The Interaction of Dietary Pectin, Inulin, and Psyllium with Copper Nanoparticle Induced Changes to the Cardiovascular System
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Experimental Protocol and Procedures
- (A)
- Control, which was further subdivided into:
- Control C (8% cellulose and 6.5 mg/kg of ionic Cu)
- Control CH (8% cellulose and 13 mg/kg of ionic Cu)
- (B)
- Supplemented, which was further subdivided into:
- CN (8% cellulose and 6.5 mg/kg of CuNPs)
- CNH (8% cellulose and 13 mg/kg of CuNPs)
- PN (6% pectin and 6.5 mg/kg of CuNPs)
- PNH (6% pectin and 13 mg/kg of CuNPs)
- JN (6% inulin and 6.5 mg/kg of CuNPs)
- JNH (6% inulin and 13 mg/kg of CuNPs)
- SN (6% psyllium and 6.5 mg/kg of CuNPs)
- SNH (6% psyllium and 13 mg/kg of CuNPs)
2.3. Blood Plasma Analysis
2.4. Markers of Antioxidant Status in Aortic Rings
2.5. Markers of Antioxidant Status in the Heart
2.6. Vascular Reactivity Studies
2.7. Retrograde Heart Perfusion: The Langendorff Heart
2.8. Data Analysis and Statistics
3. Results
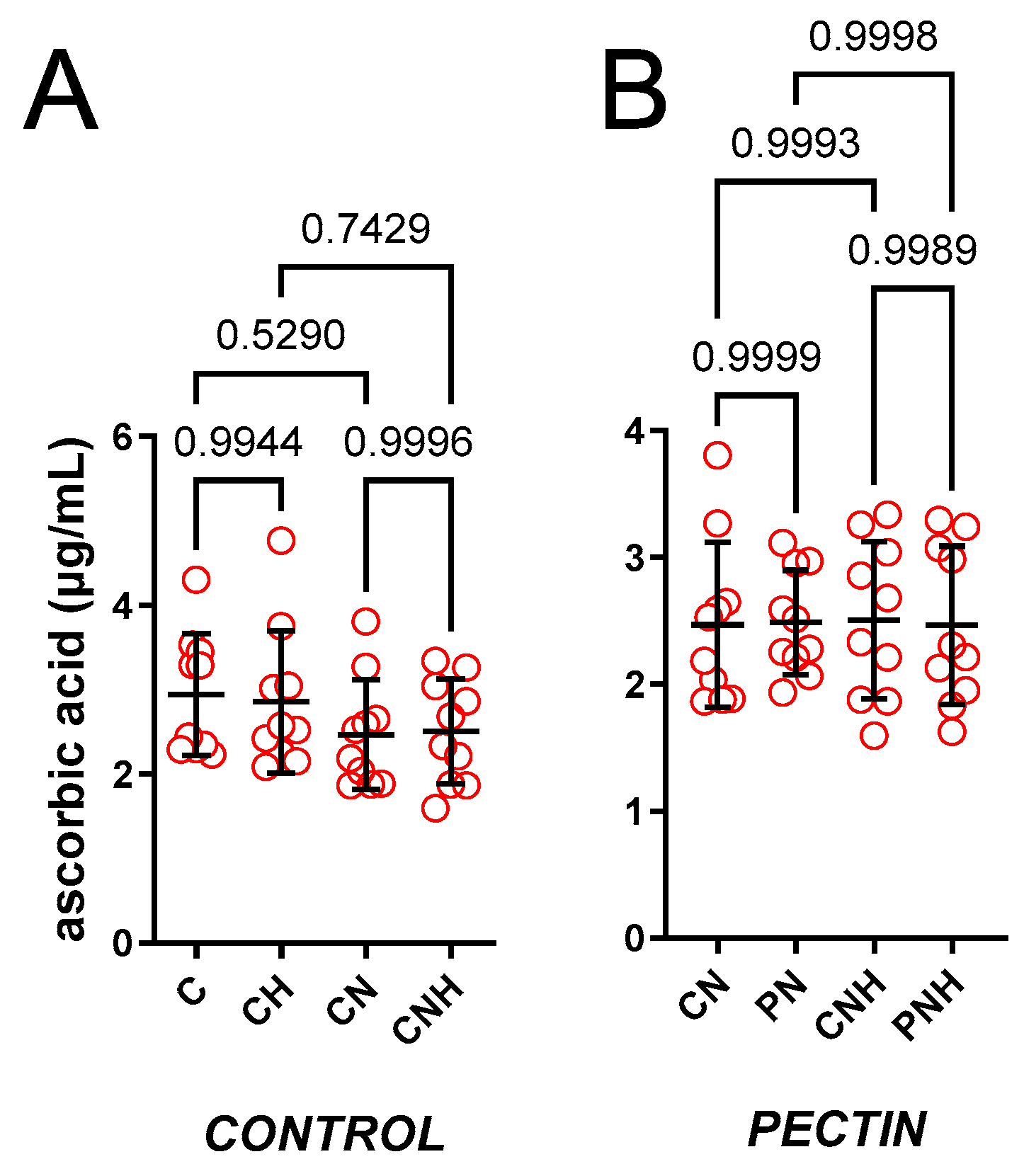
3.1. The Antioxidant Capacity of Blood Plasma
3.1.1. Blood Plasma ACW
3.1.2. Blood Plasma ACL
3.2. Heart Examination
3.2.1. Heart Malondialdehyde
3.2.2. Heart SOD
3.2.3. Heart CAT
3.2.4. Retrograde Heart Perfusion: The Langendorff Heart
3.3. Vascular Studies
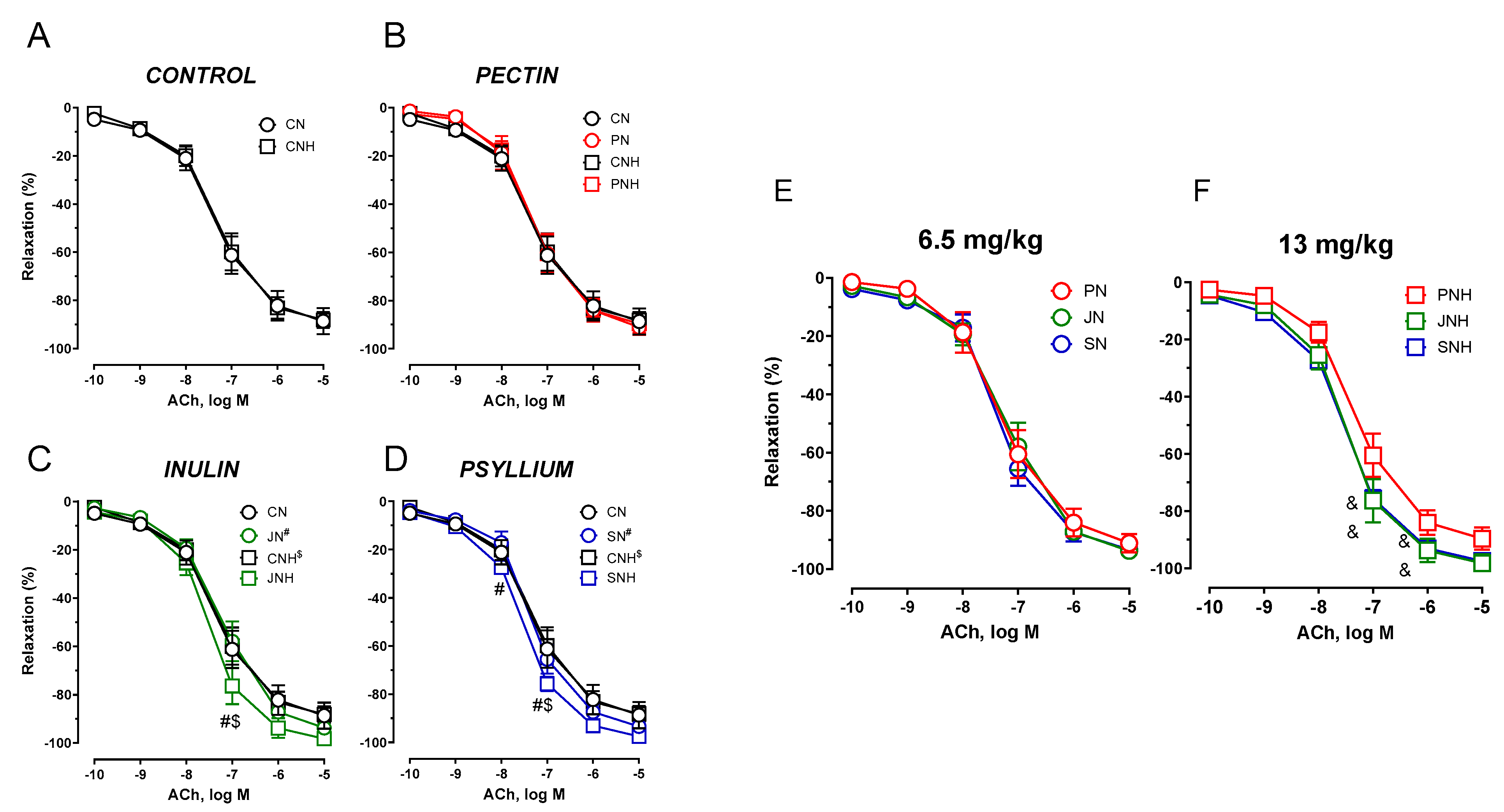
3.3.1. Vascular Reactivity
3.3.2. ELISA Studies
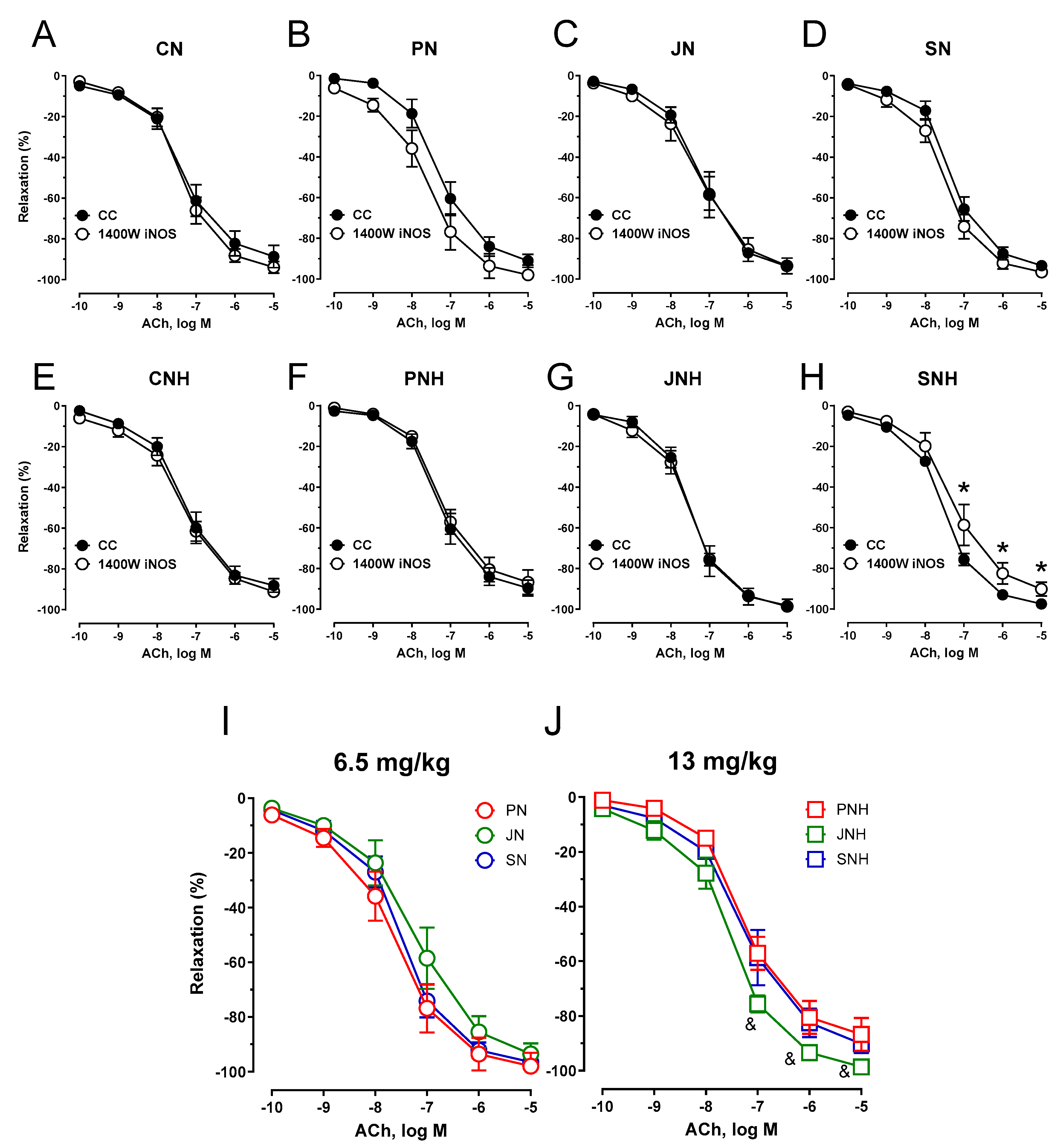

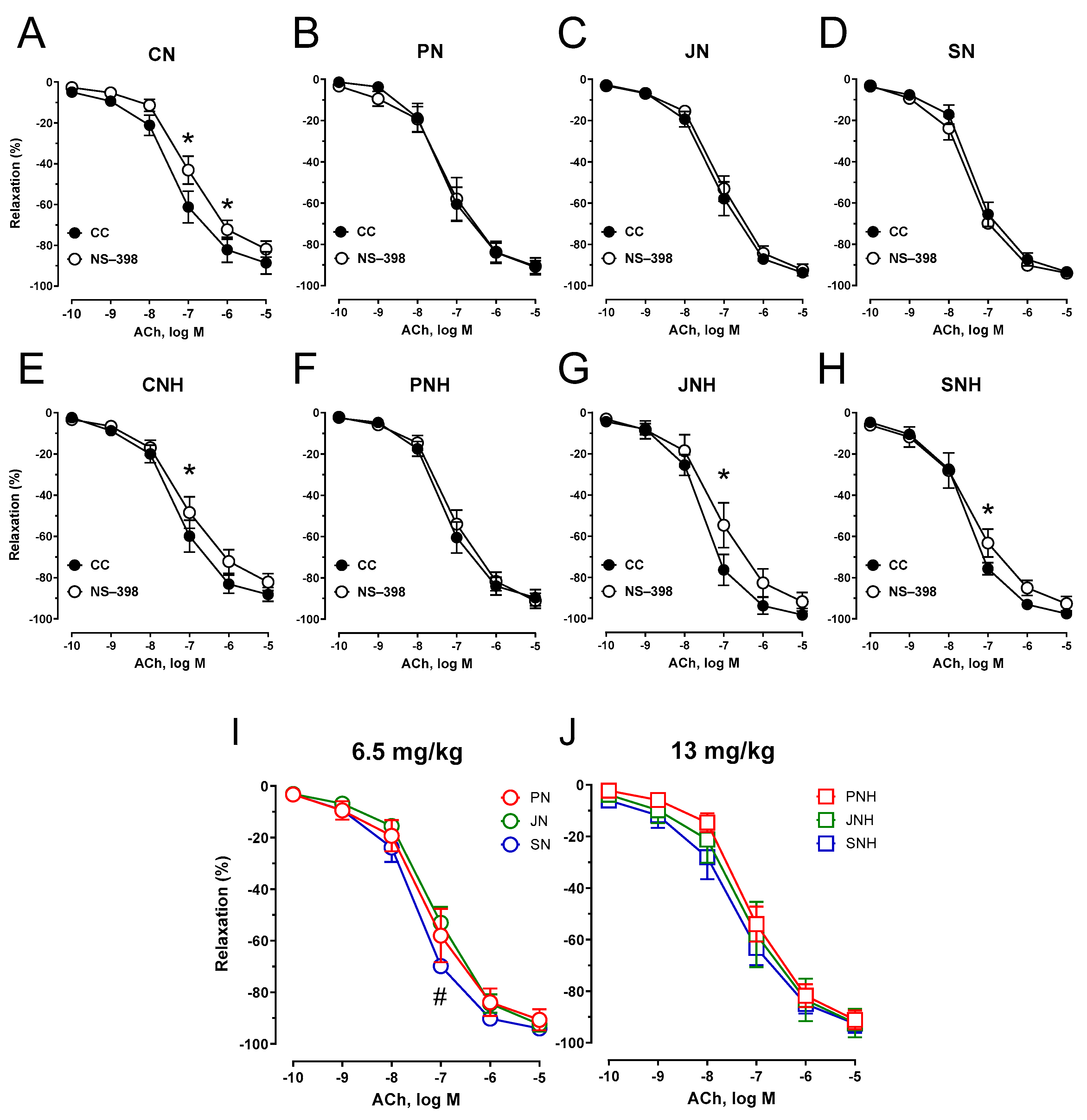

4. Discussion
4.1. High CuNP Effects
4.2. The Effect of Decreased Cellulose Content and High CuNP Dose
4.2.1. Sticky Pectin vs. Insoluble Cellulose
4.2.2. Prebiotic Inulin vs. Insoluble Cellulose
4.2.3. Swelling Psyllium vs. Insoluble Cellulose
4.3. Pectin vs. Inulin vs. Psyllium
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Bügel, S.; Harper, A.; Rock, E.; O’Connor, J.M.; Bonham, M.P.; Strain, J.J. Effect of copper supplementation on indices of copper status and certain cardiovascular disease risk markers in young healthy women. Br. J. Nutr. 2005, 94, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Shibazaki, S.; Uchiyama, S.; Tsuda, K.; Taniuchi, N. Copper deficiency caused by excessive alcohol consumption. BMJ Case Rep. 2017, 2017, bcr2017220921. [Google Scholar] [CrossRef]
- Ognik, K.; Cholewińska, E.; Tutaj, K.; Cendrowska-Pinkosz, M.; Dworzański, W.; Dworzańska, A.; Juśkiewicz, J. The effect of the source and dosage of dietary Cu on redox status in rat tissues. J. Anim. Physiol. Anim. Nutr. 2020, 104, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Gralak, M.A.; Leontowicz, M.; Morawiec, M.; Bartnikowska, E.; Kulasek, G.W. Comparison of the influence of dietary fibre sources with different proportions of soluble and insoluble fibre on Ca, Mg, Fe, Zn, Mn and Cu apparent absorption in rats. Arch. Tierernahr. 1996, 49, 293–299. [Google Scholar] [CrossRef]
- El-Zoghbi, M.; Sitohy, M.Z. Mineral absorption by albino rats as affected by some types of dietary pectins with different degrees of esterification. Food/Nahrung 2001, 45, 114–117. [Google Scholar] [CrossRef]
- Turgeon, S.L.; Brisson, G. Symposium review: The dairy matrix-Bioaccessibility and bioavailability of nutrients and physiological effects. J. Dairy Sci. 2020, 103, 6727–6736. [Google Scholar] [CrossRef]
- Lutsenko, S.; Gupta, A.; Burkhead, J.L.; Zuzel, V. Cellular multitasking: The dual role of human Cu-ATPases in cofactor delivery and intracellular copper balance. Arch. Biochem. Biophys. 2008, 476, 22–32. [Google Scholar] [CrossRef]
- Aggett, P.J.; Fairweather-Tait, S. Adaptation to high and low copper intakes: Its relevance to estimated safe and adequate daily dietary intakes. Am. J. Clin. Nutr. 1998, 67, 1061S–1063S. [Google Scholar] [CrossRef]
- Drews, L.M.; Kies, C.; Fox, H.M. Effect of dietary fiber on copper, zinc, and magnesium utilization by adolescent boys. Am. J. Clin. Nutr. 1979, 32, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Juśkiewicz, J.; Krajewska-Włodarczyk, M.; Gromadziński, L.; Socha, K.; Cholewińska, E.; Ognik, K. The Role of 20-HETE, COX, Thromboxane Receptors, and Blood Plasma Antioxidant Status in Vascular Relaxation of Copper-Nanoparticle-Fed WKY Rats. Nutrients 2021, 13, 3793. [Google Scholar] [CrossRef] [PubMed]
- Cholewińska, E.; Marzec, A.; Sołek, P.; Fotschki, B.; Listos, P.; Ognik, K.; Juśkiewicz, J. The Effect of Copper Nanoparticles and a Different Source of Dietary Fibre in the Diet on the Integrity of the Small Intestine in the Rat. Nutrients 2023, 15, 1588. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Gromadziński, L.; Cholewińska, E.; Ognik, K.; Fotschki, B.; Juśkiewicz, J. Dietary Effects of Chromium Picolinate and Chromium Nanoparticles in Wistar Rats Fed with a High-Fat, Low-Fiber Diet: The Role of Fat Normalization. Nutrients 2022, 14, 5138. [Google Scholar] [CrossRef]
- Kitala, K.; Tanski, D.; Godlewski, J.; Krajewska-Włodarczyk, M.; Gromadziński, L.; Majewski, M. Copper and Zinc Particles as Regulators of Cardiovascular System Function—A Review. Nutrients 2023, 15, 3040. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B.; Szablińska-Piernik, J.; Lahuta, L.B.; Rynkiewicz, A.; Cygański, P.; Socha, K.; Gromadziński, L.; Thoene, M.; Majewski, M. Beneficial In Vitro Effects of a Low Myo-Inositol Dose in the Regulation of Vascular Resistance and Protein Peroxidation under Inflammatory Conditions. Nutrients 2022, 14, 1118. [Google Scholar] [CrossRef]
- Majewski, M.; Lis, B.; Juśkiewicz, J.; Ognik, K.; Jedrejek, D.; Stochmal, A.; Olas, B. The composition and vascular/antioxidant properties of Taraxacum officinale flower water syrup in a normal-fat diet using an obese rat model. J. Ethnopharmacol. 2021, 265, 113393. [Google Scholar] [CrossRef]
- Majewski, M.; Klett-Mingo, M.; Verdasco-Martín, C.M.; Otero, C.; Ferrer, M. Spirulina extract improves age-induced vascular dysfunction. Pharm. Biol. 2022, 60, 627–637. [Google Scholar] [CrossRef]
- Cholewińska, E.; Juśkiewicz, J.; Majewski, M.; Smagieł, R.; Listos, P.; Fotschki, B.; Godycka-Kłos, I.; Ognik, K. Effect of Copper Nanoparticles in the Diet of WKY and SHR Rats on the Redox Profile and Histology of the Heart, Liver, Kidney, and Small Intestine. Antioxidants 2022, 11, 910. [Google Scholar] [CrossRef]
- Majewski, M.; Lis, B.; Olas, B.; Ognik, K.; Juśkiewicz, J. Dietary supplementation with copper nanoparticles influences the markers of oxidative stress and modulates vasodilation of thoracic arteries in young Wistar rats. PLoS ONE 2020, 15, e0229282. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Ognik, K.; Juśkiewicz, J. Copper nanoparticles modify the blood plasma antioxidant status and modulate the vascular mechanisms with nitric oxide and prostanoids involved in Wistar rats. Pharmacol. Rep. 2019, 71, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Ognik, K.; Zdunczyk, P.; Juskiewicz, J. Effect of dietary copper nanoparticles versus one copper (II) salt: Analysis of vasoreactivity in a rat model. Pharmacol. Rep. 2017, 69, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Ognik, K.; Juśkiewicz, J. Copper nanoparticles enhance vascular contraction induced by prostaglandin F2-alpha and decrease the blood plasma cu-zn ratio in wistar rats. J. Elem. 2019, 24, 911–922. [Google Scholar] [CrossRef]
- Majewski, M.; Ognik, K.; Juśkiewicz, J. The antioxidant status, lipid profile, and modulation of vascular function by fish oil supplementation in nano-copper and copper carbonate fed Wistar rats. J. Funct. Foods 2020, 64, 103595. [Google Scholar] [CrossRef]
- Liu, W.; Mi, S.; Ruan, Z.; Li, J.; Shu, X.; Yao, K.; Jiang, M.; Deng, Z. Dietary Tryptophan Enhanced the Expression of Tight Junction Protein ZO-1 in Intestine. J. Food Sci. 2017, 82, 562–567. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Zhang, J.; Song, H.L.; Zheng, W.P. Bone-marrow mesenchymal stem cells reduce rat intestinal ischemia-reperfusion injury, ZO-1 downregulation and tight junction disruption via a TNF-α-regulated mechanism. World J. Gastroenterol. 2013, 19, 3583–3595. [Google Scholar] [CrossRef]
- Majewski, M.; Jurgoński, A. The Effect of Hemp (Cannabis sativa L.) Seeds and Hemp Seed Oil on Vascular Dysfunction in Obese Male Zucker Rats. Nutrients 2021, 13, 2575. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewski, M.; Gromadziński, L.; Cholewińska, E.; Ognik, K.; Fotschki, B.; Juśkiewicz, J. The Interaction of Dietary Pectin, Inulin, and Psyllium with Copper Nanoparticle Induced Changes to the Cardiovascular System. Nutrients 2023, 15, 3557. https://doi.org/10.3390/nu15163557
Majewski M, Gromadziński L, Cholewińska E, Ognik K, Fotschki B, Juśkiewicz J. The Interaction of Dietary Pectin, Inulin, and Psyllium with Copper Nanoparticle Induced Changes to the Cardiovascular System. Nutrients. 2023; 15(16):3557. https://doi.org/10.3390/nu15163557
Chicago/Turabian StyleMajewski, Michał, Leszek Gromadziński, Ewelina Cholewińska, Katarzyna Ognik, Bartosz Fotschki, and Jerzy Juśkiewicz. 2023. "The Interaction of Dietary Pectin, Inulin, and Psyllium with Copper Nanoparticle Induced Changes to the Cardiovascular System" Nutrients 15, no. 16: 3557. https://doi.org/10.3390/nu15163557
APA StyleMajewski, M., Gromadziński, L., Cholewińska, E., Ognik, K., Fotschki, B., & Juśkiewicz, J. (2023). The Interaction of Dietary Pectin, Inulin, and Psyllium with Copper Nanoparticle Induced Changes to the Cardiovascular System. Nutrients, 15(16), 3557. https://doi.org/10.3390/nu15163557





