Abstract
β-alanine does not have an ergogenic effect by itself, but it does as a precursor for the synthesis of carnosine in human skeletal muscle. β-alanine and carnosine together help improve the muscles’ functionality, especially in high-intensity exercises such as combat sports. Therefore, β-alanine could be considered a nutritional ergogenic aid to improve sports performance in combat athletes. We aimed to critically review clinical trial evidence on the impact of β-alanine supplementation on sports performance, perception, and anthropometric parameters, as well as circulating biochemical markers in combat athletes. This systematic review was conducted following the specific methodological guidelines of the Preferred Report Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA), the PICOS question model, the Critical Review Form of McMaster, and the PEDro scale. Furthermore, the Cochrane risk-of-bias assessment tool was used. The search was carried out in the SCOPUS, Web of Science (WOS), and Medline (PubMed) databases for studies published from the beginning of the database until July 31, 2023. Of the 41 registers identified, only 7 met the established criteria and were included in this systematic review. Overall, performance parameters related to strength, power, total exercise work capacity, and combat-specific parameters were significantly improved (p < 0.05). Perception parameters increased non-significantly (p > 0.05). Regarding biochemical parameters, carnosine increased significantly (p < 0.05), pH decreased non-significantly (p > 0.05), and the results for blood bicarbonate and blood lactate were heterogeneous. Finally, there was a non-significant (p > 0.05) improvement in the anthropometric parameters of lean mass and fat mass. β-alanine supplementation appears to be safe and could be a suitable nutritional ergogenic aid for combat athletes.
1. Introduction
Combat sports can be classified into thre groups: grappling, hitting, and mixed. Grappling sports are characterized by holds, locks, and falls to the ground (e.g., judo, wrestling, and jiu-jitsu). Striking sports focus on the use of punches and kicks (e.g., kickboxing, boxing, Muay Thai, karate, taekwondo). Finally, mixed combat sports are those that have characteristics of both groups (e.g., hapkido, mixed martial arts [MMA]) [1]. These modalities of combat sports require the performance of explosive and high-intensity movements of both the upper and lower extremities [2]. The performance of combat sports occurs in short periods of time, from seconds to a few minutes, depending on the specific regulations of each one [3]. Overall, combat sports are sports of intermittent exertion because of the effort pattern and the “exercise-relative recovery” sequence [4]. Efforts in combat sports are characterized by intermittently demanding high work alternating short but intense explosive force and power actions that require good participation of anaerobic energy [5]. In this sense, high-intensity actions imply the intervention of anaerobic metabolism using the energy pathway of intramuscular adenosine triphosphate (ATP) and phosphocreatine (PCr) and/or short-term anaerobic glycolysis during exercise performance [1]. This metabolic situation causes high blood lactate levels after each combat. The accumulation of lactic acid can be important in a complete fight, but when several fights are carried out in a row, the acidosis caused by these can be detrimental to final performance [5]. Thus, combat sports practiced at a high level are long-term intermittent effort sports activities [4].
There is a great limitation of high-intensity maximum efforts because they can only be maintained for short periods of time [6] due to the appearance of short-term muscle fatigue, especially in the muscles involved in the exercise, which generates dysfunctions and discomfort that culminates in stopping the exercise [7]. Fatigue has metabolic consequences, such as a decrease in intramuscular PCr or an increase in lactate and a decrease in pH [7]. Muscle fatigue, then, is associated with, among other aspects, a rapid increase in the production of metabolic acids [8]. In the organism, there are immediate defense mechanisms; to avoid changes in the pH in response to changes in the acidity of body fluids, they are carried out by the buffer systems of the body, such as bicarbonate, phosphate, and hemoglobin, in addition to respiratory regulation and renal pH regulation [9]. The normal metabolism of the body continually produces acid radicals. In addition, this production increases during maximum-intensity exercises, causing situations of physiological stress, in which the buffer systems are not capable of restoring an electrolyte imbalance caused mainly by the production of lactic acid. Under these situations, athletes increase the risks of undergoing lactic acidosis, fatigue, and/or overtraining [7,8].
Therefore, it seems reasonable to implement nutritional ergogenic aids (NEAs) with a buffering capacity, helping to restore homeostatic balance and neutralize the rapid increase in the production of metabolic acids induced by high-intensity exercise [10]. NEAs have ergo-nutritional ingredients whose purpose is to help cover the specific nutritional requirements of combat sport practitioners, both for maintaining a good state of health and maximizing sports performance [11]. In this way, β-alanine is a non-essential amino acid synthesized in the liver. β-alanine does not have an ergogenic effect by itself, but it does as a precursor for the synthesis of carnosine (β-alanine and L-histidine) in human skeletal muscle [12]. Carnosine improves muscle contraction, increasing the sensitivity of myofibrillar calcium in fast fibers, and mediates 8–15% of the intramyocyte buffer capacity, reducing the limiting effect of performance related to acidosis [13]. β-alanine is a NEA with a degree of evidence A that significantly increases the concentrations of carnosine in the muscle, thus acting as an intracellular pH buffer [14,15]. In addition, it has been reported that exercise performance is improved, with more pronounced effects in activities lasting 1–4 min at doses of 4–6 g/day for at least 2–4 weeks, with a significant increase in carnosine from 20 to 30% and 40 to 60%, and after 10 weeks, an approximate increase of 80%. β-alanine has shown a moderately elevated ergogenic effect on the attenuation of neuromuscular fatigue [12,16]. Although it does not cause alterations in healthy populations at the recommended doses, a sensation of paresthesia in the extremities has been reported [17,18], as well as the appearance of itching due to L-alanine [18].
The consumption of NEAs has increased exponentially with prospects for the next decade, with an increase of between 10 and 15% [19]. Elite or recreational athletes, regardless of gender, consume NEAs equally [20]. Therefore, it is necessary to dispel doubts about the potential ergo-nutritional effect of β-alanine in combat sports, which obtained 26% of all medals at the Tokyo 2020 Olympic Games [21]. If we also add an increase in research interest in NEAs, it might be necessary to offer appropriate evidence-based advice by critically reviewing published randomized controlled trials (RCTs) on outcomes that are commonly investigated in sports nutrition science. We used the research question using the PICO model following the Evidence-Based Medicine (EBM) guidelines [22] as follows: P (population): “combat competition athletes who did not present chronic pathology”; I (intervention): “β-alanine supplementation”; C (comparison): “same conditions with placebo or control group”; O (outcomes): sports performance (strength, power, total exercise work capacity, vertical jump, and combat-specific parameters); perceptual parameters (perceptive exertion [CR-10 RPE scale], and better perceived exercise recovery [TQR scale 6–20]); anthropometric measures (lean mass, and fat mass); biochemical markers (serum carnosine, bicarbonate [HCO3]; pH and blood lactate [LAC]); and side effects (paresthesia). This systematic review was eligible for PROSPERO registration (#CRD42023426545) and was conducted and reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines [23]. These results were included as outcomes because these parameters are commonly investigated in sports science and nutrition studies to determine evidence for NEAs.
2. Materials and Methods
2.1. Search Methods
Our systematic review asked the following question: “Does β-alanine supplementation have positive impacts on sports performance, perception, and anthropometric parameters, and biochemical biomarkers for healthy combat athletes?” To answer this question, a structured search was carried out using the Scopus, Web of Science (WOS), and Medline (PubMed) database for studies published from the beginning of the database until 31 July 2023.
The search strategy contained terms related to β-alanine and free words for key concepts related that included the following: (“β-alanine OR Beta-alanine”) AND (“combat sports” or “judo” or “taekwondo” or “boxing” or “karate” or “wrestling”), AND (“supplementation” or “ergogenic aids”) AND (“combat sports” or “judo” or “taekwondo” or “boxing” or “karate” or “wrestling”) (Appendix A). In addition, we manually screened references from previous systematic reviews and meta-analyses and other sources, such as ResearchGate® (https://www.researchgate.net/) (accessed on 24 June 2023), to find possible additional studies. Two reviewers (D.F.-L. and E.M.F.). independently assessed the full texts. In addition, a third reviewer (J.M.-A.) resolved the discrepancies. To identify potential studies not included in the databases, a network graph was made using the Connected Papers website (www.connectedpapers.com, accessed on 30 June 2023).
2.2. Elegibility Criteria
We based the collection of studies applying the following selection criteria: (i) healthy adult combat athletes; (ii) studies exclusively evaluating the use of β-alanine monotherapy supplementation in combat sports; (iii) comparing it with the control group, placebo group, or sham treatment (excluding comparisons with other supplements); (iv) studies with a methodological design that corresponds exclusively to a clinical trial; (v) studies that assessed sports performance, perceptual, and anthropometric parameters, as well as biochemical biomarkers, as outcomes; (vi) studies with clear information on the dose and duration of β-alanine supplementation; (vii) studies with a risk-of-bias score ≥ 4 according to the Cochrane Collaboration tool [24]; (viii) studies with a methodological quality score ≥ 13 according to the McMaster University Occupational Therapy Evidence-Based [25]; (ix) clinical trials or randomized clinical trials with a score ≥ 6 on the Physiotherapy Evidence Database (PEDro) scale [26]; and (x) studies published in Spanish or English. Those studies that did not meet the inclusion criteria that are described were eliminated.
2.3. Methodological Quality and Risk-of-Bias Assessment
The methodological quality of the studies was evaluated by the McMaster tool [25] and PEDro scale [26]. Also, the Cochrane risk-of-bias tool was used [24].
2.4. Data Extraction
The following data were extracted from the selected studies: name of the first author; year of publication; country; study design; sample size; characteristics of the participants (gender and level of physical activity); intervention (daily amount of supplementation and timing of intake); and analyzed variables and results. This was carried out according to the CONSORT Statement rules [27]. To develop the data extraction, two components (D.F.-L. and E.M.F.) of the research team and another member (J.M.-A.) resolved the disagreements generated.
3. Results
3.1. Study Selection
A total of 64 studies were identified; 31 studies were from three electronic databases, namely Web of Science, Scopus, and Medline (PubMed), and 10 studies were obtained from other sources, such as ResearchGate®. After excluding 23 duplicates, a total of 41 articles were evaluated. After title and abstract evaluation, 18 articles were considered potential registries. After reviewing the full text and assessing potential records from databases, registries, and other sources, seven studies [28,29,30,31,32,33,34] were included in the systematic review (Figure 1).
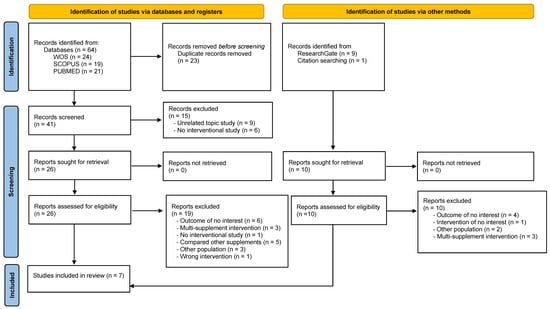
Figure 1.
Flowchart representing the identification and selection processes of relevant studies according to the PRISMA 2020 declaration [23].
Figure 2 shows the node plot that originated from the research of Halz et al. [31]. Figure 2 was made to verify the studies included in this systematic review.
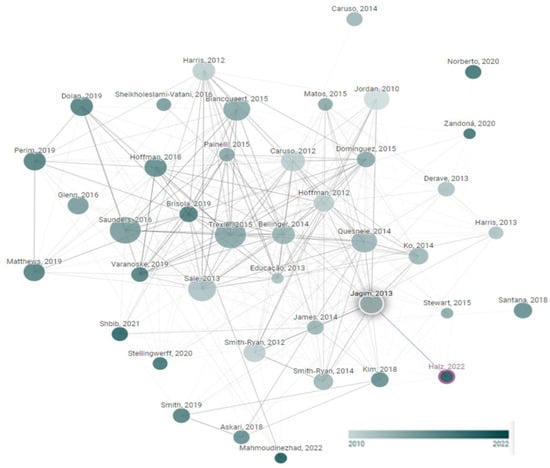
Figure 2.
Network diagram of the β-alanine supplementation trials. This graph was developed within www.connectedpapers.com and accessed on 31 July 2023.
3.2. Quality Assessment
The evaluation of the methodological quality by the McMaster [25] tool yielded the following results: three studies [29,31,33] achieved a quality of “very good” and four studies [28,30,32,34] achieve a quality of “excellent”. Seven studies [28,29,30,31,32,33,34] reunited the minimum quality criteria (Table 1).

Table 1.
Results of the methodological quality assessment of included studies—McMaster Critical Review Form for Quantitative Studies [25].
The methodological quality of the studies using the PEDro scale [26] was as follows: three studies [28,29,32] achieved ‘excellent’ quality and four studies [30,31,33,34] achieved ‘good’ quality (Table 2).

Table 2.
Evaluation of methodological quality according to PEDro scale [26].
3.3. Risk-of-Bias Assessment
Two studies [30,34] had a score of “five points”, and five studies [28,29,31,32,33] had a score of “six points” (Table 3) in terms of risk of bias according to Cochrane Bias Methods Group [24].

Table 3.
Results of the risk-of-bias assessment of included studies—Cochrane Bias Methods Group [24].
3.4. Characteristics of the Participants and Interventions
The studies [28,29,30,31,32,33,34] included in our systematic review provided a total sample of 138 participants, 135 men [28,29,30,31,32,33,34] and 3 women [34]. In this way, 54 participants practiced boxing [28,30,33], 22 competed in wrestling [32], and 47 subjects were judokas [29,31,34]. The sporting levels of the participants were amateur athletes [28,30], competition athletes [29,31,32], and elite athletes [33,34]. The administration of β-alanine was orally via capsules in all studies [28,29,30,31,32,33,34] included in the systematic review. Doses ranged from 4 g/day [32] to 6.4 g/day [29] and 0.3 g/kg/day [≈20–28 g/day] [28]. According to Kern et al. [32], the dose was administered twice a day; in three studies [31,33,34], the doses were divided into three doses a day; in two studies [29,30], the doses were divided four times a day; and in one study [28], the dose was not specified. The timing of supplementation was not specified in two studies [28,30]; in three studies [29,31,34], it was to be taken with main meals; in one study [33], it was to be taken immediately after the main meals; and in the study conducted by Kern et al. [32], it was to be taken at breakfast and lunch. Finally, the duration ranged from four weeks [28,29,30,31] to ten weeks [33] (Table 4).

Table 4.
Characteristics of athletes and β-alanine supplementation practice of the included records.
3.5. Outcome Assessment
The results of the registries selected in the systematic review are presented in Table 5.

Table 5.
Records included in the systematic review of the effect of β-alanine supplementation on sports performance, perceptual, and anthropometric parameters, as well as biochemical markers in combat sports.
3.5.1. Sport Performance
The sports performance outcomes described in the seven selected studies [28,29,30,31,32,33,34] were strength, power, total work, heart rate, jump height, blood lactate concentration, and combat-specific parameters for each sport.
- Strength
Donovan et al. [30] evaluated the cumulative strength and the average blow strength. Both parameters, cumulative and blow strength, increased significantly (p < 0.05) compared to the control group (CG), as well as the group supplemented with β-alanine (BaG) and BaG, with respect to the baseline, before the BaG was supplemented. Also, the study conducted by Kim et al. [33] evaluated knee extension force and an increase without statistical significance (p > 0.05) was only observed in the left knee in the BaG compared to the CG. However, these authors have reported a significant increase (p < 0.05) in the BaG compared to pre-supplementation.
- Power
Kern et al. [32] evaluated power through two tests: the first consisted of running 300 yards (274.32 m) (anaerobic power) and the second consisted of hanging from a bar, keeping the arms at a 90° angle (muscular power). β-alanine-supplemented wrestlers significantly (p < 0.05) improved anaerobic and muscular power relative to the CG and the study baseline for BaG.
Peak power was evaluated in two studies [28,33]. Kim et al. [33] demonstrated a significant (p < 0.05) increase in lower body peak power in the BaG compared to the CG and a non-significant (p > 0.05) increase compared to the BaG prior to intervention. However, Alabsi et al. [28] did not observe any change in maximum power. The peak power drop [(peak power − minimum)/peak power × 100] was evaluated in two studies [28,33]. Therefore, lower values indicate better sports performance. In the study carried out by Alabsi et al. [28], the drop in power decreased in a non-significant (p > 0.05) way in the BaG compared to the CG; however, it did decrease significantly (p < 0.05) in comparison with the BaG before supplementation. Kim et al. [33] reported that it decreased significantly (p < 0.05) when comparing the BaG with the placebo group, and there was no change compared to the BaG at baseline.
Mean power was analyzed in two studies [28,31]. Alabsi et al. [28] did not find differences in mean power when comparing the BaG to the CG. However, these authors [28] reported a significant increase (p < 0.05) in mean power in the BaG after 10 weeks of supplementation with β-alanine. Halz et al. [22] did not observe changes in mean lower body power in the BaG with respect to the CG, but these authors [22] reported significant increases (p < 0.05) in mean upper body power when comparing the BaG to the non-supplemented group.
- Total exercise work capacity
Halz et al. [31] assessed the total exercise work capacity on the upper and lower extremities. In both the upper and lower extremities, total exercise work capacity was significantly increased (p < 0.05) in the BaG compared to the placebo group.
- Heart rate
Heart rate was evaluated in one study conducted by Donovan et al. [30] without observing changes while comparing both groups, namely the BaG and the CG.
- Vertical Jump
Vertical jump was studied in the clinical trial of Kim et al. [33]. It increased non-significantly (p > 0.05) when comparing the BaG to CG. However, in the BaG, vertical jump increased significantly (p < 0.05) between the beginning and the end of the study.
- Combat-specific parameters
The number of projections was evaluated in two studies [29,34]. In both studies [29,34], the number of projections increased, but only in the study conducted by de Andrade et al. [29] was a significant increase (p < 0.05) in the BaG with respect to the CG reported. The number of strokes was evaluated in the study conducted by Donovan et al. [30], being significantly higher (p < 0.05) in the BaG than in the placebo group.
3.5.2. Perception Parameters
Lopez-Grueso et al. [34] evaluated two perceptual parameters, namely perceived exertion (CR-10 RPE scale), and better perceived exercise recovery (TQR 6–20). Both parameters, CR-10 RPE and TQR 6–20, increased without statistical significance (p > 0.05) in the BaG compared to the CG.
3.5.3. Anthropometric Parameters
Lean mass (skeletal muscle, other types of muscle, and non-fat components) and fat mass (group of lipids or integral fats) were evaluated in the study conducted by Kern et al. [32] in wrestlers. Lean mass increased non-significantly (p > 0.05) and fat mass decreased non-significantly (p > 0.05) in the BaG compared to the CG and compared to the BaG vs. baseline.
3.5.4. Biochemical Biomarkers
The circulating biochemical parameters evaluated were serum carnosine, HCO3, pH, and blood lactate [28,29,30,31,32,33,34].
- Serum Carnosine
Blood carnosine was measured in the study by Alabsi et al. [28], increasing significantly (p < 0.05) in the BaG compared to the CG.
- Bicarbonate (HCO3)
HCO3 was measured in two studies [29,31]. de Andrade et al. [29] observed a significant decrease (p < 0.05) in bicarbonate in the BaG with respect to its baseline. These authors [29] found no changes in bicarbonate concentration when comparing both groups, i.e., BaG vs. GC. However, in the study conducted by Halz et al. [31], HCO3 levels increased significantly (p < 0.05) in the BaG compared to the CG and with respect to baseline.
- pH
de Andrade et al. [29] showed that pH only decreased non-significantly (p > 0.05) in the BaG compared to pre-supplementation. No changes in pH were observed in the BaG vs. the CG.
- Blood Lactate
Blood lactate was measured in all selected studies [28,29,30,31,32,33,34] in this systematic review. Overall, the blood lactate concentration responses were heterogeneous when both groups were compared, namely the BaG and the CG. Four studies [28,29,33,34] did not observe changes; in two studies [30,31], a significant increase (p < 0.05) was observed; and Kern et al. [32] described a notable decrease (p > 0.05) in blood lactate concentration.
With respect to blood lactate concentration measurements when evaluating the BaG with respect to its linear base, in five studies [28,29,30,31,33], it increased significantly (p < 0.05); in one study [34], no changes were observed; and only the study conducted by Kern et al. [32] in wrestlers observed a moderate decrease (p > 0.05) in blood lactate concentration.
3.5.5. Adverse Effects
Two studies [29,34] reported cases of mild paresthesia.
4. Discussion
Our systematic review aimed to evaluate the effects of β-alanine supplementation on sports performance, anthropometric, and perception parameters, as well as biochemical markers, in healthy adults practicing combat sports. A total of seven studies [28,29,30,31,32,33,34] met the inclusion criteria, with 138 participants, 135 men [28,29,30,31,32,33,34], and 3 women [34]. In general, all the selected studies [28,29,30,31,32,33,34] showed significant improvements in sports performance in terms of strength, power, and total work capacity, better perception of recovery from physical exertion, and an increase in lean mass and decrease in fat mass in combat athletes after periods of β-alanine supplementation. On the other hand, β-alanine supplementation did not show conclusive evidence on the results related to certain circulating biochemical parameters and blood lactate concentration. Supplementation with β-alanine was shown to be safe since there were four dropouts [33,34] due to injury, not related to the supplementation, although mild paresthesia was manifested [29,34].
4.1. β-Alanine Supplementation
β-alanine supplementation was administered by oral capsules [28,29,30,31,32,33,34] and β-alanine is doping-free [35]. Doses ranged from 4 g/day [32] to 6.4 g/day [29] and 0.3 g/kg/day [≈20–28 g/day] [28]. Recently, Sport Integrity Australia [36] recommended that β-alanine supplementation should be started with a loading phase of 3.2 g per day for eight weeks, or 6.4 g per day for four weeks, followed by a maintenance β-alanine supplementation of 1.2 g per day. In this sense, Naderi et al. [18] reported that β-alanine supplementation of 1.2 g/day could maintain muscle carnosine in the range of 30% to 50% above pre-supplementation levels. It should be considered that intracellular carnosine levels are mainly determined by the availability of extracellular β-alanine [36]. Even more so, histidine could be supplemented to enhance intracellular carnosine stores [37].
The timing of β-alanine supplementation in the studies included in the review was with main meals [29,31,34], immediately after main meals [33], and with breakfast and lunch [32]. β-alanine supplementation during carbohydrate- and protein-rich meals markedly increased muscle carnosine content compared with β-alanine supplementation between meals. Perhaps insulin could induce β-alanine uptake by stimulating a greater carnosine load in muscle through the action of Na+/K+ pumps present in skeletal muscle myocytes [38].
Mild paresthesia in the extremities [29,34] was the only reported side effect of β-alanine supplementation. Paranesthesia is a consequence of an increase in the sensitivity of neuropathic pain-transmitting nociceptive neurons, which causes redness and an itching sensation on the skin [13,39]. Paranesthesia could be attenuated by fractionated lower doses (1.6 g per dose, in six–eight doses) or sustained release formulas [13] and consuming them with main meals to help improve absorption and better manage potential side effects [13,40].
4.2. Sports Performance
Increases in metabolic acids during intense physical activity is because muscle contraction substantially increases intracellular hydrogen ions and the extraordinary metabolic demands that are covered predominantly by anaerobic glycolysis, producing lactic acid [41]. Consequently, there is a decrease in the pH of the muscles that are exercised, which limits contractile function and muscle metabolism, significantly decreasing tolerance to exercise [42]. Faced with this situation, acid-base imbalance, the organism intrinsically possesses a capacity to fight against acidosis through the buffer or damping system [9]. However, high-intensity exercise exceeds this buffering capacity and, therefore, muscle fatigue is triggered, impairing the athlete’s sports performance [42].
Thus, β-alanine may improve performance by reducing acidity [43]. However, the results were conflicting, with some showing better performance in high-intensity exercise and others finding no difference [44,45]. One of the limiting factors in the efficacy of β-alanine in sports performance is acidosis. β-alanine could improve performance in physical actions that cause an extreme intramuscular acidotic environment [44]. However, the improvement in sports performance is limited when the exercise protocol does not produce severe muscle acidosis [46].
There was a significant (p < 0.05) performance improvement in strength [30], power [31,32,33], total exercise work capacity [31], and combat-specific parameters [29,30] with respect to the non-supplemented group. In the studies [29,30,31,32,33] included in our systematic review, the duration of the exercises or tests were from one to four minutes. Therefore, the main way of obtaining energy was anaerobic glycolytic, characterized by the production of LAC, creating an extreme acidotic environment [9]. Therefore, when comparing our results with other studies that investigate β-alanine supplementation in athletes, we must consider whether it is the main metabolic pathway for obtaining energy to play sports. Consistent with the results of our systematic review, the performance improvement associated with β-alanine supplementation also occurs in other sports with similar exercise times [47,48]. Ducker et al. [47] demonstrated a significant (p < 0.05) improvement in athletes who competed in 800 m races after four weeks of β-alanine supplementation with respect to the group. Also, in climbers after four weeks of supplementation with 4 g/day of β-alanine, performance improved during continuous climbs lasting one minute and repeated episodes of movements involving the upper extremities [48].
In this sense, Saunders et al. [45] described a significant (p < 0.05) improvement in sports performance in studies involving exercises between one minute to four minutes, with no improvement in exercises > one minute and with a slight improvement in exercises ranging from four minutes to 10 min. In exercises > one-minute duration, the main way of obtaining energy is the phosphagen system using phosphocreatine and, to a lesser extent, anaerobic glycolysis [9]. Therefore, in exercises lasting > one-minute, benefits in sports performance from the use of β-alanine are not evident [46,49,50] because this exercise duration is unlikely to be restricted by intracellular H+ increase [51].
On the other hand, regarding the physical exercises whose duration ranges from 4 to 10 min, both anaerobic glycolysis and the aerobic pathways are involved in its development [9]. Therefore, in these types of exercises in which anaerobic glycolysis is partly involved, slight benefits on sports performance are observed after β-alanine supplementation [52,53]. This could be justified because muscle carnosine would be the primary mechanism of the metabolic demand of exercise, as a pH buffer, which would only involve the anaerobic glycolysis pathway [52].
The use of β-alanine supplements does not appear to improve strength [44]. In athletes, increases in strength have been described after the use of creatine plus β-alanine supplement combinations [50], but not with β-alanine in monotherapy [49,50] or with other buffering supplements, such as HCO3 [54]. However, strength-related parameters improved from β-alanine supplementation compared to CG [30,33]. Donovan et al. [30] observed a significant (p < 0.05) improvement in cumulative force and mean punch force in boxers. Also, Kim et al. [33] observed a substantial increase in knee extension strength and vertical jump between the BaG and the CG. These findings may come as a surprise because strength performance is not limited by acidosis [18].
In three studies [31,32,33] included in the systematic review, significant (p < 0.05) improvements in power in the BaG vs. the CG were reported. Kim et al. [33] reported significant (p < 0.05) positive effects with a 6% improvement in maximal lower body power and a smaller 3.2% upper body power drop. Halz et al. [22] observed significant (p < 0.05) increases in mean power in the upper body. Additionally, Kern et al. [26] reported a significant (p < 0.05) improvement in anaerobic muscle power performance. Furthermore, in one study [28] included in the systematic review, significant improvements (p < 0.05) were described over a drop in maximum power and significant increases (p < 0.05) of 20% in mean power in the BaG compared to before intervention. These results are consistent with the study conducted by Van Thienen et al. [55], in which after eight weeks of oral supplementation with β-alanine, increases of 5% and 11.5% in mean power and maximum power were observed, respectively. However, other studies [49,50,56] did not show positive effects of β-alanine supplementation on power performance in upper arm flexion [49], squat exercises [50], or anaerobic muscle power during repeated sprint [56]. These differences could be because β-alanine improves performance in exercises that generate an extreme intramuscular acidotic environment [44]. But the probability of the effect of β-alanine decreases ostensibly with lower levels of acidosis [46]. PCr will be very present, and therefore, acidosis will not be the limiting factor in this type of exercise. The incomplete resynthesis of PCr has the greatest effect on fatigue and/or decreased performance than the accumulation of H+ [57].
Supplementation with β-alanine attenuates the appearance of muscular fatigue that would potentially improve total physical work capacity [44,45]. In judokas, significant (p < 0.05) increases in the total work in the upper and lower extremities [31] and significant (p < 0.05) increases in the total number of projections and projections per combat [29] in the BaG group with respect to the CG have been demonstrated. In addition, Lopez-Grueso et al. [34] described a remarkable tendency to increase in the BaG group, with respect to the CG, the number of total projections of judo. In boxers, the number of blows was evaluated in the study carried out by Donovan et al. [34], being significantly higher (p < 0.05) in the BaG group than in the placebo group.
4.3. Anthropometric Parameters
An eight-week study, included in this systematic review, supplemented β-alanine (4 g/day), to collegiate wrestlers, and lean mass increased non-significantly (p > 0.05), while fat mass decreased non-significantly (p > 0.05) in the BaG compared to the CG and compared to the BaG vs. baseline [32]. These results are similar to those reported in a six-week study in athletic women, where the BaG (6 g β-alanine) saw an increase in lean mass, while the CG did not [58]. Similarly, a three-week study in 46 healthy men, supplemented with four 1.5 g doses of β-alanine (6 g/day), reported a significant increase in lean mass comparing the start of the study with the end of the study [59].
β-alanine may promote lean mass gains, but its mechanism is unknown. Perhaps the buffering capacity of β-alanine [43] makes it possible to support a greater volume of training, causing a greater stimulus; this leads to greater adaptations, and consequently, to an increase in muscle mass. Although, the anthropometric benefits could be a consequence of the exercise, since in the three studies [32,58,59], β-alanine supplementation was combined with an exercise regimen. This was reported previously in 2006 by Hoffman et al. [60]. These results are of interest for combat sports because the categories are separated by weight; the use of β-alanine can be a very interesting strategy to lower body fat and maintain or even increase lean mass [61].
4.4. Perception Parameters
β-alanine seems to reduce the perception of fatigue and delay voluntary exhaustion in women [62], older people (55–92 years) [62], and college athletes [63]. In the study included in our systematic review on judokas conducted by López-Grueso et al. [34], a discord between these parameters of subjective perception showing a non-significant (p > 0.05) increase in CR-10 RPE and a tendency (p > 0.05) to increase in the TQR 6–20 were shown. These discrepancies could be due to physiological differences between men and women, who also differ by at least one intensity of perceived exertion of exercise. In this sense [64], Hoffman et al. [63] also described discordance between fatigue as measured by subjective ratings and fatigue as measured by a Wingate anaerobic test.
Furthermore, we should consider that perception is a biased parameter subject to subjectivity, feeling more tired when we lose than when we win. In this way, improvements in performance associated with β-alanine supplementation could determine a tendency to decrease the perception and sensation of fatigue [34].
4.5. Biochemical Biomarkers
In two studies [30,31], blood LAC had a significant increase (p < 0.05) in the BaG compared to the CG. Also, in five studies [28,29,30,31,33], blood LAC increased significantly (p < 0.05) in the BaG with respect to baseline. Blood LAC may be not the cause of H+ accumulation; the metabolic environment that causes a decrease in pH also increases lactate production, making LAC a good marker of conditions that induce metabolic acidosis [65], thus facilitating β-alanine action [44]. This increase in post-exercise LAC could be associated with β-alanine supplementation by counteracting the accumulation of H+, helping to maintain intramuscular pH during intense exercise [13]. Higher blood lactate levels could allow exercise to be carried out at a higher intensity for longer periods because it improves the buffering capacity [65]. This would allow the athlete to tolerate higher exercise loads, without the onset of fatigue at higher lactate levels [31]. Perhaps this could lead to a certain relationship between the improvements in performance parameters and the increase in LAC in the blood, as in the five studies [28,29,30,31,33] included in the systematic review.
Blood carnosine increased significantly (p < 0.05) in the BaG compared to the CG [28]. Increasing the intramuscular availability of β-alanine through supplementation is adequate to increase the endogenous synthesis of carnosine by carnosine synthetase [12]. Under normal physiological conditions, intramuscular β-alanine is below 40 μM (saturation point of carnosine synthetase), and therefore, the availability of β-alanine is the limiting factor of carnosine synthesis [66]. High concentrations of carnosine in the muscle are effective as an intracellular pH buffer [14,15]. In this way, de Andrade et al. [29] showed that pH only decreased non-significantly (p > 0.05) in the BaG compared to pre-supplementation.
HCO3 levels increased significantly (p < 0.05) in the BaG compared to the CG and with respect to baseline [31]. The increases in HCO3 could be explained by the activation of this H+ buffer pathway [9]. The progress of exercise until fatigue produces a substantial amount of H+ in the blood [8] that is quickly captured by bicarbonate, forming carbonic acid (H2CO3), which quickly dissociates, giving rise to carbon dioxide (CO2) and water (H2O). This CO2 is driven to the lungs and expelled through breathing [9].
4.6. Adverse Effects
Two studies [29,34] included in our systematic review showed mild paresthesia. There are potential side effects associated with β-alanine, especially if a person takes it in large doses, although they are not severe. These may include skin rashes and paresthesia, a tingling sensation on the skin [17,18].
4.7. Limitations
The total sample of participants was small (n = 138), and only three female athletes were included. A small number of manuscripts were included because they met the inclusion criteria. In the seven records included, there is great variability in the β-alanine supplementation regimes, the sports modality, and the sports level of the athletes. In addition, the results of sports performance, perceptual, and anthropometric parameters, as well as biochemical markers were heterogeneous, which prevented the development of a meta-analysis. Also, the high risk of bias that could undermine confidence in the results should be considered because the studies included in this systematic review could overestimate or underestimate the true effect of β-alanine supplementation. For all the above, we recommend interpreting the results with caution.
4.8. Strengths
The systematic review was carried out following the PRISMA rules [23], and the search was conducted in three databases, namely PubMed, SCOPUS and WOS, and ResearchGate®. Two methodological quality tools were used, namely McMaster [25] and PEDro [26]. We also used the Cochrane risk-of-bias assessment instrument [24]; in addition, this review was recorded in PROSPERO (#CRD42023426545).
5. Conclusions and Perspectives
β-alanine supplementation in a dose range of 4 g/day to 6 g/day for at least four weeks can improve athletic performance for high-intensity exercises lasting between 60 s and 240 s, which intramuscularly induce an extremely acidic environment. Taken together, the results described in our systematic review showed that β-alanine supplementation is safe with potential effects on performance in strength, power, and total exercise work capacity, as well as combat-specific parameters in combat athletes. Also, supplementation with β-alanine improves lean mass, decreases fat mass, and improves the feeling of recovery after a fight. These benefits were associated with the availability of β-alanine and carnosine, which is the product that forms β-alanine, to buffer H+ and with some antioxidant capacity. Therefore, β-alanine could be a suitable NEA for combat athletes seeking to improve their sports performance and anthropometric parameters, but more evidence is needed to confirm these findings. Considering the described results, supplementation with β-alanine could be beneficial in sports with the physiological characteristics simulating combat sports, such as high-intensity intermittent exercises and high-intensity exercises of more than one minute and less than four minutes and when fatigue is established as CrossFit, artistic and rhythmic gymnastics, middle-distance running in athletics, swimming, and rowing.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors want to thank Spanish Nutrition Society “SEÑ” for their support and involvement in this study. CIBEROBN is an initiative of Instituto de Salud Carlos III, Spain.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
The search sequences carried out in the Scopus, Web of Science and PubMed databases were as follows:
- Scopus
(TITLE-ABS-KEY (beta AND alanine) AND (TITLE-ABS-KEY (combat AND sport), (TITLE-ABS-KEY (beta AND alanine) AND (TITLE-ABS-KEY (judo), (TITLE-ABS-KEY (beta AND alanine) AND (TITLE-ABS-KEY (karate), (TITLE-ABS-KEY (beta AND alanine) AND (TITLE-ABS-KEY (taekwondo), (TITLE-ABS-KEY (beta AND alanine) AND (TITLE-ABS-KEY (boxing), (TITLE-ABS-KEY (beta AND alanine) AND (TITLE-ABS-KEY (boxer), (TITLE-ABS-KEY (beta AND alanine) AND (TITLE-ABS-KEY (wrestling), (TITLE-ABS-KEY (b AND alanine) AND (TITLE-ABS-KEY (combat AND sport), (TITLE-ABS-KEY (b AND alanine) AND (TITLE-ABS-KEY (judo), (TITLE-ABS-KEY (b AND alanine) AND (TITLE-ABS-KEY (karate), (TITLE-ABS-KEY (b AND alanine) AND (TITLE-ABS-KEY (taekwondo), (TITLE-ABS-KEY (b AND alanine) AND (TITLE-ABS-KEY (boxing), (TITLE-ABS-KEY (b AND alanine) AND (TITLE-ABS-KEY (boxer), (TITLE-ABS-KEY (b AND alanine) AND (TITLE-ABS-KEY (wrestling), (TITLE-ABS-KEY (supplementation AND alanine) AND (TITLE-ABS-KEY (combat AND sport), (TITLE-ABS-KEY (supplementation AND alanine) AND (TITLE-ABS-KEY (judo), (TITLE-ABS-KEY (supplementation AND alanine) AND (TITLE-ABS-KEY (karate), (TITLE-ABS-KEY (supplementation AND alanine) AND (TITLE-ABS-KEY (taekwondo), (TITLE-ABS-KEY (supplementation AND alanine) AND (TITLE-ABS-KEY (boxing), (TITLE-ABS-KEY (supplementation AND alanine) AND (TITLE-ABS-KEY (boxer), (TITLE-ABS-KEY (supplementation AND alanine) AND (TITLE-ABS-KEY (wrestling), (TITLE-ABS-KEY (ergogenic aid AND alanine) AND (TITLE-ABS-KEY (combat AND sport), (TITLE-ABS-KEY (ergogenic aid AND alanine) AND (TITLE-ABS-KEY (judo), (TITLE-ABS-KEY (ergogenic aid AND alanine) AND (TITLE-ABS-KEY (karate), (TITLE-ABS-KEY (ergogenic aid AND alanine) AND (TITLE-ABS-KEY (taekwondo), (TITLE-ABS-KEY (ergogenic aid AND alanine) AND (TITLE-ABS-KEY (boxing), (TITLE-ABS-KEY (ergogenic aid AND alanine) AND (TITLE-ABS-KEY (boxer), (TITLE-ABS-KEY (ergogenic aid AND alanine) AND (TITLE-ABS-KEY (wrestling).
- Web of Science
(ALL=(beta alanine)) AND ALL=(combat sport), (ALL=(beta alanine)) AND ALL=(boxing), (ALL=(beta alanine)) AND ALL=(boxer), (ALL=(beta alanine)) AND ALL=(judo), (ALL=(beta alanine)) AND ALL=(taekwondo), (ALL=(beta alanine)) AND ALL=(karate), (ALL=(supplementation)) AND ALL=(combat sport), (ALL=(supplementation)) AND ALL=(judo), (ALL=(supplementation)) AND ALL=(taekwondo), (ALL=(supplementation)) AND ALL=(wrestling), (ALL=(supplementation)) AND ALL=(boxing), (ALL=(supplementation)) AND ALL=(boxer), (ALL=(supplementation)) AND ALL=(karate), (ALL=(ergogenic aid)) AND ALL=(combat sport), (ALL=(ergogenic aid)) AND ALL=(judo), (ALL=(ergogenic aid)) AND ALL=(boxing), (ALL=(ergogenic aid)) AND ALL=(boxer), (ALL=(ergogenic aid)) AND ALL=(taekwondo)
- Pubmed
(beta alanine) AND (combat sport), (beta alanine) AND (judo), (beta alanine) AND (karate), (beta alanine) AND (taekwondo), (beta alanine) AND (boxing), (beta alanine) AND (boxer), (beta alanine) AND (wrestling), (b alanine) AND (combat sport), (b alanine) AND (judo), (b alanine) AND (karate), (b alanine) AND (taekwondo), (b alanine) AND (boxing), (b alanine) AND (boxer), (b alanine) AND (wrestling), (supplementation) AND (combat sport), (supplementation) AND (judo), (supplementation) AND (karate), (supplementation) AND (taekwondo), (supplementation) AND (boxing), (supplementation) AND (boxer), (supplementation) AND (wrestling), (ergogenic aid) AND (combat sport), (ergogenic aid) AND (judo), (ergogenic aid) AND (karate), (ergogenic aid) AND (taekwondo), (ergogenic aid) AND (boxing), (ergogenic aid) AND (boxer), (ergogenic aid) AND (wrestling).
References
- Barley, O.R.; Chapman, D.W.; Guppy, S.N.; Abbiss, C.R. Considerations When Assessing Endurance in Combat Sport Athletes. Front. Physiol. 2019, 10, 205. [Google Scholar] [CrossRef]
- Chaabène, H.; Hachana, Y.; Franchini, E.; Mkaouer, B.; Chamari, K. Physical and physiological profile of elite karate athletes. Sports Med. 2012, 42, 829–843. [Google Scholar] [PubMed]
- Coelho-E-Silva, M.J.; Sousa-E-Silva, P.; Morato, V.S.; Costa, D.C.; Martinho, D.V.; Rama, L.M.; Valente-Dos-Santos, J.; Werneck, A.O.; Tavares, O.M.; Conde, J.; et al. Allometric Modeling of Wingate Test among Adult Male Athletes from Combat Sports. Medicina 2020, 56, 480. [Google Scholar] [CrossRef] [PubMed]
- Ruddock, A.; James, L.; French, D.; Rogerson, D.; Driller, M.; Hembrough, D. High-Intensity Conditioning for Combat Athletes: Practical Recommendations. Appl. Sci. 2021, 11, 10658. [Google Scholar] [CrossRef]
- James, L.P.; Haff, G.G.; Kelly, V.G.; Beckman, E.M. Towards a Determination of the Physiological Characteristics Distinguishing Successful Mixed Martial Arts Athletes: A Systematic Review of Combat Sport Literature. Sports Med. 2016, 46, 1525–1551. [Google Scholar] [CrossRef] [PubMed]
- Atakan, M.M.; Li, Y.; Koşar, Ş.N.; Turnagöl, H.H.; Yan, X. Evidence-Based Effects of High-Intensity Interval Training on Exercise Capacity and Health: A Review with Historical Perspective. Int. J. Environ. Res. Public. Health. 2021, 18, 7201. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.J.; Qin, Z.; Wang, P.Y.; Sun, Y.; Liu, X. Muscle fatigue: General understanding and treatment. Exp. Mol. Med. 2017, 49, e384. [Google Scholar] [CrossRef] [PubMed]
- Westerblad, H.; Allen, D.G.; Lännergren, J. Muscle fatigue: Lactic acid or inorganic phosphate the major cause? News Physiol. Sci. 2002, 17, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Hamm, L.; Nakhoul, N.; Hering-Smith, K.S. Acid-Base Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 2232. [Google Scholar] [CrossRef]
- Lancha Junior, A.H.; de Salles Painelli, V.; Saunders, B.; Artioli, G.G. Nutritional Strategies to Modulate Intracellular and Extracellular Buffering Capacity During High-Intensity Exercise. Sports Med. 2015, 45, S71–S81. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Del Valle Soto, M.; Adams, D.P.; Gutiérrez-Abejón, E.; Seco-Calvo, J. Impact of Optimal Timing of Intake of Multi-Ingredient Performance Supplements on Sports Performance, Muscular Damage, and Hormonal Behavior across a Ten-Week Training Camp in Elite Cyclists: A Randomized Clinical Trial. Nutrients 2021, 13, 3746. [Google Scholar] [CrossRef] [PubMed]
- Hobson, R.M.; Saunders, B.; Ball, G.; Harris, R.C.; Sale, C. Effects of β-alanine supplementation on exercise performance: A meta-analysis. Amino Acids. 2012, 43, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Tallon, M.J.; Dunnett, M.; Boobis, L.; Coakley, J.; Kim, H.J.; Fallowfield, J.L.; Hill, C.A.; Sale, C.; Wise, J.A. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids. 2006, 30, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef] [PubMed]
- Trexler, E.T.; Smith-Ryan, A.E.; Stout, J.R.; Hoffman, J.R.; Wilborn, C.D.; Sale, C.; Kreider, R.B.; Jäger, R.; Earnest, C.P.; Bannock, L.; et al. International society of sports nutrition position stand: Beta-Alanine. J. Int. Soc. Sports Nutr. 2015, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Varanoske, A.; Stout, J.R. Effects of β-Alanine Supplementation on Carnosine Elevation and Physiological Performance. Adv. Food Nutr. Res. 2018, 84, 183–206. [Google Scholar] [PubMed]
- Church, D.D.; Hoffman, J.R.; Varanoske, A.N.; Wang, R.; Baker, K.M.; La Monica, M.B.; Beyer, K.S.; Dodd, S.J.; Oliveira, L.P.; Harris, R.C.; et al. Comparison of Two β-Alanine Dosing Protocols on Muscle Carnosine Elevations. J. Am. Coll. Nutr. 2017, 36, 608–616. [Google Scholar] [CrossRef]
- Naderi, A.; de Oliveira, E.P.; Ziegenfuss, T.N.; Willems, M.E.T. Timing, optimal dose and intake duration of dietary supplements with evidence-based use in sports nutrition. J. Exerc. Nutr. Biochem. 2016, 20, 1–12. [Google Scholar] [CrossRef]
- Sports Nutrition Market Size, Share & Trends Analysis Report by Product Type (Sports Drink, Sports Supplements, Sports Food), by Distribution Channel (E-commerce, Brick and Mortar), by Region, and Segment Forecasts, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/sports-nutrition-market (accessed on 24 August 2023).
- Fernández-Lázaro, D.; Seco-Calvo, J.; Pascual-Fernández, J.; Domínguez-Ortega, C.; Del Valle Soto, M.; Mielgo-Ayuso, J. 6-Week Supplementation with Tribulus terrestris L. to Trained Male CrossFit® Athletes on Muscle, Inflammation, and Antioxidant Biomarkers: A Randomized, Single-Blind, Placebo-Controlled Trial. Int. J. Environ. Res. Public. Health 2022, 19, 16158. [Google Scholar] [CrossRef] [PubMed]
- International Olympic Committee. Tokyo 2020 Summer Olympics—Athletes, Medals and Results. Available online: https://olympics.com/es/olympic-games/tokyo-2020 (accessed on 9 August 2023).
- Straus, S.E.; Glasziou, P.; Richardson, W.S.H.R. Evidence-Based Medicine: How to Practice and Teach It, 4th ed.; Churchill Livingstone Elsevier: Edinburgh, UK; Available online: https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-ebm/ (accessed on 31 March 2023).
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Stewart, C.; Pollock, N.; Letts, L.; Bosch, J.; Westmorland, M. Guidelines for Critical Review of Qualitative Studies; McMaster University Occupational Therapy Evidence-Based Practice Research Group: Hamilton, ON, Canada, 1998; pp. 1–9. [Google Scholar]
- Moseley, A.M.; Elkins, M.R.; Van der Wees, P.J.; Pinheiro, M.B. Using research to guide practice: The Physiotherapy Evidence Database (PEDro). Braz. J. Phys. Ther. 2020, 24, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alabsi, K.; Rashidlamir, A.; Dokht, E.H. The Effect of 4 Weeks of Strength Training and Beta-Alanine Supplementation on Anaerobic Power and Carnosine Level in Boxer Players. J. Sci. Sport. Exerc. 2023, 5, 62–69. [Google Scholar] [CrossRef]
- de Andrade Kratz, C.; de Salles Painelli, V.; de Andrade Nemezio, K.M.; da Silva, R.P.; Franchini, E.; Zagatto, A.M.; Gualano, B.; Artioli, G.G. Beta-alanine supplementation enhances judo-related performance in highly-trained athletes. J. Sci. Med. Sport. 2017, 20, 403–408. [Google Scholar] [CrossRef]
- Donovan, T.; Ballam, T.; Morton, J.P.; Close, G.L. β-alanine improves punch force and frequency in amateur boxers during a simulated contest. Int. J. Sport. Nutr. Exerc. Metab. 2012, 22, 331–337. [Google Scholar] [CrossRef]
- Halz, M.; Kaszuba, M.; Helbin, J.; Krzysztofik, S.; Suchanecka, A.; Zając, A. Beta-alanine supplementation and anaerobic performance in highly trained judo athletes. Balt. J. Health Phys. Act. 2022, 14, 1. [Google Scholar] [CrossRef]
- Kern, B.D.; Robinson, T.L. Effects of β-alanine supplementation on performance and body composition in collegiate wrestlers and football players. J. Strength Cond. Res. 2011, 25, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Song, H.S.; Yoon, D.H.; Fukuda, D.H.; Kim, S.H.; Park, D.H. The effects of 10 weeks of β-alanine supplementation on peak power, power drop, and lactate response in Korean national team boxers. J. Exerc. Rehabil. 2018, 14, 985–992. [Google Scholar] [CrossRef]
- Lopez-Grueso, R.; Marco, A.A.; Marín, J.M.S.; Carretero, C.M. Beta-alanine supplementation seems to increase physical performance and acute recovery in competitive judokas. Eur. J. Hum. Mov. 2014, 33, 123–136. [Google Scholar]
- World Anti-Doping Agency. Prohibited List. Available online: https://www.wada-ama.org/sites/default/files/resources/files/2022list_final_en.pdf (accessed on 10 August 2023).
- Sport Integrity Australia (Australian Gov). Supplements in Sport. Available online: https://www.sportintegrity.gov.au/what-we-do/anti-doping/supplements-sport (accessed on 10 August 2023).
- Tamaki, N.; Tsunemori, F.; Wakabayashi, M.; Hama, T. Effect of histidine-free and -excess diets on anserine and carnosine contents in rat gastrocnemius muscle. J. Nutr. Sci. Vitaminol. 1977, 23, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; Blancquaert, L.; Everaert, I.; Bex, T.; Taes, Y.; Calders, P.; Achten, E.; Derave, W. Meal and beta-alanine coingestion enhances muscle carnosine loading. Med. Sci. Sports Exerc. 2013, 45, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Crozier, R.A.; Ajit, S.K.; Kaftan, E.J.; Pausch, M.H. MrgD activation inhibits KCNQ/M-currents and contributes to enhanced neuronal excitability. J. Neurosci. 2007, 27, 4492–4496. [Google Scholar] [CrossRef] [PubMed]
- Décombaz, J.; Beaumont, M.; Vuichoud, J.; Bouisset, F.; Stellingwerff, T. Effect of slow-release β-alanine tablets on absorption kinetics and paresthesia. Amino Acids. 2012, 43, 67–76. [Google Scholar] [CrossRef]
- Woodward, M.; Debold, E.P. Acidosis and phosphate directly reduce myosin’s force-generating capacity through distinct molecular mechanisms. Front. Physiol. 2018, 10, 377831. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Domínguez-Ortega, C.; Busto, N.; Santamaría-Peláez, M.; Roche, E.; Gutiérez-Abejón, E.; Mielgo-Ayuso, J. Influence of N-Acetylcysteine Supplementation on Physical Performance and Laboratory Biomarkers in Adult Males: A Systematic Review of Controlled Trials. Nutrients 2023, 15, 2463. [Google Scholar] [CrossRef]
- Blancquaert, L.; Everaert, I.; Derave, W. Beta-alanine supplementation, muscle carnosine and exercise performance. Curr. Opin. Clin. Nutr. Metab. Care. 2015, 18, 63–70. [Google Scholar] [CrossRef]
- Artioli, G.G.; Gualano, B.; Smith, A.; Stout, J.; Lancha, A.H. Role of β-alanine supplementation on muscle carnosine and exercise performance. Med. Sci. Sports Exerc. 2010, 42, 1162–1173. [Google Scholar] [CrossRef]
- Saunders, B.; Elliott-Sale, K.; Artioli, G.G.; Swinton, P.A.; Dolan, E.; Roschel, H.; Sale, C.; Gualano, B. β-alanine supplementation to improve exercise capacity and performance: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 658–669. [Google Scholar] [CrossRef]
- Derave, W.; Ozdemir, M.S.; Harris, R.C.; Pottier, A.; Reyngoudt, H.; Koppo, K.; Wise, J.A.; Achten, E. Beta-Alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters. J. Appl. Physiol. 2007, 103, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Ducker, K.J.; Dawson, B.; Wallman, K.E. Effect of beta-alanine supplementation on 800-m running performance. Int. J. Sport. Nutr. Exerc. Metab. 2013, 23, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Sas-Nowosielski, K.; Wyciślik, J.; Kaczka, P. Beta-Alanine Supplementation and Sport Climbing Performance. Int. J. Environ. Res. Public. Health. 2021, 18, 5370. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, I.P.; Harris, R.C.; Kim, H.J.; Kim, C.K.; Dang, V.H.; Lam, T.Q.; Bui, T.T.; Smith, M.; Wise, J.A. The effects of 10 weeks of resistance training combined with beta-alanine supplementation on whole body strength, force production, muscular endurance and body composition. Amino Acids. 2008, 34, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.; Ratamess, N.A.; Ross, R.; Kang, J.; Magrelli, J.; Neese, K.; Faigenbaum, A.D.; Wise, J.A. Beta-alanine and the hormonal response to exercise. Int. J. Sports Med. 2008, 29, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R.; Michaelis, J.; Syrris, P. Non-enzymatic glycosylation of the dipeptide L-carnosine, a potential anti-protein-cross-linking agent. FEBS Lett. 1995, 371, 81–85. [Google Scholar] [CrossRef] [PubMed]
- James, R.M.; Cooper, S.B.; Robertson, J.; Martin, D.; Harris, R.C.; Sale, C. Effect of β-alanine supplementation on 20 km cycling time trial performance. Rev. Bras. Educ. Fís. Esporte. 2014, 28, 395–403. [Google Scholar] [CrossRef][Green Version]
- Hobson, R.M.; Harris, R.C.; Martin, D.; Smith, P.; Macklin, B.; Gualano, B.; Sale, C. Effect of beta-alanine, with and without sodium bicarbonate, on 2000-m rowing performance. Int. J. Sport. Nutr. Exerc. Metab. 2013, 23, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Portington, K.J.; Pascoe, D.D.; Webster, M.J.; Anderson, L.H.; Rutland, R.R.; Gladden, L.B. Effect of induced alkalosis on exhaustive leg press performance. Med. Sci. Sports Exerc. 1998, 30, 523–528. [Google Scholar] [CrossRef]
- Van Thienen, R.; Van Proeyen, K.; Eynde, B.V.; Puype, J.; Lefere, T.; Hespel, P. Beta-alanine improves sprint performance in endurance cycling. Med. Sci. Sports Exerc. 2009, 41, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, K.M.; Wright, G.A.; Glenn Brice, A.; Doberstein, S.T. The effect of beta-alanine supplementation on power performance during repeated sprint activity. J. Strength Cond. Res. 2010, 24, 79–87. [Google Scholar] [CrossRef]
- Mendez-Villanueva, A.; Edge, J.; Suriano, R.; Hamer, P.; Bishop, D. The Recovery of Repeated-Sprint Exercise Is Associated with PCr Resynthesis, while Muscle pH and EMG Amplitude Remain Depressed. PLoS ONE 2012, 7, 0051977. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.A.; Smith, A.E.; Kendall, K.L.; Stout, J.R.; Cramer, J.T. Six weeks of high-intensity interval training with and without beta-alanine supplementation for improving cardiovascular fitness in women. J. Strength Cond. Res. 2010, 24, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.E.; Walter, A.A.; Graef, J.L.; Kendall, K.L.; Moon, J.R.; Lockwood, C.M.; Fukuda, D.H.; Beck, T.W.; Cramer, J.T.; Stout, J.R. Effects of beta-alanine supplementation and high-intensity interval training on endurance performance and body composition in men; a double-blind trial. J. Int. Soc. Sports Nutr. 2009, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.; Ratamess, N.; Kang, J.; Mangine, G.; Faigenbaum, A.; Stout, J. Effect of creatine and beta-alanine supplementation on performance and endocrine responses in strength/power athletes. Int. J. Sport. Nutr. Exerc. Metab. 2006, 16, 430–446. [Google Scholar] [CrossRef] [PubMed]
- Januszko, P.; Lange, E. Nutrition, supplementation and weight reduction in combat sports: A review. AIMS Public Health 2021, 8, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.R.; Cramer, J.T.; Zoeller, R.F.; Torok, D.; Costa, P.; Hoffman, J.R.; Harris, R.C.; O’Kroy, J. Effects of beta-alanine supplementation on the onset of neuromuscular fatigue and ventilatory threshold in women. Amino Acids. 2007, 32, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Ratamess, N.A.; Faigenbaum, A.D.; Ross, R.; Kang, J.; Stout, J.R.; Wise, J.A. Short-duration beta-alanine supplementation increases training volume and reduces subjective feelings of fatigue in college football players. Nutr. Res. 2008, 28, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Rascon, J.; Trujillo, E.; Morales-Acuña, F.; Gurovivh, A.N. Differences between Males and Females in Determining Exercise Intensity. Int. J. Exerc. Sci. 2020, 13, 1305. [Google Scholar]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef] [PubMed]
- Perim, P.; Marticorena, F.M.; Ribeiro, F.; Barreto, G.; Gobbi, N.; Kerksick, C.; Dolan, E.; Saunders, B. Can the Skeletal Muscle Carnosine Response to Beta-Alanine Supplementation Be Optimized? Front. Nutr. 2019, 6, 135. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).























































