Novel Multi-Ingredient Supplement Facilitates Weight Loss and Improves Body Composition in Overweight and Obese Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Screening & Recruitment
2.2. Experimental Design
2.3. Nutritional Supplementation
2.4. Anthropometric Measurements and Body Composition
2.5. Measurement of Blood Pressure
2.6. Venous Blood Sampling and Analysis
2.7. Isolation of Extracellular Vesicles
2.8. miRNA Extraction and RT-PCR
2.9. Metabolic Measures and Resting Metabolic Rate (RMR)
2.10. Maximal Voluntary VO2peak Fitness Test
2.11. Maximal Voluntary Handgrip Assessment
2.12. Questionnaires
2.13. Dietary Intake
2.14. Activity Tracking
2.15. Sample Size Calculation
2.16. Statistical Analysis
3. Results
3.1. Study Information and Compliance
3.2. Baseline Characteristics
3.3. Co-Primary Outcomes Utilizing Modified ITT
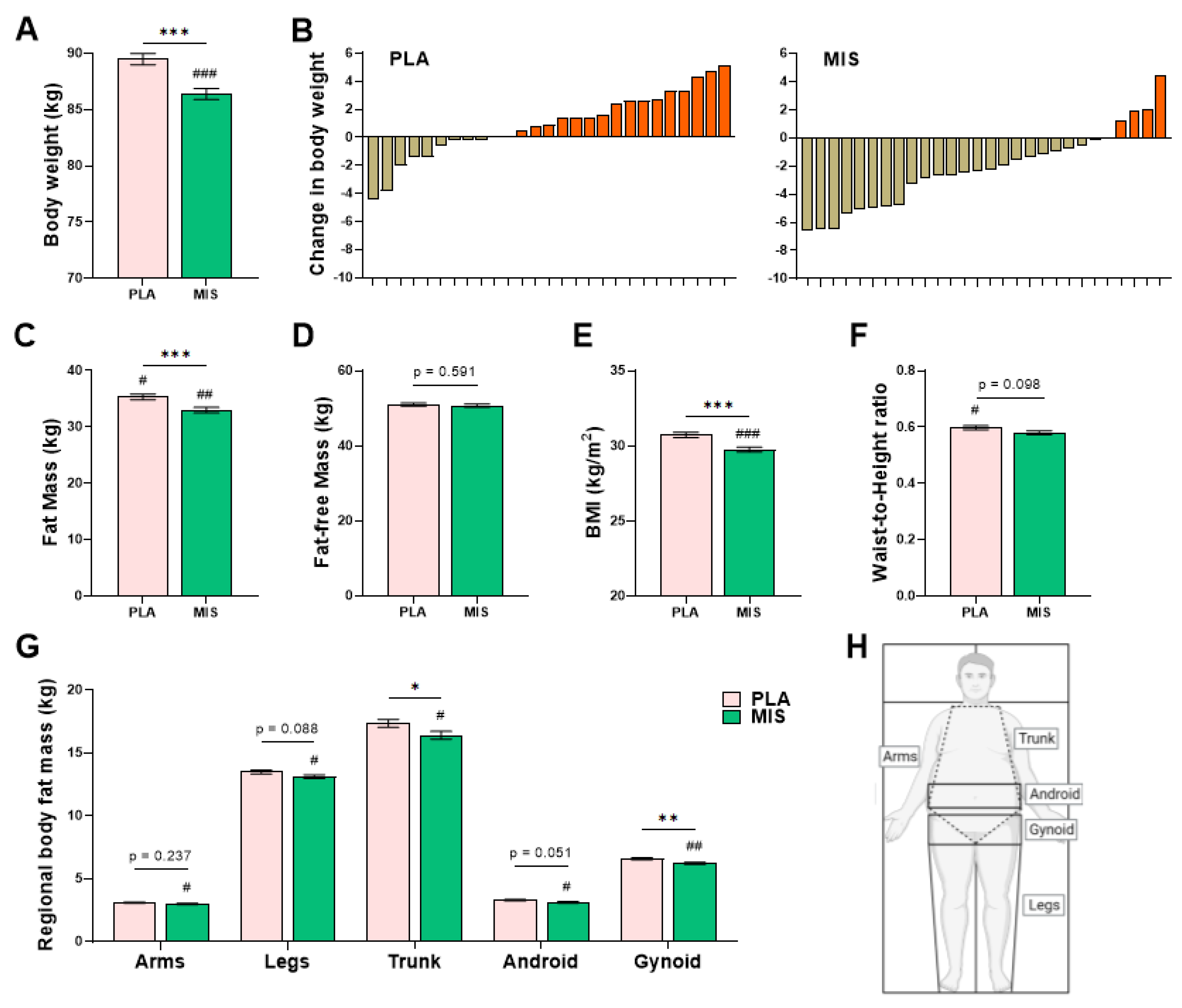
3.4. Anthropometry and Body Composition
3.5. Clinical Biochemistry
3.6. Markers of Dyslipidemia and Glucose Metabolism
3.7. Hematology
3.8. Indices of Physical Health, Metabolism and Function
3.9. Diet, Activity Level, and Self-Reported Quality of Life
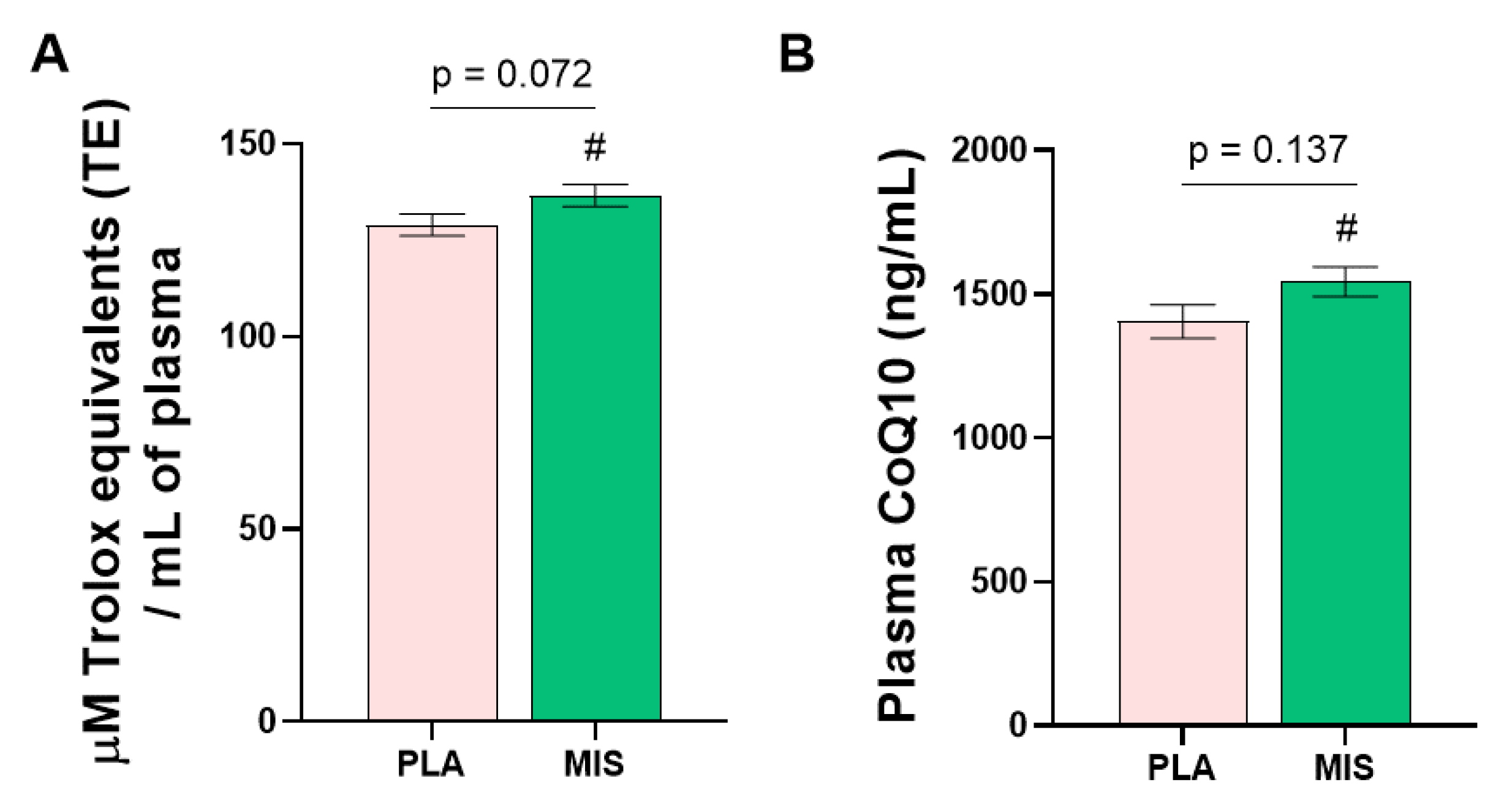
3.10. Molecular Signalling Factors and Antioxidant Capacity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018 Key Findings Data from the National Health and Nutrition Examination Survey; Centers for Disease Control and Prevention: Antlanta, GA, USA, 2017. [Google Scholar]
- Roberto, C.A.; Swinburn, B.; Hawkes, C.; Huang, T.T.K.; Costa, S.A.; Ashe, M.; Zwicker, L.; Cawley, J.H.; Brownell, K.D. Patchy progress on obesity prevention: Emerging examples, entrenched barriers, and new thinking. Lancet 2015, 385, 2400–2409. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, M.; Bentham, J.; Stevens, G.A.; Zhou, B.; Danaei, G.; Lu, Y.; Bixby, H.; Cowan, M.J.; Riley, L.M.; Hajifathalian, K.; et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Bancej, C.; Jayabalasingham, B.; Wall, R.W.; Rao, D.P.; Do, M.T.; de Groh, M.; Jayaraman, G.C. Trends and projections of obesity among Canadians. Health Promot. Chronic Dis. Prev. Can. 2015, 35, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in obesity among adults in the United States, 2005 to 2014. JAMA J. Am. Med. Assoc. 2016, 315, 2284–2291. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Willett, W.C.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Rosner, B.; Speizer, F.E.; Hennekens, C.H. Weight, weight change, and coronary heart disease in women. Risk within the “normal” weight range. JAMA 1995, 273, 461–465. [Google Scholar] [CrossRef]
- Manson, J.E.; Willett, W.C.; Stampfer, M.J.; Colditz, G.A.; Hunter, D.J.; Hankinson, S.E.; Hennekens, C.H.; Speizer, F.E. Body Weight and Mortality among Women. N. Engl. J. Med. 1995, 333, 677–685. [Google Scholar] [CrossRef]
- Pajares, F. Self-Efficacy Beliefs in Academic Settings. Rev. Educ. Res. 1996, 66, 543–578. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Bautista, L.; Franzosi, M.G.; Commerford, P.; Lang, C.C.; Rumboldt, Z.; Onen, C.L.; Lisheng, L.; et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet 2005, 366, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Brawer, R.; Brisbon, N.; Plumb, J. Obesity and Cancer. Prim. Care Clin. Off. Pract. 2009, 36, 509–531. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. From farm to fat cell: Why aren’t we all fat? Metabolism 2015, 64, 349–353. [Google Scholar] [CrossRef]
- Colley, R.C.; Garriguet, D.; Janssen, I.; Craig, C.L.; Clarke, J.; Tremblay, M.S. Physical Activity of Canadian Adults:Accelerometer Results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2011, 22, 7. [Google Scholar]
- Tappe, M.K.; Duda, J.L.; Ehrnwald, P.M. Perceived barriers to exercise among adolescents. J. Sch. Health 1989, 59, 153–155. [Google Scholar] [CrossRef]
- Muktabhant, B.; Lawrie, T.A.; Lumbiganon, P.; Laopaiboon, M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst. Rev. 2015, 2015, CD007145. [Google Scholar] [CrossRef]
- Mc Morrow, L.; Ludbrook, A.; Macdiarmid, J.I.; Olajide, D. Perceived barriers towards healthy eating and their association with fruit and vegetable consumption. J. Public Health 2016, 39, fdw038. [Google Scholar] [CrossRef]
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef] [PubMed]
- McMurray, F.; Patten, D.A.; Harper, M.E. Reactive Oxygen Species and Oxidative Stress in Obesity—Recent Findings and Empirical Approaches. Obesity 2016, 24, 2301–2310. [Google Scholar] [CrossRef]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015, 6, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Berg, A.H.; Iyengar, P.; Lam, T.K.T.; Giacca, A.; Combs, T.P.; Rajala, M.W.; Du, X.; Rollman, B.; Li, W.; et al. The hyperglycemia-induced inflammatory response in adipocytes: The role of reactive oxygen species. J. Biol. Chem. 2005, 280, 4617–4626. [Google Scholar] [CrossRef]
- Talior, I.; Yarkoni, M.; Bashan, N.; Eldar-Finkelman, H. Increased glucose uptake promotes oxidative stress and PKC-δ activation in adipocytes of obese, insulin-resistant mice. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E295–E302. [Google Scholar] [CrossRef] [PubMed]
- Harijith, A.; Ebenezer, D.L.; Natarajan, V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front. Physiol. 2014, 5, 352. [Google Scholar] [CrossRef]
- Herder, C.; Schneitler, S.; Rathmann, W.; Haastert, B.; Schneitler, H.; Winkler, H.; Bredahl, R.; Hahnloser, E.; Martin, S. Low-grade inflammation, obesity, and insulin resistance in adolescents. J. Clin. Endocrinol. Metab. 2007, 92, 4569–4574. [Google Scholar] [CrossRef]
- Johnson, A.R.; Justin Milner, J.; Makowski, L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, G.; Siani, A. Role of microRNAs in obesity and obesity-related diseases. Genes Nutr. 2017, 12, 23. [Google Scholar] [CrossRef]
- Ji, C.; Guo, X. The clinical potential of circulating microRNAs in obesity. Nat. Rev. Endocrinol. 2019, 15, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Landrier, J.F.; Derghal, A.; Mounien, L. MicroRNAs in Obesity and Related Metabolic Disorders. Cells 2019, 8, 859. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.M.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Lei, L.M.; Lin, X.; Xu, F.; Shan, S.K.; Guo, B.; Li, F.X.Z.; Zheng, M.H.; Wang, Y.; Xu, Q.S.; Yuan, L.Q. Exosomes and Obesity-Related Insulin Resistance. Front. Cell Dev. Biol. 2021, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Shah, A.; Nakamura, T. Extracellular Vesicles: A Potential Novel Regulator of Obesity and Its Associated Complications. Children 2018, 5, 152. [Google Scholar] [CrossRef]
- Cordero, M.D.; Alcocer-Gómez, E.; De Miguel, M.; Cano-García, F.J.; Luque, C.M.; Fernández-Riejo, P.; Fernández, A.M.M.; Sánchez-Alcazar, J.A. Coenzyme Q10: A novel therapeutic approach for Fibromyalgia? Case series with 5 patients. Mitochondrion 2011, 11, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Sangsefidi, Z.S.; Yaghoubi, F.; Hajiahmadi, S.; Hosseinzadeh, M. The effect of coenzyme Q10 supplementation on oxidative stress: A systematic review and meta-analysis of randomized controlled clinical trials. Food Sci. Nutr. 2020, 8, 1766–1776. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.K. Vitamin E Regulation of Mitochondrial Superoxide Generation. Neurosignals 2001, 10, 112–124. [Google Scholar] [CrossRef]
- Alcalá, M.; Sánchez-Vera, I.; Sevillano, J.; Herrero, L.; Serra, D.; Ramos, M.P.; Viana, M. Vitamin E reduces adipose tissue fibrosis, inflammation, and oxidative stress and improves metabolic profile in obesity. Obesity 2015, 23, 1598–1606. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Slowinska-Lisowska, M.; Szygula, Z.; Witkowski, K.; Stefaniak, T.; Dziubek, W. Assessment of the antioxidant effectiveness of alpha-lipoic acid in healthy men exposed to muscle-damaging exercise. J. Physiol. Pharmacol. 2009, 60, 139–143. [Google Scholar]
- Martins, V.D.; Manfredini, V.; Peralba, M.C.R.; Benfato, M.S. Alpha-lipoic acid modifies oxidative stress parameters in sickle cell trait subjects and sickle cell patients. Clin. Nutr. 2009, 28, 192–197. [Google Scholar] [CrossRef]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Wootton-Beard, P.C.; Brandt, K.; Fell, D.; Warner, S.; Ryan, L. Effects of a beetroot juice with high neobetanin content on the early-phase insulin response in healthy volunteers. J. Nutr. Sci. 2014, 3, e9. [Google Scholar] [CrossRef]
- Tesoriere, L.; Fazzari, M.; Angileri, F.; Gentile, C.; Livrea, M.A. In vitro digestion of betalainic foods. Stability and bioaccessibility of betaxanthins and betacyanins and antioxidative potential of food digesta. J. Agric. Food Chem. 2008, 56, 10487–10492. [Google Scholar] [CrossRef]
- Godard, M.P.; Johnson, B.A.; Richmond, S.R. Body Composition and Hormonal Adaptations Associated with Forskolin Consumption in Overweight and Obese Men. Obes. Res. 2005, 13, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Litosch, I.; Hudson, T.H.; Mills, I.; Li, S.Y.; Fain, J.N. Forskolin as an activator of cyclic AMP accumulation and lipolysis in rat adipocytes. Mol. Pharmacol. 1982, 22, 109–115. [Google Scholar]
- Thom, E. The Effect of Chlorogenic Acid Enriched Coffee on Glucose Absorption in Healthy Volunteers and Its Effect on Body Mass When Used Long-term in Overweight and Obese People. J. Int. Med. Res. 2007, 35, 900–908. [Google Scholar] [CrossRef]
- Revuelta-Iniesta, R.; Al-Dujaili, E.A.S. Consumption of Green Coffee Reduces Blood Pressure and Body Composition by Influencing 11 β-HSD1 Enzyme Activity in Healthy Individuals: A Pilot Crossover Study Using Green and Black Coffee. BioMed Res. Int. 2014, 2014, 482704. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Xie, Z.; Zhou, Y.; Zhang, Y.; Wan, X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur. J. Clin. Nutr. 2014, 68, 1075–1087. [Google Scholar] [CrossRef]
- Barrea, L.; Altieri, B.; Polese, B.; De Conno, B.; Muscogiuri, G.; Colao, A.; Savastano, S. Nutritionist and obesity: Brief overview on efficacy, safety, and drug interactions of the main weight-loss dietary supplements. Int. J. Obes. Suppl. 2019, 9, 32–49. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Manta, K.; Bujak, A.L.; Simone, A.C.; Fuda, M.R.; Nilsson, M.I.; Hettinga, B.P.; Hughes, M.C.; Perry, C.G.R.; Tarnopolsky, M.A. A Novel Multi-Ingredient Supplement Activates a Browning Program in White Adipose Tissue and Mitigates Weight Gain in High-Fat Diet-Fed Mice. Nutrients 2021, 13, 3726. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X.; Becker, D.M.; Bouchard, C.; Carleton, R.A.; Colditz, G.A.; Dietz, W.H.; Foreyt, J.P.; Garrison, R.J.; Grundy, S.M.; Hansen, B.C.; et al. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: Executive summary. Am. J. Clin. Nutr. 1998, 68, 899–917. [Google Scholar]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef] [PubMed]
- De Koning, L.; Merchant, A.T.; Pogue, J.; Anand, S.S. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: Meta-regression analysis of prospective studies. Eur. Heart J. 2007, 28, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Brazier, J.E.; Harper, R.; Jones, N.M.B.; O’Cathain, A.; Thomas, K.J.; Usherwood, T.; Westlake, L. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. Br. Med. J. 1992, 305, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.W.; McPherson, G.C.; Ramsay, C.R.; Campbell, M.K. The method of minimization for allocation to clinical trials: A review. Control. Clin. Trials 2002, 23, 662–674. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Treatment allocation by minimisation. BMJ 2005, 330, 843. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Warburton, D.E.R.; Janssen, I.; Paterson, D.H.; Latimer, A.E.; Rhodes, R.E.; Kho, M.E.; Hicks, A.; LeBlanc, A.G.; Zehr, L.; et al. New Canadian physical activity guidelines. Appl. Physiol. Nutr. Metab. 2011, 36, 36–46. [Google Scholar] [CrossRef]
- Nishida, C.; Ko, G.T.; Kumanyika, S. Body fat distribution and noncommunicable diseases in populations: Overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. Eur. J. Clin. Nutr. 2010, 64, 2–5. [Google Scholar] [CrossRef]
- Albanese, C.V.; Diessel, E.; Genant, H.K. Clinical applications of body composition measurements using DXA. J. Clin. Densitom. 2003, 6, 75–85. [Google Scholar] [CrossRef]
- Prior, B.M.; Cureton, K.J.; Modlesky, C.M.; Evans, E.M.; Sloniger, M.A.; Saunders, M.; Lewis, R.D. In vivo validation of whole body composition estimates from dual-energy X-ray absorptiometry. J. Appl. Physiol. 1997, 83, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Kharb, S. Dual energy X-ray absorptiometry: Pitfalls in measurement and interpretation of bone mineral density. Indian J. Endocrinol. Metab. 2013, 17, 203. [Google Scholar] [CrossRef]
- Salgado, A.L.F.D.A.; De Carvalho, L.; Oliveira, A.C.; Dos Santos, V.N.; Vieira, J.G.; Parise, E.R. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq. Gastroenterol. 2010, 47, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Weir, J.B.D.V. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E. The theoretical bases of indirect calorimetry: A review. Metabolism 1988, 37, 287–301. [Google Scholar] [CrossRef]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- Weijs, P.J.M. Validity of predictive equations for resting energy expenditure in US and Dutch overweight and obese class I and II adults aged 18–65 y. Am. J. Clin. Nutr. 2008, 88, 959–970. [Google Scholar] [CrossRef]
- Frankenfield, D.C.; Rowe, W.A.; Smith, J.S.; Cooney, R.N. Validation of several established equations for resting metabolic rate in obese and nonobese people. J. Am. Diet. Assoc. 2003, 103, 1152–1159. [Google Scholar] [CrossRef]
- Lins, L.; Carvalho, F.M. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016, 4, 205031211667172. [Google Scholar] [CrossRef]
- Hays, R.D.; Morales, L.S. The RAND-36 measure of health-related quality of life. Ann. Med. 2001, 33, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.J.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Blissmer, B.; Riebe, D.; Dye, G.; Ruggiero, L.; Greene, G.; Caldwell, M. Health-related quality of life following a clinical weight loss intervention among overweight and obese adults: Intervention and 24 month follow-up effects. Health Qual. Life Outcomes 2006, 4, 43. [Google Scholar] [CrossRef]
- Toolsee, N.A.; Aruoma, O.I.; Gunness, T.K.; Kowlessur, S.; Dambala, V.; Murad, F.; Googoolye, K.; Daus, D.; Indelicato, J.; Rondeau, P.; et al. Effectiveness of green tea in a randomized human cohort: Relevance to diabetes and its complications. BioMed Res. Int. 2013, 2013, 412379. [Google Scholar] [CrossRef]
- Verheggen, R.J.H.M.; Maessen, M.F.H.; Green, D.J.; Hermus, A.R.M.M.; Hopman, M.T.E.; Thijssen, D.H.T. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: Distinct effects on body weight and visceral adipose tissue. Obes. Rev. 2016, 17, 664–690. [Google Scholar] [CrossRef]
- Elobeid, M.A.; Padilla, M.A.; McVie, T.; Thomas, O.; Brock, D.W.; Musser, B.; Lu, K.; Coffey, C.S.; Desmond, R.A.; St-Onge, M.P.; et al. Missing data in randomized clinical trials for weight loss: Scope of the problem, state of the field, and performance of statistical methods. PLoS ONE 2009, 4, e6624. [Google Scholar] [CrossRef]
- The Emerging Risk Factors Collaboration; Wormser, D.; Kaptoge, S.; Di Angelantonio, E.; Wood, A.M.; Pennells, L.; Thompson, A.; Sarwar, N.; Kizer, J.R.; Lawlor, D.A.; et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet 2011, 377, 1085–1095. [Google Scholar] [CrossRef]
- MacMahon, S.; Baigent, C.; Duffy, S.; Rodgers, A.; Tominaga, S.; Chambless, L.; De Backer, G.; De Bacquer, D.; Kornitzer, M.; Whincup, P.; et al. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef]
- Jayedi, A.; Khan, T.A.; Aune, D.; Emadi, A.; Shab-Bidar, S. Body fat and risk of all-cause mortality: A systematic review and dose-response meta-analysis of prospective cohort studies. Int. J. Obes. 2022, 46, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J. Am. Coll. Cardiol. 2014, 63, 2985–3023. [Google Scholar] [CrossRef]
- Chaston, T.B.; Dixon, J.B.; O’Brien, P.E. Changes in fat-free mass during significant weight loss: A systematic review. Int. J. Obes. 2007, 31, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.; Scheen, A. Weight management in type 2 diabetes: Current and emerging approaches to treatment. Diabetes Care 2015, 38, 1161–1172. [Google Scholar] [CrossRef]
- Sorli, C.; Harashima, S.; Tsoukas, G.M.; Unger, J.; Karsbøl, J.D.; Hansen, T.; Bain, S.C. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): A double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017, 5, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Jakher, H.; Chang, T.I.; Tan, M.; Mahaffey, K.W. Canagliflozin review—Safety and efficacy profile in patients with T2DM. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 209–215. [Google Scholar] [CrossRef] [PubMed]
- McCrimmon, R.J.; Catarig, A.M.; Frias, J.P.; Lausvig, N.L.; le Roux, C.W.; Thielke, D.; Lingvay, I. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: A substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia 2020, 63, 473–485. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Ida, S.; Kaneko, R.; Imataka, K.; Okubo, K.; Shirakura, Y.; Azuma, K.; Fujiwara, R.; Murata, K. Effects of Antidiabetic Drugs on Muscle Mass in Type 2 Diabetes Mellitus. Curr. Diabetes Rev. 2020, 17, 293–303. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, J.H.; Jeong, K.W.; Choi, C.S.; Jun, H.S. Amelioration of muscle wasting by glucagon-like peptide-1 receptor agonist in muscle atrophy. J. Cachexia Sarcopenia Muscle 2019, 10, 903–918. [Google Scholar] [CrossRef]
- Ross, R. Reduction in Obesity and Related Comorbid Conditions after Diet-Induced Weight Loss or Exercise-Induced Weight Loss in Men. Ann. Intern. Med. 2000, 133, 92. [Google Scholar] [CrossRef]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Mittendorfer, B.; Magkos, F.; Fabbrini, E.; Mohammed, B.S.; Klein, S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity 2009, 17, 1872–1877. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.J.; McGlory, C.; Damas, F.; Mazara, N.; Baker, S.K.; Phillips, S.M. Pronounced energy restriction with elevated protein intake results in no change in proteolysis and reductions in skeletal muscle protein synthesis that are mitigated by resistance exercise. FASEB J. 2018, 32, 265–275. [Google Scholar] [CrossRef]
- Carbone, J.W.; Pasiakos, S.M.; Vislocky, L.M.; Anderson, J.M.; Rodriguez, N.R. Effects of short-term energy deficit on muscle protein breakdown and intramuscular proteolysis in normal-weight young adults. Appl. Physiol. Nutr. Metab. 2014, 39, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Cava, E.; Yeat, N.C.; Mittendorfer, B. Preserving healthy muscle during weight loss. Adv. Nutr. 2017, 8, 511–519. [Google Scholar] [CrossRef]
- Yang, C.S.; Landau, J.M. Effects of tea consumption nutrition health. J. Nutr. 2000, 130, 2409–2412. [Google Scholar] [CrossRef]
- Chantre, P.; Lairon, D. Recent findings of green tea extract AR25 (exolise) and its activity for the treatment of obesity. Phytomedicine 2002, 9, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Komine, Y.; Soga, S.; Meguro, S.; Hase, T.; Tanaka, Y.; Tokimitsu, I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am. J. Clin. Nutr. 2005, 81, 122–129. [Google Scholar] [CrossRef]
- Nagao, T.; Hase, T.; Tokimitsu, I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity 2007, 15, 1473–1483. [Google Scholar] [CrossRef]
- Hase, T.; Komine, Y.; Meguro, S.; Takeda, Y.; Takahashi, H.; Matsui, Y.; Inaoka, S.; Katsuragi, Y.; Tokimitsu, I.; Shimasaki, H.; et al. Anti-obesity Effects of Tea Catechins in Humans. J. Oleo Sci. 2001, 50, 599–605. [Google Scholar] [CrossRef]
- Wang, H.; Wen, Y.; Du, Y.; Yan, X.; Guo, H.; Rycroft, J.A.; Boon, N.; Kovacs, E.M.R.; Mela, D.J. Effects of catechin enriched green tea on body composition. Obesity 2010, 18, 773–779. [Google Scholar] [CrossRef]
- Dulloo, A.G.; Seydoux, J.; Girardier, L.; Chantre, P.; Vandermander, J. Green tea and thermogenesis: Interactions between catechin-polyphenols, caffeine and sympathetic activity. Int. J. Obes. 2000, 24, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Duret, C.; Rohrer, D.; Girardier, L.; Mensi, N.; Fathi, M.; Chantre, P.; Vandermander, J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 1999, 70, 1040–1045. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, W.; Wang, X.; Wu, X.; Tian, W. Novel inhibitors of fatty-acid synthase from green tea (Camellia sinensis Xihu Longjing) with high activity and a new reacting site. Biotechnol. Appl. Biochem. 2006, 43, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Diepvens, K.; Kovacs, E.M.R.; Nijs, I.M.T.; Vogels, N.; Westerterp-Plantenga, M.S. Effect of green tea on resting energy expenditure and substrate oxidation during weight loss in overweight females. Br. J. Nutr. 2005, 94, 1026–1034. [Google Scholar] [CrossRef]
- Henderson, S.; Magu, B.; Rasmussen, C.; Lancaster, S.; Kerksick, C.; Smith, P.; Melton, C.; Cowan, P.; Greenwood, M.; Earnest, C.; et al. Effects of Coleus Forskohlii Supplementation on Body Composition and Hematological Profiles in Mildly Overweight Women. J. Int. Soc. Sports Nutr. 2005, 2, 54. [Google Scholar] [CrossRef]
- Stohs, S.J.; Badmaev, V. A Review of Natural Stimulant and Non-stimulant Thermogenic Agents. Phytother. Res. 2016, 30, 732–740. [Google Scholar] [CrossRef]
- Shivaprasad, H.N.; Gopalakrishna, S.; Mariyanna, B.; Thekkoot, M.; Reddy, R.; Tippeswamy, B.S. Effect of Coleus forskohliiextract on cafeteria diet-induced obesity in rats. Pharmacogn. Res. 2014, 6, 42–45. [Google Scholar] [CrossRef]
- Loftus, H.L.; Astell, K.J.; Mathai, M.L.; Su, X.Q. Coleus forskohlii extract supplementation in conjunction with a hypocaloric diet reduces the risk factors of metabolic syndrome in overweight and obese subjects: A randomized controlled trial. Nutrients 2015, 7, 9508–9522. [Google Scholar] [CrossRef]
- Del Castillo, M.D.; Ames, J.M.; Gordon, M.H. Effect of roasting on the antioxidant activity of coffee brews. J. Agric. Food Chem. 2002, 50, 3698–3703. [Google Scholar] [CrossRef]
- Arion, W.J.; Canfield, W.K.; Ramos, F.C.; Schindler, P.W.; Burger, H.J.; Hemmerle, H.; Schubert, G.; Below, P.; Herling, A.W. Chlorogenic acid and hydroxynitrobenzaldehyde: New inhibitors of hepatic glucose 6-phosphatase. Arch. Biochem. Biophys. 1997, 339, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Seki, E.; Aitani, M. Inhibitory effect of green coffee bean extract on fat accumulation and body weight gain in mice. BMC Complement. Altern. Med. 2006, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.C.; MacDonald, J.R.; Mahoney, D.J.; Parise, G.; Beal, M.F.; Tarnopolsky, M.A. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve 2007, 35, 235–242. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Alcala, M.; Calderon-Dominguez, M.; Serra, D.; Herrero, L.; Ramos, M.P.; Viana, M. Short-term vitamin E treatment impairs reactive oxygen species signaling required for adipose tissue expansion, resulting in fatty liver and insulin resistance in obese mice. PLoS ONE 2017, 12, e0186579. [Google Scholar] [CrossRef] [PubMed]
- Carbonelli, M.; Renzo, L.; Bigioni, M.; Daniele, N.; De Lorenzo, A.; Fusco, M. α-Lipoic Acid Supplementation: A Tool for Obesity Therapy? Curr. Pharm. Des. 2010, 16, 840–846. [Google Scholar] [CrossRef]
- Salehi, B.; Berkay Yılmaz, Y.; Antika, G.; Boyunegmez Tumer, T.; Fawzi Mahomoodally, M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta Gen. Subj. 2009, 1790, 1149–1160. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Lee, S.G.; Davis, C.G.; Kenny, A.; Koo, S.I.; Chun, O.K. Plasma total antioxidant capacity is associated with dietary intake and plasma level of antioxidants in postmenopausal women. J. Nutr. Biochem. 2012, 23, 1725–1731. [Google Scholar] [CrossRef]
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006, 40, 445–453. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016, 15, 817–828. [Google Scholar] [CrossRef]
- Ali, N.; Sumon, A.H.; Fariha, K.A.; Asaduzzaman, M.; Kathak, R.R.; Molla, N.H.; Mou, A.D.; Barman, Z.; Hasan, M.; Miah, R.; et al. Assessment of the relationship of serum liver enzymes activity with general and abdominal obesity in an urban Bangladeshi population. Sci. Rep. 2021, 11, 6640. [Google Scholar] [CrossRef]
- Ruhl, C.E.; Everharty, J.E. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology 2003, 124, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Arora, C.; Malhotra, A.; Ranjan, P.; Singh, V.; Singh, N.; Shalimar; Dwivedi, S.N.; Vikram, N.K. Effect of intensive weight-loss intervention on metabolic, ultrasound and anthropometric parameters among patients with obesity and non-alcoholic fatty liver disease: An RCT. Eur. J. Clin. Nutr. 2022, 76, 1332–1338. [Google Scholar] [CrossRef]
- Gasteyger, C.; Larsen, T.M.; Vercruysse, F.; Astrup, A. Effect of a dietary-induced weight loss on liver enzymes in obese subjects. Am. J. Clin. Nutr. 2008, 87, 1141–1147. [Google Scholar] [CrossRef]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jørgensen, S.B.; Steinberg, G.R. GDF15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 2021, 17, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Um, S.H.; Seo, D.S.; Joo, S.K.; Bae, J.M.; Park, J.H.; Chang, M.S.; Kim, J.H.; Lee, J.; Jeong, W., II; et al. Growth differentiation factor 15 predicts advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease. Liver Int. 2018, 38, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Galuppo, B.; Agazzi, C.; Pierpont, B.; Chick, J.; Li, Z.; Caprio, S.; Santoro, N. Growth differentiation factor 15 (GDF15) is associated with non-alcoholic fatty liver disease (NAFLD) in youth with overweight or obesity. Nutr. Diabetes 2022, 12, 9. [Google Scholar] [CrossRef]
- Coll, A.P.; Chen, M.; Taskar, P.; Rimmington, D.; Patel, S.; Tadross, J.A.; Cimino, I.; Yang, M.; Welsh, P.; Virtue, S.; et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 2020, 578, 444–448. [Google Scholar] [CrossRef]
- Day, E.A.; Ford, R.J.; Smith, B.K.; Mohammadi-Shemirani, P.; Morrow, M.R.; Gutgesell, R.M.; Lu, R.; Raphenya, A.R.; Kabiri, M.; McArthur, A.G.; et al. Metformin-induced increases in GDF15 are important for suppressing appetite and promoting weight loss. Nat. Metab. 2019, 1, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Townsend, L.K.; DesOrmeaux, G.J.; Frangos, S.M.; Batchuluun, B.; Dumont, L.; Kuhre, R.E.; Ahmadi, E.; Hu, S.; Rebalka, I.A.; et al. GDF15 promotes weight loss by enhancing energy expenditure in muscle. Nature 2023, 619, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, M.; Clemmensen, C.; Sjøberg, K.A.; Carl, C.S.; Jeppesen, J.F.; Wojtaszewski, J.F.P.; Kiens, B.; Richter, E.A. Exercise increases circulating GDF15 in humans. Mol. Metab. 2018, 9, 187–191. [Google Scholar] [CrossRef]
- Cai, L.; Li, C.; Wang, Y.; Mo, Y.; Yin, J.; Ma, X. Increased serum GDF15 related to improvement in metabolism by lifestyle intervention among young overweight and obese adults. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 1195–1202. [Google Scholar] [CrossRef]
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front. Physiol. 2021, 11, 1757. [Google Scholar] [CrossRef] [PubMed]
- Verweij, F.J.; Bebelman, M.P.; Jimenez, C.R.; Garcia-Vallejo, J.J.; Janssen, H.; Neefjes, J.; Knol, J.C.; de Goeij-de Haas, R.; Piersma, S.R.; Baglio, S.R.; et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J. Cell Biol. 2018, 217, 1129–1142. [Google Scholar] [CrossRef]
- Heyn, G.S.; Corrêa, L.H.; Magalhães, K.G. The Impact of Adipose Tissue–Derived miRNAs in Metabolic Syndrome, Obesity, and Cancer. Front. Endocrinol. 2020, 11, 563816. [Google Scholar] [CrossRef]
- Tryndyak, V.P.; Latendresse, J.R.; Montgomery, B.; Ross, S.A.; Beland, F.A.; Rusyn, I.; Pogribny, I.P. Plasma microRNAs are sensitive indicators of inter-strain differences in the severity of liver injury induced in mice by a choline- and folate-deficient diet. Toxicol. Appl. Pharmacol. 2012, 262, 52–59. [Google Scholar] [CrossRef]
- Yamada, H.; Suzuki, K.; Ichino, N.; Ando, Y.; Sawada, A.; Osakabe, K.; Sugimoto, K.; Ohashi, K.; Teradaira, R.; Inoue, T.; et al. Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clin. Chim. Acta 2013, 424, 99–103. [Google Scholar] [CrossRef]
- Wang, R.; Hong, J.; Cao, Y.; Shi, J.; Gu, W.; Ning, G.; Zhang, Y.; Wang, W. Elevated circulating microRNA-122 is associated with obesity and insulin resistance in young adults. Eur. J. Endocrinol. 2015, 172, 291–300. [Google Scholar] [CrossRef]
- Povero, D.; Eguchi, A.; Li, H.; Johnson, C.D.; Papouchado, B.G.; Wree, A.; Messer, K.; Feldstein, A.E. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS ONE 2014, 9, e113651. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xiong, Y.; Sheng, Q.; Zhao, S.; Wattacheril, J.; Flynn, C.R. A micro-RNA expression signature for human NAFLD progression. J. Gastroenterol. 2016, 51, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Otgonsuren, M.; Younoszai, Z.; Allawi, H.; Raybuck, B.; Younossi, Z. Circulating miRNA in patients with non-alcoholic fatty liver disease and coronary artery disease. BMJ Open Gastroenterol. 2016, 3, e000096. [Google Scholar] [CrossRef]
- Sunadome, H.; Matsumoto, H.; Izuhara, Y.; Nagasaki, T.; Kanemitsu, Y.; Ishiyama, Y.; Morimoto, C.; Oguma, T.; Ito, I.; Murase, K.; et al. Correlation between eosinophil count, its genetic background and body mass index: The Nagahama Study. Allergol. Int. 2020, 69, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.S.; Kim, H.J.; Kang, E.S.; Ahn, C.W.; Lim, S.K.; Lee, H.C.; Cha, B.S. The association of total and differential white blood cell count with metabolic syndrome in type 2 diabetic patients. Diabetes Res. Clin. Pract. 2006, 73, 284–291. [Google Scholar] [CrossRef]
- Tsuboi, N.; Koike, K.; Hirano, K.; Utsunomiya, Y.; Kawamura, T.; Hosoya, T. Clinical features and long-term renal outcomes of Japanese patients with obesity-related glomerulopathy. Clin. Exp. Nephrol. 2013, 17, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Kambham, N.; Markowitz, G.S.; Valeri, A.M.; Lin, J.; D’Agati, V.D. Obesity-related glomerulopathy: An emerging epidemic. Kidney Int. 2001, 59, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Streja, E.; Molnar, M.Z.; Lukowsky, L.R.; Krishnan, M.; Kovesdy, C.P.; Greenland, S. Mortality prediction by surrogates of body composition: An examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. Am. J. Epidemiol. 2012, 175, 793–803. [Google Scholar] [CrossRef]
- Foster, M.C.; Hwang, S.J.; Larson, M.G.; Lichtman, J.H.; Parikh, N.I.; Vasan, R.S.; Levy, D.; Fox, C.S. Overweight, Obesity, and the Development of Stage 3 CKD: The Framingham Heart Study. Am. J. Kidney Dis. 2008, 52, 39–48. [Google Scholar] [CrossRef]
- Hall, M.E.; Cohen, J.B.; Ard, J.D.; Egan, B.M.; Hall, J.E.; Lavie, C.J.; Ma, J.; Ndumele, C.E.; Schauer, P.R.; Shimbo, D. Weight-Loss Strategies for Prevention and Treatment of Hypertension: A Scientific Statement from the American Heart Association. Hypertension 2021, 78, E38–E50. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.M.; Ahmadi, S.F.; Kalantar-Zadeh, K. The dual roles of obesity in chronic kidney disease: A review of the current literature. Curr. Opin. Nephrol. Hypertens. 2016, 25, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, V.T.N.; Schei, J.; Jenssen, T.G.; Melsom, T.; Eriksen, B.O. Central obesity associates with renal hyperfiltration in the non-diabetic general population: A cross-sectional study. BMC Nephrol. 2016, 17, 172. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Lawler, E.V.; Mackenzie, H.S. The hyperfiltration theory: A paradigm shift in nephrology. Kidney Int. 1996, 49, 1774–1777. [Google Scholar] [CrossRef]
- Chagnac, A.; Weinstein, T.; Herman, M.; Hirsh, J.; Gafter, U.; Ori, Y. The effects of weight loss on renal function in patients with severe obesity. J. Am. Soc. Nephrol. 2003, 14, 1480–1486. [Google Scholar] [CrossRef]
- Gerchman, F.; Tong, J.; Utzschneider, K.M.; Zraika, S.; Udayasankar, J.; McNeely, M.J.; Carr, D.B.; Leonetti, D.L.; Young, B.A.; De Boer, I.H.; et al. Body mass index is associated with increased creatinine clearance by a mechanism independent of body fat distribution. J. Clin. Endocrinol. Metab. 2009, 94, 3781–3788. [Google Scholar] [CrossRef]

| Ingredients | PLA | MIS |
|---|---|---|
| Forskolin (mg) | 0 | 50 |
| Green Coffee Bean Extract (mg) | 0 | 500 |
| Green Tea Extract (mg) | 0 | 500 |
| Beet Root Extract (mg) | 0 | 500 |
| α-Lipoic Acid (mg) | 0 | 400 |
| Vitamin E (IU) | 0 | 200 |
| Coenzyme Q10 (mg) | 0 | 200 |
| Microcrystalline Cellulose (g) | 2.15 | 0 |
| Variable (Unit) | Placebo (PLA; n = 27) | Active (MIS; n = 28) |
|---|---|---|
| Sex, n (M/F) | 12/15 | 11/17 |
| Age (years) | 25.5 ± 1.5 | 26.4 ± 1.6 |
| Body weight (kg) | 87.8 ± 3.5 | 89.4 ± 2.8 |
| BMI (kg/m2) | 29.8 ± 1.0 | 31.1 ± 0.8 |
| Waist-to-Height Ratio (a.u) | 0.57 ± 0.01 | 0.59 ± 0.01 |
| Body Fat percentage (%) | 40.2 ± 1.7 | 40.3 ± 1.5 |
| Fat mass (kg) | 34.2 ± 2.3 | 34.6 ± 1.7 |
| Fat-free mass (kg) | 50.2 ± 0.1 | 51.4 ± 0.1 |
| Variable (Unit) | Placebo (n) | Active (n) | p Value |
|---|---|---|---|
| Weight (kg) | 89.5 ± 0.5 (27) | 86.4 ± 0.5 (28) *** | <0.001 |
| BMI (kg/m2) | 30.7 ± 0.2 (27) | 29.7 ± 0.2 (28) *** | <0.001 |
| Waist to Height ratio (a.u.) | 0.60 ± 0.01 (27) * | 0.58 ± 0.01 (28) | 0.098 |
| Total Fat-Free Mass (kg) | 51.0 ± 0.4 (27) | 50.7 ± 0.4 (28) | 0.591 |
| Total Fat Mass (kg) §,† | 35.4 ± 0.5 (27) | 33.0 ± 0.5 (28) ** | <0.001 |
| BCI (a.u.) | 1.57 ± 0.04 (27) | 1.69 ± 0.04 (28) * | 0.034 |
| Arm Fat Mass (kg) | 3.10 ± 0.06 (27) | 3.01 ± 0.06 (28) * | 0.237 |
| Leg Fat Mass (kg) | 13.5 ± 0.2 (27) | 13.1 ± 0.2 (28) * | 0.088 |
| Trunk Fat Mass (kg) | 17.4 ± 0.3 (27) | 16.4 ± 0.3 (28) * | 0.039 |
| Android Fat (kg) | 3.31 ± 0.06 (27) | 3.14 ± 0.06 (28) * | 0.051 |
| Gynoid Fat (kg) | 6.60 ± 0.09 (27) | 6.22 ± 0.08 (28) ** | 0.002 |
| Variable (Unit) | Placebo (n) | Active (n) | p Value |
|---|---|---|---|
| Clinical biochemistry | |||
| ALT (U/L) | 30.3 ± 2.4 (21) | 21.1 ± 2.4 (21) * | 0.008 |
| AST (U/L) | 32.9 ± 1.6 (18) | 27.3 ± 1.6 (18) * | 0.017 |
| GGT (U/L) ‡ | 29.3 ± 1.4 (21) | 29.5 ± 1.4 (21) | 0.913 |
| CRP (mg/L) ‡ | 3.4 ± 1.0 (21) | 4.2 ± 1.0 (21) | 0.804 |
| Creatinine (µmol/L) §,† | 80.0 ± 1.4 (21) | 83.4 ± 1.4 (21) * | 0.089 |
| Bilirubin (µmol/L) ‡ | 12.3 ± 1.0 (21) | 11.3 ± 1.0 (21) | 0.362 |
| Fasting blood lipids & glucose homeostasis | |||
| Total Cholesterol (mmol/L) | 4.8 ± 0.1 (21) | 4.9 ± 0.1 (21) | 0.526 |
| LDL (mmol/L) | 2.9 ± 0.1 (21) | 3.0 ± 0.1 (21) | 0.342 |
| HDL (mmol/L) | 1.3 ± 0.03 (21) | 1.2 ± 0.03 (21) | 0.311 |
| Triglycerides (mmol/L) | 1.4 ± 0.1 (21) | 1.4 ± 0.1 (21) | 0.924 |
| Glucose (mmol/L) | 5.0 ± 0.1 (21) | 5.0 ± 0.1 (21) | 0.942 |
| Insulin (pmol/L) | 80.1 ± 5.5 (21) | 81.0 ± 5.5 (21) | 0.908 |
| HOMA IR (a.u.) | 2.6 ± 0.2 (21) | 2.6 ± 0.2 (21) | 0.991 |
| Variable (Unit) | Placebo (n) | Active (n) | p Value |
|---|---|---|---|
| Hemoglobin | 142.9 ± 1.19 (21) | 143.7 ± 1.19 (21) | 0.650 |
| Hb (g/L) | |||
| Hematocrit | 0.4 ± 0.0 (21) | 0.4 ± 0.0 (21) | 0.977 |
| HCT (%) | |||
| Mean Corpuscular Volume | 87.3 ± 0.3 (21) | 86.9 ± 0.3 (21) | 0.309 |
| MCV (fL) | |||
| Mean Corpuscular Hb | 28.8 ± 0.1 (21) | 28.9 ± 0.1 (21) | 0.694 |
| MCH (pg) | |||
| Mean Corpuscular Hb Conc. | 330.9 ± 1.5 (21) | 331.3 ± 1.5 (21) | 0.835 |
| MCHC (g/L) | |||
| Mean Platelet Volume §,† | 10.5 ± 0.1 (21) | 10.6 ± 0.1 (21) | 0.472 |
| MPV (fL) | |||
| Red Cell Distribution Width | 12.9 ± 0.09 (21) | 13.1 ± 0.09 (21) | 0.172 |
| RDW (%) | |||
| Leukocyte Count | 6.4 ± 0.3 (21) | 6.5 ± 0.3 (21) | 0.783 |
| LKCS (×109/L) | |||
| Erythrocyte Count | 5.0 ± 0.04 (21) | 5.0 ± 0.04 (21) | 0.864 |
| ERCS (×1012/L) | |||
| Platelet Count ‡ | 253.6 ± 6.5 (21) | 254.9 ± 6.5 (21) | 0.880 |
| (×109/L) | |||
| Neutrophil Count | 3.5 ± 0.2 (21) | 3.8 ± 0.2 (21) | 0.370 |
| (×109/L) | |||
| Lymphocyte Count | 2.1 ± 0.1 (21) | 2.0 ± 0.1 (21) | 0.273 |
| (×109/L) | |||
| Monocyte Count ‡ | 0.5 ± 0.0 (21) | 0.5 ± 0.0 (21) | 0.555 |
| (×109/L) | |||
| Eosinophil Count §,† | 0.19 ± 0.02 (21) * | 0.13 ± 0.02 (21) | 0.007 |
| (×109/L) | |||
| Basophil Count §,† | 0.03 ± 0.01 (21) | 0.04 ± 0.01 (21) | 0.521 |
| (×109/L) |
| Variable (Unit) | Placebo (n) | Active (n) | p Value |
|---|---|---|---|
| Indices of health | |||
| Systolic Blood Pressure (mmHg) | 121 ± 2 (21) | 127 ± 2 (21) | 0.08 |
| Diastolic Blood Pressure (mmHg) | 78 ± 2 (17) | 81 ± 2 (17) | 0.101 |
| Resting Heart Rate (bpm) | 77 ± 3 (21) | 78 ± 3 (21) | 0.655 |
| Bone mineral density (g/cm2) | 1.24 ± 0.01 (27) | 1.24 ± 0.01 (28) | 0.734 |
| Indices of physical fitness | |||
| Relative VO2peak §,† (mL/kg/min−1) | 29.6 ± 1.2 (19) | 30.1 ± 1.1 (21) | 0.526 |
| Relative Grip Strength (kg/kg bw−1) | 0.42 ± 0.01 (21) * | 0.47 ± 0.01 (21) * | 0.002 |
| Indices of metabolic rate | |||
| Resting metabolic rate (Kcal/day) | 1808.3 ± 44.5 (21) | 1827.6 ± 44.5 (21) | 0.761 |
| Estimated metabolic rate (Kcal/day) | 1736.3 ± 5.0 (27) | 1705.0 ± 4.9 (28) * | <0.001 |
| Variable (Unit) | Placebo (n) | Active (n) | p Value |
|---|---|---|---|
| GDF15 (pg/mL) | 411.1 ± 17.0 (20) | 466.0 ± 17.0 (20) * | 0.028 |
| FGF21 (µg/mL) §,† | 253.5 ± 48.0 (20) | 313.4 ± 48.0 (20) | 0.192 |
| IL-6 (pg/mL) §,† | 110.1 ± 11.3 (18) | 103.8 ± 10.7 (20) | 0.633 |
| Leptin (ng/mL) | 28.5 ± 2.0 (20) | 25.1 ± 1.9 (19) | 0.225 |
| ORAC (µM trolox equivalents/mL) | 129.0 ± 2.9 (20) | 136.6 ± 2.9 (20) * | 0.072 |
| CoQ10 (µg/mL) §,† | 1405.3 ± 58.9 (16) | 1544.5 ± 51.2 (21) * | 0.137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nederveen, J.P.; Mastrolonardo, A.J.; Xhuti, D.; Di Carlo, A.; Manta, K.; Fuda, M.R.; Tarnopolsky, M.A. Novel Multi-Ingredient Supplement Facilitates Weight Loss and Improves Body Composition in Overweight and Obese Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 3693. https://doi.org/10.3390/nu15173693
Nederveen JP, Mastrolonardo AJ, Xhuti D, Di Carlo A, Manta K, Fuda MR, Tarnopolsky MA. Novel Multi-Ingredient Supplement Facilitates Weight Loss and Improves Body Composition in Overweight and Obese Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients. 2023; 15(17):3693. https://doi.org/10.3390/nu15173693
Chicago/Turabian StyleNederveen, Joshua P., Alexander J. Mastrolonardo, Donald Xhuti, Alessia Di Carlo, Katherine Manta, Matthew R. Fuda, and Mark A. Tarnopolsky. 2023. "Novel Multi-Ingredient Supplement Facilitates Weight Loss and Improves Body Composition in Overweight and Obese Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial" Nutrients 15, no. 17: 3693. https://doi.org/10.3390/nu15173693
APA StyleNederveen, J. P., Mastrolonardo, A. J., Xhuti, D., Di Carlo, A., Manta, K., Fuda, M. R., & Tarnopolsky, M. A. (2023). Novel Multi-Ingredient Supplement Facilitates Weight Loss and Improves Body Composition in Overweight and Obese Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients, 15(17), 3693. https://doi.org/10.3390/nu15173693







