Effects on Fetal Metabolic Programming and Endocannabinoid System of a Normocaloric Diet during Pregnancy and Lactation of Female Mice with Pregestational Obesity
Abstract
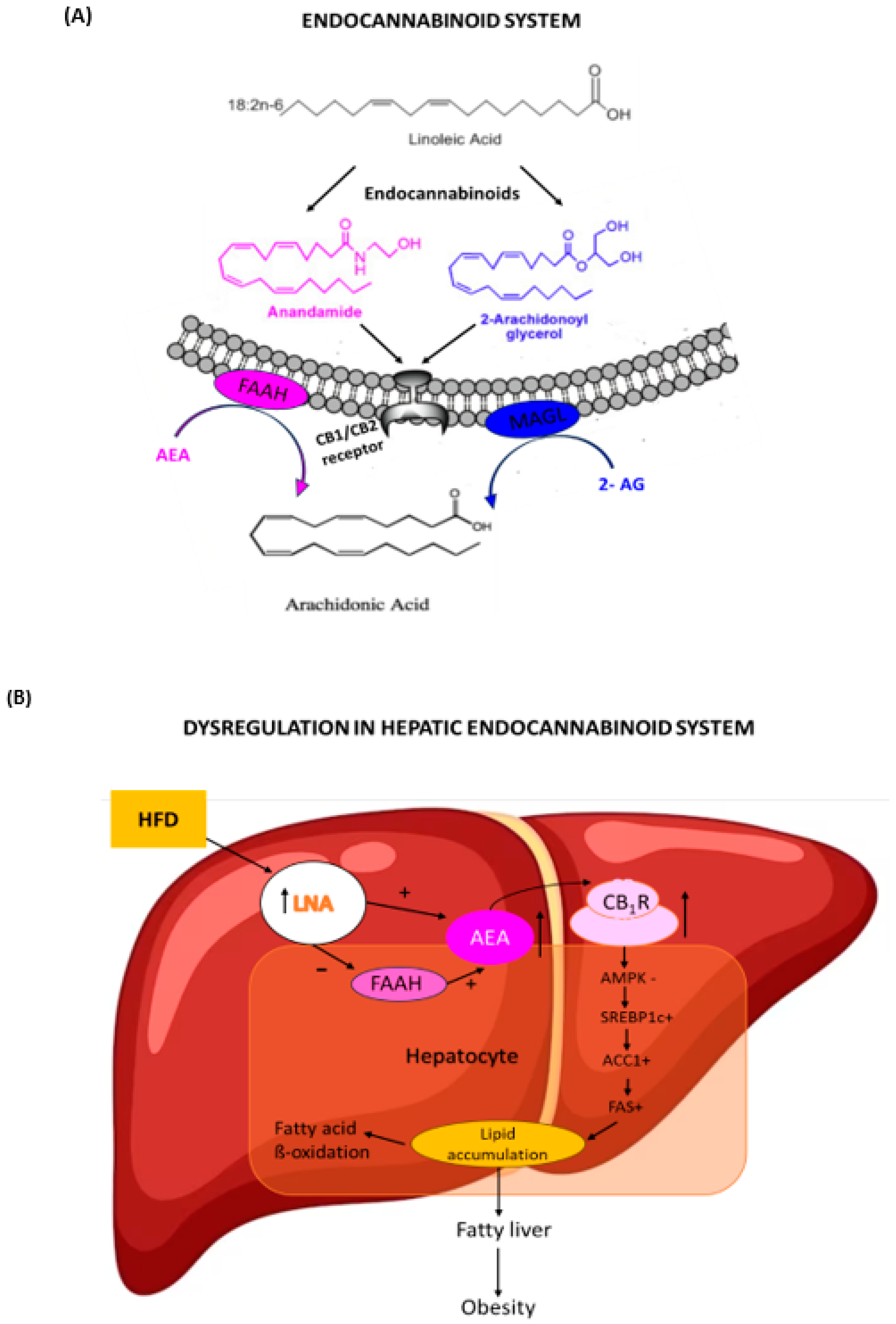
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
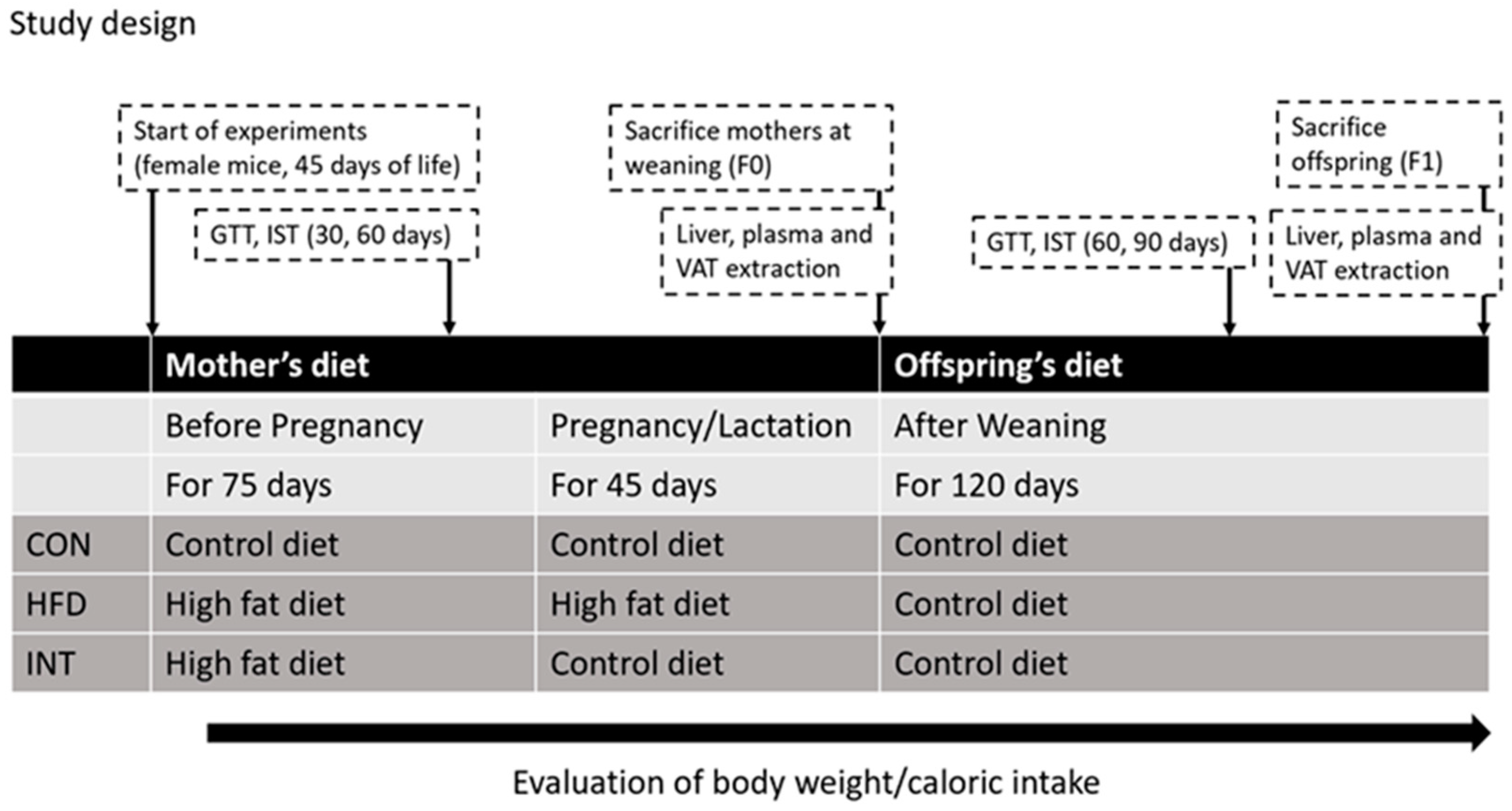
2.2. Experimental Design
- (a)
- CON-F0 group (control group): females with control diet before and during pregnancy and lactation (n = 5); and their offspring CON-F1 group (n = 10);
- (b)
- HFD-F0 group (high fat diet group): females with HFD before and during pregnancy and lactation (n = 4); and their offspring HFD-F1 group (n = 10);
- (c)
- INT-F0 group (intervention group): females with HFD until mating and control diet during pregnancy and lactation (intervention group, n = 6); and their offspring INT-F1 group (n = 10).
2.3. Diets
2.4. Evaluation of Body Parameters
2.4.1. Measurement of Body Weight and Intake
2.4.2. Weight Measurement of Visceral Fat and Liver
2.5. Evaluation of Metabolic Parameters
2.5.1. Glucose Tolerance Test and Insulin Sensitivity Test
2.5.2. Plasma and Tissue Collection
2.5.3. Determination of Plasmatic Hormone Levels
- -
- Corticosterone was evaluated with the ELISA kit from the Cayman Chemical Com-pany, MI, USA, No. 501320.
- -
- Insulin with ELISA kit EMD Millipore Corporation, MI, USA, Cat. # EZRMI-13K.
- -
- Adiponectin with ELISA kit EMD Millipore Corporation, MI, USA, Cat. # EZMADP-60K.
- -
- Leptin with EMD ELISA kit Millipore Corporation, MI, USA, Cat. # EZML-82K.
2.5.4. Determination of Plasmatic and Hepatic Lipids
2.5.5. Determination of Hepatic Fatty Acid Profile
Extraction of Fat
Preparation of Fatty Acid Methyl Esters
Analysis of Fatty Acid Methyl Esters
2.6. Determination of Gene Expression
2.6.1. Total RNA Extraction
2.6.2. cDNA Synthesis
2.6.3. qPCR in Real Time
2.7. Determination of the Amount of Total Protein
2.7.1. Protein Extraction and Quantification
- Protein precipitation
2.7.2. Western Blot Analysis
- Incubation with antibodies
- -
- Anti-FAAH antibody (CaymanChem Co., Ann Arbor, Michigan, USA), developed in rabbit, used with a dilution of 1:500.
- -
- Anti-MAGL antibody (CaymanChem Co., Michigan, USA), developed in rabbit, at a concentration of 5 µg/mL, using a 1:120 dilution.
- -
- The peroxidase-conjugated anti-rabbit secondary antibody (Rockland Immunochemicals, Gilbertsville, PA, USA), at a concentration of 2 mg/mL, was used at a dilution of 1:5000.
- Revealed
2.8. Statistical Analysis
3. Results
3.1. Hepatic Fatty Acid Profile
3.2. Body, Hormonal, Biochemical, and Metabolic Parameters in Offspring
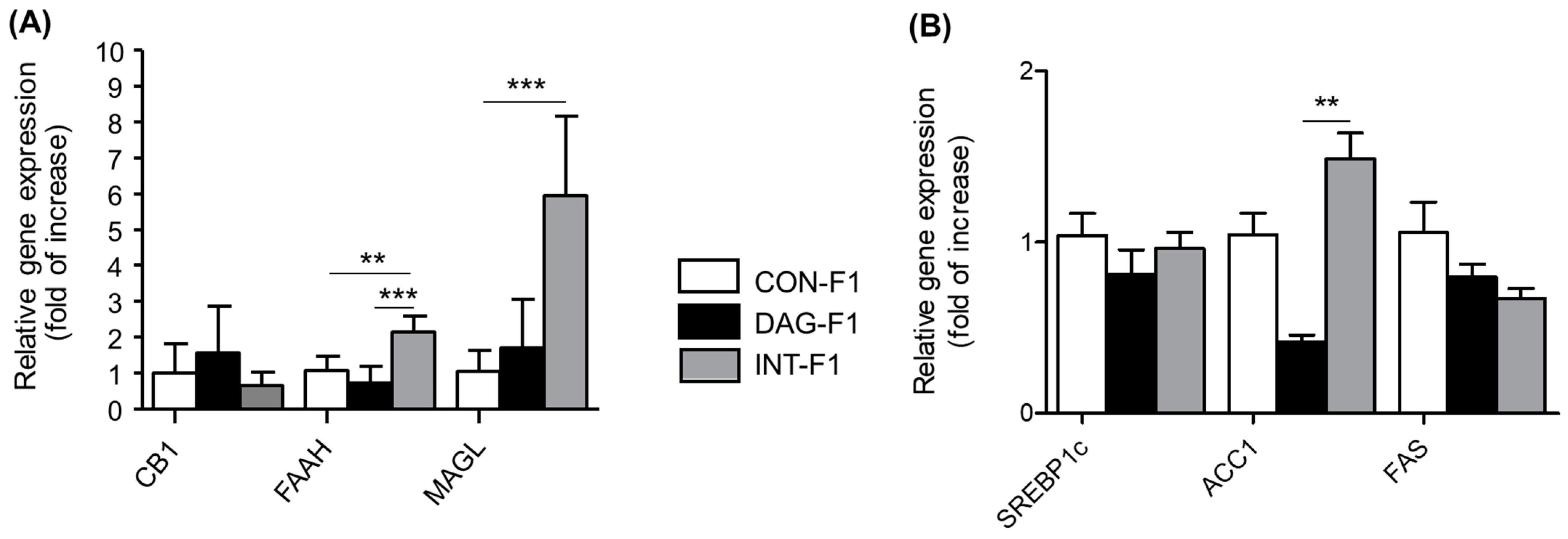
3.3. Gene Expression of mRNA of Components of the Endocannabinoid System and Target Genes of the CB1 Receptor in Liver Tissue of Offspring
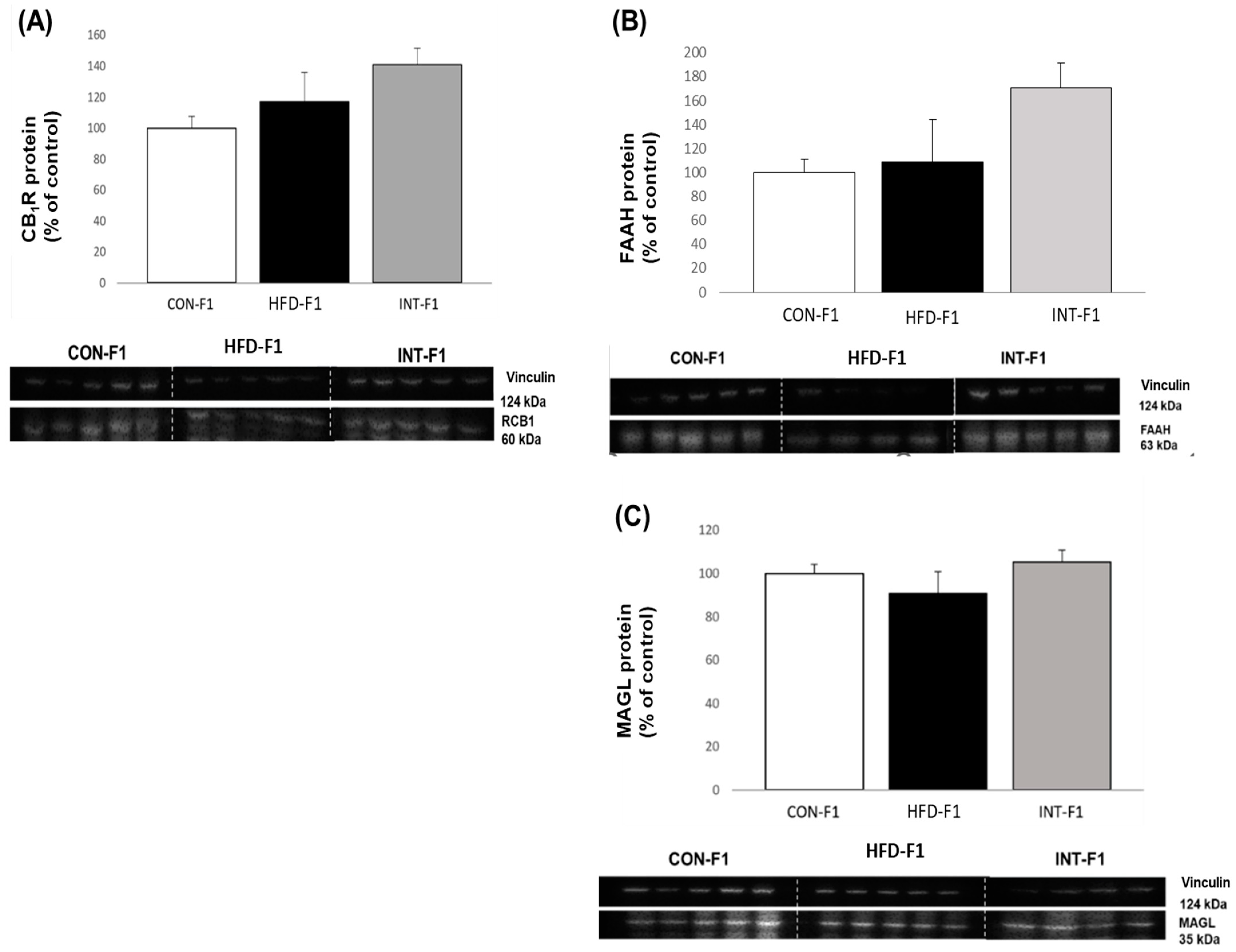
3.4. Western Blot Analysis of Components of the Endocannabinoid System in Liver Tissue in Offspring
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD. The Heavy Burden of Obesity: The Economics of Prevention; Organisation for Economic Co-Operation and Development: Paris, France, 2019; ISBN 978-92-64-48458-0. [Google Scholar]
- Products—Data Briefs—Number 392—November 2020. Available online: https://www.cdc.gov/nchs/products/databriefs/db392.htm (accessed on 26 August 2021).
- Ott, R.; Stupin, J.H.; Loui, A.; Eilers, E.; Melchior, K.; Rancourt, R.C.; Schellong, K.; Ziska, T.; Dudenhausen, J.W.; Henrich, W.; et al. Maternal Overweight is not an Independent Risk Factor for Increased Birth Weight, Leptin and Insulin in Newborns of Gestational Diabetic Women: Observations from the Prospective “EaCH” Cohort Study. BMC Pregnancy Childbirth 2018, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. Estado Mundial de la Infancia 2019: Niños, Alimentos y Nutrición—Crecer Bien en un Mundo en Transformación; United Nations: New York, NY, USA, 2020; ISBN 978-92-1-004949-8. [Google Scholar]
- Dodd, J.M.; Grivell, R.M.; Nguyen, A.-M.; Chan, A.; Robinson, J.S. Maternal and Perinatal Health Outcomes by Body Mass Index Category: Maternal and Perinatal Health Outcomes by BMI Category. Aust. New Zealand J. Obstet. Gynaecol. 2011, 51, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, H.M.; Mercer, B.M.; Catalano, P.M. The Influence of Obesity and Diabetes on the Prevalence of Macrosomia. Am. J. Obstet. Gynecol. 2004, 191, 964–968. [Google Scholar] [CrossRef]
- Catalano, P.M.; Presley, L.; Minium, J.; Hauguel-de Mouzon, S. Fetuses of Obese Mothers Develop Insulin Resistance in Utero. Diabetes Care 2009, 32, 1076–1080. [Google Scholar] [CrossRef]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic Syndrome in Childhood: Association with Birth Weight, Maternal Obesity, and Gestational Diabetes Mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef]
- Casanello, P.; Krause, B.J.; Castro-Rodriguez, J.A.; Uauy, R. Fetal programming of chronic diseases: Current concepts and epigenetics. Rev. Chil. Pediatr. 2015, 86, 135–137. [Google Scholar] [CrossRef]
- Alfaradhi, M.Z.; Ozanne, S.E. Developmental Programming in Response to Maternal Overnutrition. Front. Genet. 2011, 2, 27. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanuš, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S. Central and Peripheral Cannabinoid Receptors as Therapeutic Targets in the Control of Food Intake and Body Weight. In Appetite Control; Joost, H.-G., Ed.; Handbook of Experimental Pharmacology; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2012; Volume 209, pp. 357–381. ISBN 978-3-642-24715-6. [Google Scholar]
- Pagotto, U.; Marsicano, G.; Cota, D.; Lutz, B.; Pasquali, R. The Emerging Role of the Endocannabinoid System in Endocrine Regulation and Energy Balance. Endocr. Rev. 2006, 27, 73–100. [Google Scholar] [CrossRef]
- Alvheim, A.R.; Malde, M.K.; Osei-Hyiaman, D.; Hong, Y.H.; Pawlosky, R.J.; Madsen, L.; Kristiansen, K.; Frøyland, L.; Hibbeln, J.R. Dietary Linoleic Acid Elevates Endogenous 2-AG and Anandamide and Induces Obesity. Obesity 2012, 20, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Hoffler, U.; Hobbie, K.; Wilson, R.; Bai, R.; Rahman, A.; Malarkey, D.; Travlos, G.; Ghanayem, B.I. Diet-Induced Obesity Is Associated with Hyperleptinemia, Hyperinsulinemia, Hepatic Steatosis, and Glomerulopathy in C57Bl/6J Mice. Endocrine 2009, 36, 311–325. [Google Scholar] [CrossRef]
- Osei-Hyiaman, D.; DePetrillo, M.; Pacher, P.; Liu, J.; Radaeva, S.; Bátkai, S.; Harvey-White, J.; Mackie, K.; Offertáler, L.; Wang, L.; et al. Endocannabinoid Activation at Hepatic CB1 Receptors Stimulates Fatty Acid Synthesis and Contributes to Diet-Induced Obesity. J. Clin. Investig. 2005, 115, 1298–1305. [Google Scholar] [CrossRef]
- Di Marzo, V.; Matias, I. Endocannabinoid Control of Food Intake and Energy Balance. Nat. Neurosci. 2005, 8, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Cota, D.; Marsicano, G.; Tschöp, M.; Grübler, Y.; Flachskamm, C.; Schubert, M.; Auer, D.; Yassouridis, A.; Thöne-Reineke, C.; Ortmann, S.; et al. The Endogenous Cannabinoid System Affects Energy Balance via Central Orexigenic Drive and Peripheral Lipogenesis. J. Clin. Investig. 2003, 112, 423–431. [Google Scholar] [CrossRef]
- Valenzuela, C.A.; Castillo, V.A.; Aguirre, C.A.; Ronco, A.M.; Llanos, M.N. The CB 1 Receptor Antagonist SR141716A Reverses Adult Male Mice Overweight and Metabolic Alterations Induced by Early Stress. Obesity 2011, 19, 29–35. [Google Scholar] [CrossRef]
- Ramírez-López, M.T.; Arco, R.; Decara, J.; Vázquez, M.; Noemí Blanco, R.; Alén, F.; Suárez, J.; Gómez de Heras, R.; Rodríguez de Fonseca, F. Exposure to a Highly Caloric Palatable Diet during the Perinatal Period Affects the Expression of the Endogenous Cannabinoid System in the Brain, Liver and Adipose Tissue of Adult Rat Offspring. PLoS ONE 2016, 11, e0165432. [Google Scholar] [CrossRef]
- Dalrymple, K.V.; Flynn, A.C.; Relph, S.A.; O’Keeffe, M.; Poston, L. Lifestyle Interventions in Overweight and Obese Pregnant or Postpartum Women for Postpartum Weight Management: A Systematic Review of the Literature. Nutrients 2018, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Agha, M.; Agha, R.A.; Sandell, J. Interventions to Reduce and Prevent Obesity in Pre-Conceptual and Pregnant Women: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e95132. [Google Scholar] [CrossRef] [PubMed]
- Vesco, K.K.; Karanja, N.; King, J.C.; Gillman, M.W.; Leo, M.C.; Perrin, N.; McEvoy, C.T.; Eckhardt, C.L.; Smith, K.S.; Stevens, V.J. Efficacy of a Group-Based Dietary Intervention for Limiting Gestational Weight Gain among Obese Women: A Randomized Trial: Weight Management in Pregnancy. Obesity 2014, 22, 1989–1996. [Google Scholar] [CrossRef]
- Matusiak, K.; Barrett, H.L.; Callaway, L.K.; Nitert, M.D. Periconception Weight Loss: Common Sense for Mothers, but What about for Babies? J. Obes. 2014, 2014, 204295. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Olson, P.; Rasmussen, D.; Keith, B.; Williamson, M.; Zhang, K.K.; Xie, L. A Short-Term Transition from a High-Fat Diet to a Normal-Fat Diet before Pregnancy Exacerbates Female Mouse Offspring Obesity. Int. J. Obes. 2016, 40, 564–572. [Google Scholar] [CrossRef]
- Loizzo, A.; Loizzo, S.; Galietta, G.; Caiola, S.; Spampinato, S.; Campana, G.; Seghieri, G.; Ghirlanda, G.; Franconi, F. Overweight and Metabolic and Hormonal Parameter Disruption Are Induced in Adult Male Mice by Manipulations During Lactation Period. Pediatr. Res. 2006, 59, 111–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Srinivasan, M.; Katewa, S.D.; Palaniyappan, A.; Pandya, J.D.; Patel, M.S. Maternal High-Fat Diet Consumption Results in Fetal Malprogramming Predisposing to the Onset of Metabolic Syndrome-like Phenotype in Adulthood. Am. J. Physiol.-Endocrinol. Metab. 2006, 291, E792–E799. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sloboda, D.M.; Vickers, M.H. Maternal Obesity and Developmental Programming of Metabolic Disorders in Offspring: Evidence from Animal Models. Exp. Diabetes Res. 2011, 2011, 592408. [Google Scholar] [CrossRef]
- Hartil, K.; Vuguin, P.M.; Kruse, M.; Schmuel, E.; Fiallo, A.; Vargas, C.; Warner, M.J.; Durand, J.L.; Jelicks, L.A.; Charron, M.J. Maternal Substrate Utilization Programs the Development of the Metabolic Syndrome in Male Mice Exposed to High Fat In Utero. Pediatr. Res. 2009, 66, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.N.; Woollett, L.A.; Barbour, N.; Prasad, P.D.; Powell, T.L.; Jansson, T. High-fat Diet before and during Pregnancy Causes Marked Up-regulation of Placental Nutrient Transport and Fetal Overgrowth in C57/BL6 Mice. FASEB J. 2009, 23, 271–278. [Google Scholar] [CrossRef]
- Tellechea, M.L.; Mensegue, M.F.; Pirola, C.J. The Association between High Fat Diet around Gestation and Metabolic Syndrome-Related Phenotypes in Rats: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 5086. [Google Scholar] [CrossRef] [PubMed]
- Ornellas, F.; Mello, V.S.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Sexual Dimorphism in Fat Distribution and Metabolic Profile in Mice Offspring from Diet-Induced Obese Mothers. Life Sci. 2013, 93, 454–463. [Google Scholar] [CrossRef]
- Ribaroff, G.A.; Wastnedge, E.; Drake, A.J.; Sharpe, R.M.; Chambers, T.J.G. Animal Models of Maternal High Fat Diet Exposure and Effects on Metabolism in Offspring: A Meta-Regression Analysis. Obes. Rev. 2017, 18, 673–686. [Google Scholar] [CrossRef]
- Dunn, G.A.; Bale, T.L. Maternal High-Fat Diet Promotes Body Length Increases and Insulin Insensitivity in Second-Generation Mice. Endocrinology 2009, 150, 4999–5009. [Google Scholar] [CrossRef]
- Oben, J.A.; Patel, T.; Mouralidarane, A.; Samuelsson, A.M.; Matthews, P.; Pombo, J.; Morgan, M.; Mckee, C.; Soeda, J.; Novelli, M.; et al. Maternal Obesity Programmes Offspring Development of Non-Alcoholic Fatty Pancreas Disease. Biochem. Biophys. Res. Commun. 2010, 394, 24–28. [Google Scholar] [CrossRef]
- Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Adiponectin: Action, Regulation and Association to Insulin Sensitivity. Obes. Rev. 2005, 6, 13–21. [Google Scholar] [CrossRef]
- Wesolowski, S.R.; Mulligan, C.M.; Janssen, R.C.; Baker, P.R.; Bergman, B.C.; D’Alessandro, A.; Nemkov, T.; Maclean, K.N.; Jiang, H.; Dean, T.A.; et al. Switching Obese Mothers to a Healthy Diet Improves Fetal Hypoxemia, Hepatic Metabolites, and Lipotoxicity in Non-Human Primates. Mol. Metab. 2018, 18, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Gregoraszczuk, E.; Slupecka, M.; Wolinski, J.; Hejmej, A.; Bilinska, B.; Fiedor, E.; Piwnicka, N.; Rak, A. Maternal High-Fat Diet during Pregnancy and Lactation Had Gender Difference Effect on Adiponectin in Rat Offspring. J. Physiol. Pharmacol. 2016, 67, 543–553. [Google Scholar]
- Walker, B.R. Cortisol?Cause and Cure for Metabolic Syndrome? Diabet. Med. 2006, 23, 1281–1288. [Google Scholar] [CrossRef]
- Pasquali, R.; Vicennati, V.; Gambineri, A.; Pagotto, U. Sex-Dependent Role of Glucocorticoids and Androgens in the Pathophysiology of Human Obesity. Int. J. Obes. 2008, 32, 1764–1779. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Vicennati, V.; Cacciari, M.; Pagotto, U. The Hypothalamic-Pituitary-Adrenal Axis Activity in Obesity and the Metabolic Syndrome. Ann. New York Acad. Sci. 2006, 1083, 111–128. [Google Scholar] [CrossRef]
- Mueller, B.; Bale, T. Impact of Prenatal Stress on Long Term Body Weight Is Dependent on Timing and Maternal Sensitivity. Physiol. Behav. 2006, 88, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Carrier, E.J.; Ho, W.-S.V.; Shi, L.; Patel, S.; Gorzalka, B.B.; Hillard, C.J. Prolonged Glucocorticoid Treatment Decreases Cannabinoid CB1 Receptor Density in the Hippocampus. Hippocampus 2008, 18, 221–226. [Google Scholar] [CrossRef]
- Weidenfeld, J.; Feldman, S.; Mechoulam, R. Effect of the Brain Constituent Anandamide, a Cannabinoid Receptor Agonist, on the Hypothalamo-Pituitary-Adrenal Axis in the Rat. Neuroendocrinology 1994, 59, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Roelke, C.T.; Rademacher, D.J.; Cullinan, W.E.; Hillard, C.J. Endocannabinoid Signaling Negatively Modulates Stress-Induced Activation of the Hypothalamic-Pituitary-Adrenal Axis. Endocrinology 2004, 145, 5431–5438. [Google Scholar] [CrossRef] [PubMed]
- D’Asti, E.; Long, H.; Tremblay-Mercier, J.; Grajzer, M.; Cunnane, S.C.; Di Marzo, V.; Walker, C.-D. Maternal Dietary Fat Determines Metabolic Profile and the Magnitude of Endocannabinoid Inhibition of the Stress Response in Neonatal Rat Offspring. Endocrinology 2010, 151, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.R. Nutritional and Hormonal Factors Influencing Desaturation of Essential Fatty Acids. Prog. Lipid Res. 1981, 20, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Videla, L.A.; Hernandez-Rodas, M.C.; Metherel, A.H.; Valenzuela, R. Influence of the Nutritional Status and Oxidative Stress in the Desaturation and Elongation of N-3 and n-6 Polyunsaturated Fatty Acids: Impact on Non-Alcoholic Fatty Liver Disease. Prostaglandins Leukot. Essent. Fat. Acids 2022, 181, 102441. [Google Scholar] [CrossRef] [PubMed]
- González-Mañán, D.; Tapia, G.; Gormaz, J.G.; D’Espessailles, A.; Espinosa, A.; Masson, L.; Varela, P.; Valenzuela, A.; Valenzuela, R. Bioconversion of α-Linolenic Acid to n-3 LCPUFA and Expression of PPAR-Alpha, Acyl Coenzyme A Oxidase 1 and Carnitine Acyl Transferase I Are Incremented after Feeding Rats with α-Linolenic Acid-Rich Oils. Food Funct. 2012, 3, 765. [Google Scholar] [CrossRef]
- Domenichiello, A.F.; Kitson, A.P.; Chen, C.T.; Trépanier, M.-O.; Stavro, P.M.; Bazinet, R.P. The Effect of Linoleic Acid on the Whole Body Synthesis Rates of Polyunsaturated Fatty Acids from α-Linolenic Acid and Linoleic Acid in Free-Living Rats. J. Nutr. Biochem. 2016, 30, 167–176. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.A.; Valenzuela, R.; Hernandez-Rodas, M.C.; Marambio, M.; Espinosa, A.; Mayer, S.; Romero, N.; Barrera, M.; Sc, C.; Valenzuela, A.; et al. Supplementation with Antioxidant-Rich Extra Virgin Olive Oil Prevents Hepatic Oxidative Stress and Reduction of Desaturation Capacity in Mice Fed a High-Fat Diet: Effects on Fatty Acid Composition in Liver and Extrahepatic Tissues. Nutrition 2016, 32, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Salem, N.; Sinclair, A.J.; Cunnane, S.C. α-Linolenic Acid Supplementation and Conversion to n-3 Long-Chain Polyunsaturated Fatty Acids in Humans. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef]
- Lauritzen, L.; Carlson, S.E. Maternal Fatty Acid Status during Pregnancy and Lactation and Relation to Newborn and Infant Status: Transfer of Fatty Acids from Mother to Child. Matern. Child Nutr. 2011, 7, 41–58. [Google Scholar] [CrossRef]
- Lewis, R.M.; Desoye, G. Placental Lipid and Fatty Acid Transfer in Maternal Overnutrition. Ann. Nutr. Metab. 2017, 70, 228–231. [Google Scholar] [CrossRef]
- Kowalski, G.M.; Hamley, S.; Selathurai, A.; Kloehn, J.; De Souza, D.P.; O’Callaghan, S.; Nijagal, B.; Tull, D.L.; McConville, M.J.; Bruce, C.R. Reversing Diet-Induced Metabolic Dysregulation by Diet Switching Leads to Altered Hepatic de Novo Lipogenesis and Glycerolipid Synthesis. Sci. Rep. 2016, 6, 27541. [Google Scholar] [CrossRef] [PubMed]
- Boekelheide, K.; Blumberg, B.; Chapin, R.E.; Cote, I.; Graziano, J.H.; Janesick, A.; Lane, R.; Lillycrop, K.; Myatt, L.; States, J.C.; et al. Predicting Later-Life Outcomes of Early-Life Exposures. Environ. Health Perspect. 2012, 120, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Perez, P.A.; DiPatrizio, N.V. Impact of Maternal Western Diet-Induced Obesity on Offspring Mortality and Peripheral Endocannabinoid System in Mice. PLoS ONE 2018, 13, e0205021. [Google Scholar] [CrossRef] [PubMed]


| Enzyme | Sequence | T |
|---|---|---|
| AAC1 Fw qRT-PCR | 5′-CCT CCG TCA GCT CAG ATA CA-3′ | 56.0 °C |
| ACC1 Rev qRT-PCR | 5′-TTT ACT AGG TGC AAG CCA GAC A-3′ | 56.2 °C |
| FAS1 Fw qRT-PCR | 5′-GGA GGT GGT GAT AGC CGG TAT-3′ | 58.5 °C |
| FAS1 Rev qRT-PCR | 5′-TGG GTA ATC CAT AGA GCC CAG-3′ | 56.0 °C |
| FAS2 Fw qRT-PCR | 5′-GGC ACT GAC TGT CTG TTT TCC A-3′ | 57.5 °C |
| FAS2 Rev qRT-PCR | 5′-GTA AAA ATG ACA CAG TCC AGA CAC TTC-3′ | 56.0 °C |
| YWHAZ Fw qRT-PCR (housekeeping) | 5′-TTG ATC CCC AAT GCT TCG C-3′ | 55.9 °C |
| YWHAZ Rev qRT-PCR (housekeeping) | 5′-CAG CAA CCT CGG CCA AGT AA-3′ | 57.8 °C |
| Groups of Study | ||||
|---|---|---|---|---|
| Control-F0 | High Fat Diet-F0 | Intervention-F0 | p Value | |
| Fatty Acid | Fatty Acid Composition (FAME) | |||
| C16:0 | 44.2 ± 15.2 | 80.2 ± 19.9 | 51.4 ± 17.1 | NS |
| C18:0 | 15.4 ± 5.5 a | 26.2 ± 2.7 b | 17.4 ± 3.8 | 0.0336 |
| C18:1n-9 | 112.8 ± 51.1 | 112.1 ± 23.0 | 111.9 ± 36.0 | NS |
| C18:2n-6 (LA) | 20.8 ± 6.0 a | 68.4 ± 19.0 b | 22.6 ± 6.6 | 0.0380 |
| C18:3n-3 (ALA) | 0.6 ± 0.2 | 1.9 ± 0.7 b | 0.6 ± 0.3 a | 0.0422 |
| C20:4n-6 (AA) | 14.1 ± 3.1 | 23.3 ± 1.9 b | 13.0 ± 6.4 a | 0.0436 |
| C20:5n-3 (EPA) | 0.2 ± 0.06 | 0.4 ± 0.1 | 0.3 ± 0.06 | NS |
| C22:6n-3 (DHA) | 7.7 ± 1.5 a | 15.0 ± 2.3 b | 9.1 ± 2.0 | 0.0380 |
| SFA | 63.0 ± 25.0 | 108.0 ± 22.9 | 70.3 ± 21.0 | NS |
| MUFA | 124.0 ± 55.1 | 117.0 ± 23.0 | 133.0 ± 39.3 | NS |
| PUFA | 45.7 ± 11.1 a | 114.2 ± 25.3 b | 48.4 ± 5.7 | 0.0380 |
| LCPUFA | 22.7 ± 1.6 a | 39.5 ± 1.4 b | 23.2 ± 1.2 | 0.0363 |
| n-6 LCPUFA | 14.8 ± 1.5 | 24.1 ± 1.2 b | 13.8 ± 1.0a | 0.0423 |
| n-3 LCPUFA | 7.9 ± 0.6 a | 15.4 ± 0.5 b | 9.4 ± 0.04 | 0.0380 |
| n-6/n-3 LCPUFA ratio | 1.9 ± 0.05 | 1.6 ± 0.04 | 1.5 ± 0.05 | NS |
| Variable | Groups of Study | |||
|---|---|---|---|---|
| CON-F1 (n = 10) | HFD-F1 (n = 5) | INT-F1 (n = 10) | p Value | |
| Body weight (g) | 32.3 ± 0.8 | 29.1 ± 0.8 | 29.9 ± 0.9 | 0.08 |
| Liver weight (g) | 1.2 ± 0.04 a | 0.96 ± 0.13 b | 1.14 ± 0.04 a | 0.05 |
| Visceral fat weight (g) | 1.09 ± 0.05 | 0.84 ± 0.16 | 0.89 ± 0.07 | 0.15 |
| Accumulated energy intake (kcal) | 4815.9 ± 133.4 | 4677.3 ± 126.3 | 5680 ± 52.09 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrera, C.; Castillo, V.; Valenzuela, R.; Valenzuela, C.A.; Garcia-Diaz, D.F.; Llanos, M. Effects on Fetal Metabolic Programming and Endocannabinoid System of a Normocaloric Diet during Pregnancy and Lactation of Female Mice with Pregestational Obesity. Nutrients 2023, 15, 3531. https://doi.org/10.3390/nu15163531
Barrera C, Castillo V, Valenzuela R, Valenzuela CA, Garcia-Diaz DF, Llanos M. Effects on Fetal Metabolic Programming and Endocannabinoid System of a Normocaloric Diet during Pregnancy and Lactation of Female Mice with Pregestational Obesity. Nutrients. 2023; 15(16):3531. https://doi.org/10.3390/nu15163531
Chicago/Turabian StyleBarrera, Cynthia, Valeska Castillo, Rodrigo Valenzuela, Carina A. Valenzuela, Diego F. Garcia-Diaz, and Miguel Llanos. 2023. "Effects on Fetal Metabolic Programming and Endocannabinoid System of a Normocaloric Diet during Pregnancy and Lactation of Female Mice with Pregestational Obesity" Nutrients 15, no. 16: 3531. https://doi.org/10.3390/nu15163531
APA StyleBarrera, C., Castillo, V., Valenzuela, R., Valenzuela, C. A., Garcia-Diaz, D. F., & Llanos, M. (2023). Effects on Fetal Metabolic Programming and Endocannabinoid System of a Normocaloric Diet during Pregnancy and Lactation of Female Mice with Pregestational Obesity. Nutrients, 15(16), 3531. https://doi.org/10.3390/nu15163531







