-glucosidase inhibitory activities, and in vivo anti-hyperglycaemic activity of S. norvegica. J. Ethnopharmacol. 2019, 242, 112061. [Google Scholar] [CrossRef]
- Hasbal, G.; Yilmaz Ozden, T.; Can, A. In vitro Antidiabetic Activities of Two Sorbus Species. Eur. J. Biol. 2017, 76, 57–60. [Google Scholar] [CrossRef]
- Flores-Bocanegra, L.; Pérez-Vásquez, A.; Torres-Piedra, M.; Bye, R.; Linares, E.; Mata, R. α-Glucosidase inhibitors from Vauquelinia corymbosa. Molecules 2015, 20, 15330–15342. [Google Scholar] [CrossRef] [PubMed]
- Alongi, M.; Verardo, G.; Gorassini, A.; Anese, M. Effect of pasteurization on in vitro α-glucosidase inhibitory activity of apple juice. LWT 2018, 98, 366–371. [Google Scholar] [CrossRef]
- Boath, A.S.; Stewart, D.; McDougall, G.J. Berry components inhibit α-glucosidase in vitro: Synergies between acarbose and polyphenols from black currant and rowanberry. Food Chem. 2012, 135, 929–936. [Google Scholar] [CrossRef]
- Grussu, D.; Stewart, D.; McDougall, G.J. Berry polyphenols inhibit α-amylase in vitro: Identifying active components in rowanberry and raspberry. J. Agric. Food Chem. 2011, 59, 2324–2331. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, J.; Meng, X. Aronia melanocarpa anthocyanin extracts are an effective regulator of suppressor of cytokine signaling 3-dependent insulin resistance in HepG2 and C2C12 cells. J. Funct. Foods 2020, 75, 104258. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Pawlik, N. Japanese quince (Chaenomeles japonica L.) fruit polyphenolic extract modulates carbohydrate metabolism in HepG2 cells via AMP-activated protein kinase. Acta Biochim. Pol. 2018, 65, 67–78. [Google Scholar] [CrossRef]
- Manzano, M.; Giron, M.D.; Vilchez, J.D.; Sevillano, N.; El-Azem, N.; Rueda, R.; Salto, R.; Lopez-Pedrosa, J.M. Apple polyphenol extract improves insulin sensitivity in vitro and in vivo in animal models of insulin resistance. Nutr. Metab. 2016, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- You, M.K.; Kim, H.J.; Rhyu, J.; Kim, H.A. Pear pomace ethanol extract improves insulin resistance through enhancement of insulin signaling pathway without lipid accumulation. Nutr. Res. Pract. 2017, 11, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Castro-Acosta, M.L.; Stone, S.G.; Mok, J.E.; Mhajan, R.K.; Fu, C.I.; Lenihan-Geels, G.N.; Corpe, C.P.; Hall, W.L. Apple and blackcurrant polyphenol-rich drinks decrease postprandial glucose, insulin and incretin response to a high-carbohydrate meal in healthy men and women. J. Nutr. Biochem. 2017, 49, 53–62. [Google Scholar] [CrossRef]
- Manzano, S.; Williamson, G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol. Nutr. Food Res. 2010, 54, 1773–1780. [Google Scholar] [CrossRef]
- Mela, D.J.; Cao, X.Z.; Dobriyal, R.; Fowler, M.I.; Lin, L.; Joshi, M.; Mulder, T.J.P.; Murray, P.G.; Peters, H.P.F.; Vermeer, M.A.; et al. The effect of 8 plant extracts and combinations on post-prandial blood glucose and insulin responses in healthy adults: A randomized controlled trial. Nutr. Metab. 2020, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Kim, M.; Park, Y.; Bae, M.; Kang, H.; Hu, S.; Pham, T.X.; Carpenter, R.; Lee, J.; Lee, O.; et al. Anthocyanin-Rich Aronia Berry Extract Mitigates High-Fat and High-Sucrose Diet-Induced Adipose Tissue Inflammation by Inhibiting Nuclear Factor-j B Activation. J. Med. Food 2021, 24, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Zakłos-Szyda, M.; Majewska, I.; Redzynia, M.; Koziołkiewicz, M. Antidiabetic Effect of Polyphenolic Extracts from Selected Edible Plants as α-Amylase, α-Glucosidase and PTP1B Inhibitors, and β Pancreatic Cells Cytoprotective Agents—A Comparative Study. Curr. Top. Med. Chem. 2015, 15, 2431–2444. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Sun, L.; Fang, Z.; Chen, L.; Zhao, P.; Wang, Z.; Guo, Y. Effect of young apple (Malus domestica Borkh. cv. Red Fuji) polyphenols on alleviating insulin resistance. Food Biosci. 2020, 36, 100637. [Google Scholar] [CrossRef]
- Mueller, M.; Jungbauer, A. Culinary plants, herbs and spices—A rich source of PPARγ ligands. Food Chem. 2009, 117, 660–667. [Google Scholar] [CrossRef]
- Kim, D.J.; Chung, M.J.; You, J.K.; Seo, D.J.; Kim, J.M.; Choe, M. Effect of medicinal plant water extracts on glucose-regulating enzyme activities in goto-kakizaki rat liver cytosol. J. Korean Soc. Food Sci. Nutr. 2009, 38, 1331–1335. [Google Scholar] [CrossRef]
- Kraft, T.F.B.; Dey, M.; Rogers, R.B.; Ribnicky, D.M.; Gipp, D.M.; Cefalu, W.T.; Raskin, I.; Lila, M.A. Phytochemical composition and metabolic performance-enhancing activity of dietary berries traditionally used by native North Americans. J. Agric. Food Chem. 2008, 56, 654–660. [Google Scholar] [CrossRef]
- Kazeem, M.I.; Adeyemi, A.A.; Adenowo, A.F.; Akinsanya, M.A. Carica papaya Linn. fruit extract inhibited the activities of aldose reductase and sorbitol dehydrogenase: Possible mechanism for amelioration of diabetic complications. Futur. J. Pharm. Sci. 2020, 6, 96. [Google Scholar] [CrossRef]
- Yu, C.H.J.; Migicovsky, Z.; Song, J.; Rupasinghe, H.P.V. (Poly)phenols of apples contribute to in vitro antidiabetic properties: Assessment of Canada’s Apple Biodiversity Collection. Plants People Planet. 2022, 5, 225–240. [Google Scholar] [CrossRef]
- Rutkowska, M.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Zakrzewska, A.; Magiera, A.; Olszewska, M.A. Novel insight into biological activity and phytochemical composition of Sorbus aucuparia L. fruits: Fractionated extracts as inhibitors of protein glycation and oxidative/nitrative damage of human plasma components. Food Res. Int. 2021, 147, 110526. [Google Scholar] [CrossRef]
- Termentzi, A.; Alexiou, P.; Demopoulos, V.J.; Kokkalou, E. The aldose reductase inhibitory capacity of Sorbus domestica fruit extracts depends on their phenolic content and may be useful for the control of diabetic complications. Pharmazie 2008, 63, 693–696. [Google Scholar] [CrossRef]
- Cohen, G.N. Biosynthesis of the Amino Acids of the Glutamic Acid Family and Its Regulation. In Microbial Biochemistry; Springer: Dordrecht, The Netherland, 2014; ISBN 9789048194360. [Google Scholar]
- Gupta, P.; Taiyab, A.; Hassan, M.I. Emerging role of protein kinases in diabetes mellitus: From mechanism to therapy. Adv. Protein Chem. Struct. Biol. 2021, 124, 47–85. [Google Scholar] [CrossRef]
- Han, H.S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef]
- Jang, H.R.; Lee, H.-Y. Mechanisms linking gut microbial metabolites to insulin resistance. World J. Diabetes 2021, 12, 730–744. [Google Scholar] [CrossRef]
- Mathebula, S.D. Polyol pathway: A possible mechanism of diabetes complications in the eye. Afr. Vis. Eye Health 2015, 74, 13. [Google Scholar] [CrossRef]
- Mirra, P.; Nigro, C.; Prevenzano, I.; Leone, A.; Raciti, G.A.; Formisano, P.; Beguinot, F.; Miele, C. The destiny of glucose from a MicroRNA perspective. Front. Endocrinol. 2018, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Schultze, S.M.; Hemmings, B.A.; Niessen, M.; Tschopp, O. PI3K/AKT, MAPK and AMPK signalling: Protein kinases in glucose homeostasis. Expert. Rev. Mol. Med. 2012, 14, e1. [Google Scholar] [CrossRef]
- Soto-Avellaneda, A.; Morrison, B.E. Signaling and other functions of lipids in autophagy: A review. Lipids Health Dis. 2020, 19, 214. [Google Scholar] [CrossRef]
- Titchenell, P.M.; Lazar, M.A.; Birnbaum, M.J. Unraveling the Regulation of Hepatic Metabolism by Insulin. Trends Endocrinol. Metab. 2017, 28, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Gusdon, A.M.; Liu, H.; Qu, S. Effects of glucagon-like peptide-1 receptor agonists on non-alcoholic fatty liver disease and inflammation. World J. Gastroenterol. 2014, 20, 14821–14830. [Google Scholar] [CrossRef]
- Lachowicz, S.; Wiśniewski, R.; Ochmian, I.; Drzymała, K.; Pluta, S. Anti-microbiological, anti-hyperglycemic and anti-obesity potency of natural antioxidants in fruit fractions of saskatoon berry. Antioxidants 2019, 8, 397. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, T.; Melzig, M.F. Medicinal Plants Traditionally Used for Treatment of Obesity and Diabetes Mellitus—Screening for Pancreatic Lipase and α-Amylase Inhibition. Phyther. Res. 2016, 30, 260–266. [Google Scholar] [CrossRef]
- Siegień, J.; Buchholz, T.; Popowski, D.; Granica, S.; Osińska, E.; Melzig, M.F.; Czerwińska, M.E. Pancreatic lipase and α-amylase inhibitory activity of extracts from selected plant materials after gastrointestinal digestion in vitro. Food Chem. 2021, 355, 129414. [Google Scholar] [CrossRef]
- Worsztynowicz, P.; Napierała, M.; Białas, W.; Grajek, W.; Olkowicz, M. Pancreatic α-amylase and lipase inhibitory activity of polyphenolic compounds present in the extract of black chokeberry (Aronia melanocarpa L.). Process Biochem. 2014, 49, 1457–1463. [Google Scholar] [CrossRef]
- Miao, J.; Li, X.; Zhao, C.; Gao, X.; Wang, Y.; Cheng, K.; Gao, W. Solvents effect on active chemicals and activities of antioxidant, anti-α-glucosidase and inhibit effect on smooth muscle contraction of isolated rat jejunum of Chaenomeles speciosa. J. Funct. Foods 2018, 40, 146–155. [Google Scholar] [CrossRef]
- Zhao, C.; Miao, J.; Li, X.; Chen, X.; Mao, X.; Wang, Y.; Hua, X.; Gao, W. Impact of in vitro simulated digestion on the chemical composition and potential health benefits of Chaenomeles speciosa and Crataegus pinnatifida. Food Biosci. 2020, 35, 100511. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, H.; Zhang, P.; Gao, L.; Yan, N.; Li, P.; Liu, X.; Du, Y.; Shen, G. Chemical composition, antioxidant activity and α-Glucosidase inhibitory activity of chaenomeles speciosa from four production areas in China. Molecules 2018, 23, 2518. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Zhao, C.; Li, X.; Chen, X.; Mao, X.; Huang, H.; Wang, T.; Gao, W. Chemical Composition and Bioactivities of Two Common Chaenomeles Fruits in China: Chaenomeles speciosa and Chaenomeles sinensis. J. Food Sci. 2016, 81, H2049–H2058. [Google Scholar] [CrossRef]
- Miao, J.; Li, X.; Zhao, C.; Gao, X.; Wang, Y.; Gao, W. Active compounds, antioxidant activity and α-glucosidase inhibitory activity of different varieties of Chaenomeles fruits. Food Chem. 2018, 248, 330–339. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Wojdyło, A.; Tkacz, K.; Nowicka, P.; Golis, T.; Bąbelewski, P. ABTS on-line antioxidant, α-amylase, α-glucosidase, pancreatic lipase, acetyl-and butyrylcholinesterase inhibition activity of chaenomeles fruits determined by polyphenols and other chemical compounds. Antioxidants 2020, 9, 60. [Google Scholar] [CrossRef]
- Al-Hallaq, E.K.; Kasabri, V.; Abdalla, S.S.; Bustanji, Y.K.; Afifi, F.U. Anti-Obesity and Antihyperglycemic Effects of Crataegus aronia Extracts: In Vitro and in Vivo Evaluations. Food Nutr. Sci. 2013, 04, 972–983. [Google Scholar] [CrossRef]
- Renda, G.; Özel, A.; Barut, B.; Korkmaz, B.; Yayli, N. In vitro protection by crataegus microphylla extracts against oxidative damage and enzyme inhibition effects. Turk. J. Pharm. Sci. 2018, 15, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Son, H.J.; Huang, C.; Lee, S.K.; Lohakare, J.; Wang, M.H. Comparison of Crataegus pinnatifida Bunge var. typica Schneider and C. pinnatifida Bunge fruits for antioxidant, anti-α-glucosidase, and anti-inflammatory activities. Food Sci. Biotechnol. 2010, 19, 769–775. [Google Scholar] [CrossRef]
- Lin, Y.T.; Lin, H.R.; Yang, C.S.; Liaw, C.C.; Sung, P.J.; Kuo, Y.H.; Cheng, M.J.; Chen, J.J. Antioxidant and Anti-α-Glucosidase Activities of Various Solvent Extracts and Major Bioactive Components from the Fruits of Crataegus pinnatifida. Antioxidants 2022, 11, 320. [Google Scholar] [CrossRef]
- Sakhri, F.Z.; Zerizer, S.; Bensouici, C. Evaluation of the Antioxidant, Antidiabetic and Immunomodulatory Activity of Cydonia oblonga Fruit Extract. Chiang Mai Univ. J. Nat. Sci. 2021, 20, e2021052. [Google Scholar] [CrossRef]
- De Bellis, R.; Chiarantini, L.; Potenza, L.; Gorassini, A.; Verardo, G.; De Marco, R.; Benayada, L.; Stocchi, V.; Cristina Albertini, M.; Fraternale, D. High production of secondary metabolites and biological activities of Cydonia oblonga Mill. pulp fruit callus. J. Funct. Foods 2022, 94, 105133. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Tkacz, K.; Turkiewicz, I.P.; Nowicka, P.; Hernández, F.; Lech, K.; Carbonell-Barrachina, Á.A.; Wojdyło, A. Inhibition of enzymes associated with metabolic and neurological disorder by dried pomegranate sheets as a function of pomegranate cultivar and fruit puree. J. Sci. Food Agric. 2021, 101, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
- Besnard, M.; Megard, D.; Rousseau, I.; Zaragoza, M.C.; Martinez, N.; Mit Javila, M.T.; Inisan, C. Polyphenolic apple extract: Characterisation, safety and potential effect on human glucose metabolism. Agro Food Ind. Hi Tech. 2008, 19, 16–19. [Google Scholar]
- Prpa, E.J.; Corpe, C.P.; Atkinson, B.; Blackstone, B.; Leftley, E.S.; Parekh, P.; Philo, M.; Kroon, P.A.; Hall, W.L. Apple polyphenol-rich drinks dose-dependently decrease early-phase postprandial glucose concentrations following a high-carbohydrate meal: A randomized controlled trial in healthy adults and in vitro studies. J. Nutr. Biochem. 2020, 85, 108466. [Google Scholar] [CrossRef]
- Cianfruglia, L.; Morresi, C.; Bacchetti, T.; Armeni, T.; Ferretti, G. Protection of polyphenols against glyco-oxidative stress: Involvement of glyoxalase pathway. Antioxidants 2020, 9, 1006. [Google Scholar] [CrossRef]
- Schulze, C.; Bangert, A.; Kottra, G.; Geillinger, K.E.; Schwanck, B.; Vollert, H.; Blaschek, W.; Daniel, H. Inhibition of the intestinal sodium-coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans. Mol. Nutr. Food Res. 2014, 58, 1795–1808. [Google Scholar] [CrossRef] [PubMed]
- Alongi, M.; Verardo, G.; Gorassini, A.; Lemos, M.A.; Hungerford, G.; Cortella, G.; Anese, M. Phenolic content and potential bioactivity of apple juice as affected by thermal and ultrasound pasteurization. Food Funct. 2019, 10, 7366–7377. [Google Scholar] [CrossRef]
- Ankolekar, C.; Sarkar, D.; Greene, D.; Shetty, K. Using Biological Elicitation to Improve Type 2 Diabetes Targeted Food Quality of Stored Apple. Front. Sustain. Food Syst. 2021, 5, 709384. [Google Scholar] [CrossRef]
- Barbosa, A.C.L.; Pinto, M.D.S.; Sarkar, D.; Ankolekar, C.; Greene, D.; Shetty, K. Influence of varietal and ph variation on antihyperglycemia and antihypertension properties of long-term stored apples using in vitro assay models. J. Food Biochem. 2012, 36, 479–493. [Google Scholar] [CrossRef]
- Gong, T.; Yang, X.; Bai, F.; Li, D.; Zhao, T.; Zhang, J.; Sun, L.; Guo, Y. Young apple polyphenols as natural α-glucosidase inhibitors: In vitro and in silico studies. Bioorg. Chem. 2020, 96, 103625. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Aslam, N.; Sheikh, A. Malus domestica as an Inhibitor of Glycation. Sch. Acad. J. Biosci. Sch. Acad. J. Biosci. 2014, 2, 2321–68831. [Google Scholar]
- Wang, X.; Han, M.; Zhang, M.; Wang, Y.; Ren, Y.; Yue, T.; Gao, Z. In vitro evaluation of the hypoglycemic properties of lactic acid bacteria and its fermentation adaptability in apple juice. LWT 2021, 136, 110363. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Han, M.; Liang, J.; Zhang, M.; Bai, X.; Yue, T.; Gao, Z. Evaluating the changes in phytochemical composition, hypoglycemic effect, and influence on mice intestinal microbiota of fermented apple juice. Food Res. Int. 2022, 155, 110998. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Campiglia, P.; Stiuso, P.; Ritieni, A.; Novellino, E. Nutraceutical potential of polyphenolic fractions from Annurca apple (M. pumila Miller cv Annurca). Food Chem. 2013, 140, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Nasu, R.; Miura, M.; Gomyo, T. Effects of fruit, spices and herbs on α glucosidase activity and glycemic index. Food Sci. Technol. Res. 2005, 11, 77–81. [Google Scholar] [CrossRef]
- Dorsey, P.G.; Greenspan, P. Inhibition of nonenzymatic protein glycation by pomegranate and other fruit juices. J. Med. Food 2014, 17, 447–454. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Johargy, A.; Gammoh, S.; Ereifej, K.; Almajoul, A.; Al-Karaki, G.; Kubow, S.; Ghozlan, K.A. Phenolic contents, in vitro antioxidant activities and biological properties, and HPLC profiles of free and conjugated phenolics extracted from onion, pomegranate, grape, and apple. Int. J. Food Prop. 2017, 20, 1823–1837. [Google Scholar] [CrossRef]
- Żołnierczyk, A.K.; Ciałek, S.; Styczyńska, M.; Oziembłowski, M. Functional properties of fruits of common medlar (Mespilus germanica L.) extract. Appl. Sci. 2021, 11, 7528. [Google Scholar] [CrossRef]
- Wu, H.; Xu, B. Inhibitory effects of onion against α-glucosidase activity and its correlation with phenolic antioxidants. Int. J. Food Prop. 2014, 17, 599–609. [Google Scholar] [CrossRef]
- Isbilir, S.S.; Kabala, S.I.; Yagar, H. Assessment of in vitro antioxidant and antidiabetic capacities of medlar (Mespilus germanica). Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 384–389. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Zhou, B.; Li, H.; Zeng, J.; Gao, W. Anti-diabetic activity in type 2 diabetic mice and α-glucosidase inhibitory, antioxidant and anti-inflammatory potential of chemically profiled pear peel and pulp extracts (Pyrus spp.). J. Funct. Foods 2015, 13, 276–288. [Google Scholar] [CrossRef]
- Pavlović, M.O.; Aradski, A.A.; Savić, A.; Janković, S.; Milutinović, M.; Marin, P.D.; Duletić-Lausević, S. Traditional varieties and wild pear from Serbia: A link among antioxidant, antidiabetic and cytotoxic activities of fruit peel and flesh. Bot. Serbica 2021, 45, 203–213. [Google Scholar] [CrossRef]
- Pucel, N.; Sarkar, D.; Labbe, R.G.; Khanongnuch, C.; Shetty, K. Improving Health Targeted Food Quality of Blackberry: Pear Fruit Synergy Using Lactic Acid Bacterial Fermentation. Front. Sustain. Food Syst. 2021, 5, 703672. [Google Scholar] [CrossRef]
- Ankolekar, C.; Pinto, M.; Greene, D.; Shetty, K. In vitro bioassay based screening of antihyperglycemia and antihypertensive activities of Lactobacillus acidophilus fermented pear juice. Innov. Food Sci. Emerg. Technol. 2012, 13, 221–230. [Google Scholar] [CrossRef]
- Barbosa, A.C.L.; Sarkar, D.; Pinto, M.D.S.; Ankolekar, C.; Greene, D.; Shetty, K. Type 2 diabetes relevant bioactive potential of freshly harvested and long-term stored pears using in vitro assay models. J. Food Biochem. 2013, 37, 677–686. [Google Scholar] [CrossRef]
- Sarkar, D.; Ankolekar, C.; Pinto, M.; Shetty, K. Dietary functional benefits of Bartlett and Starkrimson pears for potential management of hyperglycemia, hypertension and ulcer bacteria Helicobacter pylori while supporting beneficial probiotic bacterial response. Food Res. Int. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Ci, Z.; Jiang, C.; Cui, Y.; Kojima, M. Suppressive Effect of Polyphenols from Immature Pear Fruits on Blood Glucose Levels. J. Food Nutr. Res. 2018, 6, 445–449. [Google Scholar] [CrossRef]
- He, M.; Zeng, J.; Zhai, L.; Liu, Y.; Wu, H.; Zhang, R.; Li, Z.; Xia, E. Effect of In Vitro Simulated Gastrointestinal Digestion on Polyphenol and Polysaccharide Content and Their Biological Activities among 22 Fruit Juices; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 102, ISBN 8676922896. [Google Scholar]
- Harris, C.S.; Cuerrier, A.; Lamont, E.; Haddad, P.S.; Arnason, J.T.; Bennett, S.A.L.; Johns, T. Investigating Wild Berries as a Dietary Approach to Reducing the Formation of Advanced Glycation Endproducts: Chemical Correlates of In Vitro Antiglycation Activity. Plant Foods Hum. Nutr. 2014, 69, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Hasbal, G.; Yilmaz-Ozden, T.; Sen, M.; Yanardag, R.; Can, A. In vitro investigation of Sorbus domestica as an enzyme inhibitor. Istanb. J. Pharm. 2020, 50, 28–32. [Google Scholar]
- Chen, J.; Meng, X. Aronia melanocarpa Anthocyanin Extracts Improve Hepatic Structure and Function in High-Fat Diet-/Streptozotocin-Induced T2DM Mice. J. Agric. Food Chem. 2022, 70, 11531–11543. [Google Scholar] [CrossRef] [PubMed]
- Alaghawani, W.; Naser, I. Study the hypoglycemic effect of Crataegus laevigata in diabetic rats. Int. J. Pharm. Clin. Res. 2013, 5, 145–149. [Google Scholar]
- Liu, S.; Yu, J.; Fu, M.; Wang, X.; Chang, X. Regulatory effects of hawthorn polyphenols on hyperglycemic, inflammatory, insulin resistance responses, and alleviation of aortic injury in type 2 diabetic rats. Food Res. Int. 2021, 142, 110239. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, C.; Bhargava, A. Anti-diabetic and hypolipidemic activity of fruits of Pyrus communis L. in hyperglycemic rats. Asian J. Pharm. Clin. Res. 2013, 6, 108–111. [Google Scholar]
- Huang, F.; Zhao, R.; Xia, M.; Shen, G.X. Impact of cyanidin-3-glucoside on gut microbiota and relationship with metabolism and inflammation in high fat-high sucrose diet-induced insulin resistant mice. Microorganisms 2020, 8, 1238. [Google Scholar] [CrossRef]
- Zhao, R.; Khafipour, E.; Sepehri, S.; Huang, F.; Beta, T.; Shen, G.X. Impact of Saskatoon berry powder on insulin resistance and relationship with intestinal microbiota in high fat–high sucrose diet-induced obese mice. J. Nutr. Biochem. 2019, 69, 130–138. [Google Scholar] [CrossRef]
- Zhao, R.; Huang, F.; Shen, G.X. Dose-responses relationship in glucose lowering and gut dysbiosis to saskatoon berry powder supplementation in high fat-high sucrose diet-induced insulin resistant mice. Microorganisms 2021, 9, 1553. [Google Scholar] [CrossRef]
- Zhao, R.; Xiang, B.; Dolinsky, V.W.; Xia, M.; Shen, G.X. Saskatoon berry powder reduces hepatic steatosis and insulin resistance in high fat-high sucrose diet-induced obese mice. J. Nutr. Biochem. 2021, 95, 108778. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeon, Y.E.; Jung, J.I.; Kim, S.M.; Hong, S.H.; Lee, J.; Hwang, J.S.; Hwang, M.O.; Kwon, K.; Kim, E.J. Anti-obesity effect of Cydonia oblonga Miller extract in high-fat diet-induced obese C57BL/6 mice. J. Funct. Foods 2022, 89, 104945. [Google Scholar] [CrossRef]
- Boqué, N.; Campión, J.; de la Iglesia, R.; de la Garza, A.L.; Milagro, F.I.; San Román, B.; Bañuelos, Ó.; Martínez, J.A. Screening of polyphenolic plant extracts for anti-obesity properties in Wistar rats. J. Sci. Food Agric. 2013, 93, 1226–1232. [Google Scholar] [CrossRef]
- Han, X.; Zhao, W.; Zhou, Q.; Chen, H.; Yuan, J.; Zhang, X.F.; Zhang, Z. Procyanidins from Hawthorn (Crataegus pinnatifida) Alleviates Lipid Metabolism Disorder via Inhibiting Insulin Resistance and Oxidative Stress, Normalizing Gut Microbiota Structure and Intestinal Barrier, Further Suppressing Hepatic Inflammation and Li. Food Funct. 2022, 13, 7901–7917. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.D.; Kang, S.H.; Moon, K.H.; Lee, J.H.; Kim, D.G.; Kim, W.; Kim, J.S.; Ahn, B.Y.; Jin, J.S. The Effect of Aronia Berry on Type 1 Diabetes In Vivo and In Vitro. J. Med. Food 2018, 21, 244–253. [Google Scholar] [CrossRef]
- He, K.; Li, X.; Chen, X.; Ye, X.; Huang, J.; Jin, Y.; Li, P.; Deng, Y.; Jin, Q.; Shi, Q.; et al. Evaluation of antidiabetic potential of selected traditional Chinese medicines in STZ-induced diabetic mice. J. Ethnopharmacol. 2011, 137, 1135–1142. [Google Scholar] [CrossRef]
- Ogura, K.; Ogura, M.; Shoji, T.; Sato, Y.; Tahara, Y.; Yamano, G.; Sato, H.; Sugizaki, K.; Fujita, N.; Tatsuoka, H.; et al. Oral Administration of Apple Procyanidins Ameliorates Insulin Resistance via Suppression of Pro-Inflammatory Cytokine Expression in Liver of Diabetic ob/ob Mice. J. Agric. Food Chem. 2016, 64, 8857–8865. [Google Scholar] [CrossRef]
- Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z. Ingestion of black chokeberry fruit extract leads to intestinal and systemic changes in a rat model of prediabetes and hyperlipidemia. Plant Foods Hum. Nutr. 2008, 63, 176–182. [Google Scholar] [CrossRef]
- Yamane, T.; Kozuka, M.; Konda, D.; Nakano, Y.; Nakagaki, T.; Ohkubo, I.; Ariga, H. Improvement of blood glucose levels and obesity in mice given aronia juice by inhibition of dipeptidyl peptidase IV and α-glucosidase. J. Nutr. Biochem. 2016, 31, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Xin, G.; Zhang, B.; Wang, Y.; Ning, C.; Meng, X. Beneficial effects of Aronia melanocarpa berry extract on hepatic insulin resistance in type 2 diabetes mellitus rats. J. Food Sci. 2020, 85, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Anderson, R.A. An extract of chokeberry attenuates weight gain and modulates insulin, adipogenic and inflammatory signalling pathways in epididymal adipose tissue of rats fed a fructose-rich diet. Br. J. Nutr. 2012, 108, 581–587. [Google Scholar] [CrossRef]
- Shih, C.C.; Lin, C.H.; Lin, Y.J.; Wu, J.B. Validation of the antidiabetic and hypolipidemic effects of hawthorn by assessment of gluconeogenesis and lipogenesis related genes and AMP-activated protein kinase phosphorylation. Evid. Based Complement. Altern. Med. 2013, 2013, 597067. [Google Scholar] [CrossRef]
- Yamane, T.; Imai, M.; Handa, S.; Yamada, K.; Sakamoto, T.; Ishida, T.; Inui, H.; Yamamoto, Y.; Nakagaki, T.; Nakano, Y. Reduction of blood glucose and HbA1c levels by cyanidin 3,5-diglucoside in KKAy mice. J. Funct. Foods 2019, 58, 21–26. [Google Scholar] [CrossRef]
- Heidarianpour, A.; Keshvari, M.; Shahidi, S.; Zarei, M. Modulation of GPC-4 and GPLD1 serum levels by improving glycemic indices in type 2 diabetes: Resistance training and hawthorn extract intervention. Heliyon 2023, 9, e15537. [Google Scholar] [CrossRef] [PubMed]
- du Preez, R.; Wanyonyi, S.; Mouatt, P.; Panchal, S.K.; Brown, L. Saskatoon berry Amelanchier alnifolia regulates glucose metabolism and improves cardiovascular and liver signs of diet-induced metabolic syndrome in rats. Nutrients 2020, 12, 931. [Google Scholar] [CrossRef]
- Zahoor, M.; Jan, M.R.; Naz, S. Investigation of repressive and enhancive effects of fruit extracts on the activity of glucose-6-phophatase. Pak. J. Pharm. Sci. 2016, 29, 1985–1991. [Google Scholar]
- Nagahora, N.; Ito, Y.; Nagasawa, T. Dietary Chinese quince polyphenols suppress generation of α-dicarbonyl compounds in diabetic KK-Ay mice. J. Agric. Food Chem. 2013, 61, 6629–6635. [Google Scholar] [CrossRef]
- Zhao, R.; Xie, X.; Le, K.; Li, W.; Moghadasian, M.H.; Beta, T.; Shen, G.X. Endoplasmic reticulum stress in diabetic mouse or glycated LDL-treated endothelial cells: Protective effect of Saskatoon berry powder and cyanidin glycans. J. Nutr. Biochem. 2015, 26, 1248–1253. [Google Scholar] [CrossRef]
- Zhao, R.; Le, K.; Li, W.; Ren, S.; Moghadasian, M.H.; Beta, T.; Shen, G.X. Effects of Saskatoon berry powder on monocyte adhesion to vascular wall of leptin receptor-deficient diabetic mice. J. Nutr. Biochem. 2014, 25, 851–857. [Google Scholar] [CrossRef]
- Moghadasian, M.H.; Masisi, K.; Le, K.; Beta, T.; Shen, G.; Fischer, G. The Potential Anti-Diabetic Effects of Saskatoon Berry in Experimental Mouse Models. Austin J. Nutr. Food Sci. 2019, 7, 1111. [Google Scholar] [CrossRef]
- Baum, J.I.; Howard, L.R.; Prior, R.L.; Lee, S.O. Effect of Aronia melanocarpa (Black Chokeberry) supplementation on the development of obesity in mice fed a high-fat diet. J. Berry Res. 2016, 6, 203–212. [Google Scholar] [CrossRef]
- Bhaswant, M.; Shafie, S.R.; Mathai, M.L.; Mouatt, P.; Brown, L. Anthocyanins in chokeberry and purple maize attenuate diet-induced metabolic syndrome in rats. Nutrition 2017, 41, 24–31. [Google Scholar] [CrossRef]
- Oprea, E.; Manolescu, B.N.; Fǎrcǎşanu, I.C.; Mladin, P.; Mihele, D. Studies concerning antioxidant and hypoglycaemic activity of Aronia melanocarpa fruits. Farmacia 2014, 62, 254–263. [Google Scholar]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Tancheva, S.; Belcheva, A. Hypoglycemic and hypolipidemic effects of Aronia melanocarpa fruit juice in streptozotocin-induced diabetic rats. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Shimizu, H.; Okazaki, Y.; Sakaguchi, H.; Taira, T.; Suzuki, T.; Chiji, H. Anthocyanin-rich phytochemicals from aronia fruits inhibit visceral fat accumulation and hyperglycemia in high-fat diet-induced dietary obese rats. J. Oleo Sci. 2015, 64, 1243–1250. [Google Scholar] [CrossRef]
- Lipińska, P.; Jóźwik, A. Hepatoprotective, Hypoglycemic, and Hypolipidemic Effect of Chokeberry Pomace on Polish Merino Lambs. Anim. Biotechnol. 2018, 29, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Ljubuncic, P.; Azaizeh, H.; Cogan, U.; Bomzon, A. The effects of a decoction prepared from the leaves and unripe fruits of crataegus aronia in streptozotocin-induced diabetic rats. J. Complement. Integr. Med. 2006, 3, 6. [Google Scholar] [CrossRef]
- Asgari, M.; Salehi, I.; Ranjbar, K.; Khosravi, M. Biomedicine & Pharmacotherapy Interval training and Crataegus persica ameliorate diabetic nephropathy via miR-126/Nrf-2 mediated inhibition of stress oxidative in rats with diabetes after myocardial ischemia-reperfusion injury. Biomed. Pharmacother. 2022, 153, 113411. [Google Scholar] [CrossRef] [PubMed]
- Valipour Chahardahcharic, S.; Setorki, M. The effect of hydroalcoholic extract of crataegus monogyna on hyperglycemia, oxidative stress and pancreatic tissue damage in streptozotocin-induced diabetic rats. J. Herbmed Pharmacol. 2018, 7, 294–299. [Google Scholar] [CrossRef]
- Aierken, A.; Buchholz, T.; Chen, C.; Zhang, X.; Melzig, M.F. Hypoglycemic effect of hawthorn in type II diabetes mellitus rat model. J. Sci. Food Agric. 2017, 97, 4557–4561. [Google Scholar] [CrossRef]
- Pirmoghani, A.; Salehi, I.; Moradkhani, S.; Karimi, S.A.; Salehi, S. Effect of Crataegus extract supplementation on diabetes induced memory deficits and serum biochemical parameters in male rats. IBRO Rep. 2019, 7, 90–96. [Google Scholar] [CrossRef]
- Zarrinkalam, E.; Ranjbar, K.; Salehi, I.; Kheiripour, N.; Komaki, A. Resistance training and hawthorn extract ameliorate cognitive deficits in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018, 97, 503–510. [Google Scholar] [CrossRef]
- Fathy, S.M.; Drees, E.A. Protective effects of Egyptian cloudy apple juice and apple peel extract on lipid peroxidation, antioxidant enzymes and inflammatory status in diabetic rat pancreas. BMC Complement. Altern. Med. 2016, 16, 8. [Google Scholar] [CrossRef]
- Wusu, D.; Kazeem, M.; Lawal, O.; Opoku, A. Antidiabetic Effects of Some Tropical Fruit Extracts in Fructose Induced Insulin Resistant Wistar Rats. Br. J. Pharm. Res. 2015, 7, 230–235. [Google Scholar] [CrossRef]
- Snyder, S.M.; Zhao, B.; Luo, T.; Kaiser, C.; Cavender, G.; Hamilton-Reeves, J.; Sullivan, D.K.; Shay, N.F. Consumption of quercetin and quercetin-containing apple and cherry extracts affects blood glucose concentration, hepatic metabolism, and gene expression patterns in obese C57BL/6J high fat-fed mice. J. Nutr. 2016, 146, 1001–1007. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, G.; Zhang, X.; Gao, J.; Zhou, Z.; Fan, J. Polyphenols from Sorbus aucuparia ameliorate insulin resistance and metabolic disorders in diabetic mice. Curr. Top. Nutraceutical Res. 2016, 14, 227–233. [Google Scholar]
- Simeonov, S.B.; Botushanov, N.P.; Karahanian, E.B.; Pavlova, M.B.; Husianitis, H.K.; Troev, D.M. Effects of Aronia melanocarpa juice as part of the dietary regimen in patients with diabetes mellitus. Folia Med. 2002, 44, 20–23. [Google Scholar]
- Milutinović, M.; Radovanović, R.V.; Šavikin, K.; Radenković, S.; Arvandi, M.; Pešić, M.; Kostić, M.; Miladinović, B.; Branković, S.; Kitić, D. Chokeberry juice supplementation in type 2 diabetic patients—Impact on health status. J. Appl. Biomed. 2019, 17, 218–224. [Google Scholar] [CrossRef]
- Tasic, N.; Jakovljevic, V.L.J.; Mitrovic, M.; Djindjic, B.; Tasic, D.; Dragisic, D.; Citakovic, Z.; Kovacevic, Z.; Radoman, K.; Zivkovic, V.; et al. Black chokeberry Aronia melanocarpa extract reduces blood pressure, glycemia and lipid profile in patients with metabolic syndrome: A prospective controlled trial. Mol. Cell. Biochem. 2021, 476, 2663–2673. [Google Scholar] [CrossRef]
- Yamane, T.; Kozuka, M.; Wada-Yoneta, M.; Sakamoto, T.; Nakagaki, T.; Nakano, Y.; Ohkubo, I. Aronia juice suppresses the elevation of postprandial blood glucose levels in adult healthy Japanese. Clin. Nutr. Exp. 2017, 12, 20–26. [Google Scholar] [CrossRef]
- Makarova, E.; Górnaś, P.; Konrade, I.; Tirzite, D.; Cirule, H.; Gulbe, A.; Pugajeva, I.; Seglina, D.; Dambrova, M. Acute anti-hyperglycaemic effects of an unripe apple preparation containing phlorizin in healthy volunteers: A preliminary study. J. Sci. Food Agric. 2014, 95, 560–568. [Google Scholar] [CrossRef]
- Shoji, T.; Yamada, M.; Miura, T.; Nagashima, K.; Ogura, K.; Inagaki, N.; Maeda-Yamamoto, M. Chronic administration of apple polyphenols ameliorates hyperglycaemia in high-normal and borderline subjects: A randomised, placebo-controlled trial. Diabetes Res. Clin. Pract. 2017, 129, 43–51. [Google Scholar] [CrossRef]
- Lu, X.; Lu, J.; Fan, Z.; Liu, A.; Zhao, W.; Wu, Y.; Zhu, R. Both isocarbohydrate and hypercarbohydrate fruit preloads curbed postprandial glycemic excursion in healthy subjects. Nutrients 2021, 13, 2470. [Google Scholar] [CrossRef] [PubMed]
- Yuliwati, N.; Nugroho, R.F. The Potential of Strawberry, Rome Beauty Apple, and New Combination on Fasting Blood as Supporting Diet Therapy in Patients with Type II Diabetes Mellitus. Glob. Med. Health Commun. 2021, 9, 69–75. [Google Scholar] [CrossRef]
- Gancheva, S.; Ivanova, I.; Atanassova, A.; Gancheva-tomova, D.; Eftimov, M.; Moneva, K.; Zhelyazkova-savova, M. Effects of Aronia melanocarpa fruit juice on oxidative stress, energy homeostasis, and liver function in overweight and healthy—Weight individuals. Scr. Sci. Med. 2021, 53, 39–46. [Google Scholar] [CrossRef]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Possible role for apple juice phenolic compounds in the acute modification of glucose tolerance and gastrointestinal hormone secretion in humans. J. Sci. Food Agric. 2002, 82, 1800–1805. [Google Scholar] [CrossRef]
- Sołtys, A.; Galanty, A.; Podolak, I. Ethnopharmacologically Important But Underestimated Genus Sorbus: A Comprehensive Review; Springer: Amsterdam, The Netherlands, 2020; Volume 19, ISBN 0123456789. [Google Scholar]
- Sidor, A.; Gramza-Michałowska, A. Gramza-Michałowska Black Chokeberry Aronia melanocarpa L.—A Qualitative Composition, Phenolic Profile and Antioxidant Potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuca, K.; Valis, M.; Wu, W. Malus domestica: A Review on Nutritional Features, Chemical Composition, Traditional and Medicinal Value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Y.; Gong, X.; Li, Z.; Wang, H.; Ma, L.; Tuerhong, M.; Abudukeremu, M.; Ohizumi, Y.; Xu, J.; et al. Preparation, characterization, and antitumor activity of Chaenomeles speciosa polysaccharide-based selenium nanoparticles. Arab. J. Chem. 2022, 15, 103943. [Google Scholar] [CrossRef]
- Zlobin, A.A.; Martinson, E.A.; Litvinets, S.G.; Ovechkina, I.A.; Durnev, E.A.; Ovodova, R.G. Pectin polysaccharides of rowan Sorbus aucuparia L. Russ. J. Bioorganic Chem. 2012, 38, 702–706. [Google Scholar] [CrossRef]
- Gyurova, D.K.; Enikova, R.K. Dried Fruits—Brief Characteristics of Their Nutritional Values. Author’s Own Data for Dietary Fibers Content. J. Food Nutr. Sci. 2014, 2, 105–109. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Srichaikul, K.; Kendall, C.W.; Sievenpiper, J.L.; Abdulnour, S.; Mirrahimi, A.; Meneses, C.; Nishi, S.; He, X.; Lee, S.; et al. The Relation of Low Glycaemic Index Fruit Consumption to Glycaemic Control and Risk Factors for Coronary Heart Disease in Type 2 Diabetes. Diabetologia 2011, 54, 271–279. [Google Scholar] [CrossRef]
- Monro, J.A. Glycaemic Glucose Equivalent: Combining Carbohydrate Content, Quantity and Glycaemic Index of Foods for Precision in Glycaemia Management. Asia Pac. J. Clin. Nutr. 2002, 11, 217–225. [Google Scholar] [CrossRef] [PubMed]

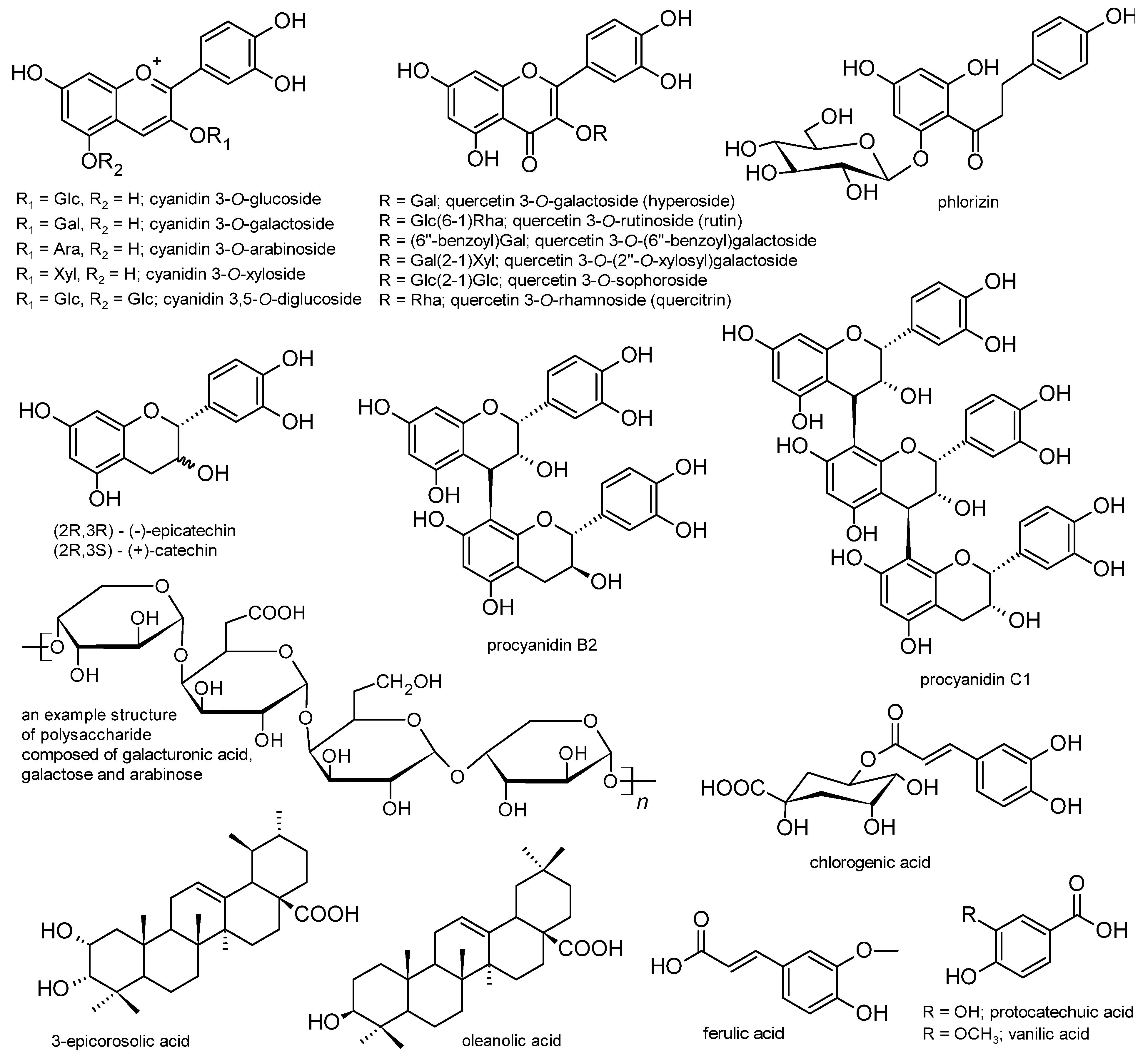
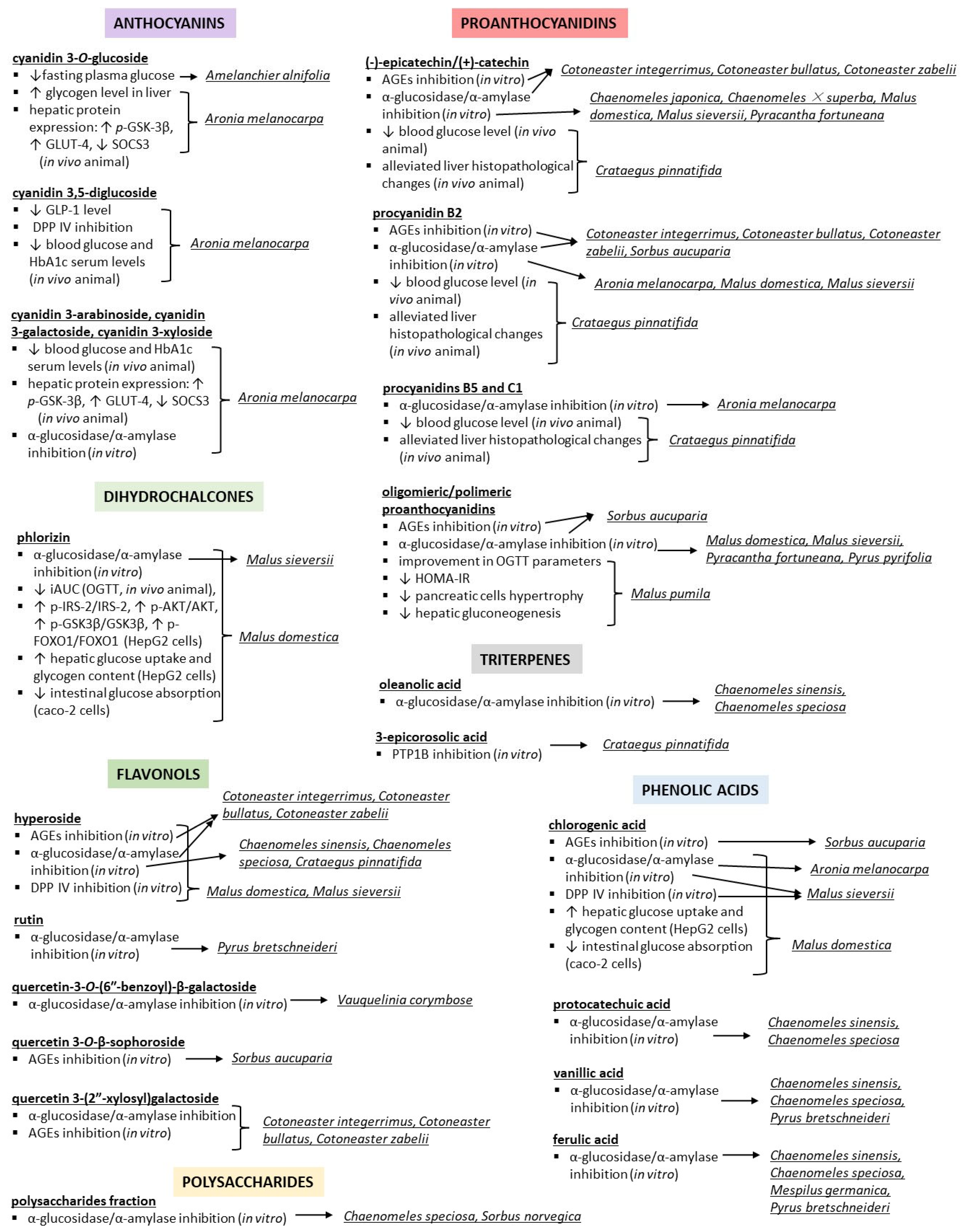
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutkowska, M.; Olszewska, M.A. Anti-Diabetic Potential of Polyphenol-Rich Fruits from the Maleae Tribe—A Review of In Vitro and In Vivo Animal and Human Trials. Nutrients 2023, 15, 3756. https://doi.org/10.3390/nu15173756
Rutkowska M, Olszewska MA. Anti-Diabetic Potential of Polyphenol-Rich Fruits from the Maleae Tribe—A Review of In Vitro and In Vivo Animal and Human Trials. Nutrients. 2023; 15(17):3756. https://doi.org/10.3390/nu15173756
Chicago/Turabian StyleRutkowska, Magdalena, and Monika A. Olszewska. 2023. "Anti-Diabetic Potential of Polyphenol-Rich Fruits from the Maleae Tribe—A Review of In Vitro and In Vivo Animal and Human Trials" Nutrients 15, no. 17: 3756. https://doi.org/10.3390/nu15173756
APA StyleRutkowska, M., & Olszewska, M. A. (2023). Anti-Diabetic Potential of Polyphenol-Rich Fruits from the Maleae Tribe—A Review of In Vitro and In Vivo Animal and Human Trials. Nutrients, 15(17), 3756. https://doi.org/10.3390/nu15173756