Poultry Consumption and Human Cardiometabolic Health-Related Outcomes: A Narrative Review
Abstract
1. Introduction
2. Nutritional Content of Chicken/Poultry
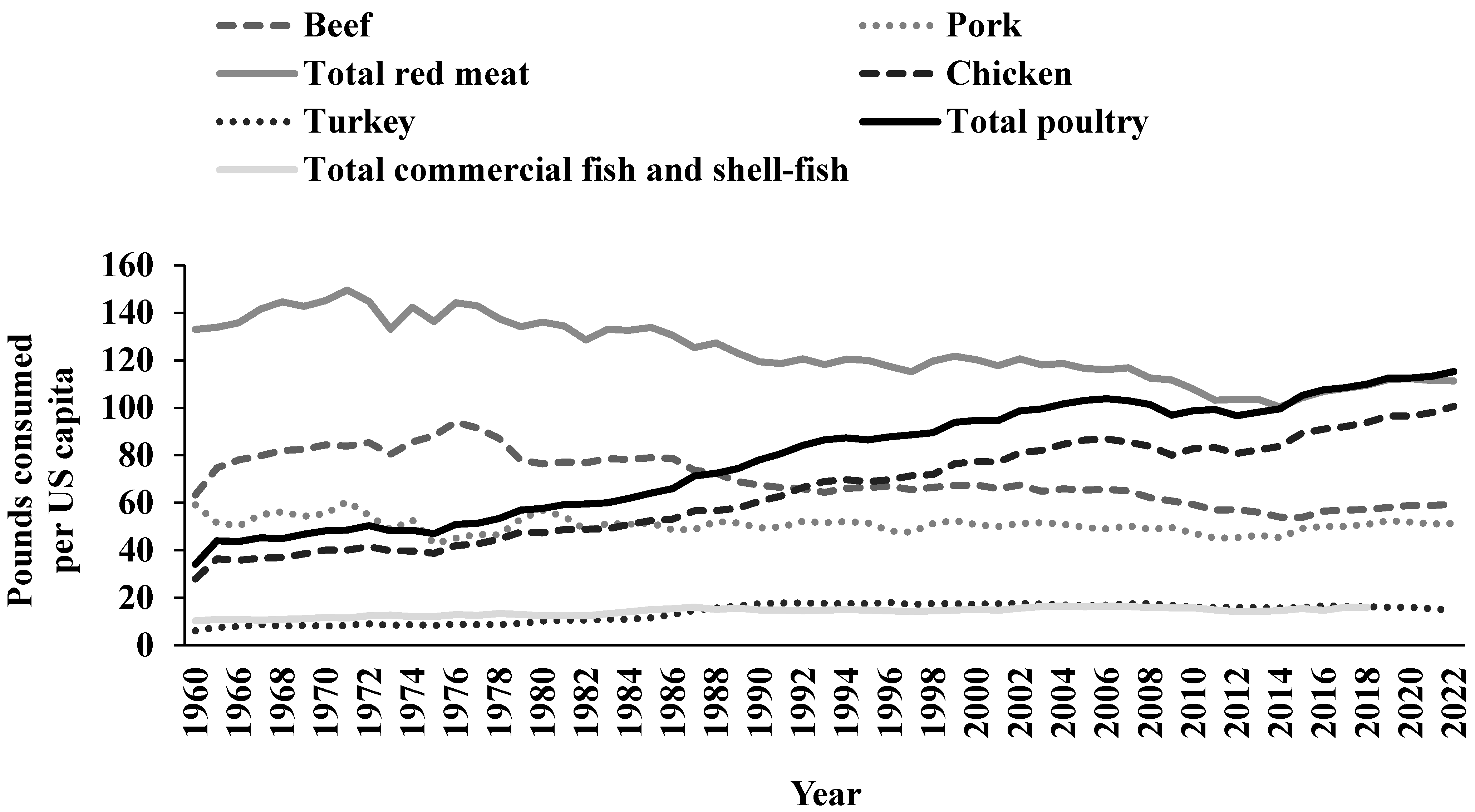
3. Chicken and Poultry Consumption in the US
4. Dietary Guidance in the United States
5. Experimental Approaches and Considerations When Studying the Influence of Poultry Intake on Human Health
5.1. Experimental Approaches
5.2. Potential Explanatory Variables
5.2.1. Definitions
5.2.2. Processing
5.2.3. Cooking Methods
6. Poultry Consumption and Body Weight and Body Composition
6.1. RCTs
6.2. Observational Studies
6.2.1. Observational Studies in Adults
6.2.2. Observational Studies in Children and Adolescents
7. Poultry Consumption and CVD
7.1. RCTs
7.2. Observational Studies
7.2.1. CVD
7.2.2. Stroke
7.2.3. Hypertension
8. Poultry Consumption and T2DM
8.1. RCTs
8.1.1. Acute Feeding RCTs
8.1.2. Chronic Feeding RCTs
8.2. Observational Studies
9. Conclusions
Future Research Recommendations
- (1)
- There is a need for future experimental and observational research to include definitions and detailed descriptions of the different types and amounts of poultry products consumed.Rationale: The research that currently exists on poultry is complicated by the lack of a clear definition of poultry and limited descriptions of the types and forms of poultry used among studies. Importantly, “muscle food categories and descriptions are substantively different within and between experimental and observational studies and do not match regulatory definitions” [20]. New research with greater consideration and more detailed descriptions of multiple factors, including the amounts, types, and forms of chicken/poultry consumed; the health, medical, and dietary characteristics of the research cohorts; and other confounding factors will help improve our understanding of the influence of chicken/poultry on body weight and body composition, CVD, and T2DM.
- (2)
- Chronic feeding RCTs are warranted assessing the effects of consuming unprocessed and processed poultry products in the context of healthy and unhealthy dietary patterns on body weight and body composition, CVD, and T2DM health-related outcomes.Rationale: Of 59 RCTs identified in our scoping review [4], only seven included assessments of body weight and body composition, 17 included CVD risk factors, and 11 included T2DM risk factors. Accounting for overlap among studies, there were 26 unique RCTs. Zero RCTs specifically assessed processed chicken/poultry, 23 RCTs assessed unprocessed chicken/poultry, and 3 RCTs were indeterminate. Most chronic feeding RCTs did not document (e.g., using the Healthy Eating Index) the healthfulness of the dietary pattern.
- (3)
- Observational studies are warranted examining associations between processed poultry consumption or poultry cooking methods and body weight and body composition, CVD, or T2DM health-related outcomes in humans across the life course.Rationale: Our scoping review identified only four of 366 (1%) observational studies assessed the influence of processed poultry on human health outcomes [4]. Of these four observational studies identified and included in this review, three were on body weight or BMI and one was on T2DM risk. Zero observational studies were identified that included investigating associations between processed poultry consumption and CVD. In addition, only 14% of 366 observational studies reported the cooking method used [4].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Chicken Council: Per Capita Consumption of Poultry and Livestock, 1965 to Estimated 2021, in Pounds. Available online: https://www.Nationalchickencouncil.Org/Statistic/per-Capita-Consumption-Poultry/ (accessed on 23 January 2023).
- Papp, R.E.; Hasenegger, V.; Ekmekcioglu, C.; Schwingshackl, L. Association of Poultry Consumption with Cardiovascular Diseases and All-Cause Mortality: A Systematic Review and Dose Response Meta-Analysis of Prospective Cohort Studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 2366–2387. [Google Scholar] [CrossRef] [PubMed]
- OECD; FAO. OECD-FAO Agricultural Outlook 2023–2032; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Connolly, G.; Clark, C.M.; Campbell, R.E.; Byers, A.W.; Reed, J.B.; Campbell, W.W. Poultry Consumption and Human Health: How Much Is Really Known? A Systematically Searched Scoping Review and Research Perspective. Adv. Nutr. 2022, 13, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Corsello, G.; Cricelli, C.; Ferrara, N.; Ghiselli, A.; Lucchin, L.; Poli, A. Role of Poultry Meat in a Balanced Diet Aimed at Maintaining Health and Wellbeing: An Italian Consensus Document. Food Nutr. Res. 2015, 59, 27606. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture and U.S. Department of Health and Human Services: Washington, DC, USA, 2020. Available online: https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf (accessed on 23 January 2023).
- US Department of Agriculture (USDA) FoodData Central. Available online: https://fdc.nal.usda.gov/Index.Html (accessed on 25 January 2023).
- World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; AICR: Washington, DC, USA, 2007. [Google Scholar]
- Pearson, A.M.; Gillett, T.A. Processed Meats; Springer: New York, NY, USA, 1996. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Agarwal, S.; Lieberman, H.R.; Fulgoni, V.L. Sources and Amounts of Animal, Dairy, and Plant Protein Intake of US Adults in 2007–2010. Nutrients 2015, 7, 7058–7069. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Rehm, C.D.; Rogers, G.; Ruan, M.; Wang, D.D.; Hu, F.B.; Mozaffarian, D.; Zhang, F.F.; Bhupathiraju, S.N. Trends in Dietary Carbohydrate, Protein, and Fat Intake and Diet Quality among US Adults, 1999–2016. JAMA 2019, 322, 1178–1187. [Google Scholar] [CrossRef]
- Hoy, K.; Clemens, J.; Moshfegh, A. Estimated Protein Intake from Animal and Plant Foods by U.S. Adults, What We Eat in America, NHANES, 2015–2016. Curr. Dev. Nutr. 2021, 5, 133. [Google Scholar] [CrossRef]
- Phillips, S.M.; Fulgoni, V.L.; Heaney, R.P.; Nicklas, T.A.; Slavin, J.L.; Weaver, C.M. Commonly Consumed Protein Foods Contribute to Nutrient Intake, Diet Quality, and Nutrient Adequacy. Am. J. Clin. Nutr. 2015, 101, 1346S–1352S. [Google Scholar] [CrossRef]
- Bowman, S.A.; Clemens, J.C.; Friday, J.E.; Moshfegh, A.J. Food Patterns Equivalents Database 2017–2018: Methodology and User Guide; Food Surveys Research Group: Beltsville, MD, USA, 2020. Available online: http://www.ars.usda.gov/nea/bhnrc/fsrg (accessed on 23 January 2023).
- O’Connor, L.E.; Campbell, W.W. Nutritional Composition and the Value of Pig Meat. In Achieving Sustainable Production of Pig Meat Volume 1: Safety, Quality and Sustainability, 1st ed.; Matthew, A., Ed.; Burleigh Dodds Science Publishing: London, UK, 2017; Chapter 8; pp. 175–192. ISBN 9781351114493. [Google Scholar] [CrossRef]
- Hébert, J.R.; Frongillo, E.A.; Adams, S.A.; Turner-McGrievy, G.M.; Hurley, T.G.; Miller, D.R.; Ockene, I.S. Perspective: Randomized Controlled Trials Are not a Panacea for Diet-Related Research. Adv. Nutr. 2016, 7, 423–432. [Google Scholar] [CrossRef]
- Booth, C.M.; Tannock, I.F. Randomised Controlled Trials and Population-Based Observational Research: Partners in the Evolution of Medical Evidence. Br. J. Cancer 2014, 110, 551–555. [Google Scholar] [CrossRef]
- Faraoni, D.; Schaefer, S.T. Randomized Controlled Trials vs. Observational Studies: Why Not Just Live Together? BMC Anesthesiol. 2016, 16, 102. [Google Scholar] [CrossRef]
- Barton, S. Which Clinical Studies Provide the Best Evidence? BMJ 2000, 321, 255–256. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Gifford, C.L.; Woerner, D.R.; Sharp, J.L.; Belk, K.E.; Campbell, W.W. Dietary Meat Categories and Descriptions in Chronic Disease Research Are Substantively Different within and between Experimental and Observational Studies: A Systematic Review and Landscape Analysis. Adv. Nutr. 2020, 11, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Michas, G.; Mozaffarian, D. Unprocessed Red and Processed Meats and Risk of Coronary Artery Disease and Type 2 Diabetes—An Updated Review of the Evidence. Curr. Atheroscler. Rep. 2012, 14, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-S.; Oh, K.; Kim, H.C. Dietary Assessment Methods in Epidemiologic Studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef]
- Gifford, C.L.; O’Connor, L.E.; Campbell, W.W.; Woerner, D.R.; Belk, K.E. Broad and Inconsistent Muscle Food Classification Is Problematic for Dietary Guidance in the U.S. Nutrients 2017, 9, 1027. [Google Scholar] [CrossRef]
- Dalle Zotte, A. Perception of Rabbit Meat Quality and Major Factors Influencing the Rabbit Carcass and Meat Quality. Livest. Prod. Sci. 2002, 75, 11–32. [Google Scholar] [CrossRef]
- Cavani, C.; Petracci, M.; Trocino, A.; Xiccato, G. Advances in Research on Poultry and Rabbit Meat Quality. Ital. J. Anim. Sci. 2009, 8, 741–750. [Google Scholar] [CrossRef]
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djoussé, L.; Engler, M.B.; Kris-Etherton, P.M.; Mozaffarian, D.; Siscovick, D.S.; Lichtenstein, A.H. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory from the American Heart Association. Circulation 2018, 138, e35–e47. [Google Scholar] [CrossRef]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and Processed Meat Consumption and Risk of Incident Coronary Heart Disease, Stroke, and Diabetes Mellitus: A Systematic Review and Meta-Analysis. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef]
- Förstermann, U. Oxidative Stress in Vascular Disease: Causes, Defense Mechanisms and Potential Therapies. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 338–349. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.; Clifton, P. A Review of Potential Metabolic Etiologies of the Observed Association between Red Meat Consumption and Development of Type 2 Diabetes Mellitus. Metabolism 2015, 64, 768–779. [Google Scholar] [CrossRef]
- Abete, I.; Romaguera, D.; Vieira, A.R.; Lopez De Munain, A.; Norat, T. Association between Total, Processed, Red and White Meat Consumption and All-Cause, CVD and IHD Mortality: A Meta-Analysis of Cohort Studies. Br. J. Nutr. 2014, 112, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and Management of Type 2 Diabetes: Dietary Components and Nutritional Strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Etemadi, A.; Sinha, R.; Ward, M.H.; Graubard, B.I.; Inoue-Choi, M.; Dawsey, S.M.; Abnet, C.C. Mortality from Different Causes Associated with Meat, Heme Iron, Nitrates, and Nitrites in the NIH-AARP Diet and Health Study: Population Based Cohort Study. BMJ 2017, 357, j1957. [Google Scholar] [CrossRef] [PubMed]
- White, D.L.; Collinson, A. Red Meat, Dietary Heme Iron, and Risk of Type 2 Diabetes: The Involvement of Advanced Lipoxidation Endproducts. Adv. Nutr. 2013, 4, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, P.; Cerami, A. Protein Glycation, Diabetes, and Aging. Recent. Prog. Horm. Res. 2001, 56, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zong, G.; Wu, K.; Hu, Y.; Li, Y.; Willett, W.C.; Eisenberg, D.M.; Hu, F.B.; Sun, Q. Meat Cooking Methods and Risk of Type 2 Diabetes: Results from Three Prospective Cohort Studies. Diabetes Care 2018, 41, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, B.; Snetselaar, L.G.; Robinson, J.G.; Wallace, R.B.; Peterson, L.L.; Bao, W. Association of Fried Food Consumption with All Cause, Cardiovascular, and Cancer Mortality: Prospective Cohort Study. BMJ 2019, 364, k5420. [Google Scholar] [CrossRef]
- Krishnan, S.; Coogan, P.F.; Boggs, D.A.; Rosenberg, L.; Palmer, J.R. Consumption of Restaurant Foods and Incidence of Type 2 Diabetes in African American Women. Am. J. Clin. Nutr. 2010, 91, 465–471. [Google Scholar] [CrossRef]
- Stierman, B.; Afful, J.; Carroll, M.D.; Chen, T.C.; Davy, O.; Fink, S.; Fryar, C.D.; Gu, Q.; Hales, C.M.; Hughes, J.P.; et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files-Development of Files and Prevalence Estimates for Selected Health Outcomes. Natl. Health Stat. Rep. 2021, 158, 1–21. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, Childhood Obesity Facts. Available online: https://www.cdc.gov/obesity/data/childhood.html (accessed on 28 January 2023).
- Finkelstein, E.A.; Trogdon, J.G.; Cohen, J.W.; Dietz, W. Annual Medical Spending Attributable to Obesity: Payer-and Service-Specific Estimates. Health Aff. 2009, 28, w822–w831. [Google Scholar] [CrossRef]
- Finkelstein, E.A.; DiBonaventura, M.D.C.; Burgess, S.M.; Hale, B.C. The Costs of Obesity in the Workplace. J. Occup. Environ. Med. 2010, 52, 971–976. [Google Scholar] [CrossRef]
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; McFarlane, S.I.; Jean-Louis, G.; Myers, A.K. Energy Imbalance: Obesity, Associated Comorbidities, Prevention, Management and Public Health Implications. Adv. Obes. Weight. Manag. Control 2020, 10, 141–161. [Google Scholar] [CrossRef]
- Niemiro, G.M.; Rewane, A.; Algotar, A.M. Exercise and Fitness Effect on Obesity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; Bookshelf ID: NBK539893. [Google Scholar]
- Fock, K.M.; Khoo, J. Diet and Exercise in Management of Obesity and Overweight. J. Gastroenterol. Hepatol. 2013, 28, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Westerterp-Plantenga, M.S.; Lemmens, S.G.; Westerterp, K.R. Dietary Protein—Its Role in Satiety, Energetics, Weight Loss and Health. Br. J. Nutr. 2012, 108, S105–S112. [Google Scholar] [CrossRef] [PubMed]
- Drummen, M.; Tischmann, L.; Gatta-Cherifi, B.; Adam, T.; Westerterp-Plantenga, M. Dietary Protein and Energy Balance in Relation to Obesity and Co-Morbidities. Front. Endocrinol. 2018, 9, 443. [Google Scholar] [CrossRef]
- Hudson, J.L.; Wang, Y.; Bergia Iii, R.E.; Campbell, W.W. Protein Intake Greater than the RDA Differentially Influences Whole-Body Lean Mass Responses to Purposeful Catabolic and Anabolic Stressors: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020, 11, 548–558. [Google Scholar] [CrossRef]
- Kim, J.E.; O’Connor, L.E.; Sands, L.P.; Slebodnik, M.B.; Campbell, W.W. Effects of Dietary Protein Intake on Body Composition Changes after Weight Loss in Older Adults: A Systematic Review and Meta-Analysis. Nutr. Rev. 2016, 74, 210–224. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Westman, E.; Mattes, R.D.; Wolfe, R.R.; Astrup, A.; Westerterp-Plantenga, M. Protein, Weight Management, and Satiety. Am. J. Clin. Nutr. 2008, 87, 1558S–1561S. [Google Scholar] [CrossRef]
- Magkos, F. The Role of Dietary Protein in Obesity. Rev. Endocr. Metab. Disord. 2020, 21, 329–340. [Google Scholar] [CrossRef]
- Murphy, K.J.; Parker, B.; Dyer, K.A.; Davis, C.R.; Coates, A.M.; Buckley, J.D.; Howe, P.R.C. A Comparison of Regular Consumption of Fresh Lean Pork, Beef and Chicken on Body Composition: A Randomized Cross-over Trial. Nutrients 2014, 6, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Mahon, A.K.; Flynn, M.G.; Stewart, L.K.; McFarlin, B.K.; Iglay, H.B.; Mattes, R.D.; Lyle, R.M.; Considine, R.V.; Campbell, W.W. Protein Intake during Energy Restriction: Effects on Body Composition and Markers of Metabolic and Cardiovascular Health in Postmenopausal Women. J. Am. Coll. Nutr. 2007, 26, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Melanson, K.; Gootman, J.; Myrdal, A.; Kline, G.; Rippe, J.M. Weight Loss and Total Lipid Profile Changes in Overweight Women Consuming Beef or Chicken as the Primary Protein Source. Nutrition 2003, 19, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Sharp, M.H.; Lowery, R.P.; Shields, K.A.; Lane, J.R.; Gray, J.L.; Partl, J.M.; Hayes, D.W.; Wilson, G.J.; Hollmer, C.A.; Minivich, J.R.; et al. The Effects of Beef, Chicken, or Whey Protein After Workout on Body Composition and Muscle Performance. J. Strength. Cond. Res. 2018, 32, 2233–2242. [Google Scholar] [CrossRef] [PubMed]
- Keogh, C.; Li, C.; Gao, Z. Evolving Consumer Trends for Whey Protein Sports Supplements: The Heckman Ordered Probit Estimation. Agric. Food. Econ. 2019, 7, 6. [Google Scholar] [CrossRef]
- Gilsing, A.M.J.; Weijenberg, M.P.; Hughes, L.A.E.; Ambergen, T.; Dagnelie, P.C.; Goldbohm, R.A.; van den Brandt, P.A.; Schouten, L.J. Longitudinal Changes in BMI in Older Adults Are Associated with Meat Consumption Differentially, by Type of Meat Consumed. J. Nutr. 2012, 142, 340–349. [Google Scholar] [CrossRef]
- Linde, J.A.; Utter, J.; Jeffery, R.W.; Sherwood, N.E.; Pronk, N.P.; Boyle, R.G. Specific Food Intake, Fat and Fiber Intake, and Behavioral Correlates of BMI among Overweight and Obese Members of a Managed Care Organization. Int. J. Behav. Nutr.Phys. Act. 2006, 3, 42. [Google Scholar] [CrossRef][Green Version]
- Weinrich, S.P.; Priest, J.; Reynolds, W.; Godley, P.A.; Tuckson, W.; Weinrich, M. Body Mass Index and Intake of Selected Foods in African American Men. Public Health Nurs. 2007, 24, 217–229. [Google Scholar] [CrossRef]
- Satija, A.; Taylor, F.C.; Khurana, S.; Tripathy, V.; Khandpur, N.; Bowen, L.; Prabhakaran, D.; Kinra, S.; Reddy, S.; Ebrahim, S. Differences in Consumption of Food Items between Obese and Normal-Weight People in India. Natl. Med. J. India 2012, 25, 10–13. [Google Scholar]
- León-Muñoz, L.M.; García-Esquinas, E.; Soler-Vila, H.; Guallar-Castillón, P.; Banegas, J.R.; Rodríguez-Artalejo, F. Unhealthy Eating Behaviors and Weight Gain: A Prospective Study in Young and Middle-Age Adults. Obesity 2016, 24, 1178–1184. [Google Scholar] [CrossRef]
- Giglio, B.M.; Duarte, V.I.R.; Galvão, A.F.; Marini, A.C.B.; Schincaglia, R.M.; Mota, J.F.; Souza, L.B.; Pimentel, G.D. High-Protein Diet Containing Dairy Products Is Associated with Low Body Mass Index and Glucose Concentrations: A Cross-Sectional Study. Nutrients 2019, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, T.A.; Yang, S.-J.; Baranowski, T.; Zakeri, I.; Berenson, G. Eating Patterns and Obesity in Children. The Bogalusa Heart Study. Am. J. Prev. Med. 2003, 25, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.; Buyken, A.; von Berg, A.; Berdel, D.; Lehmann, I.; Hoffmann, B.; Koletzko, S.; Koletzko, B.; Heinrich, J.; Standl, M. Prospective Associations of Meat Consumption during Childhood with Measures of Body Composition during Adolescence: Results from the GINIplus and LISAplus Birth Cohorts. Nutr. J. 2016, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Bilger, M.; van Dam, R.M.; Finkelstein, E.A. Consumption of Specific Foods and Beverages and Excess Weight Gain among Children and Adolescents. Health Aff. 2015, 34, 1940–1948. [Google Scholar] [CrossRef]
- Gunes, F.E.; Bekiroglu, N.; Imeryuz, N.; Agirbasli, M. Relation between Eating Habits and a High Body Mass Index among Freshman Students: A Cross-Sectional Study. J. Am. Coll. Nutr. 2012, 31, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Musaiger, A.O.; Hammad, S.S.; Tayyem, R.F.; Qatatsheh, A.A. Socio-Demographic and Dietary Factors Associated with Obesity among Female University Students in Jordan. Int. J. Adolesc. Med. Health 2015, 27, 299–305. [Google Scholar] [CrossRef]
- Mohammadbeigi, A.; Asgarian, A.; Moshir, E.; Heidari, H.; Afrashteh, S.; Khazaei, S.; Ansari, H. Fast Food Consumption and Overweight/Obesity Prevalence in Students and Its Association with General and Abdominal Obesity. J. Prev. Med. Hyg. 2018, 59, E236–E240. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Joseph, J.J.; Deedwania, P.; Acharya, T.; Aguilar, D.; Bhatt, D.L.; Chyun, D.A.; Di Palo, K.E.; Golden, S.H.; Sperling, L.S. Comprehensive Management of Cardiovascular Risk Factors for Adults with Type 2 Diabetes: A Scientific Statement from the American Heart Association. Circulation 2022, 145, e722–e759. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health Effects of Dietary Risks in 195 Countries, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, National Center for Health Statistics. About Multiple Cause of Death, 1999–2020. CDC WONDER Online Database Website. Centers for Disease Control and Prevention: Atlanta, GA, USA. 2022. Available online: https://wonder.cdc.gov/wonder/help/mcd.html (accessed on 28 February 2023).
- Ahmad, F.B.; Anderson, R.N. The Leading Causes of Death in the US for 2020. JAMA 2021, 325, 1829–1830. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update a Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention; National Center for Health Statistics. About Underlying Cause of Death 1999–2019. CDC WONDER Online Database. Available online: https://wonder.cdc.gov/ucd-icd10.html (accessed on 28 February 2023).
- Bergeron, N.; Chiu, S.; Williams, P.T.; M King, S.; Krauss, R.M. Effects of Red Meat, White Meat, and Nonmeat Protein Sources on Atherogenic Lipoprotein Measures in the Context of Low Compared with High Saturated Fat Intake: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2019, 110, 24–33. [Google Scholar] [CrossRef]
- Beauchesne-Rondeau, É.; Gascon, A.; Bergeron, J.; Jacques, H. Plasma Lipids and Lipoproteins in Hypercholesterolemic Men Fed a Lipid-Lowering Diet Containing Lean Beef, Lean Fish, or Poultry. Am. J. Clin. Nutr. 2003, 77, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.W.; Dunn, J.K.; Pownall, H.J.; Braucht, D.J.; McMann, M.C.; Herd, J.A.; Harris, K.B.; Savell, J.W.; Cross, H.R.; Gotto, A.M. Effects of Beef and Chicken Consumption on Plasma Lipid Levels in Hypercholesterolemic Men. Arch. Intern. Med. 1994, 154, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Gallego, R.; Perez-Calahorra, S.; Cenarro, A.; Bea, A.M.; Andres, E.; Horno, J.; Ros, E.; Civeira, F. Effect of Lean Red Meat from Lamb v. Lean White Meat from Chicken on the Serum Lipid Profile: A Randomised, Cross-over Study in Women. Br. J. Nutr. 2012, 107, 1403–1407. [Google Scholar] [CrossRef]
- Lee, J.E.; McLerran, D.F.; Rolland, B.; Chen, Y.; Grant, E.J.; Vedanthan, R.; Inoue, M.; Tsugane, S.; Gao, Y.T.; Tsuji, I.; et al. Meat Intake and Cause-Specific Mortality: A Pooled Analysis of Asian Prospective Cohort Studies. Am. J. Clin. Nutr. 2013, 98, 1032–1041. [Google Scholar] [CrossRef]
- Takata, Y.; Shu, X.O.; Gao, Y.T.; Li, H.; Zhang, X.; Gao, J.; Cai, H.; Yang, G.; Xiang, Y.B.; Zheng, W. Red Meat and Poultry Intakes and Risk of Total and Cause-Specific Mortality: Results from Cohort Studies of Chinese Adults in Shanghai. PLoS ONE 2013, 8, e56963. [Google Scholar] [CrossRef]
- Nagao, M.; Iso, H.; Yamagishi, K.; Date, C.; Tamakoshi, A. Meat Consumption in Relation to Mortality from Cardiovascular Disease among Japanese Men and Women. Eur. J. Clin. Nutr. 2012, 66, 687–693. [Google Scholar] [CrossRef]
- Key, T.J.; Appleby, P.N.; Bradbury, K.E.; Sweeting, M.; Wood, A.; Johansson, I.; Kühn, T.; Steur, M.; Weiderpass, E.; Wennberg, M.; et al. Consumption of Meat, Fish, Dairy Products, and Eggs and Risk of Ischemic Heart Disease. Circulation 2019, 139, 2835–2845. [Google Scholar] [CrossRef]
- Haring, B.; Gronroos, N.; Nettleton, J.A.; Wyler Von Ballmoos, M.C.; Selvin, E.; Alonso, A. Dietary Protein Intake and Coronary Heart Disease in a Large Community Based Cohor: Results from the Atherosclerosis Risk in Communities (ARIC) Study. PLoS ONE 2014, 9, e109552. [Google Scholar] [CrossRef]
- Ogilvie, R.P.; Lutsey, P.L.; Heiss, G.; Folsom, A.R.; Steffen, L.M. Dietary Intake and Peripheral Arterial Disease Incidence in Middle-Aged Adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2017, 105, 651–659. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, K.; Son, J.; Jang, J.; Kang, R.; Chung, H.K.; Lee, K.W.; Lee, S.M.; Lim, H.; Shin, M.J. Unprocessed Meat Consumption and Incident Cardiovascular Diseases in Korean Adults: The Korean Genome and Epidemiology Study (KoGES). Nutrients 2017, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.M.; Sun, Q.; Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Willett, W.C. Major Dietary Protein Sources and Risk of Coronary Heart Disease in Women. Circulation 2010, 122, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Campos, H.; Baylin, A. Red Meat Intake Is Positively Associated with Non-Fatal Acute Myocardial Infarction in the Costa Rica Heart Study. Br. J. Nutr. 2017, 118, 303–311. [Google Scholar] [CrossRef][Green Version]
- Zhong, V.W.; Van Horn, L.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Allen, N.B. Associations of Processed Meat, Unprocessed Red Meat, Poultry, or Fish Intake With Incident Cardiovascular Disease and All-Cause Mortality. JAMA. Intern. Med. 2020, 180, 503. [Google Scholar] [CrossRef]
- Papier, K.; Fensom, G.K.; Knuppel, A.; Appleby, P.N.; Tong, T.Y.N.; Schmidt, J.A.; Travis, R.C.; Key, T.J.; Perez-Cornago, A. Meat Consumption and Risk of 25 Common Conditions: Outcome-Wide Analyses in 475,000 Men and Women in the UK Biobank Study. BMC Med. 2021, 19, 53. [Google Scholar] [CrossRef]
- Mohammadi, H.; Jayedi, A.; Ghaedi, E.; Golbidi, D.; Shab-bidar, S. Dietary Poultry Intake and the Risk of Stroke: A Dose–Response Meta-Analysis of Prospective Cohort Studies. Clin. Nutr. ESPEN 2018, 23, 25–33. [Google Scholar] [CrossRef]
- Sauvaget, C.; Nagano, J.; Allen, N.; Grant, E.J.; Beral, V. Intake of Animal Products and Stroke Mortality in the Hiroshima/Nagasaki Life Span Study. Int. J. Epidemiol. 2003, 32, 536–543. [Google Scholar] [CrossRef]
- Tong, T.Y.N.; Appleby, P.N.; Key, T.J.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjønneland, A.; Katzke, V.; Kühn, T.; Boeing, H.; et al. The Associations of Major Foods and Fibre with Risks of Ischaemic and Haemorrhagic Stroke: A Prospective Study of 418 329 Participants in the EPIC Cohort across Nine European Countries. Eur. Heart J. 2020, 41, 2632–2640. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Meat Consumption and Risk of Metabolic Syndrome: Results from the Korean Population and a Meta-Analysis of Observational Studies. Nutrients 2018, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.M.; Pan, A.; Rexrode, K.M.; Stampfer, M.; Hu, F.B.; Mozaffarian, D.; Willett, W.C. Dietary Protein Sources and the Risk of Stroke in Men and Women. Stroke 2012, 43, 637–664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, D. Red Meat, Poultry, and Egg Consumption with the Risk of Hypertension: A Meta-Analysis of Prospective Cohort Studies. J. Hum. Hypertens. 2018, 32, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Borgia, L.; Curhan, G.C.; Willett, W.C.; Hu, F.B.; Satijad, A.; Forman, J.P. Long-Term Intake of Animal Flesh and Risk of Developing Hypertension in Three Prospective Cohort Studies. J. Hypertens. 2015, 33, 2231–2238. [Google Scholar] [CrossRef]
- Griep, L.M.O.; Seferidi, P.; Stamler, J.; Van Horn, L.; Chan, Q.; Tzoulaki, I.; Steffen, L.M.; Miura, K.; Ueshima, H.; Okuda, N.; et al. Relation of Unprocessed, Processed Red Meat and Poultry Consumption to Blood Pressure in East Asian and Western Adults. J. Hypertens. 2016, 34, 1721–2729. [Google Scholar] [CrossRef]
- Miura, K.; Greenland, P.; Stamler, J.; Liu, K.; Daviglus, M.L.; Nakagawa, H. Relation of Vegetable, Fruit, and Meat Intake to 7-Year Blood Pressure Change in Middle-Aged Men: The Chicago Western Electric Study. Am. J. Epidemiol. 2004, 159, 572–580. [Google Scholar] [CrossRef]
- Steffen, L.M.; Kroenke, C.H.; Yu, X.; Pereira, M.A.; Slattery, M.L.; Van Horn, L.; Gross, M.D.; Jacobs, D.R. Associations of Plant Food, Dairy Product, and Meat Intakes with 15-y Incidence of Elevated Blood Pressure in Young Black and White Adults: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am. J. Clin. Nutr. 2005, 82, 1169–1177. [Google Scholar] [CrossRef]
- Golzarand, M.; Bahadoran, Z.; Mirmiran, P.; Azizi, F. Protein Foods Group and 3-Year Incidence of Hypertension: A Prospective Study from Tehran Lipid and Glucose Study. J. Ren. Nutr. 2016, 26, 219–225. [Google Scholar] [CrossRef]
- Wang, L.; Manson, J.E.; Buring, J.E.; Sesso, H.D. Meat Intake and the Risk of Hypertension in Middle-Aged and Older Women. J. Hypertens. 2008, 26, 215–222. [Google Scholar] [CrossRef]
- Takashima, Y.; Iwase, Y.; Yoshida, M.; Kokaze, A.; Takagi, Y.; Tsubono, Y.; Tsugane, S.; Takahashi, T.; Litoi, Y.; Akabane, M.; et al. Relationship of Food Intake and Dietary Patterns with Blood Pressure Levels among Middle-Aged Japanese Men. J. Epidemiol. 1998, 8, 106–115. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report Website. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 18 February 2023).
- Zhou, B.; Lu, Y.; Hajifathalian, K.; Bentham, J.; Di Cesare, M.; Danaei, G.; Bixby, H.; Cowan, M.J.; Ali, M.K.; Taddei, C.; et al. Worldwide Trends in Diabetes since 1980: A Pooled Analysis of 751 Population-Based Studies with 4.4 Million Participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- Sun, L.; Ranawana, D.V.; Leow, M.K.S.; Henry, C.J. Effect of Chicken, Fat and Vegetable on Glycaemia and Insulinaemia to a White Rice-Based Meal in Healthy Adults. Eur. J. Nutr. 2014, 53, 1719–1726. [Google Scholar] [CrossRef]
- Quek, R.; Bi, X.; Henry, C.J. Impact of Protein-Rich Meals on Glycaemic Response of Rice. Br. J. Nutr. 2016, 115, 1194–1201. [Google Scholar] [CrossRef]
- Hätönen, K.A.; Virtamo, J.; Eriksson, J.G.; Sinkko, H.K.; Sundvall, J.E.; Valsta, L.M. Protein and Fat Modify the Glycaemic and Insulinaemic Responses to a Mashed Potato-Based Meal. Br. J. Nutr. 2011, 106, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, F.Q.; Neil, B.J.; Westphal, S.A. The Insulin and Glucose Responses to Meals of Glucose plus Various Proteins in Type II Diabetic Subjects. Metabolism 1988, 37, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Sithamparapillai, A.; Brimble, K.S.; Banfield, L.; Morton, R.W.; Phillips, S.M. Changes in Kidney Function Do Not Differ between Healthy Adults Consuming Higher- Compared with Lower- or Normal-Protein Diets: A Systematic Review and Meta-Analysis. J. Nutr. 2018, 148, 1760–1775. [Google Scholar] [CrossRef]
- Walker, J.D.; Dodds, R.A.; Murrells, T.J.; Bending, J.J.; Mattock, M.B.; Keen, H.; Viberti, G.C. Restriction of Dietary Protein and Progression of Renal Failure in Diabetic Nephropathy. Lancet 1989, 334, 1411–1415. [Google Scholar] [CrossRef]
- Hansen, H.P.; Christensen, P.K.; Tauber-Lassen, E.; Klausen, A.; Jensen, B.R.; Parving, H.H. Low-Protein Diet and Kidney Function in Insulin-Dependent Diabetic Patients with Diabetic Nephropathy. Kidney Int. 1999, 55, 621–628. [Google Scholar] [CrossRef]
- Ko, G.J.; Rhee, C.M.; Kalantar-Zadeh, K.; Joshi, S. The Effects of High-Protein Diets on Kidney Health and Longevity. J. Am. Soc. Nephrol. 2020, 31, 1667–1679. [Google Scholar] [CrossRef]
- Gross, J.L.; Zelmanovitz, T.; Moulin, C.C.; De Mello, V.; Perassolo, M.; Leitao, C.; Hoefel, A.; Paggi, A.; Azevedo, M.J. Effect of a Chicken-Based Diet on Renal Function and Lipid Profile in Patients with Type 2 Diabetes: A Randomized Crossover Trial. Diabetes Care 2002, 25, 645–651. [Google Scholar] [CrossRef]
- De Mello, V.D.F.; Zelmanovitz, T.; Perassolo, M.S.; Azevedo, M.J.; Gross, J.L. Withdrawal of Red Meat from the Usual Diet Reduces Albuminuria and Improves Serum Fatty Acid Profile in Type 2 Diabetes Patients with Macroalbuminuria. Am. J. Clin. Nutr. 2006, 83, 1032–1038. [Google Scholar] [CrossRef]
- de Mello, V.D.F.; Zelmanovitz, T.; Azevedo, M.J.; de Paula, T.P.; Gross, J.L. Long-Term Effect of a Chicken-Based Diet Versus Enalapril on Albuminuria in Type 2 Diabetic Patients with Microalbuminuria. J. Ren. Nutr. 2008, 18, 440–447. [Google Scholar] [CrossRef]
- Fan, M.; Li, Y.; Wang, C.; Mao, Z.; Zhou, W.; Zhang, L.; Yang, X.; Cui, S.; Li, L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: Adose-Response Meta-Analysis of Prospective Studies. Nutrients 2019, 11, 2783. [Google Scholar] [CrossRef] [PubMed]
- Feskens, E.J.M.; Sluik, D.; van Woudenbergh, G.J. Meat Consumption, Diabetes, and Its Complications. Curr. Diab. Rep. 2013, 13, 298–306. [Google Scholar] [CrossRef] [PubMed]
- The InterAct Consortium. Association between Dietary Meat Consumption and Incident Type 2 Diabetes: The EPIC-InterAct Study. Diabetologia 2013, 56, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Talaei, M.; Wang, Y.L.; Yuan, J.M.; Pan, A.; Koh, W.P. Meat, Dietary Heme Iron, and Risk of Type 2 Diabetes MellitusThe Singapore Chinese Health Study. Am. J. Epidemiol. 2017, 186, 824–833. [Google Scholar] [CrossRef]
- Villegas, R.; Xiao, O.S.; Gao, Y.T.; Yang, G.; Cai, H.; Li, H.; Zheng, W. The Association of Meat Intake and the Risk of Type 2 Diabetes May Be Modified by Body Weight. Int. J. Med. Sci. 2006, 3, 152–159. [Google Scholar] [CrossRef]
- Montonen, J.; Järvinen, R.; Heliövaara, M.; Reunanen, A.; Aromaa, A.; Knekt, P. Food Consumption and the Incidence of Type II Diabetes Mellitus. Eur. J. Clin. Nutr. 2005, 59, 152–159. [Google Scholar] [CrossRef]
- Sluik, D.; Boeing, H.; Li, K.; Kaaks, R.; Johnsen, N.F.; Tjønneland, A.; Arriola, L.; Barricarte, A.; Masala, G.; Grioni, S.; et al. Lifestyle Factors and Mortality Risk in Individuals with Diabetes Mellitus: Are the Associations Different from Those in Individuals without Diabetes? Diabetologia 2014, 57, 63–72. [Google Scholar] [CrossRef]
- Würtz, A.M.L.; Jakobsen, M.U.; Bertoia, M.L.; Hou, T.; Schmidt, E.B.; Willett, W.C.; Overvad, K.; Sun, Q.; Manson, J.A.E.; Hu, F.B.; et al. Replacing the Consumption of Red Meat with Other Major Dietary Protein Sources and Risk of Type 2 Diabetes Mellitus: A Prospective Cohort Study. Am. J. Clin. Nutr. 2021, 113, 612–621. [Google Scholar] [CrossRef]
- Ibsen, D.B.; Steur, M.; Imamura, F.; Overvad, K.; Schulze, M.B.; Bendinelli, B.; Guevara, M.; Agudo, A.; Amiano, P.; Aune, D.; et al. Replacement of Red and Processed Meat with Other Food Sources of Protein and the Risk of Type 2 Diabetes in European Populations: The EPIC-InterAct Study. Diabetes Care 2020, 43, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, D.B.; Warberg, C.K.; Würtz, A.M.L.; Overvad, K.; Dahm, C.C. Substitution of Red Meat with Poultry or Fish and Risk of Type 2 Diabetes: A Danish Cohort Study. Eur. J. Nutr. 2019, 58, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Satija, A.; Blondin, S.A.; Janiszewski, M.; Emlen, E.; O’Connor, L.E.; Campbell, W.W.; Hu, F.B.; Willett, W.C.; Stampfer, M.J. Meta-Analysis of Randomized Controlled Trials of Red Meat Consumption in Comparison with Various Comparison Diets on Cardiovascular Risk Factors. Circulation 2019, 139, 1828–1845. [Google Scholar] [CrossRef] [PubMed]
| Values per 100 g Cooked Product | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unprocessed Poultry Products | Processed Poultry Products | ||||||||||||||
| Nutrient | Chicken Breast, Skinless, Boneless, Roasted | Dark Chicken Meat, Skinless, Boneless, Roasted | Chicken Drumstick, Skinless, Boneless, Roasted | Chicken Drumstick, Skin-on | Chicken Thigh, Skinless | Chicken Thigh, Skin-on, Roasted | Chicken Wing, Skinless | Chicken Wing, Skin-on, Roasted | Ground Turkey, 93% Lean, 7% Fat, Pan-Broiled | Turkey Patty, 93%, 7% Fat, Broiled | Turkey patty, 85%, 15% fat, broiled | Chicken Nuggets, White Meat, Breaded, Precooked, Frozen | Chicken Tenders, Breaded, Precooked, Frozen | Chicken, Luncheon Meat | Turkey, Luncheon Meat |
| Calories (kcal) | 165 | 205 | 155 | 191 | 179 | 232 | 203 | 254 | 213 | 207 | 249 | 261 | 240 | 98 | 106 |
| Protein (g) | 31.0 | 27.4 | 24.2 | 23.4 | 24.8 | 23.3 | 30.5 | 23.8 | 27.1 | 25.9 | 25.9 | 14.4 | 14.6 | 17.4 | 14.8 |
| Total fat (g) | 3.6 | 9.7 | 5.7 | 10.2 | 8.2 | 14.7 | 8.1 | 16.9 | 11.6 | 11.4 | 16.2 | 15.4 | 13.6 | 1.9 | 3.8 |
| Saturated fat (g) | 1.0 | 2.7 | 2.1 | 2.7 | 2.3 | 4.1 | 2.3 | 5.0 | 3.0 | 3.0 | 4.1 | 3.4 | 2.4 | 0.6 | 0.9 |
| Monounsaturated fat (g) | 1.2 | 3.6 | 3.1 | 4.2 | 3.4 | 6.3 | 2.6 | 7.7 | 3.8 | 3.9 | 5.5 | 4.7 | 4.1 | 0.7 | 1.1 |
| Polyunsaturated fat (g) | 0.8 | 2.3 | 1.6 | 2.1 | 1.7 | 3.0 | 1.8 | 3.6 | 3.5 | 3.5 | 4.2 | 6.2 | 6.2 | 0.5 | 1.0 |
| Carbohydrates (g) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 16.2 | 14.9 | 0.0 | 0.0 |
| Cholesterol (mg) | 85 | 93 | 128 | 130 | 133 | 133 | 85 | 141 | 104 | 106 | 105 | 34 | 36 | 51 | 49 |
| Sodium (mg) | 74 | 93 | 128 | 123 | 106 | 102 | 92 | 98 | 90 | 91 | 81 | 538 | 527 | 1032 | 898 |
| Vitamin D (µg) | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 |
| Calcium (mg) | 15 | 15 | 11 | 11 | 9 | 9 | 16 | 18 | 31 | 29 | 48 | 38 | 39 | 11 | 14 |
| Iron (mg) | 1.0 | 1.3 | 1.1 | 1.1 | 1.1 | 1.1 | 1.2 | 0.8 | 1.6 | 1.7 | 2.0 | 1.4 | 0.8 | 0.4 | 0.4 |
| Potassium (mg) | 256 | 240 | 256 | 247 | 269 | 253 | 210 | 212 | 304 | 247 | 242 | 281 | 281 | 360 | 371 |
| Vitamin B2 [riboflavin] (mg) | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.3 | 0.2 | 0 | 0.1 | 0.1 | 0.1 | 0.1 |
| Vitamin B3 [niacin] (mg) | 13.7 | 6.5 | 5.6 | 5.4 | 6.2 | 5.8 | 7.3 | 6.3 | 8.1 | 6.6 | 7 | 6.7 | 5.9 | 9.1 | 7.2 |
| Vitamin B5 [pantothenic acid] (mg) | 1.0 | 1.2 | 1.1 | 1.1 | 1.3 | 1.2 | 1.0 | 0.9 | 1.4 | 1.3 | 1 | 0.9 | 0.9 | 1.0 | 0.6 |
| Vitamin B6 (mg) | 0.6 | 0.4 | 0.4 | 0.4 | 0.5 | 0.4 | 0.6 | 0.6 | 0.5 | 0.5 | 0.4 | 0.3 | 0.3 | 0.4 | 0.4 |
| Vitamin B12 (µg) | 0.3 | 0.3 | 0.4 | 0.4 | 0.4 | 0.4 | 0.3 | 0.4 | 1.9 | 1.8 | 1.4 | 0.2 | 0.2 | 0.1 | 0.4 |
| Phosphorus (mg) | 228 | 179 | 205 | 200 | 230 | 216 | 166 | 147 | 259 | 210 | 235 | 213 | 200 | 257 | 249 |
| Magnesium (mg) | 29 | 23 | 24 | 22 | 24 | 22 | 21 | 19 | 29 | 25 | 25 | 35 | 29 | 26 | 19 |
| Zinc (mg) | 1.0 | 2.8 | 2.6 | 2.4 | 1.9 | 1.7 | 2.1 | 1.6 | 3.8 | 3.7 | 3.3 | 0.8 | 0.6 | 0.5 | 0.9 |
| Selenium (µg) | 27.6 | 18.0 | 25.2 | 28.1 | 27.1 | 25.3 | 24.7 | 25.5 | 28.4 | 27.5 | 35.1 | 17.0 | 19.3 | 13.2 | 13.0 |
| Choline (mg) | 85.3 | 74.0 | 63.4 | 67.8 | 71.8 | 67.6 | 79.6 | 111.3 | 78.7 | 77.7 | 79.1 | 45.5 | 46.1 | 44.2 | 30.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Connolly, G.; Campbell, W.W. Poultry Consumption and Human Cardiometabolic Health-Related Outcomes: A Narrative Review. Nutrients 2023, 15, 3550. https://doi.org/10.3390/nu15163550
Connolly G, Campbell WW. Poultry Consumption and Human Cardiometabolic Health-Related Outcomes: A Narrative Review. Nutrients. 2023; 15(16):3550. https://doi.org/10.3390/nu15163550
Chicago/Turabian StyleConnolly, Gavin, and Wayne W. Campbell. 2023. "Poultry Consumption and Human Cardiometabolic Health-Related Outcomes: A Narrative Review" Nutrients 15, no. 16: 3550. https://doi.org/10.3390/nu15163550
APA StyleConnolly, G., & Campbell, W. W. (2023). Poultry Consumption and Human Cardiometabolic Health-Related Outcomes: A Narrative Review. Nutrients, 15(16), 3550. https://doi.org/10.3390/nu15163550






