The Mechanism of the Gut-Brain Axis in Regulating Food Intake
Abstract
1. Introduction
2. The Role of “First-Order Neural Nuclei” in Food Intake Regulation
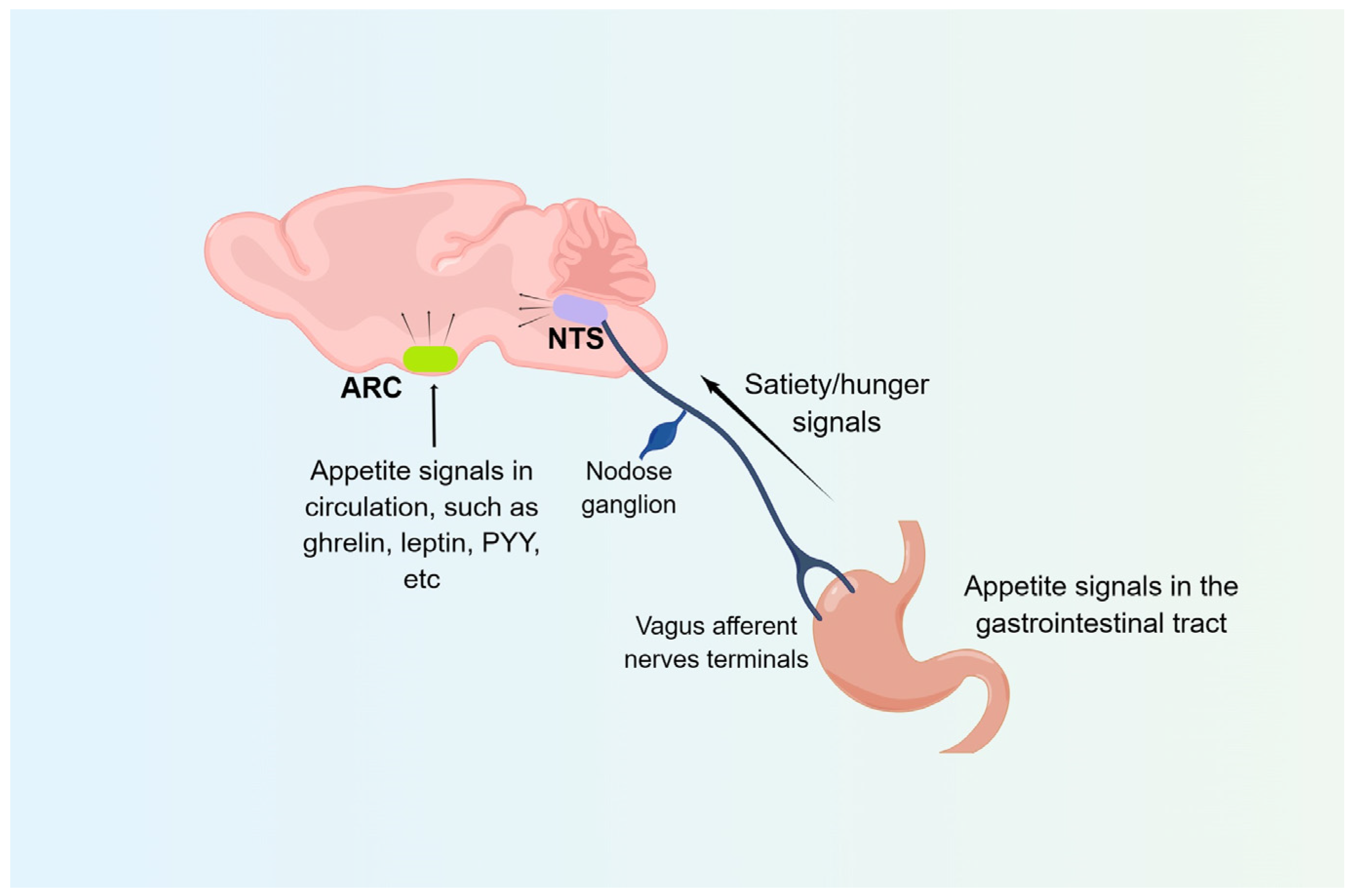
2.1. Arcuate Nucleus (ARC)
2.2. Nucleus Tractus Solitarii (NTS)
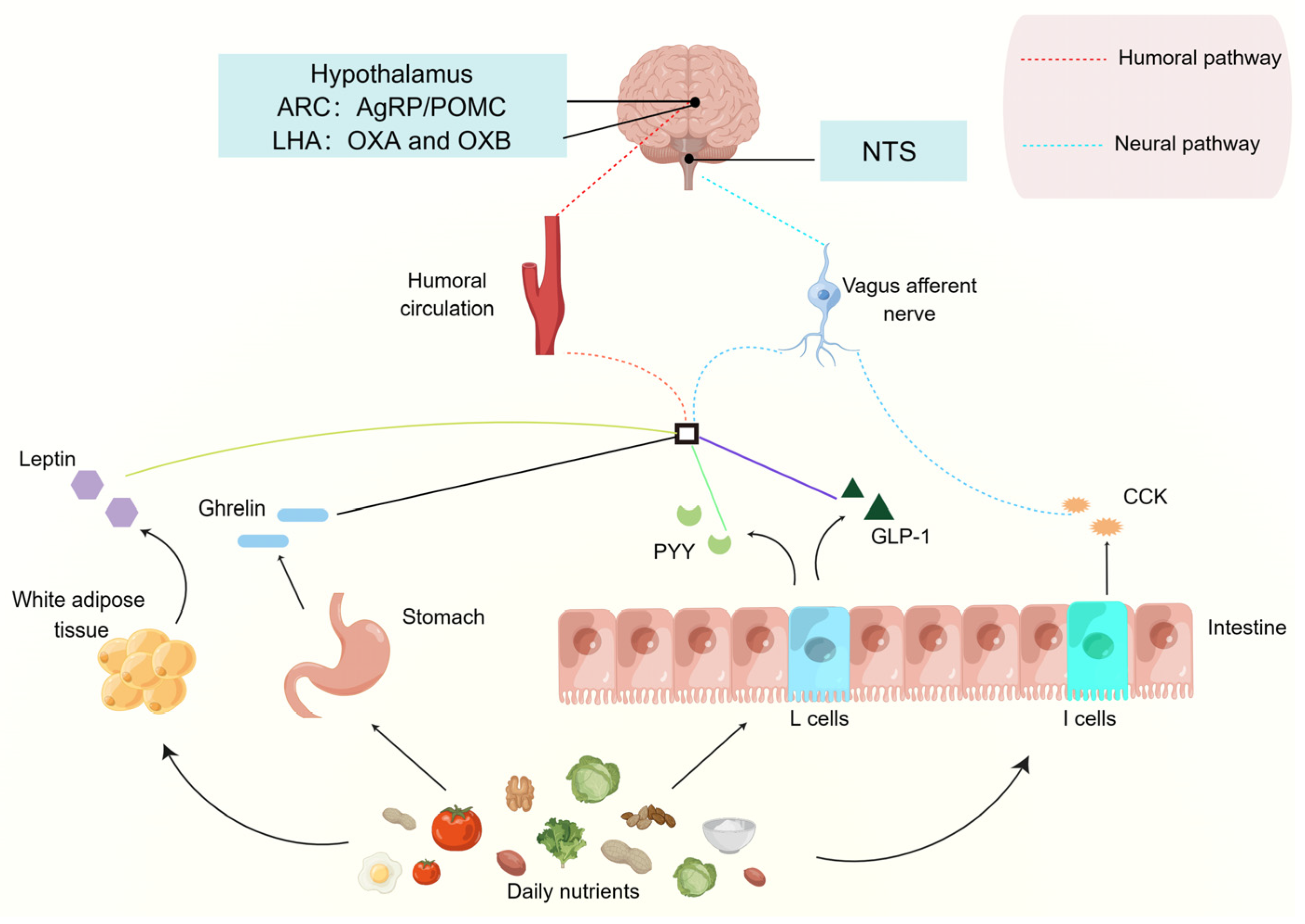
3. The Effect of Typical Gut-Brain Peptides on Food Intake Regulation
3.1. Orexin
3.2. Ghrelin
3.3. CCK
3.4. GLP-1
3.5. Peptide YY (PYY)
3.6. Leptin
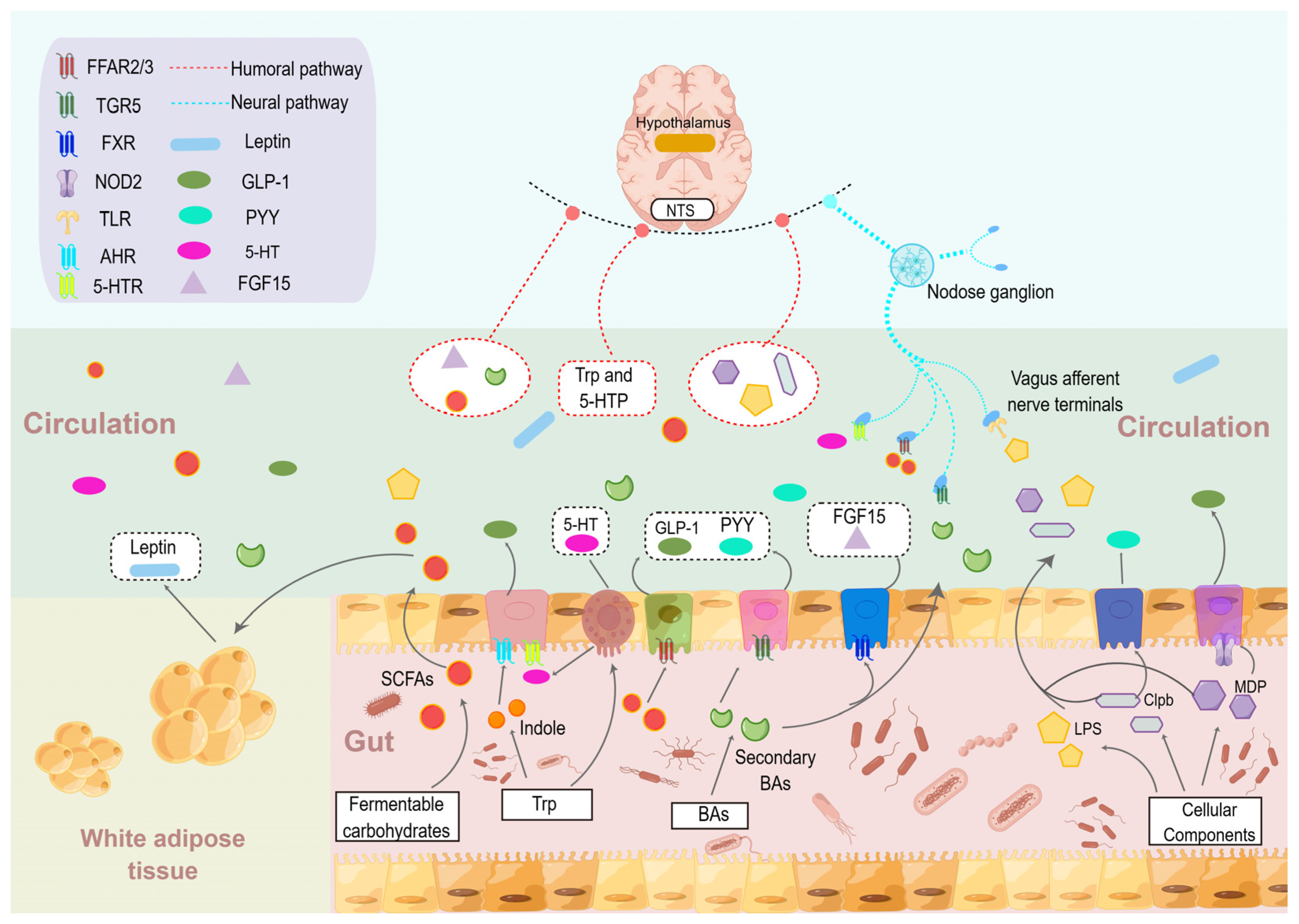
4. The Influence of Gut Microbes and Their Metabolites in Food Intake Regulation
4.1. SCFAs
4.2. BAs
4.3. Tryptophan-Derived Metabolites
4.4. Bacterial Proteins and Cellular Components of Gut Microbe
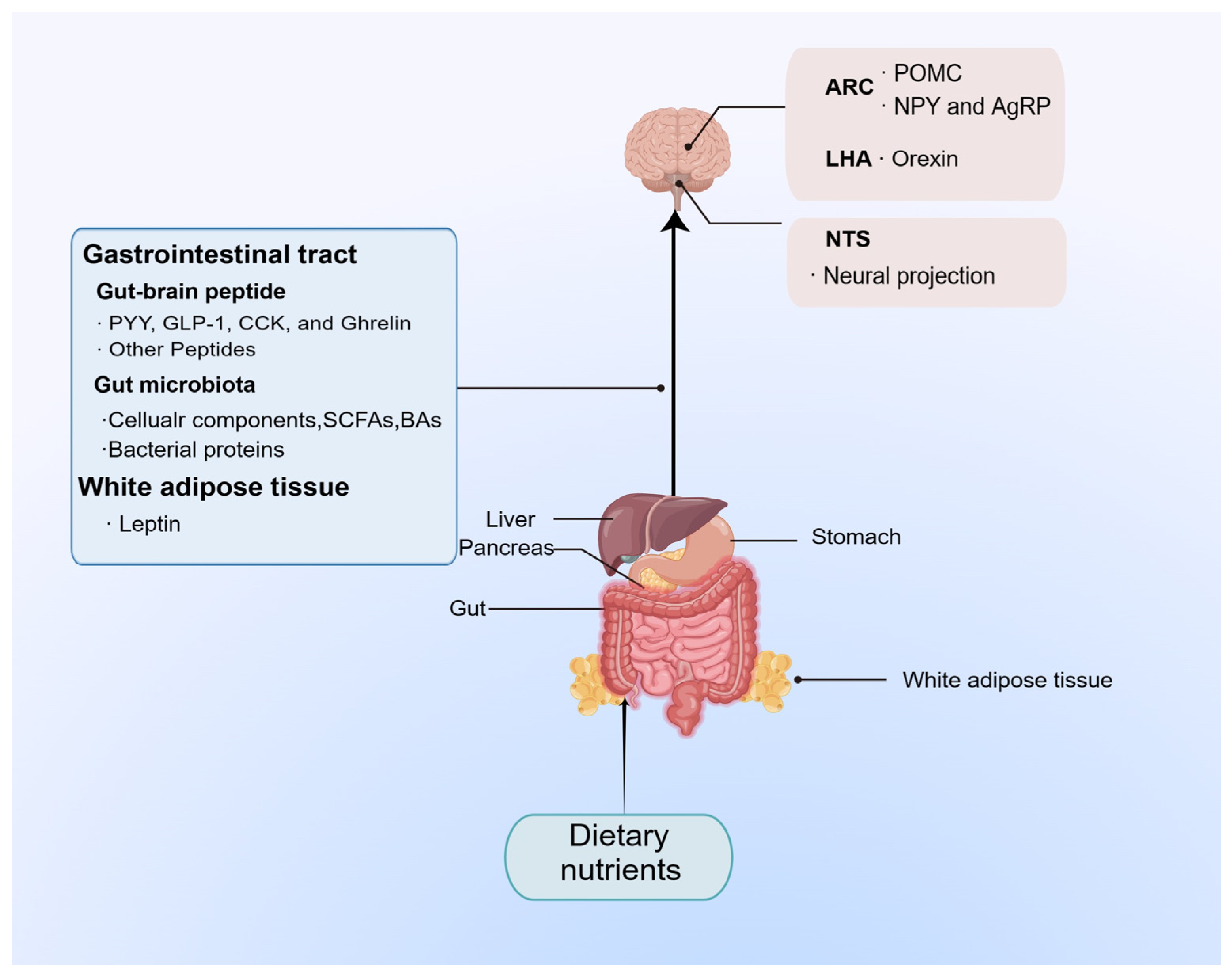
5. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Packard, A.E.B.; Ghosal, S.; Herman, J.P.; Woods, S.C.; Ulrich-Lai, Y.M. Chronic Variable Stress Improves Glucose Tolerance in Rats with Sucrose-Induced Prediabetes. Psychoneuroendocrinology 2014, 47, 178–188. [Google Scholar] [CrossRef]
- Mithieux, G. Crosstalk between Gastrointestinal Neurons and the Brain in the Control of Food Intake. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Fetissov, S.O.; Averina, O.V.; Danilenko, V.N. Neuropeptides in the Microbiota-Brain Axis and Feeding Behavior in Autism Spectrum Disorder. Nutrition 2019, 61, 43–48. [Google Scholar] [CrossRef]
- Roh, E.; Choi, K.M. Hormonal Gut–Brain Signaling for the Treatment of Obesity. Int. J. Mol. Sci. 2023, 24, 3384. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Vohra, M.S.; Benchoula, K.; Serpell, C.J.; Hwa, W.E. AgRP/NPY and POMC Neurons in the Arcuate Nucleus and Their Potential Role in Treatment of Obesity. Eur. J. Pharmacol. 2022, 915, 174611. [Google Scholar] [CrossRef]
- Wen, S.; Wang, C.; Gong, M.; Zhou, L. An Overview of Energy and Metabolic Regulation. Sci. China Life Sci. 2019, 62, 771–790. [Google Scholar] [CrossRef]
- Bouret, S.G. Development of Hypothalamic Circuits That Control Food Intake and Energy Balance. In Appetite and Food Intake: Central Control; Harris, R.B.S., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-2316-9. [Google Scholar]
- Essner, R.A.; Smith, A.G.; Jamnik, A.A.; Ryba, A.R.; Trutner, Z.D.; Carter, M.E. AgRP Neurons Can Increase Food Intake during Conditions of Appetite Suppression and Inhibit Anorexigenic Parabrachial Neurons. J. Neurosci. 2017, 37, 8678–8687. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, Q.; Purohit, N.M.; Davenport, J.B.; Brennan, C.; Patel, R.K.; Godschall, E.; Zwiefel, L.S.; Spano, A.; Campbell, J.N.; et al. Food-Induced Dopamine Signaling in AgRP Neurons Promotes Feeding. Cell Rep. 2022, 41, 111718. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yoon, N.A.; Liu, Z.-W.; Song, J.E.; Horvath, T.L.; Kim, J.D.; Diano, S. Drp1 Is Required for AgRP Neuronal Activity and Feeding. eLife 2021, 10, e64351. [Google Scholar] [CrossRef]
- Luquet, S.; Perez, F.A.; Hnasko, T.S.; Palmiter, R.D. NPY/AgRP Neurons Are Essential for Feeding in Adult Mice but Can Be Ablated in Neonates. Science 2005, 310, 683–685. [Google Scholar] [CrossRef]
- Clark, A.J.L.; Lowry, P. 60 YEARS OF POMC: POMC: The Consummate Peptide Hormone Precursor. J. Mol. Endocrinol. 2016, 56, E1–E2. [Google Scholar] [CrossRef][Green Version]
- Dores, R.M.; Baron, A.J. Evolution of POMC: Origin, Phylogeny, Posttranslational Processing, and the Melanocortins. Ann. N. Y. Acad. Sci. 2011, 1220, 34–48. [Google Scholar] [CrossRef]
- Tao, Y.-X. Mutations in the Melanocortin-3 Receptor (MC3R) Gene: Impact on Human Obesity or Adiposity. Curr. Opin. Investig. Drugs 2010, 11, 1092–1096. [Google Scholar]
- Biebermann, H.; Kühnen, P.; Kleinau, G.; Krude, H. The Neuroendocrine Circuitry Controlled by POMC, MSH, and AGRP. Handb. Exp. Pharmacol. 2012, 209, 47–75. [Google Scholar] [CrossRef]
- Koch, M.; Varela, L.; Kim, J.G.; Kim, J.D.; Hernández-Nuño, F.; Simonds, S.E.; Castorena, C.M.; Vianna, C.R.; Elmquist, J.K.; Morozov, Y.M.; et al. Hypothalamic POMC Neurons Promote Cannabinoid-Induced Feeding. Nature 2015, 519, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Yada, T. Vagal Afferents Sense Meal-Associated Gastrointestinal and Pancreatic Hormones: Mechanism and Physiological Role. Neuropeptides 2012, 46, 291–297. [Google Scholar] [CrossRef]
- Miller, G.D. Appetite Regulation: Hormones, Peptides, and Neurotransmitters and Their Role in Obesity. Am. J. Lifestyle Med. 2019, 13, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Kaelberer, M.M.; Rupprecht, L.E.; Liu, W.W.; Weng, P.; Bohórquez, D.V. Neuropod Cells: The Emerging Biology of Gut-Brain Sensory Transduction. Annu. Rev. Neurosci. 2020, 43, 337–353. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A Gut-Brain Neural Circuit for Nutrient Sensory Transduction. Science 2018, 361, eaat5236. [Google Scholar] [CrossRef]
- Cheng, W.; Gordian, D.; Ludwig, M.Q.; Pers, T.H.; Seeley, R.J.; Myers, M.G. Hindbrain Circuits in the Control of Eating Behaviour and Energy Balance. Nat. Metab. 2022, 4, 826–835. [Google Scholar] [CrossRef]
- Cheng, W.; Gonzalez, I.; Pan, W.; Tsang, A.H.; Adams, J.; Ndoka, E.; Gordian, D.; Khoury, B.; Roelofs, K.; Evers, S.S.; et al. Calcitonin Receptor Neurons in the Mouse Nucleus Tractus Solitarius Control Energy Balance via the Non-Aversive Suppression of Feeding. Cell Metab. 2020, 31, 301–312.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Zhou, J.; Feng, Q.; Zhang, J.-E.; Lin, S.; Bao, J.; Wu, P.; Luo, M. Acute and Long-Term Suppression of Feeding Behavior by POMC Neurons in the Brainstem and Hypothalamus, Respectively. J. Neurosci. 2013, 33, 3624–3632. [Google Scholar] [CrossRef]
- Murphy, S.; Collis Glynn, M.; Dixon, T.N.; Grill, H.J.; McNally, G.P.; Ong, Z.Y. Nucleus of the Solitary Tract A2 Neurons Control Feeding Behaviors via Projections to the Paraventricular Hypothalamus. Neuropsychopharmacology 2023, 48, 351–361. [Google Scholar] [CrossRef] [PubMed]
- de Lartigue, G. Role of the Vagus Nerve in the Development and Treatment of Diet-Induced Obesity. J. Physiol. 2016, 594, 5791–5815. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, M.; Wang, L.; Zhang, L.; Xu, D.; Cao, P.; Wang, F.; Herzog, H.; Song, S.; Zhan, C. A Vagal-NTS Neural Pathway That Stimulates Feeding. Curr. Biol. 2020, 30, 3986–3998.e5. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors That Regulate Feeding Behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef]
- Milbank, E.; López, M. Orexins/Hypocretins: Key Regulators of Energy Homeostasis. Front. Endocrinol. 2019, 10, 830. [Google Scholar] [CrossRef]
- Soya, S.; Sakurai, T. Evolution of Orexin Neuropeptide System: Structure and Function. Front. Neurosci. 2020, 14, 691. [Google Scholar] [CrossRef]
- Edwards, C.M.; Abusnana, S.; Sunter, D.; Murphy, K.G.; Ghatei, M.A.; Bloom, S.R. The Effect of the Orexins on Food Intake: Comparison with Neuropeptide Y, Melanin-Concentrating Hormone and Galanin. J. Endocrinol. 1999, 160, R7–R12. [Google Scholar] [CrossRef]
- Gotter, A.L.; Webber, A.L.; Coleman, P.J.; Renger, J.J.; Winrow, C.J. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin Receptor Function, Nomenclature and Pharmacology. Pharmacol. Rev. 2012, 64, 389–420. [Google Scholar] [CrossRef]
- Jin, T.; Jiang, Z.; Luan, X.; Qu, Z.; Guo, F.; Gao, S.; Xu, L.; Sun, X. Exogenous Orexin-A Microinjected Into Central Nucleus of the Amygdala Modulates Feeding and Gastric Motility in Rats. Front. Neurosci. 2020, 14, 274. [Google Scholar] [CrossRef] [PubMed]
- Takamata, A.; Nishimura, Y.; Oka, A.; Nagata, M.; Kosugi, N.; Eguchi, S.; Negishi, H.; Morimoto, K. Endogenous Androgens Diminish Food Intake and Activation of Orexin A Neurons in Response to Reduced Glucose Availability in Male Rats. Nutrients 2022, 14, 1235. [Google Scholar] [CrossRef] [PubMed]
- Morello, G.; Imperatore, R.; Palomba, L.; Finelli, C.; Labruna, G.; Pasanisi, F.; Sacchetti, L.; Buono, L.; Piscitelli, F.; Orlando, P.; et al. Orexin-A Represses Satiety-Inducing POMC Neurons and Contributes to Obesity via Stimulation of Endocannabinoid Signaling. Proc. Natl. Acad. Sci. USA 2016, 113, 4759–4764. [Google Scholar] [CrossRef] [PubMed]
- Schalla, M.A.; Stengel, A. The Role of the Gastric Hormones Ghrelin and Nesfatin-1 in Reproduction. Int. J. Mol. Sci. 2021, 22, 11059. [Google Scholar] [CrossRef]
- Jiao, Z.-T.; Luo, Q. Molecular Mechanisms and Health Benefits of Ghrelin: A Narrative Review. Nutrients 2022, 14, 4191. [Google Scholar] [CrossRef]
- Al Massadi, O.; López, M.; Tschöp, M.; Diéguez, C.; Nogueiras, R. Current Understanding of the Hypothalamic Ghrelin Pathways Inducing Appetite and Adiposity. Trends Neurosci. 2017, 40, 167–180. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin Is a Growth-Hormone-Releasing Acylated Peptide from Stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Price, M.L.; Ley, C.D.; Gorvin, C.M. The Emerging Role of Heterodimerisation and Interacting Proteins in Ghrelin Receptor Function. J. Endocrinol. 2021, 252, R23–R39. [Google Scholar] [CrossRef]
- Nunez-Salces, M.; Li, H.; Feinle-Bisset, C.; Young, R.L.; Page, A.J. The Regulation of Gastric Ghrelin Secretion. Acta Physiol. 2021, 231, e13588. [Google Scholar] [CrossRef]
- Holubová, M.; Spolcová, A.; Demianová, Z.; Sýkora, D.; Fehrentz, J.A.; Martinez, J.; Stofková, A.; Jurčovičová, J.; Drápalová, J.; Lacinová, Z.; et al. Ghrelin Agonist JMV 1843 Increases Food Intake, Body Weight and Expression of Orexigenic Neuropeptides in Mice. Physiol. Res. 2013, 62, 435–444. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, J.; Li, D.; Zhou, X.; Li, X. Tryptophan Enhances Ghrelin Expression and Secretion Associated with Increased Food Intake and Weight Gain in Weanling Pigs. Domest. Anim. Endocrinol. 2007, 33, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-R.; Chen, H.; Zhou, J.-J.; Pradhan, G.; Sun, Y.; Pan, H.-L.; Li, D.-P. Ghrelin Receptors Mediate Ghrelin-Induced Excitation of Agouti-Related Protein/Neuropeptide Y but Not pro-Opiomelanocortin Neurons. J. Neurochem. 2017, 142, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Leyva, S.; Diano, S. Hormonal Regulation of the Hypothalamic Melanocortin System. Front. Physiol. 2014, 5, 480. [Google Scholar] [CrossRef]
- Bruschetta, G.; Jin, S.; Kim, J.D.; Diano, S. Prolyl Carboxypeptidase in Agouti-Related Peptide Neurons Modulates Food Intake and Body Weight. Mol. Metab. 2018, 10, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Caron, J.; Domenger, D.; Dhulster, P.; Ravallec, R.; Cudennec, B. Protein Digestion-Derived Peptides and the Peripheral Regulation of Food Intake. Front. Endocrinol. 2017, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Moran, T.H.; Kinzig, K.P. Gastrointestinal Satiety Signals II. Cholecystokinin. Am. J. Physiol. -Gastrointest. Liver Physiol. 2004, 286, G183–G188. [Google Scholar] [CrossRef]
- Wren, A.M.; Bloom, S.R. Gut Hormones and Appetite Control. Gastroenterology 2007, 132, 2116–2130. [Google Scholar] [CrossRef]
- Wang, L.; Ding, L.; Zhu, W.; Hang, S. Soybean Protein Hydrolysate Stimulated Cholecystokinin Secretion and Inhibited Feed Intake through Calcium-Sensing Receptors and Intracellular Calcium Signalling in Pigs. Food Funct. 2021, 12, 9286–9299. [Google Scholar] [CrossRef]
- Panda, V.; Shinde, P. Appetite Suppressing Effect of Spinacia Oleracea in Rats: Involvement of the Short Term Satiety Signal Cholecystokinin. Appetite 2017, 113, 224–230. [Google Scholar] [CrossRef]
- Penney, C.C.; Volkoff, H. Peripheral Injections of Cholecystokinin, Apelin, Ghrelin and Orexin in Cavefish (Astyanax Fasciatus Mexicanus): Effects on Feeding and on the Brain Expression Levels of Tyrosine Hydroxylase, Mechanistic Target of Rapamycin and Appetite-Related Hormones. Gen. Comp. Endocrinol. 2014, 196, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Broberger, C.; Holmberg, K.; Shi, T.J.; Dockray, G.; Hökfelt, T. Expression and Regulation of Cholecystokinin and Cholecystokinin Receptors in Rat Nodose and Dorsal Root Ganglia. Brain Res. 2001, 903, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Ndjim, M.; Poinsignon, C.; Parnet, P.; Le Dréan, G. Loss of Vagal Sensitivity to Cholecystokinin in Rats Born with Intrauterine Growth Retardation and Consequence on Food Intake. Front. Endocrinol. 2017, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Yahashi, S.; Azuma, M.; Matsuda, K. The Anorexigenic Effect of Cholecystokinin Octapeptide in a Goldfish Model Is Mediated by the Vagal Afferent and Subsequently through the Melanocortin- and Corticotropin-Releasing Hormone-Signaling Pathways. Peptides 2010, 31, 2130–2134. [Google Scholar] [CrossRef]
- Fan, W.; Ellacott, K.L.J.; Halatchev, I.G.; Takahashi, K.; Yu, P.; Cone, R.D. Cholecystokinin-Mediated Suppression of Feeding Involves the Brainstem Melanocortin System. Nat. Neurosci. 2004, 7, 335–336. [Google Scholar] [CrossRef]
- D’Agostino, G.; Lyons, D.J.; Cristiano, C.; Burke, L.K.; Madara, J.C.; Campbell, J.N.; Garcia, A.P.; Land, B.B.; Lowell, B.B.; Dileone, R.J.; et al. Appetite Controlled by a Cholecystokinin Nucleus of the Solitary Tract to Hypothalamus Neurocircuit. eLife 2016, 5, e12225. [Google Scholar] [CrossRef]
- Holt, M.K.; Richards, J.E.; Cook, D.R.; Brierley, D.I.; Williams, D.L.; Reimann, F.; Gribble, F.M.; Trapp, S. Preproglucagon Neurons in the Nucleus of the Solitary Tract Are the Main Source of Brain GLP-1, Mediate Stress-Induced Hypophagia, and Limit Unusually Large Intakes of Food. Diabetes 2019, 68, 21–33. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Metabolic Messengers: Glucagon-like Peptide 1. Nat. Metab. 2021, 3, 142–148. [Google Scholar] [CrossRef]
- Holst, J.J.; Deacon, C.F. Glucagon-like Peptide-1 Mediates the Therapeutic Actions of DPP-IV Inhibitors. Diabetologia 2005, 48, 612–615. [Google Scholar] [CrossRef]
- Lu, K.; Chen, X.; Deng, X.; Long, J.; Yan, J. Potential Role of Hypothalamic and Plasma Ghrelin in the Feeding Behavior of Obese Type 2 Diabetic Rats with Intraventricular Glucagon-Like Peptide-1 Receptor Agonist Intervention. Obes. Facts 2021, 14, 10–20. [Google Scholar] [CrossRef]
- Hong, X.; Zhang, H.; Liang, H.; Li, D.; Huang, J.; Li, Z.; Jiang, S.; Zhang, W.; Xu, G. Exendin-4 Decreases Ghrelin Levels through MTOR Signaling. Mol. Cell. Endocrinol. 2016, 437, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Carty, J.; Goldstein, N.; He, Z.; Hwang, E.; Chau, D.; Wallace, B.; Kabahizi, A.; Lieu, L.; Peng, Y.; et al. Time and Metabolic State-Dependent Effects of GLP-1R Agonists on NPY/AgRP and POMC Neuronal Activity in Vivo. Mol. Metab. 2021, 54, 101352. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Wang, L.; Xia, B.; Liu, J.; Tahiri, A.; El Ouaamari, A.; Wheeler, M.B.; Pang, Z.P. Activation of Arcuate Nucleus Glucagon-like Peptide-1 Receptor-Expressing Neurons Suppresses Food Intake. Cell Biosci. 2022, 12, 178. [Google Scholar] [CrossRef]
- Katsurada, K.; Maejima, Y.; Nakata, M.; Kodaira, M.; Suyama, S.; Iwasaki, Y.; Kario, K.; Yada, T. Endogenous GLP-1 Acts on Paraventricular Nucleus to Suppress Feeding: Projection from Nucleus Tractus Solitarius and Activation of Corticotropin-Releasing Hormone, Nesfatin-1 and Oxytocin Neurons. Biochem. Biophys. Res. Commun. 2014, 451, 276–281. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Chen, L.; Yang, W.; Xie, A.-M. GLP-1 Suppresses Feeding Behaviors and Modulates Neuronal Electrophysiological Properties in Multiple Brain Regions. Front. Mol. Neurosci. 2021, 14, 793004. [Google Scholar] [CrossRef] [PubMed]
- Adrian, T.E.; Ferri, G.L.; Bacarese-Hamilton, A.J.; Fuessl, H.S.; Polak, J.M.; Bloom, S.R. Human Distribution and Release of a Putative New Gut Hormone, Peptide YY. Gastroenterology 1985, 89, 1070–1077. [Google Scholar] [CrossRef]
- Keire, D.A.; Whitelegge, J.P.; Souda, P.; Faull, K.F.; Bassilian, S.; Reidelberger, R.D.; Haver, A.C.; Reeve, J.R. PYY(1-36) Is the Major Form of PYY in Rat Distal Small Intestine: Quantification Using High-Resolution Mass Spectrometry. Regul. Pept. 2010, 165, 151–157. [Google Scholar] [CrossRef]
- Lafferty, R.A.; Flatt, P.R.; Irwin, N. C-Terminal Degradation of PYY Peptides in Plasma Abolishes Effects on Satiety and Beta-Cell Function. Biochem. Pharmacol. 2018, 158, 95–102. [Google Scholar] [CrossRef]
- Østergaard, S.; Kofoed, J.; Paulsson, J.F.; Madsen, K.G.; Jorgensen, R.; Wulff, B.S. Design of Y2 Receptor Selective and Proteolytically Stable PYY3-36 Analogues. J. Med. Chem. 2018, 61, 10519–10530. [Google Scholar] [CrossRef]
- Adrian, T.E.; Bacarese-Hamilton, A.J.; Smith, H.A.; Chohan, P.; Manolas, K.J.; Bloom, S.R. Distribution and Postprandial Release of Porcine Peptide YY. J. Endocrinol. 1987, 113, 11–14. [Google Scholar] [CrossRef]
- Amin, A.; Neophytou, C.; Thein, S.; Martin, N.M.; Alamshah, A.; Spreckley, E.; Bloom, S.R.; Murphy, K.G. L-Arginine Increases Postprandial Circulating GLP-1 and PYY Levels in Humans. Obesity 2018, 26, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Maroni, M.J.; Capri, K.M.; Cushman, A.V.; Deane, H.V.; Concepcion, H.; DeCourcey, H.; Seggio, J.A. The Timing of Fasting Leads to Different Levels of Food Consumption and PYY3-36 in Nocturnal Mice. Hormones 2020, 19, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, M.; Salinas, C.B.G.; Rehfeld, J.F.; Secher, A.; Raun, K.; Wulff, B.S. PYY(3-36) and Exendin-4 Reduce Food Intake and Activate Neuronal Circuits in a Synergistic Manner in Mice. Neuropeptides 2019, 73, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Dischinger, U.; Hasinger, J.; Königsrainer, M.; Corteville, C.; Otto, C.; Fassnacht, M.; Hankir, M.; Seyfried, F.J.D. Toward a Medical Gastric Bypass: Chronic Feeding Studies With Liraglutide + PYY3-36 Combination Therapy in Diet-Induced Obese Rats. Front. Endocrinol. 2020, 11, 598843. [Google Scholar] [CrossRef] [PubMed]
- Broberger, C.; Landry, M.; Wong, H.; Walsh, J.N.; Hökfelt, T. Subtypes Y1 and Y2 of the Neuropeptide Y Receptor Are Respectively Expressed in Pro-Opiomelanocortin- and Neuropeptide-Y-Containing Neurons of the Rat Hypothalamic Arcuate Nucleus. Neuroendocrinology 1997, 66, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.S.; Nunn, N.; Chambers, A.P.; Østergaard, S.; Wulff, B.S.; Luckman, S.M. Modified Peptide YY Molecule Attenuates the Activity of NPY/AgRP Neurons and Reduces Food Intake in Male Mice. Endocrinology 2019, 160, 2737–2747. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut Hormone PYY(3-36) Physiologically Inhibits Food Intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef]
- Batterham, R.L.; Bloom, S.R. The Gut Hormone Peptide YY Regulates Appetite. Ann. N. Y. Acad. Sci. 2003, 994, 162–168. [Google Scholar] [CrossRef]
- Koda, S.; Date, Y.; Murakami, N.; Shimbara, T.; Hanada, T.; Toshinai, K.; Niijima, A.; Furuya, M.; Inomata, N.; Osuye, K.; et al. The Role of the Vagal Nerve in Peripheral PYY3-36-Induced Feeding Reduction in Rats. Endocrinology 2005, 146, 2369–2375. [Google Scholar] [CrossRef]
- Abbott, C.R.; Monteiro, M.; Small, C.J.; Sajedi, A.; Smith, K.L.; Parkinson, J.R.C.; Ghatei, M.A.; Bloom, S.R. The Inhibitory Effects of Peripheral Administration of Peptide YY(3-36) and Glucagon-like Peptide-1 on Food Intake Are Attenuated by Ablation of the Vagal-Brainstem-Hypothalamic Pathway. Brain Res. 2005, 1044, 127–131. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Tartaglia, L.A.; Dembski, M.; Weng, X.; Deng, N.; Culpepper, J.; Devos, R.; Richards, G.J.; Campfield, L.A.; Clark, F.T.; Deeds, J.; et al. Identification and Expression Cloning of a Leptin Receptor, OB-R. Cell 1995, 83, 1263–1271. [Google Scholar] [CrossRef]
- Friedman, J.M.; Mantzoros, C.S. 20 Years of Leptin: From the Discovery of the Leptin Gene to Leptin in Our Therapeutic Armamentarium. Metabolism 2015, 64, 1–4. [Google Scholar] [CrossRef]
- Friedman, J.M. Leptin and the Endocrine Control of Energy Balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef]
- de Lartigue, G.; Ronveaux, C.C.; Raybould, H.E. Deletion of Leptin Signaling in Vagal Afferent Neurons Results in Hyperphagia and Obesity. Mol. Metab. 2014, 3, 595–607. [Google Scholar] [CrossRef]
- Zhou, Y.; Rui, L. Leptin Signaling and Leptin Resistance. Front. Med. 2013, 7, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.L.; Cho, K.W.; Rui, L. Critical Role of the Src Homology 2 (SH2) Domain of Neuronal SH2B1 in the Regulation of Body Weight and Glucose Homeostasis in Mice. Endocrinology 2010, 151, 3643–3651. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.A.L.; Bornath, D.P.D.; McCarthy, S.F.; Hazell, T.J. Leptin and Energy Balance: Exploring Leptin’s Role in the Regulation of Energy Intake and Energy Expenditure. Nutr. Neurosci. 2022, 1–9. [Google Scholar] [CrossRef]
- Rautmann, A.W.; de La Serre, C.B. Microbiota’s Role in Diet-Driven Alterations in Food Intake: Satiety, Energy Balance, and Reward. Nutrients 2021, 13, 3067. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Huangfu, W.; Yang, X.; Xu, J.; Zhang, Y.; Wang, Z.; Zhu, X.; Wang, C.; Shi, Y.; Cui, Y. “King of the Forage”-Alfalfa Supplementation Improves Growth, Reproductive Performance, Health Condition and Meat Quality of Pigs. Front. Vet. Sci. 2022, 9, 1025942. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Du, Y.; Li, N.; Geng, H.; Ali, Q.; Li, X.; Gao, Y.; Wang, Y.; Xing, R.; Wu, J.; et al. Effects of Alfalfa Meal on Quality and Function of Pork Meatballs. Food Sci. Nutr. 2022, 10, 2601–2610. [Google Scholar] [CrossRef]
- Ali, Q.; Ma, S.; La, S.; Guo, Z.; Liu, B.; Gao, Z.; Farooq, U.; Wang, Z.; Zhu, X.; Cui, Y.; et al. Microbial Short-Chain Fatty Acids: A Bridge between Dietary Fibers and Poultry Gut Health—A Review. Anim. Biosci. 2022, 35, 1461–1478. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G Protein-Coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Bolognini, D.; Dedeo, D.; Milligan, G. Metabolic and Inflammatory Functions of Short-Chain Fatty Acid Receptors. Curr. Opin. Endocr. Metab. Res. 2021, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C.K. Short-Chain Fatty Acids in Control of Energy Metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The Short-Chain Fatty Acid Acetate Reduces Appetite via a Central Homeostatic Mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Zaibi, M.S.; Stocker, C.J.; O’Dowd, J.; Davies, A.; Bellahcene, M.; Cawthorne, M.A.; Brown, A.J.H.; Smith, D.M.; Arch, J.R.S. Roles of GPR41 and GPR43 in Leptin Secretory Responses of Murine Adipocytes to Short Chain Fatty Acids. FEBS Lett. 2010, 584, 2381–2386. [Google Scholar] [CrossRef]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The Short Chain Fatty Acid Propionate Stimulates GLP-1 and PYY Secretion via Free Fatty Acid Receptor 2 in Rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. SCFAs Strongly Stimulate PYY Production in Human Enteroendocrine Cells. Sci. Rep. 2018, 8, 74. [Google Scholar] [CrossRef]
- Li, Z.; Yi, C.-X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F.P.; et al. Butyrate Reduces Appetite and Activates Brown Adipose Tissue via the Gut-Brain Neural Circuit. Gut 2018, 67, 1269–1279. [Google Scholar] [CrossRef]
- Cook, T.M.; Gavini, C.K.; Jesse, J.; Aubert, G.; Gornick, E.; Bonomo, R.; Gautron, L.; Layden, B.T.; Mansuy-Aubert, V. Vagal Neuron Expression of the Microbiota-Derived Metabolite Receptor, Free Fatty Acid Receptor (FFAR3), Is Necessary for Normal Feeding Behavior. Mol. Metab. 2021, 54, 101350. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.-J.; Hylemon, P.B. Consequences of Bile Salt Biotransformations by Intestinal Bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Cardoso, V.F.; Corlianò, M.; Singaraja, R.R. Bile Acids: A Communication Channel in the Gut-Brain Axis. Neuromol. Med. 2021, 23, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Trammell, S.A.J.; Wewer Albrechtsen, N.J.; Schoonjans, K.; Albrechtsen, R.; Gillum, M.P.; Kuhre, R.E.; Holst, J.J. Bile Acids Drive Colonic Secretion of Glucagon-like-Peptide 1 and Peptide-YY in Rodents. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G574–G584. [Google Scholar] [CrossRef] [PubMed]
- Kuhre, R.E.; Wewer Albrechtsen, N.J.; Larsen, O.; Jepsen, S.L.; Balk-Møller, E.; Andersen, D.B.; Deacon, C.F.; Schoonjans, K.; Reimann, F.; Gribble, F.M.; et al. Bile Acids Are Important Direct and Indirect Regulators of the Secretion of Appetite- and Metabolism-Regulating Hormones from the Gut and Pancreas. Mol. Metab. 2018, 11, 84–95. [Google Scholar] [CrossRef]
- Liu, S.; Marcelin, G.; Blouet, C.; Jeong, J.H.; Jo, Y.-H.; Schwartz, G.J.; Chua, S. A Gut-Brain Axis Regulating Glucose Metabolism Mediated by Bile Acids and Competitive Fibroblast Growth Factor Actions at the Hypothalamus. Mol. Metab. 2018, 8, 37–50. [Google Scholar] [CrossRef]
- Higashi, T.; Watanabe, S.; Tomaru, K.; Yamazaki, W.; Yoshizawa, K.; Ogawa, S.; Nagao, H.; Minato, K.; Maekawa, M.; Mano, N. Unconjugated Bile Acids in Rat Brain: Analytical Method Based on LC/ESI-MS/MS with Chemical Derivatization and Estimation of Their Origin by Comparison to Serum Levels. Steroids 2017, 125, 107–113. [Google Scholar] [CrossRef]
- Perino, A.; Velázquez-Villegas, L.A.; Bresciani, N.; Sun, Y.; Huang, Q.; Fénelon, V.S.; Castellanos-Jankiewicz, A.; Zizzari, P.; Bruschetta, G.; Jin, S.; et al. Central Anorexigenic Actions of Bile Acids Are Mediated by TGR5. Nat. Metab. 2021, 3, 595–603. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.-Y.; Lee, A.; Lu, Y.-X.; Zhou, S.-Y.; Owyang, C. Satiety Induced by Bile Acids Is Mediated via Vagal Afferent Pathways. JCI Insight 2020, 5, e132400. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Liu, N.; Sun, S.; Wang, P.; Sun, Y.; Hu, Q.; Wang, X. The Mechanism of Secretion and Metabolism of Gut-Derived 5-Hydroxytryptamine. Int. J. Mol. Sci. 2021, 22, 7931. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome Axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut Microbes Promote Colonic Serotonin Production through an Effect of Short-Chain Fatty Acids on Enterochromaffin Cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Voigt, J.-P.; Fink, H. Serotonin Controlling Feeding and Satiety. Behav. Brain Res. 2015, 277, 14–31. [Google Scholar] [CrossRef]
- Ripken, D.; van der Wielen, N.; Wortelboer, H.M.; Meijerink, J.; Witkamp, R.F.; Hendriks, H.F.J. Nutrient-Induced Glucagon like Peptide-1 Release Is Modulated by Serotonin. J. Nutr. Biochem. 2016, 32, 142–150. [Google Scholar] [CrossRef]
- Tian, P.; O’Riordan, K.J.; Lee, Y.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a Psychobiotic Therapy for Depression: Bifidobacterium Breve CCFM1025 Reverses Chronic Stress-Induced Depressive Symptoms and Gut Microbial Abnormalities in Mice. Neurobiol. Stress 2020, 12, 100216. [Google Scholar] [CrossRef]
- Heisler, L.K.; Jobst, E.E.; Sutton, G.M.; Zhou, L.; Borok, E.; Thornton-Jones, Z.; Liu, H.Y.; Zigman, J.M.; Balthasar, N.; Kishi, T.; et al. Serotonin Reciprocally Regulates Melanocortin Neurons to Modulate Food Intake. Neuron 2006, 51, 239–249. [Google Scholar] [CrossRef]
- Tiligada, E.; Wilson, J.F. Regulation of α-Melanocyte-Stimulating Hormone Release from Superfused Slices of Rat Hypothalamus by Serotonin and the Interaction of Serotonin with the Dopaminergic System Inhibiting Peptide Release. Brain Res. 1989, 503, 225–228. [Google Scholar] [CrossRef]
- Jones, L.A.; Sun, E.W.; Martin, A.M.; Keating, D.J. The Ever-Changing Roles of Serotonin. Int. J. Biochem. Cell Biol. 2020, 125, 105776. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J. Indole as an Intercellular Signal in Microbial Communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef] [PubMed]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.-L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018, 28, 737–749.e4. [Google Scholar] [CrossRef] [PubMed]
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial Metabolite Indole Modulates Incretin Secretion from Intestinal Enteroendocrine L Cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Fetissov, S.O.; Legrand, R.; Lucas, N. Bacterial Protein Mimetic of Peptide Hormone as a New Class of Protein- Based Drugs. Curr. Med. Chem. 2019, 26, 546–553. [Google Scholar] [CrossRef]
- Ericson, M.D.; Schnell, S.M.; Freeman, K.T.; Haskell-Luevano, C. A Fragment of the Escherichia Coli ClpB Heat-Shock Protein Is a Micromolar Melanocortin 1 Receptor Agonist. Bioorg. Med. Chem. Lett. 2015, 25, 5306–5308. [Google Scholar] [CrossRef]
- Fetissov, S.O.; Hamze Sinno, M.; Coëffier, M.; Bole-Feysot, C.; Ducrotté, P.; Hökfelt, T.; Déchelotte, P. Autoantibodies against Appetite-Regulating Peptide Hormones and Neuropeptides: Putative Modulation by Gut Microflora. Nutrition 2008, 24, 348–359. [Google Scholar] [CrossRef]
- Breton, J.; Legrand, R.; Akkermann, K.; Järv, A.; Harro, J.; Déchelotte, P.; Fetissov, S.O. Elevated Plasma Concentrations of Bacterial ClpB Protein in Patients with Eating Disorders. Int. J. Eat. Disord. 2016, 49, 805–808. [Google Scholar] [CrossRef]
- Molfino, A.; Amabile, M.I.; Imbimbo, G.; Emiliani, A.; Ramaccini, C.; Lahaye, E.; Takagi, K.; Fetissov, S.O. Plasma Enterobacterial ClpB Levels and ClpB- and α-MSH-Reactive Immunoglobulins in Lung Cancer Patients with and without Anorexia. Nutrition 2020, 78, 110952. [Google Scholar] [CrossRef]
- Arnoriaga-Rodríguez, M.; Mayneris-Perxachs, J.; Burokas, A.; Pérez-Brocal, V.; Moya, A.; Portero-Otin, M.; Ricart, W.; Maldonado, R.; Fernández-Real, J.-M. Gut Bacterial ClpB-like Gene Function Is Associated with Decreased Body Weight and a Characteristic Microbiota Profile. Microbiome 2020, 8, 59. [Google Scholar] [CrossRef]
- Legrand, R.; Lucas, N.; Dominique, M.; Azhar, S.; Deroissart, C.; Le Solliec, M.-A.; Rondeaux, J.; Nobis, S.; Guérin, C.; Léon, F.; et al. Commensal Hafnia Alvei Strain Reduces Food Intake and Fat Mass in Obese Mice-a New Potential Probiotic for Appetite and Body Weight Management. Int. J. Obes. 2020, 44, 1041–1051. [Google Scholar] [CrossRef]
- Lucas, N.; Legrand, R.; Deroissart, C.; Dominique, M.; Azhar, S.; Le Solliec, M.-A.; Léon, F.; do Rego, J.-C.; Déchelotte, P.; Fetissov, S.O.; et al. Hafnia Alvei HA4597 Strain Reduces Food Intake and Body Weight Gain and Improves Body Composition, Glucose, and Lipid Metabolism in a Mouse Model of Hyperphagic Obesity. Microorganisms 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Tennoune, N.; Chan, P.; Breton, J.; Legrand, R.; Chabane, Y.N.; Akkermann, K.; Järv, A.; Ouelaa, W.; Takagi, K.; Ghouzali, I.; et al. Bacterial ClpB Heat-Shock Protein, an Antigen-Mimetic of the Anorexigenic Peptide α-MSH, at the Origin of Eating Disorders. Transl. Psychiatry 2014, 4, e458. [Google Scholar] [CrossRef]
- Dominique, M.; Breton, J.; Guérin, C.; Bole-Feysot, C.; Lambert, G.; Déchelotte, P.; Fetissov, S. Effects of Macronutrients on the In Vitro Production of ClpB, a Bacterial Mimetic Protein of α-MSH and Its Possible Role in Satiety Signaling. Nutrients 2019, 11, 2115. [Google Scholar] [CrossRef] [PubMed]
- Dominique, M.; Lucas, N.; Legrand, R.; Bouleté, I.-M.; Bôle-Feysot, C.; Deroissart, C.; Léon, F.; Nobis, S.; do Rego, J.-C.; Lambert, G.; et al. Effects of Bacterial CLPB Protein Fragments on Food Intake and PYY Secretion. Nutrients 2021, 13, 2223. [Google Scholar] [CrossRef] [PubMed]
- Dominique, M.; Boulete, I.; Bole-Feysot, C.; Leon, F.; Do Rego, J.-C.; Fetissov, S.O.; Déchelotte, P.; Lambert, G.; Legrand, R.; Lucas, N. Rôle de la protéine bactérienne ClpB et d’un de ses fragments peptidiques dans la régulation de la prise alimentaire. Nutr. Clin. Métabolisme 2019, 33, 23–24. [Google Scholar] [CrossRef]
- Breton, J.; Tennoune, N.; Lucas, N.; Francois, M.; Legrand, R.; Jacquemot, J.; Goichon, A.; Guérin, C.; Peltier, J.; Pestel-Caron, M.; et al. Gut Commensal E. Coli Proteins Activate Host Satiety Pathways Following Nutrient-Induced Bacterial Growth. Cell Metab. 2016, 23, 324–334. [Google Scholar] [CrossRef]
- Manco, M.; Putignani, L.; Bottazzo, G.F. Gut Microbiota, Lipopolysaccharides, and Innate Immunity in the Pathogenesis of Obesity and Cardiovascular Risk. Endocr. Rev. 2010, 31, 817–844. [Google Scholar] [CrossRef]
- Breen, D.M.; Jagarlapudi, S.; Patel, A.; Zou, C.; Joaquim, S.; Li, X.; Kang, L.; Pang, J.; Hales, K.; Ziso-Qejvanaj, E.; et al. Growth Differentiation Factor 15 Neutralization Does Not Impact Anorexia or Survival in Lipopolysaccharide-Induced Inflammation. iScience 2021, 24, 102554. [Google Scholar] [CrossRef]
- Taksande, B.G.; Chopde, C.T.; Umekar, M.J.; Kotagale, N.R. Agmatine Attenuates Lipopolysaccharide Induced Anorexia and Sickness Behavior in Rats. Pharmacol. Biochem. Behav. 2015, 132, 108–114. [Google Scholar] [CrossRef]
- Yousefi, M.; Jonaidi, H.; Sadeghi, B. Influence of Peripheral Lipopolysaccharide (LPS) on Feed Intake, Body Temperature and Hypothalamic Expression of Neuropeptides Involved in Appetite Regulation in Broilers and Layer Chicks. Br. Poult. Sci. 2021, 62, 110–117. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, D.; Jiao, H.C.; Zhao, J.P.; Lin, H. Lipopolysaccharide Inhibits Hypothalamic Agouti-Related Protein Gene Expression via Activating Mechanistic Target of Rapamycin Signaling in Chicks. Gen. Comp. Endocrinol. 2021, 313, 113876. [Google Scholar] [CrossRef] [PubMed]
- Pradhananga, S.; Tashtush, A.A.; Allen-Vercoe, E.; Petrof, E.O.; Lomax, A.E. Protease-Dependent Excitation of Nodose Ganglion Neurons by Commensal Gut Bacteria. J. Physiol. 2020, 598, 2137–2151. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.J.; Plata-Salamán, C.R.; Langhans, W. Subdiaphragmatic Vagal Deafferentation Fails to Block Feeding-Suppressive Effects of LPS and IL-1 Beta in Rats. Am. J. Physiol. 1997, 273, R1193–R1198. [Google Scholar] [CrossRef]
- Fosset, S.; Fromentin, G.; Rampin, O.; Lang, V.; Mathieu, F.; Tomé, D. Pharmacokinetics and Feeding Responses to Muramyl Dipeptide in Rats. Physiol. Behav. 2003, 79, 173–182. [Google Scholar] [CrossRef]
- Williams, L.; Alshehri, A.; Robichaud, B.; Cudmore, A.; Gagnon, J. The Role of the Bacterial Muramyl Dipeptide in the Regulation of GLP-1 and Glycemia. Int. J. Mol. Sci. 2020, 21, 5252. [Google Scholar] [CrossRef] [PubMed]
- Gabanyi, I.; Lepousez, G.; Wheeler, R.; Vieites-Prado, A.; Nissant, A.; Wagner, S.; Moigneu, C.; Dulauroy, S.; Hicham, S.; Polomack, B.; et al. Bacterial Sensing via Neuronal Nod2 Regulates Appetite and Body Temperature. Science 2022, 376, eabj3986. [Google Scholar] [CrossRef]
- Blanco, A.M.; Bertucci, J.I.; Valenciano, A.I.; Delgado, M.J.; Unniappan, S. Ghrelin Suppresses Cholecystokinin (CCK), Peptide YY (PYY) and Glucagon-like Peptide-1 (GLP-1) in the Intestine, and Attenuates the Anorectic Effects of CCK, PYY and GLP-1 in Goldfish (Carassius Auratus). Horm. Behav. 2017, 93, 62–71. [Google Scholar] [CrossRef]
- Kawai, K.; Gebremeskel, B.G.; Acosta, C.J. Systematic Review of Incidence and Complications of Herpes Zoster: Towards a Global Perspective. BMJ Open 2014, 4, e004833. [Google Scholar] [CrossRef]
- Merrick, B.; Allen, L.; Zain, N.M.M.; Forbes, B.; Shawcross, D.L.; Goldenberg, S.D. Regulation, Risk and Safety of Faecal Microbiota Transplant. Infect. Prev. Pract. 2020, 2, 100069. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, M.; Cao, S.; Liu, B.; Li, D.; Wang, Z.; Sun, H.; Cui, Y.; Shi, Y. The Mechanism of the Gut-Brain Axis in Regulating Food Intake. Nutrients 2023, 15, 3728. https://doi.org/10.3390/nu15173728
Li S, Liu M, Cao S, Liu B, Li D, Wang Z, Sun H, Cui Y, Shi Y. The Mechanism of the Gut-Brain Axis in Regulating Food Intake. Nutrients. 2023; 15(17):3728. https://doi.org/10.3390/nu15173728
Chicago/Turabian StyleLi, Shouren, Mengqi Liu, Shixi Cao, Boshuai Liu, Defeng Li, Zhichang Wang, Hao Sun, Yalei Cui, and Yinghua Shi. 2023. "The Mechanism of the Gut-Brain Axis in Regulating Food Intake" Nutrients 15, no. 17: 3728. https://doi.org/10.3390/nu15173728
APA StyleLi, S., Liu, M., Cao, S., Liu, B., Li, D., Wang, Z., Sun, H., Cui, Y., & Shi, Y. (2023). The Mechanism of the Gut-Brain Axis in Regulating Food Intake. Nutrients, 15(17), 3728. https://doi.org/10.3390/nu15173728





