Combined Treatment of Mori folium and Mori Cortex Radicis Ameliorate Obesity in Mice via UCP-1 in Brown Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Mori Folium and Mori Cortex Radicis Extracts
2.2. HPLC Analysis
2.3. Reagents and Antibodies
2.4. 3T3-L1 Cell Culture, Differentiation, and Browning Process
2.5. Cell Viability under Different Conditions
2.6. Animal Experiments
2.7. Biochemical Analysis
2.8. Histological and Immunohistochemical Analysis
2.9. RNA Isolation and Quantitative Real-Time RT-PCR
2.10. Immunoblotting
2.11. Dual-Energy X-ray Absorptiometry (DXA) Scan
2.12. Immunohistochemistry
2.13. Statistical Analysis
3. Results
3.1. Mori Folium (MF) and Mori Cortex Radics (MR) Extracts Promote the Expressions of UCP-1 and Browning-Associated Genes and Proteins in 3T3-L1 Cells
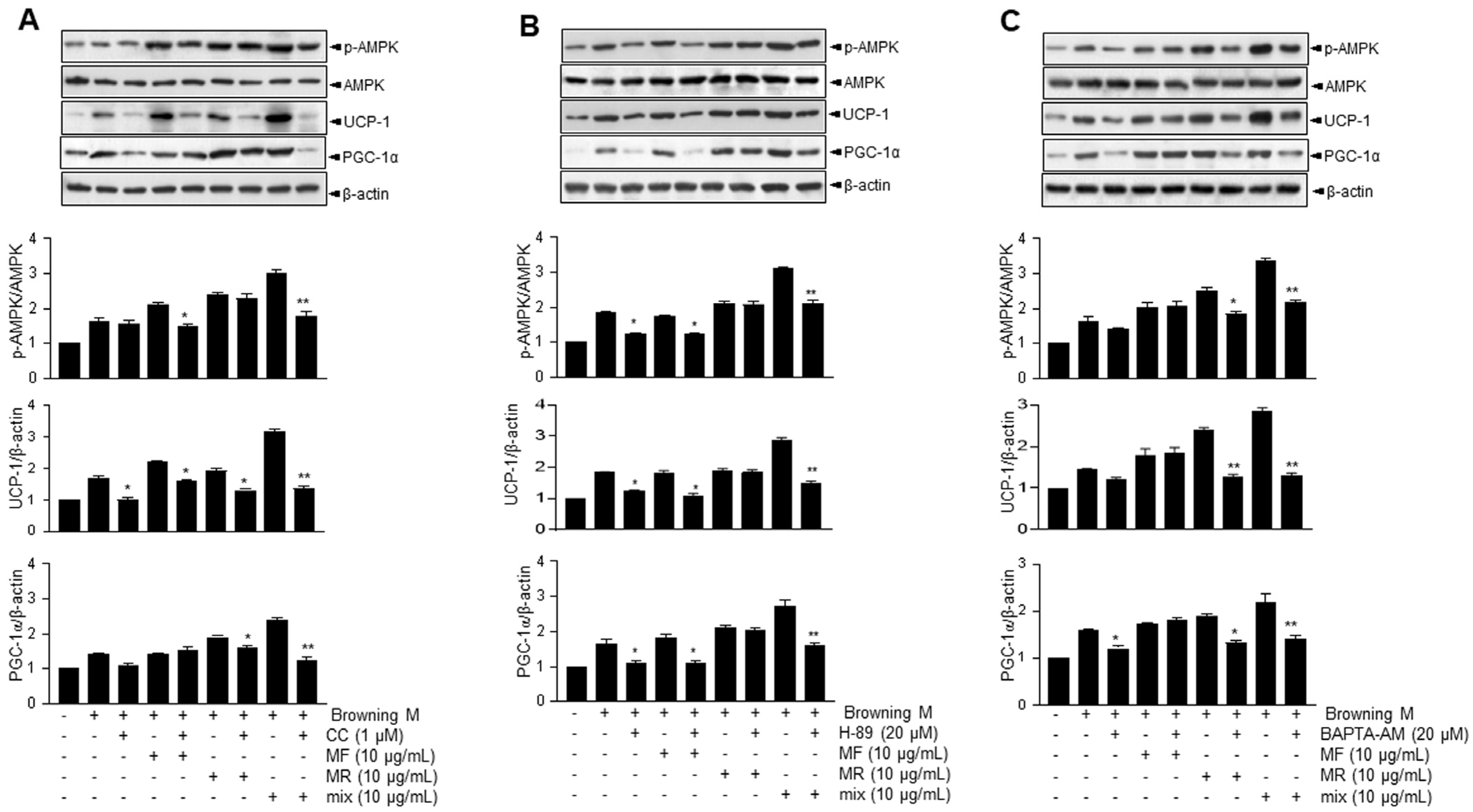
3.2. Supplementation of MF/MR Stimulates the Expression of UCP-1 through AMPK Signaling, Including Ca2+ and cAMP
3.3. Analysis of Compounds in MF, MR, and MF/MR
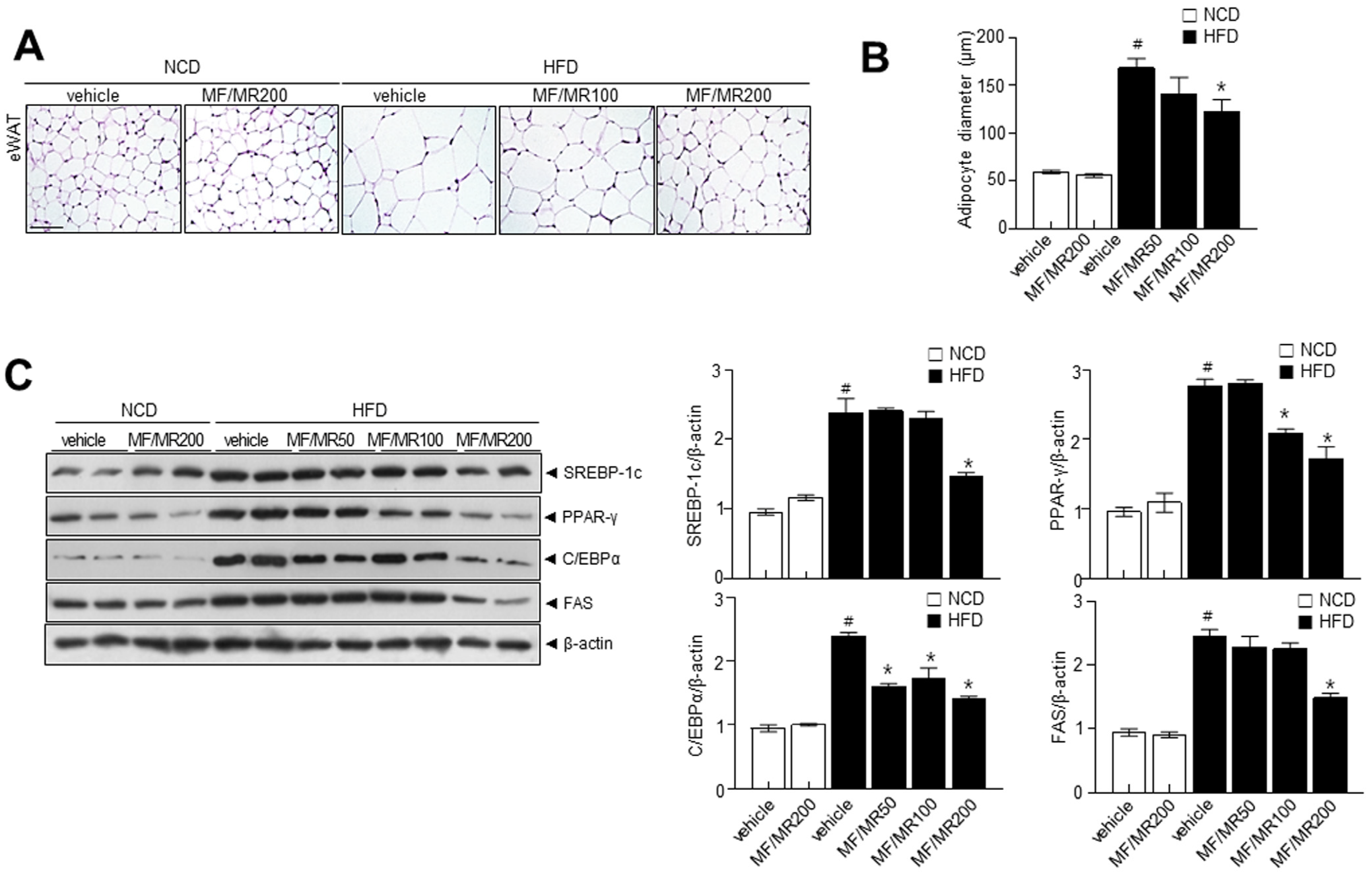
3.4. Supplementation of MF/MR Regulate Body and Adipose Tissue Weights, and Lipid Metabolism in HFD-Induced Obese Mice
3.5. Administration of MF/MR Reduce Lipid Accumulation and Increase Thermogenesis in HFD-Induced Obese Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sacks, H.; Symonds, M.E. Anatomical locations of human brown adipose tissue: Functional relevance and implications in obesity and type 2 diabetes. Diabetes 2013, 62, 1783–1790. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Cypess, A.M.; Kahn, C.R. Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov. 2010, 9, 465–482. [Google Scholar] [CrossRef] [PubMed]
- Forman, L.M.; Simmons, D.A.; Diamond, R.H. Hepatic failure in a patient taking rosiglitazone. Ann. Intern. Med. 2000, 132, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Hemmeryckx, B.; Gaekens, M.; Gallacher, D.J.; Lu, H.R.; Lijnen, H.R. Effect of rosiglitazone on liver structure and function in genetically diabetic Akita mice. Basic Clin. Pharmacol. Toxicol. 2013, 113, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; He, Q.; Yang, Y. Calculation and Evaluation of Carbon Footprint in Mulberry Production: A Case of Haining in China. Int. J. Environ. Res. Public Health 2020, 17, 1339. [Google Scholar] [CrossRef]
- Zhong, S.; Han, Z.; Li, J.; Xie, D.; Yang, Q.; Ni, J. Mechanized and Optimized Configuration Pattern of Crop-Mulberry Systems for Controlling Agricultural, Non-Point Source Pollution on Sloping Farmland in the Three Gorges Reservoir Area, China. Int. J. Environ. Res. Public Health 2020, 17, 3599. [Google Scholar] [CrossRef]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Khan, M.U.; Mohapatra, S.; Ahmad, S. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.; Anam Saeed, R.; Sahar, A.; Khan, M.I. Mulberry plant as a source of functional food with therapeutic and nutritional applications: A review. J. Food Biochem. 2022, 46, e14263. [Google Scholar] [CrossRef]
- Memete, A.R.; Timar, A.V.; Vuscan, A.N.; Miere Groza, F.; Venter, A.C.; Vicas, S.I. Phytochemical Composition of Different Botanical Parts of Morus Species, Health Benefits and Application in Food Industry. Plants 2022, 11, 152. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Parray, H.A.; Yun, J.W. Cannabidiol promotes browning in 3T3-L1 adipocytes. Mol. Cell. Biochem. 2016, 416, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 2020, 40, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Magaki, S.; Hojat, S.A.; Wei, B.; So, A.; Yong, W.H. An Introduction to the Performance of Immunohistochemistry. Methods Mol. Biol. 2019, 1897, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.T.; Wolfe, D. Tissue processing and hematoxylin and eosin staining. Methods Mol. Biol. 2014, 1180, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Peng, C.; Park, S.A.; Hoang, T.H.; Lee, H.Y.; Kim, J.; Kang, S.I.; Lee, C.H.; Lee, J.S.; Chae, H.J. Citrus Peel Extract Ameliorates High-Fat Diet-Induced NAFLD via Activation of AMPK Signaling. Nutrients 2020, 12, 673. [Google Scholar] [CrossRef]
- Yeu, J.; Ko, H.J.; Kim, D.; Ahn, Y.; Kim, J.; Lee, W.; Jung, I.; Suh, J.; Lee, S.J. Evaluation of iNSiGHT VET DXA (Dual-Energy X-ray Absorptiometry) for assessing body composition in obese rats fed with high fat diet: A follow-up study of diet induced obesity model for 8 weeks. Lab. Anim. Res. 2019, 35, 2. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamada, T. UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes. Front Endocrinol. 2020, 11, 498. [Google Scholar] [CrossRef]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Kim, N.; Nam, M.; Kang, M.S.; Lee, J.O.; Lee, Y.W.; Hwang, G.S.; Kim, H.S. Piperine regulates UCP1 through the AMPK pathway by generating intracellular lactate production in muscle cells. Sci. Rep. 2017, 7, 41066. [Google Scholar] [CrossRef]
- Hurtado de Llera, A.; Martin-Hidalgo, D.; Gil, M.C.; Garcia-Marin, L.J.; Bragado, M.J. The calcium/CaMKKalpha/beta and the cAMP/PKA pathways are essential upstream regulators of AMPK activity in boar spermatozoa. Biol. Reprod. 2014, 90, 29. [Google Scholar] [CrossRef]
- Ye, M.; Ke, Y.; Liu, B.; Yuan, Y.; Wang, F.; Bu, S.; Zhang, Y. Root bark of Morus alba ameliorates the depressive-like behaviors in diabetic rats. Neurosci. Lett. 2017, 637, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Ann, J.Y.; Eo, H.; Lim, Y. Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice. Genes Nutr. 2015, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, Y. Mulberry Fruit Extract Ameliorates Adipogenesis via Increasing AMPK Activity and Downregulating MicroRNA-21/143 in 3T3-L1 Adipocytes. J. Med. Food 2020, 23, 266–272. [Google Scholar] [CrossRef]
- Meng, Q.; Qi, X.; Fu, Y.; Chen, Q.; Cheng, P.; Yu, X.; Sun, X.; Wu, J.; Li, W.; Zhang, Q.; et al. Flavonoids extracted from mulberry (Morus alba L.) leaf improve skeletal muscle mitochondrial function by activating AMPK in type 2 diabetes. J. Ethnopharmacol. 2020, 248, 112326. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Qian, Q.; Ding, Q.; Zhou, L.; Fu, A.; Du, Z.; Wang, C.; Song, Z.; Li, S.; Dou, X. Activation of AMP-Activated Protein Kinase-Sirtuin 1 Pathway Contributes to Salvianolic Acid A-Induced Browning of White Adipose Tissue in High-Fat Diet Fed Male Mice. Front Pharmacol. 2021, 12, 614406. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front Physiol. 2018, 9, 122. [Google Scholar] [CrossRef]
- Song, Z.; Wang, Y.; Zhang, F.; Yao, F.; Sun, C. Calcium Signaling Pathways: Key Pathways in the Regulation of Obesity. Int. J. Mol. Sci. 2019, 20, 2768. [Google Scholar] [CrossRef]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Jakab, J.; Miskic, B.; Miksic, S.; Juranic, B.; Cosic, V.; Schwarz, D.; Vcev, A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabetes Metab. Syndr. Obes. 2021, 14, 67–83. [Google Scholar] [CrossRef]
- Fenzl, A.; Kiefer, F.W. Brown adipose tissue and thermogenesis. Horm. Mol. Biol. Clin. Investig. 2014, 19, 25–37. [Google Scholar] [CrossRef]
- Dempersmier, J.; Sambeat, A.; Gulyaeva, O.; Paul, S.M.; Hudak, C.S.; Raposo, H.F.; Kwan, H.Y.; Kang, C.; Wong, R.H.; Sul, H.S. Cold-inducible Zfp516 activates UCP1 transcription to promote browning of white fat and development of brown fat. Mol. Cell 2015, 57, 235–246. [Google Scholar] [CrossRef] [PubMed]




| Sample | Contents of Standards (%) | ||||
|---|---|---|---|---|---|
| Rutin | Isoquercitrin | Astragalin | Mulberroside A | Cis-Mulberroside A | |
| MF | 0.003 | 0.005 | 0.007 | ||
| MR | 10.99 | 13.15 | |||
| MF/MR | 0.002 | 0.003 | 0.004 | 6.68 | 8.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-S.; Lee, H.-Y.; Kim, H.-J.; Lee, G.-H.; Lim, Y.J.; Ko, B.M.; Kim, J.-H.; Kim, T.W.; Kim, H.K.; Kim, T.Y.; et al. Combined Treatment of Mori folium and Mori Cortex Radicis Ameliorate Obesity in Mice via UCP-1 in Brown Adipocytes. Nutrients 2023, 15, 3713. https://doi.org/10.3390/nu15173713
Kim D-S, Lee H-Y, Kim H-J, Lee G-H, Lim YJ, Ko BM, Kim J-H, Kim TW, Kim HK, Kim TY, et al. Combined Treatment of Mori folium and Mori Cortex Radicis Ameliorate Obesity in Mice via UCP-1 in Brown Adipocytes. Nutrients. 2023; 15(17):3713. https://doi.org/10.3390/nu15173713
Chicago/Turabian StyleKim, Do-Sung, Hwa-Young Lee, Hwa-Jin Kim, Geum-Hwa Lee, Young Jae Lim, Bo Mi Ko, Ji-Hyun Kim, Tae Won Kim, Hye Kyung Kim, Tae Young Kim, and et al. 2023. "Combined Treatment of Mori folium and Mori Cortex Radicis Ameliorate Obesity in Mice via UCP-1 in Brown Adipocytes" Nutrients 15, no. 17: 3713. https://doi.org/10.3390/nu15173713
APA StyleKim, D.-S., Lee, H.-Y., Kim, H.-J., Lee, G.-H., Lim, Y. J., Ko, B. M., Kim, J.-H., Kim, T. W., Kim, H. K., Kim, T. Y., Hwang, D. I., Choi, H. K., Ju, S. M., Chung, M. J., & Chae, H.-J. (2023). Combined Treatment of Mori folium and Mori Cortex Radicis Ameliorate Obesity in Mice via UCP-1 in Brown Adipocytes. Nutrients, 15(17), 3713. https://doi.org/10.3390/nu15173713







