Oral Administration of Egg- and Soy-Derived Lysophosphatidylcholine Mitigated Acetylcholine Depletion in the Brain of Scopolamine-Treated Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Protocol for Animal Experiments
2.3.1. Experiment 1 (LPC Reagent)
2.3.2. Experiment 2 (LPC-Containing Food Additives and GPC)
2.3.3. Experiment 3 (LPC-Containing Food Additives with Different Fat Contents)
2.4. ACh and Ch Measurement
2.5. LPC Measurement
2.6. Statistical Analyses
3. Results
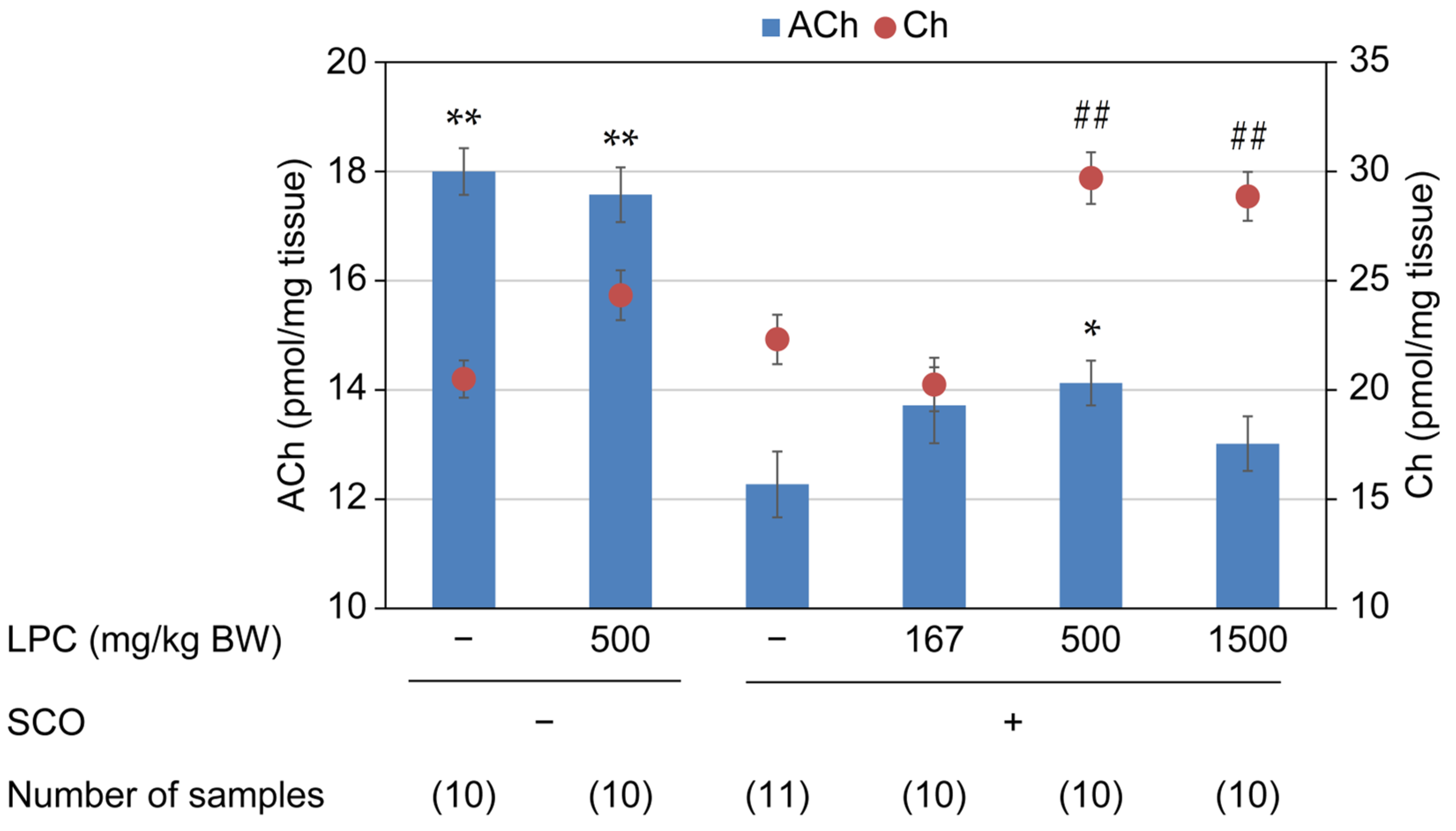
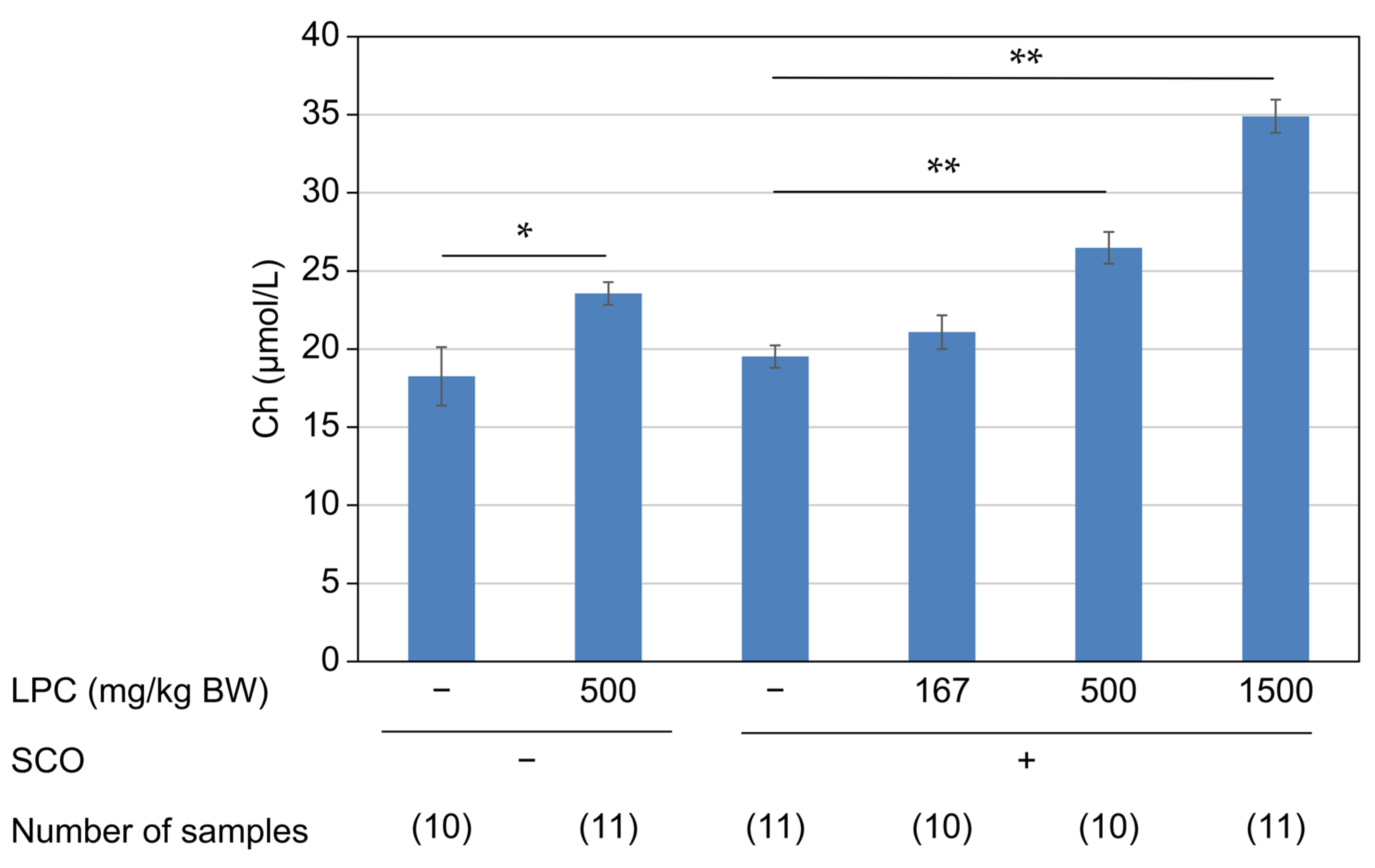
3.1. Experiment 1 (LPC Reagent)
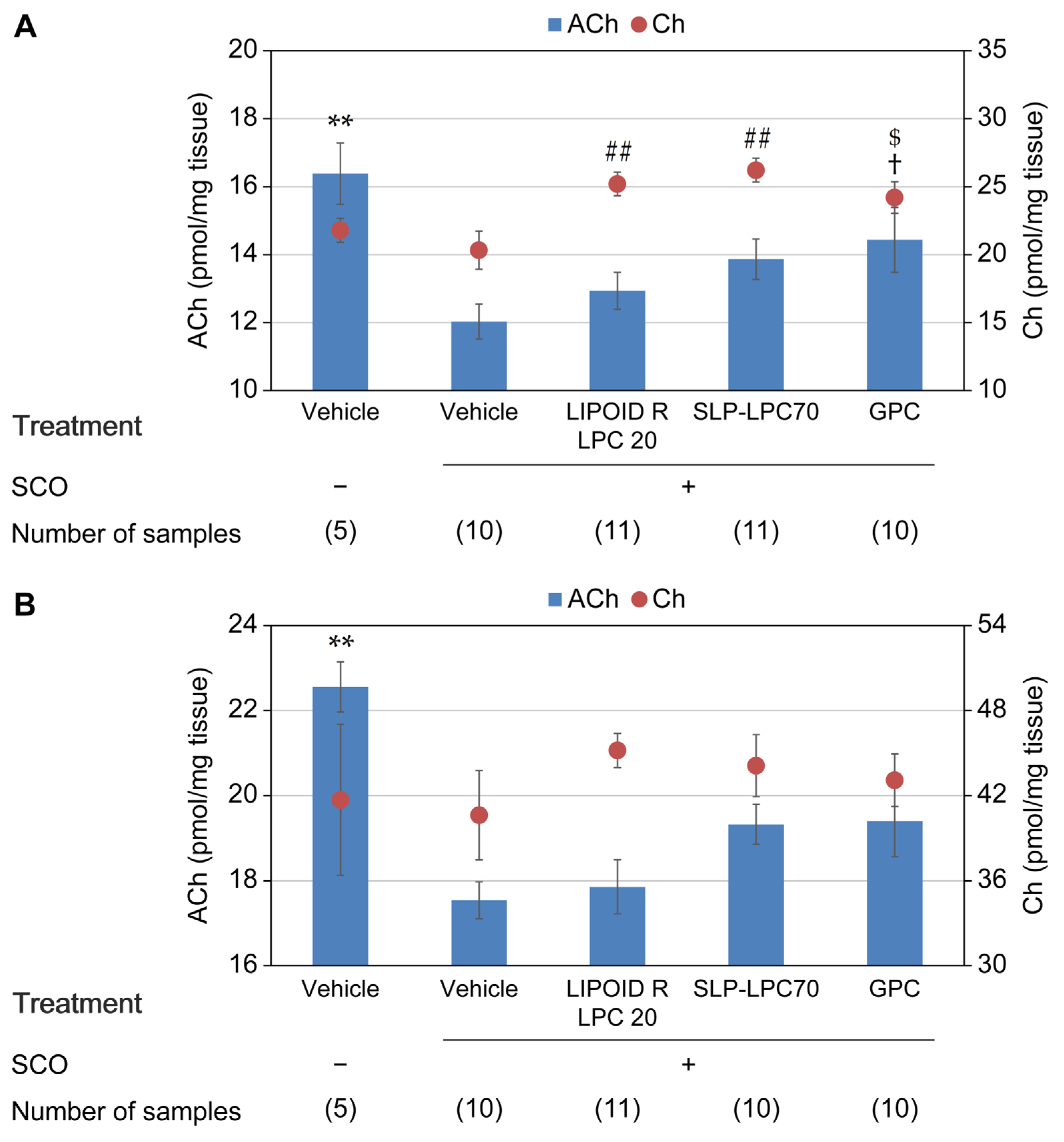
3.2. Experiment 2 (LPC-Containing Food Additives and GPC)
3.3. Experiment 3 (LPC-Containing Food Additives with Different Fat Contents)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semba, R.D. Perspective: The potential role of circulating lysophosphatidylcholine in neuroprotection against Alzheimer disease. Adv. Nutr. 2020, 11, 760–772. [Google Scholar] [CrossRef]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid. Res. 2020, 80, 101068. [Google Scholar] [CrossRef]
- Traini, E.; Bramanti, V.; Amenta, F. Choline alphoscerate (alpha-glyceryl-phosphoryl-choline) an old choline- containing phospholipid with a still interesting profile as cognition enhancing agent. Curr. Alzheimer Res. 2013, 10, 1070–1079. [Google Scholar] [CrossRef]
- Traini, E.; Carotenuto, A.; Fasanaro, A.M.; Amenta, F. Volume analysis of brain cognitive areas in Alzheimer’s disease: Interim 3-year results from the ASCOMALVA trial. J. Alzheimers Dis. 2020, 76, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Tayebati, S.K.; Amenta, F. Choline-containing phospholipids: Relevance to brain functional pathways. Clin. Chem. Lab. Med. 2013, 51, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Pillon, C.; Moliere, P.; Lagarde, M.; Lecerf, J. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Am. J. Physiol. 1994, 267, R1273–R1279. [Google Scholar] [CrossRef]
- Sugasini, D.; Yalagala, P.C.R.; Goggin, A.; Tai, L.M.; Subbaiah, P.V. Enrichment of brain docosahexaenoic acid (DHA) is highly dependent upon the molecular carrier of dietary DHA: Lysophosphatidylcholine is more efficient than either phosphatidylcholine or triacylglycerol. J. Nutr. Biochem. 2019, 74, 108231. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, M.K.; Hachem, M.; Ahmmed, F.; Rashidinejad, A.; Oz, F.; Bekhit, A.A.; Carne, A.; Bekhit, A.E.A. Marine fish-derived lysophosphatidylcholine: Properties, extraction, quantification, and brain health application. Molecules 2023, 28, 3088. [Google Scholar] [CrossRef]
- She, S.; Zhang, Q.; Shi, J.; Yang, F.; Dai, K. Roles of autotaxin/autotaxin-lysophosphatidic acid axis in the initiation and progression of liver cancer. Front. Oncol. 2022, 12, 922945. [Google Scholar] [CrossRef] [PubMed]
- Block, R.C.; Duff, R.; Lawrence, P.; Kakinami, L.; Brenna, J.T.; Shearer, G.C.; Meednu, N.; Mousa, S.; Friedman, A.; Harris, W.S.; et al. The effects of EPA, DHA, and aspirin ingestion on plasma lysophospholipids and autotaxin, Prostaglandins Leukot. Essent. Fatty Acids. 2010, 82, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Hosogaya, S.; Yatomi, Y.; Nakamura, K.; Ohkawa, R.; Okubo, S.; Yokota, H.; Ohta, M.; Yamazaki, H.; Koike, T.; Ozaki, Y. Measurement of plasma lysophosphatidic acid concentration in healthy subjects: Strong correlation with lysophospholipase D activity. Ann. Clin. Biochem. 2008, 45, 364–368. [Google Scholar] [CrossRef]
- Knuplez, E.; Marsche, G. An updated review of pro- and anti-inflammatory properties of plasma lysophosphatidylcholines in the vascular system. Int. J. Mol. Sci. 2020, 21, 4501. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, A.; Nieto, S.; Sanhueza, J.; Morgado, N.; Rojas, I.; Zañartu, P. Supplementing female rats with DHA-lysophosphatidylcholine increases docosahexaenoic acid and acetylcholine contents in the brain and improves the memory and learning capabilities of the pups. Grasas y Aceites 2010, 61, 16–23. [Google Scholar] [CrossRef]
- Law, S.H.; Chan, M.L.; Marathe, G.K.; Parveen, F.; Chen, C.H.; Ke, L.Y. An updated review of lysophosphatidylcholine metabolism in human diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef] [PubMed]
- Food US and Administration Drug. Direct Food Substances Affirmed as Generally Recognized as Safe (GRAS), Enzyme-Modified Lecithin, Code of Federal Regulations Title 21, Volume 3, 21CFR184.1063. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1063 (accessed on 27 May 2023).
- Fernandes, G.D.; Alberici, R.M.; Pereira, G.G.; Cabral, E.C.; Eberlin, M.N.; Barrera-Arellano, D. Direct characterization of commercial lecithins by easy ambient sonic-spray ionization mass spectrometry. Food Chem. 2012, 135, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.N.; Yeong, K.Y. Scopolamine, a toxin-induced experimental model, used for research in Alzheimer’s disease, CNS Neurol. Disord. Drug Targets 2020, 19, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Domino, E.F.; Mathews, B.N.; Tait, S.K.; Ortiz, A. Effects of oral phosphatidylcholine on mouse brain choline and acetylcholine, Arch. Int. Pharmacodyn. Ther. 1983, 265, 49–54. [Google Scholar]
- Lopez, C.M.; Govoni, S.; Battaini, F.; Bergamaschi, S.; Longoni, A.; Giaroni, C.; Trabucchi, M. Effect of a new cognition enhancer, alpha-glycerylphosphorylcholine, on scopolamine-induced amnesia and brain acetylcholine. Pharmacol. Biochem. Behav. 1991, 39, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Uslu, G.; Savci, V.; Buyukuysal, L.R.; Goktalay, G. CDP-choline attenuates scopolamine induced disruption of prepulse inhibition in rats: Involvement of central nicotinic mechanism. Neurosci. Lett. 2014, 569, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, Å.; Duan, R.D. Pancreatic and mucosal enzymes in choline phospholipid digestion. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G425–G445. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Kanegae, R.; Hamada, K. A novel in vitro assay model developed to measure both extracellular and intracellular acetylcholine levels for screening cholinergic agents. PLoS ONE 2021, 16, e0258420. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Spignoli, G.; Pepeu, G. Interactions between oxiracetam, aniracetam and scopolamine on behavior and brain acetylcholine. Pharmacol. Biochem. Behav. 1987, 27, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Packard, M.G.; Regenold, W.; Quirion, R.; White, N.M. Post-training injection of the acetylcholine M2 receptor antagonist AF-DX 116 improves memory. Brain Res. 1990, 524, 72–76. [Google Scholar] [CrossRef]
- Zhou, M.M.; Xue, Y.; Sun, S.H.; Wen, M.; Li, Z.J.; Xu, J.; Wang, J.F.; Yanagita, T.; Wang, Y.M.; Xue, C.H. Effects of different fatty acids composition of phosphatidylcholine on brain function of dementia mice induced by scopolamine. Lipids Health Dis. 2016, 15, 135. [Google Scholar] [CrossRef]
- Robert, C.; Couëdelo, L.; Vaysse, C.; Michalski, M.C. Vegetable lecithins: A review of their compositional diversity, impact on lipid metabolism and potential in cardiometabolic disease prevention. Biochimie 2020, 169, 121–132. [Google Scholar] [CrossRef]
- Nilsson, Å. Intestinal absorption of lecithin and lysolecithin by lymph fistula rats. Biochim. Biophys. Acta 1968, 152, 379–390. [Google Scholar] [CrossRef]
- Thiés, F.; Delachambre, M.C.; Bentejac, M.; Lagarde, M.; Lecerf, J. Unsaturated fatty acids esterified in 2-acyl-l-lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. J. Neurochem. 1992, 59, 1110–1116. [Google Scholar] [CrossRef]
- Murota, K.; Takagi, M.; Watanabe, Y.; Tokumura, A.; Ohkubo, T. Roe-derived phospholipid administration enhances lymphatic docosahexaenoic acid-containing phospholipid absorption in unanesthetized rats. Prostaglandins Leukot. Essent. Fatty Acids 2018, 139, 40–48. [Google Scholar] [CrossRef]
- Dutilh, C.E.; Groger, W. Improvement of product attributes of mayonnaise by enzymic hydrolysis of egg yolk with phospholipase A2. J. Sci. Food. Agric. 1981, 32, 451–458. [Google Scholar] [CrossRef]
- Yalagala, P.C.R.; Sugasini, D.; Dasarathi, S.; Pahan, K.; Subbaiah, P.V. Dietary lysophosphatidylcholine-EPA enriches both EPA and DHA in the brain: Potential treatment for depression. J. Lipid Res. 2019, 60, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Amenta, F.; Tayebati, S.K. Pathways of acetylcholine synthesis, transport and release as targets for treatment of adult-onset cognitive dysfunction. Curr. Med. Chem. 2008, 15, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Blusztajn, J.K.; Liscovitch, M.; Mauron, C.; Richardson, U.I.; Wurtman, R.J. Phosphatidylcholine as a precursor of choline for acetylcholine synthesis. J. Neural Transm. Suppl. 1987, 24, 247–259. [Google Scholar] [PubMed]
- Lee, H.C.; Fellenz-Maloney, M.P.; Liscovitch, M.; Blusztajn, J.K. Phospholipase D-catalyzed hydrolysis of phosphatidylcholine provides the choline precursor for acetylcholine synthesis in a human neuronal cell line. Proc. Natl. Acad. Sci. USA 1993, 90, 10086–10090. [Google Scholar] [CrossRef]
- de Wilde, M.C.; Vellas, B.; Girault, E.; Yavuz, A.C.; Sijben, J.W. Lower brain and blood nutrient status in Alzheimer’s disease: Results from meta-analyses. Alzheimer’s Dement. 2017, 3, 416–431. [Google Scholar] [CrossRef]
- Böckmann, K.A.; Franz, A.R.; Minarski, M.; Shunova, A.; Maiwald, C.A.; Schwarz, J.; Gross, M.; Poets, C.F.; Bernhard, W. Differential metabolism of choline supplements in adult volunteers. Eur. J. Nutr. 2022, 61, 219–230. [Google Scholar] [CrossRef]
- Böckmann, K.A.; Franz, A.R.; Shunova, A.; Minarski, M.; Wiechers, C.; Poets, C.F.; Bernhard, W. Different choline supplement metabolism in adults using deuterium labelling. Eur. J. Nutr. 2023, 62, 1795–1807. [Google Scholar] [CrossRef]
- Zhu, B.; Ren, H.; Xie, F.; An, Y.; Wang, Y.; Tan, Y. Trimethylamine N-oxide generated by the gut microbiota: Potential atherosclerosis treatment strategies. Curr. Pharm. Des. 2022, 28, 2914–2919. [Google Scholar] [CrossRef]
- Jope, R.S. Effects of phosphatidylcholine administration to rats on choline in blood and choline and acetylcholine in brain. J. Pharmacol. Exp. Ther. 1982, 220, 322–328. [Google Scholar]
- Hirsch, M.J.; Wurtman, R.J. Lecithin consumption increases acetylcholine concentrations in rat brain and adrenal gland. Science 1978, 202, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Abbiati, G.; Fossati, T.; Lachmann, G.; Bergamaschi, M.; Castiglioni, C. Absorption, tissue distribution and excretion of radiolabelled compounds in rats after administration of [14C]-L-alpha-glycerylphosphorylcholine. Eur. J. Drug Metab. Pharmacokinet. 1993, 18, 173–180. [Google Scholar] [CrossRef]
- McSwiney, B.A.; Spurrell, W.R. Influence of osmotic pressure upon the emptying time of the stomach. J. Physiol. 1933, 79, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Imaizumi, K.; Sugano, M. Absorption and transport of base moieties of phosphatidylcholine and phosphatidylethanolamine in rats. Biochim. Biophys. Acta 1987, 921, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Caselli, C.; Carlier, H. Linoleic acid chyloportal partition and metabolism during its intestinal absorption. Ann. Nutr. Metab. 1991, 35, 98–110. [Google Scholar] [CrossRef]


| LPC Species (%) | |||||
|---|---|---|---|---|---|
| Palmitoyl | Stearoyl | Oleoyl | Linoleoyl | Linolenoyl | |
| LPC reagent (egg-derived) | 71.9 | 21.5 | 6.6 | ND | ND |
| LIPOID R LPC 20 (rapeseed-derived) | 16.6 | ND | 53.5 | 24.9 | 5.0 |
| SLP-LPC70 (soy-derived) | 25.3 | 5.2 | 9.6 | 53.1 | 6.8 |
| SLP-PasteLyso (soy-derived) | 29.0 | 5.9 | 9.8 | 46.6 | 8.8 |
| LPC Species | Treatment | |||||
|---|---|---|---|---|---|---|
|
LPC− SCO− |
LPC500 SCO− |
LPC− SCO+ |
LPC167 SCO+ |
LPC500 SCO+ |
LPC1500 SCO+ | |
| Palmitoyl (µmol/L) | 123.3 ± 5.5 | 113.0 ± 3.9 | 120.8 ± 4.0 | 119.7 ± 4.5 | 118.0 ± 5.7 | 116.0 ± 3.4 |
| Stearoyl (µmol/L) | 70.1 ± 4.9 | 62.5 ± 3.6 | 64.7 ± 2.3 | 66.7 ± 3.1 | 64.9 ± 3.5 | 62.3 ± 2.1 |
| Oleoyl (µmol/L) | 21.8 ± 0.9 | 18.3 ± 0.8 | 19.2 ± 0.9 | 19.0 ± 1.1 | 19.0 ± 1.0 | 17.6 ± 0.8 |
| Linoleoyl (µmol/L) | 99.7 ± 4.3 | 89.0 ± 4.6 | 93.6 ± 4.5 | 92.6 ± 4.2 | 86.9 ± 3.6 | 83.4 ± 4.3 |
| Total (µmol/L) | 314.8 ± 14.6 | 282.8 ± 12.5 | 298.4 ± 10.5 | 298.0 ± 12.1 | 288.8 ± 13.3 | 279.2 ± 9.6 |
| Treatment | ||||
|---|---|---|---|---|
| SLP-LPC70 SCO+ | SLP-PasteLyso SCO+ | |||
| Frontal cortex | ACh (pmol/mg tissue) | 13.6 ± 0.4 | 14.1 ± 0.5 | |
| Ch (pmol/mg tissue) | 24.1 ± 1.4 | 25.9 ± 1.2 | ||
| Hippocampus | ACh (pmol/mg tissue) | 17.6 ± 0.3 | 18.4 ± 0.5 | |
| Ch (pmol/mg tissue) | 47.2 ± 2.7 | 48.2 ± 0.9 | ||
| Striatum | ACh (pmol/mg tissue) | 51.0 ± 1.1 | 55.3 ± 3.3 | |
| Ch (pmol/mg tissue) | 29.2 ± 1.3 | 29.6 ± 1.3 | ||
| Plasma | Ch (µmol/L) | 24.9 ± 0.6 | 22.1 ± 0.8 | * |
| Palmitoyl-LPC (µmol/L) | 120.5 ± 2.7 | 104.8 ± 3.8 | ** | |
| Stearoyl-LPC (µmol/L) | 65.5 ± 2.5 | 60.3 ± 2.3 | ||
| Oleoyl-LPC (µmol/L) | 18.7 ± 1.1 | 17.4 ± 1.0 | ||
| Linoleoyl-LPC (µmol/L) | 94.6 ± 3.8 | 110.3 ± 5.1 | * | |
| Total LPC (µmol/L) | 299.3 ± 9.0 | 292.9 ± 11.2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka-Kanegae, R.; Kimura, H.; Hamada, K. Oral Administration of Egg- and Soy-Derived Lysophosphatidylcholine Mitigated Acetylcholine Depletion in the Brain of Scopolamine-Treated Rats. Nutrients 2023, 15, 3618. https://doi.org/10.3390/nu15163618
Tanaka-Kanegae R, Kimura H, Hamada K. Oral Administration of Egg- and Soy-Derived Lysophosphatidylcholine Mitigated Acetylcholine Depletion in the Brain of Scopolamine-Treated Rats. Nutrients. 2023; 15(16):3618. https://doi.org/10.3390/nu15163618
Chicago/Turabian StyleTanaka-Kanegae, Ryohei, Hiroyuki Kimura, and Koichiro Hamada. 2023. "Oral Administration of Egg- and Soy-Derived Lysophosphatidylcholine Mitigated Acetylcholine Depletion in the Brain of Scopolamine-Treated Rats" Nutrients 15, no. 16: 3618. https://doi.org/10.3390/nu15163618
APA StyleTanaka-Kanegae, R., Kimura, H., & Hamada, K. (2023). Oral Administration of Egg- and Soy-Derived Lysophosphatidylcholine Mitigated Acetylcholine Depletion in the Brain of Scopolamine-Treated Rats. Nutrients, 15(16), 3618. https://doi.org/10.3390/nu15163618






