The Effect of the Rice Endosperm Protein Hydrolysate on the Subjective Negative Mood Status in Healthy Humans: A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial
Abstract
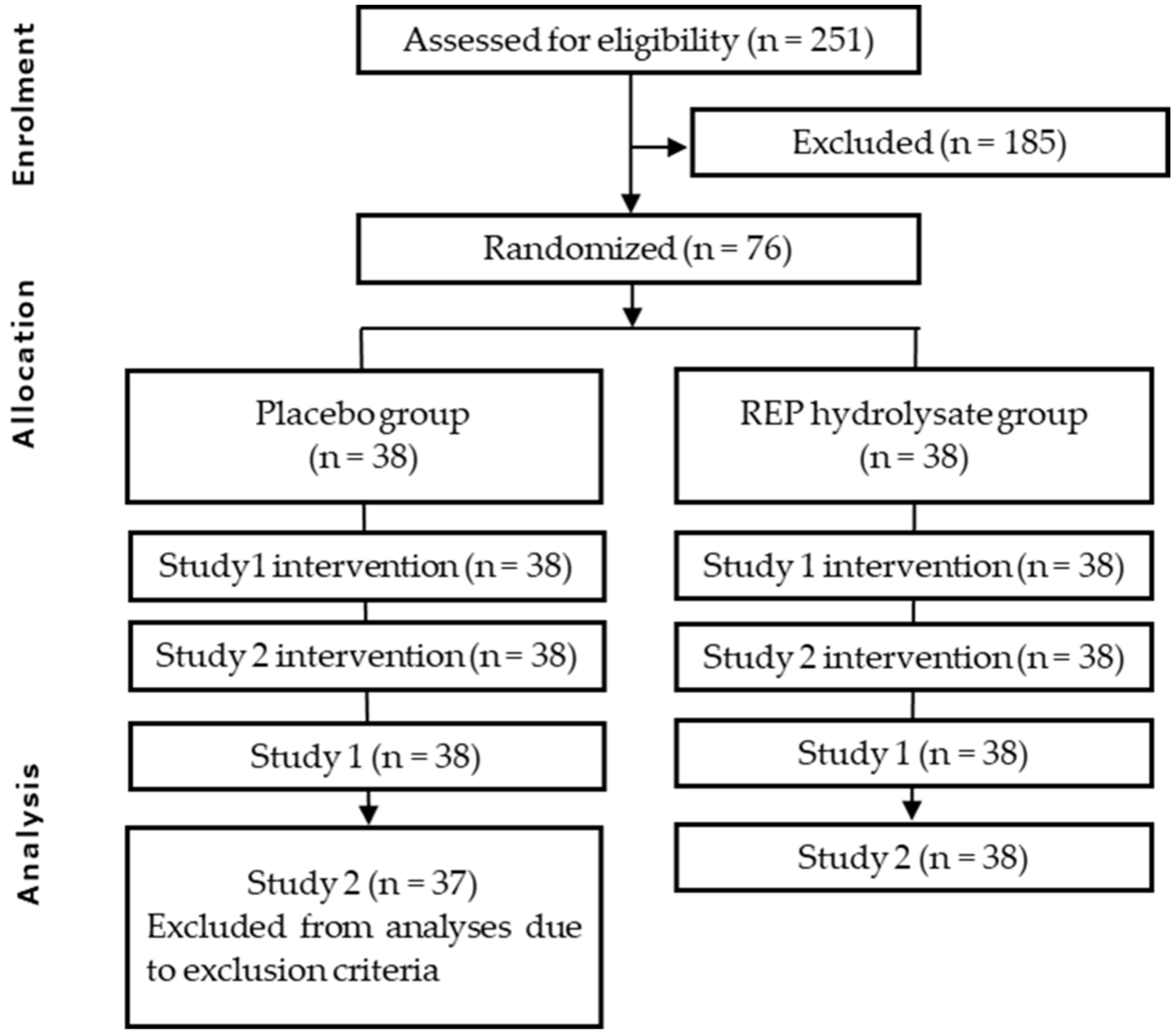
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Study 1
2.1.2. Study 2
2.2. Procedure
2.3. Participants
2.4. Test Foods
2.5. Measurements
2.5.1. Subjective Mood State Questionnaire
2.5.2. Subjective Sleep Quality Evaluation
2.5.3. Measurements of Salivary CgA Concentrations
2.6. Outcomes
2.6.1. Study 1
2.6.2. Study 2
2.7. Sample Size Calculation
2.8. Randomization and Blinding
2.9. Statistical Analyses
3. Results
3.1. Study 1
3.1.1. Subjective Mood State Questionnaire (POMS 2, Euthymia Scale)
3.1.2. Salivary CgA Concentrations (Post Hoc)
3.2. Study 2
3.2.1. Subjective Mood State Questionnaire (POMS 2, Euthymia Scale)
3.2.2. Subjective Insomnia Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An Overview of Global Rice Production, Supply, Trade, and Consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Hu, Z.; Yu, Y.; Mou, R.; Zhu, Z.; Beta, T. Phenolic Acids, Anthocyanins, Proanthocyanidins, Antioxidant Activity, Minerals and Their Correlations in Non-Pigmented, Red, and Black Rice. Food Chem. 2018, 239, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.S.; Bhattacharya, K.R. Research Note the Texture of Cooked Rice. J. Texture Stud. 1982, 13, 31–42. [Google Scholar] [CrossRef]
- Lyon, B.G.; Champagne, E.T.; Vinyard, B.T.; Windham, W.R.; Barton, F.E.; Webb, B.D.; McClung, A.M.; Moldenhauer, K.A.; Linscombe, S.; McKenzie, K.S.; et al. Effects of Degree of Milling, Drying Condition, and Final Moisture Content on Sensory Texture of Cooked Rice. Cereal Chem. J. 1999, 76, 56–62. [Google Scholar] [CrossRef]
- Roy, P.; Orikasa, T.; Okadome, H.; Nakamura, N.; Shiina, T. Processing Conditions, Rice Properties, Health and Environment. Int. J. Environ. Res. Public Health 2011, 8, 1957–1976. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. The National Health and Nutrition Survey in Japan; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2019. [Google Scholar]
- Ishikawa, Y.; Hira, T.; Inoue, D.; Harada, Y.; Hashimoto, H.; Fujii, M.; Kadowaki, M.; Hara, H. Rice Protein Hydrolysates Stimulate GLP-1 Secretion, Reduce GLP-1 Degradation, and Lower the Glycemic Response in Rats. Food Funct. 2015, 6, 2525–2534. [Google Scholar] [CrossRef]
- Hosojima, M.; Kaseda, R.; Kondo, H.; Fujii, M.; Kubota, M.; Watanabe, R.; Tanabe, N.; Kadowaki, M.; Suzuki, Y.; Saito, A. Beneficial Effects of Rice Endosperm Protein Intake in Japanese Men with Risk Factors for Metabolic Syndrome: A Randomized, Crossover Clinical Trial. BMC Nutr. 2016, 2, 25. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, Y.; Hosojima, M.; Kabasawa, H.; Kuwahara, S.; Goto, S.; Toba, K.; Kaseda, R.; Tanaka, T.; Kitamura, N.; Takihara, H.; et al. Rice Endosperm Protein Administration to Juvenile Mice Regulates Gut Microbiota and Suppresses the Development of High-Fat Diet-Induced Obesity and Related Disorders in Adulthood. Nutrients 2019, 11, 2919. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, R.; Meisel, H. Food-Derived Peptides with Biological Activity: From Research to Food Applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef]
- Daroit, D.J.; Brandelli, A. In Vivo Bioactivities of Food Protein-Derived Peptides—A Current Review. Curr. Opin. Food Sci. 2021, 39, 120–129. [Google Scholar] [CrossRef]
- Mori, Y.; Asakura, S.; Yamamoto, A.; Odagiri, S.; Yamada, D.; Sekiguchi, M.; Wada, K.; Sato, M.; Kurabayashi, A.; Suzuki, H.; et al. Characterization of Soy-Deprestatin, a Novel Orally Active Decapeptide That Exerts Antidepressant-like Effects via Gut-Brain Communication. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 568–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.-S.; Yang, W.-S.; Kim, C.-H. Beneficial Effects of Soybean-Derived Bioactive Peptides. Int. J. Mol. Sci. 2021, 22, 8570. [Google Scholar] [CrossRef] [PubMed]
- Nishi, D.; Susukida, R.; Usuda, K.; Mojtabai, R.; Yamanouchi, Y. Trends in the Prevalence of Psychological Distress and the Use of Mental Health Services from 2007 to 2016 in Japan. J. Affect. Disord. 2018, 239, 208–213. [Google Scholar] [CrossRef]
- Mojtabai, R.; Jorm, A.F. Trends in Psychological Distress, Depressive Episodes and Mental Health Treatment-Seeking in the United States: 2001–2012. J. Affect. Disord. 2015, 174, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Dyrbye, L.N.; Thomas, M.R.; Shanafelt, T.D. Systematic Review of Depression, Anxiety, and Other Indicators of Psychological Distress among U.S. And Canadian Medical Students. Acad. Med. 2006, 81, 354–373. [Google Scholar] [CrossRef]
- Marchand, A.; Demers, A.; Durand, P. Does Work Really Cause Distress? The Contribution of Occupational Structure and Work Organization to the Experience of Psychological Distress. Soc. Sci. Med. 2005, 61, 1–14. [Google Scholar] [CrossRef]
- Smyth, J.M.; Johnson, J.A.; Auer, B.J.; Lehman, E.; Talamo, G.; Sciamanna, C.N. Online Positive Affect Journaling in the Improvement of Mental Distress and Well-Being in General Medical Patients with Elevated Anxiety Symptoms: A Preliminary Randomized Controlled Trial. JMIR Ment. Health 2018, 5, e11290. [Google Scholar] [CrossRef] [Green Version]
- Fournier, J.C.; DeRubeis, R.J.; Hollon, S.D.; Dimidjian, S.; Amsterdam, J.D.; Shelton, R.C.; Fawcett, J. Antidepressant Drug Effects and Depression Severity. JAMA 2010, 303, 47. [Google Scholar] [CrossRef] [Green Version]
- Eakley, R.; Lyndon, A. Antidepressant Use during Pregnancy: Knowledge, Attitudes, and Decision-Making of Patients and Providers. J. Midwifery Women’s Health 2022, 67, 332–353. [Google Scholar] [CrossRef]
- Lang, U.E.; Beglinger, C.; Schweinfurth, N.; Walter, M.; Borgwardt, S. Nutritional Aspects of Depression. Cell. Physiol. Biochem. 2015, 37, 1029–1043. [Google Scholar] [CrossRef]
- Suzuki, T.; Miyaki, K.; Tsutsumi, A.; Hashimoto, H.; Kawakami, N.; Takahashi, M.; Shimazu, A.; Inoue, A.; Kurioka, S.; Kakehashi, M.; et al. Japanese Dietary Pattern Consistently Relates to Low Depressive Symptoms and It Is Modified by Job Strain and Worksite Supports. J. Affect. Disord. 2013, 150, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Nishi, D.; Su, K.-P.; Usuda, K.; Pei-Chen Chang, J.; Chiang, Y.-J.; Chen, H.; Chien, Y.-C.; Guu, T.-W.; Okazaki, E.; Hamazaki, K.; et al. The Efficacy of Omega-3 Fatty Acids for Depressive Symptoms among Pregnant Women in Japan and Taiwan: A Randomized, Double-Blind, Placebo-Controlled Trial (SYNCHRO; NCT01948596). Psychother. Psychosom. 2018, 88, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.A.; Forster, J.; Khan, J.; Pouchieu, C.; Dubreuil, S.; Gaudout, D.; Moras, B.; Pourtau, L.; Joffre, F.; Vaysse, C.; et al. Effects of Saffron Extract Supplementation on Mood, Well-Being, and Response to a Psychosocial Stressor in Healthy Adults: A Randomized, Double-Blind, Parallel Group, Clinical Trial. Front. Nutr. 2021, 7, 606124. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, B.; O’Neill, G.; Abu-Ghannam, N. Potential Psychoactive Effects of Microalgal Bioactive Compounds for the Case of Sleep and Mood Regulation: Opportunities and Challenges. Mar. Drugs 2022, 20, 493. [Google Scholar] [CrossRef]
- Ohinata, K.; Asakura, S.; Kaneko, K.; Kawano, K.; Shobako, M.; Jo, S.; Sato, M.; Kurabayashi, A.; Suzuki, H.; Ito, A.; et al. Rice Endosperm-Derived Antidepressant-like Peptide (REAP): An Orally Active Novel Tridecapeptide Derived from Rice Protein. Preprint 2022. [Google Scholar] [CrossRef]
- Sasai, M.; Kato, M.; Ohsawa, K.; Sashihara, K.; Nakamura, Y.; Kaneko, T. Effects of a Single Dose of Tablets Containing Lactononadecapeptide on Cognitive Function in Healthy Adults: A Randomized, Double-Blind, Cross-Over, Placebo-Controlled Trial. Biosci. Biotechnol. Biochem. 2020, 85, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, G.; De Cesaris, F.; Geppetti, P.; Cosci, F. Protocol and Methods for Testing the Efficacy of Well-Being Therapy in Chronic Migraine Patients: A Randomized Controlled Trial. Trials 2018, 19, 561. [Google Scholar] [CrossRef]
- Hokazono, H. Effects of γ-Aminobutyric Acid (GABA)—Containing Food on Mood Status and Sleep Quality in Worker—A Double-blind Randomized Controlled Trial. Jpn. Pharmacol. Ther. 2016, 44, 1445–1454. [Google Scholar]
- Yokoyama, K.; Araki, S.; Kawakami, N.; Tkakeshita, T. Production of the Japanese Edition of Profile of Mood States (POMS): Assessment of Reliability and Validity. Jpn. J. Public Health 1990, 37, 913–918. [Google Scholar]
- Konuma, H.; Hirose, H.; Yokoyama, K. Relationship of the Japanese Translation of the Profile of Mood States Second Edition (POMS 2®) to the First Edition (POMS®). Juntendo Med. J. 2015, 61, 517–519. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, N.; Carrozzino, D.; Nishi, D. Sensitivity and Concurrent Validity of the Japanese Version of the Euthymia Scale: A Clinimetric Analysis. BMC Psychiatry 2021, 21, 482. [Google Scholar] [CrossRef] [PubMed]
- Fava, G.A.; Guidi, J. The Pursuit of Euthymia. World Psychiatry 2020, 19, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Munezawa, T.; Morin, C.; Inoue, Y.; Nedate, K. Development of the Japanese Version of the Insomnia Severity Index (ISI-J). Jpn. J. Psychiatr. Treat. 2009, 24, 219–225. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Hengartner, M.P.; Plöderl, M. Statistically Significant Antidepressant-Placebo Differences on Subjective Symptom-Rating Scales Do Not Prove That the Drugs Work: Effect Size and Method Bias Matter! Front. Psychiatry 2018, 9, 517. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, T.; Morimoto-Kobayashi, Y.; Koizumi, K.; Takahashi, C.; Nakajima, S.; Kitao, S.; Taniguchi, Y.; Katayama, M.; Ogawa, Y. Secretion of a Gastrointestinal Hormone, Cholecystokinin, by Hop-Derived Bitter Components Activates Sympathetic Nerves in Brown Adipose Tissue. J. Nutr. Biochem. 2019, 64, 80–87. [Google Scholar] [CrossRef]
- Ayabe, T.; Ohya, R.; Taniguchi, Y.; Shindo, K.; Kondo, K.; Ano, Y. Matured Hop-Derived Bitter Components in Beer Improve Hippocampus-Dependent Memory through Activation of the Vagus Nerve. Sci. Rep. 2018, 8, 15372. [Google Scholar] [CrossRef] [Green Version]
- Marquette, C.; Linard, C.; Galonnier, M.; Van Uye, A.; Mathieu, J.; Gourmelon, P.; Clarençon, D. IL-1beta, TNFalpha and IL-6 Induction in the Rat Brain after Partial-Body Irradiation: Role of Vagal Afferents. Int. J. Radiat. Biol. 2003, 79, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.D.; Xu, Q.J.; Chang, R.B. Vagal Sensory Neurons and Gut-Brain Signaling. Curr. Opin. Neurobiol. 2020, 62, 133–140. [Google Scholar] [CrossRef]
- Vignes, M.; Maurice, T.; Lanté, F.; Nedjar, M.; Thethi, K.; Guiramand, J.; Récasens, M. Anxiolytic Properties of Green Tea Polyphenol (−)-Epigallocatechin Gallate (EGCG). Brain Res. 2006, 1110, 102–115. [Google Scholar] [CrossRef]
- Yoto, A.; Murao, S.; Nakamura, Y.; Yokogoshi, H. Intake of Green Tea Inhibited Increase of Salivary Chromogranin a after Mental Task Stress Loads. J. Physiol. Anthropol. 2014, 33, 20. [Google Scholar] [CrossRef] [Green Version]
- Den, R.; Toda, M.; Nagasawa, S.; Kitamura, K.; Morimoto, K. Circadian Rhythm of Human Salivary Chromogranin A. Biomed. Res. 2007, 28, 57–60. [Google Scholar] [CrossRef] [Green Version]
- Nakane, H.; Asami, O.; Yamada, Y.; Harada, T.; Matsui, N.; Kanno, T.; Yanaihara, N. Salivary Chromogranin A as an Index of Psychosomatic Stress Response. Biomed. Res. 1998, 19, 401–406. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Asakura, H.; Hayashi, T. Increased Salivary Chromogranin a in Women with Severe Negative Mood States in the Premenstrual Phase. J. Psychosom. Obstet. Gynecol. 2012, 33, 120–128. [Google Scholar] [CrossRef]
- Chojnowska, S.; Ptaszyńska-Sarosiek, I.; Kępka, A.; Knaś, M.; Waszkiewicz, N. Salivary Biomarkers of Stress, Anxiety and Depression. J. Clin. Med. 2021, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Kanamaru, Y.; Kikukawa, A.; Shimamura, K. Salivary Chromogranin-A as a Marker of Psychological Stress during a Cognitive Test Battery in Humans. Stress 2006, 9, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N. Neurological and Humoral Control of Blood Pressure. Anaesth. Intensive Care Med. 2007, 8, 221–226. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J. The Effects of a Saffron Extract (Affron®) on Menopausal Symptoms in Women during Perimenopause: A Randomised, Double-Blind, Placebo-Controlled Study. J. Menopausal Med. 2021, 27, 66. [Google Scholar] [CrossRef]
- Mazidi, M.; Shemshian, M.; Mousavi, S.H.; Norouzy, A.; Kermani, T.; Moghiman, T.; Sadeghi, A.; Mokhber, N.; Ghayour-Mobarhan, M.; Ferns, G.A.A. A Double-Blind, Randomized and Placebo-Controlled Trial of Saffron (Crocus sativus L.) in the Treatment of Anxiety and Depression. J. Complement. Integr. Med. 2016, 13, 195–199. [Google Scholar] [CrossRef]
- Ettehadi, H.; Mojabi, S.N.; Ranjbaran, M.; Shams, J.; Sahraei, H.; Hedayati, M.; Asefi, F. Aqueous Extract of Saffron (Crocus sativus) Increases Brain Dopamine and Glutamate Concentrations in Rats. J. Behav. Brain Sci. 2013, 3, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Neubauer, A.B.; Smyth, J.M.; Sliwinski, M.J. When You See It Coming: Stressor Anticipation Modulates Stress Effects on Negative Affect. Emotion 2018, 18, 342–354. [Google Scholar] [CrossRef]
- Hyun, J.; Sliwinski, M.J.; Smyth, J.M. Waking up on the Wrong Side of the Bed: The Effects of Stress Anticipation on Working Memory in Daily Life. J. Gerontol. Ser. B 2018, 74, 38–46. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Total (n = 76) | Placebo (n = 38) | Active (n = 38) |
|---|---|---|---|
| Age (years) | 50.1 ± 8.1 | 53.1 ± 6.3 | 48.7 ± 9.2 |
| Height (cm) | 164.74 ± 9.4 | 164.24 ± 8.69 | 165.08 ± 10.24 |
| Weight (kg) | 59.00 ± 10.92 | 59.74 ± 11.59 | 58.26 ± 10.32 |
| BMI (kg/m2) | 21.58 ± 2.48 | 21.90 ± 2.60 | 21.26 ± 2.34 |
| Heart rate (bpm) | 71.97 ± 10.50 | 71.37 ± 10.16 | 72.57 ± 10.93 |
| POMS2 FI | 63.1 ± 1.0 | 63.1 ± 1.1 | 63.1 ± 0.9 |
| POMS2 VA | 37.2 ± 0.5 | 37.2 ± 0.5 | 37.2 ± 0.5 |
| Euthymia Scale | 3.5 ± 0.2 | 3.5 ± 0.3 | 3.4 ± 0.3 |
| First Pre-Examination | Second Pre-Examination | Third Pre-Examination | 0 Weeks (Study 1) | 4 Weeks | Followup | |
|---|---|---|---|---|---|---|
| Health check | ● | ● | ● | |||
| Saliva collection | ● | ● | ||||
| POMS 2 | ● | ● | ● | ● | ||
| Euthymia Scale | ● | ● | ● | ● | ||
| ISI | ● | ● |
| Subscales | Placebo (n = 38) | Active (n = 38) | p Value for Change | |||
|---|---|---|---|---|---|---|
| Score | Change | Score | Change | |||
| AH | Before | 57.4 ± 1.9 | −2.0 ± 1.4 | 53.3 ± 1.7 | −2.9 ± 1.1 | 0.109 |
| After | 55.4 ± 2.2 | 50.4 ± 1.9 | ||||
| CB | Before | 58.1 ± 1.7 | −2.6 ± 1.0 | 60.2 ± 1.8 | −4.3 ± 1.1 | 0.250 |
| After | 55.5 ± 1.8 | 55.9 ± 2.3 | ||||
| DD | Before | 58.0 ± 2.0 | −3.3 ± 0.8 | 60.3 ± 2.1 | −2.6 ± 1.1 | 0.576 |
| After | 54.7 ± 2.0 | 57.7 ± 2.2 | ||||
| FI | Before | 60.8 ± 1.5 | −2.6 ± 1.0 | 60.3 ± 1.4 | −4.6 ± 1.0 | 0.175 |
| After | 58.2 ± 1.8 | 55.7 ± 1.8 | ||||
| TA | Before | 57.1 ± 1.4 | −3.1 ± 0.8 | 58.5 ± 1.6 | −5.8 ± 1.0 | 0.037 |
| After | 53.9 ± 1.7 | 52.7 ± 2.2 | ||||
| VA | Before | 37.6 ± 0.8 | +1.1 ± 0.5 | 37.1 ± 0.8 | +0.0 ± 0.6 | 0.177 |
| After | 38.6 ± 1.0 | 37.1 ± 0.9 | ||||
| F | Before | 40.6 ± 1.2 | −0.1 ± 0.8 | 39.5 ± 1.0 | +0.2 ± 0.8 | 0.801 |
| After | 40.5 ± 1.3 | 39.7 ± 1.1 | ||||
| TMD | Before | 61.8 ± 1.6 | −3.2 ± 0.8 | 61.9 ± 1.7 | −4.3 ± 0.9 | 0.340 |
| After | 58.7 ± 1.8 | 57.6 ± 2.1 | ||||
| Placebo (n = 38) | Active (n = 38) | p Value for Change | |||
|---|---|---|---|---|---|
| Score | Change | Score | Change | ||
| Before | 3.9 ± 0.4 | +0.7 ± 0.2 | 3.9 ± 0.4 | +0.7 ± 0.3 | 0.882 |
| After | 4.6 ± 0.5 | 4.7 ± 0.4 | |||
| Placebo (n = 30) | Active (n = 36) | p Value for Change | |||
|---|---|---|---|---|---|
| pmol/mg Protein | Change | pmol/mg Protein | Change | ||
| Before | 9.0 ± 1.1 | −0.8 ± 1.0 | 12.1 ± 1.7 | −4.3 ± 1.2 | 0.134 * |
| After | 8.3 ± 1.2 | 7.9 ± 0.9 | 0.034 | ||
| Subscales | Weeks | Placebo (n = 38) | Active (n = 37) | p Value for Change | ||
|---|---|---|---|---|---|---|
| Score | Change | Score | Change | |||
| AH | 0 | 57.9 ± 1.9 | −6.5 ± 1.5 | 55.2 ± 1.8 | −5.6 ± 1.6 | 0.674 |
| 4 | 51.4 ± 1.6 | 49.7 ± 1.8 | ||||
| CB | 0 | 59.3 ± 2.0 | −7.4 ± 1.4 | 61.6 ± 1.9 | −7.9 ± 1.6 | 0.802 |
| 4 | 51.9 ± 2.1 | 53.7 ± 2.3 | ||||
| DD | 0 | 59.8 ± 1.9 | −6.8 ± 1.2 | 60.4 ± 2.1 | −4.6 ± 1.3 | 0.244 |
| 4 | 53.1 ± 2.0 | 55.8 ± 2.2 | ||||
| FI | 0 | 62.6 ± 1.6 | −11.2 ± 1.7 | 63.1 ± 1.3 | −9.2 ± 1.8 | 0.421 |
| 4 | 51.5 ± 1.7 | 53.9 ± 1.9 | ||||
| TA | 0 | 58.2 ± 1.7 | −6.9 ± 1.4 | 59.9 ± 1.6 | −7.6 ± 1.8 | 0.781 |
| 4 | 51.3 ± 2.0 | 52.4 ± 2.1 | ||||
| VA | 0 | 37.3 ± 0.8 | +6.1 ± 1.1 | 37.2 ± 0.7 | +3.6 ± 1.1 | 0.107 |
| 4 | 43.4 ± 1.3 | 43.4 ± 1.3 | ||||
| F | 0 | 38.6 ± 1.0 | +5.4 ± 1.4 | 39.5 ± 1.2 | +2.4 ± 1.4 | 0.134 |
| 4 | 44.0 ± 1.5 | 41.9 ± 1.3 | ||||
| TMD | 0 | 63.2 ± 1.6 | −9.8 ± 1.4 | 63.4 ± 1.6 | −8.2 ± 1.5 | 0.442 |
| 4 | 53.5 ± 1.9 | 55.2 ± 2.1 | ||||
| Weeks | Placebo (n = 38) | Active (n = 37) | p Value for Change | |||
|---|---|---|---|---|---|---|
| Score | Change | Score | Change | |||
| Euthymia Scale | 0 | 3.5 ± 0.4 | +2.8 ± 0.3 | 3.4 ± 0.4 | +1.7 ± 0.4 | 0.034 |
| 4 | 6.4 ± 0.5 | 5.1 ± 0.5 | ||||
| Weeks | Placebo (n = 38) | Active (n = 37) | p Value for Change | |||
|---|---|---|---|---|---|---|
| Score | Change | Score | Change | |||
| ISI | 0 | 10.8 ± 0.9 | −3.2 ± 0.7 | 11.9 ± 0.9 | −3.3 ± 0.5 | 0.927 |
| 4 | 7.6 ± 0.9 | 8.7 ± 0.8 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, R.; Nishi, D.; Sato, M.; Ito, A.; Uchiyama, K.; Higuchi, Y.; Takahashi, H.; Ohinata, K. The Effect of the Rice Endosperm Protein Hydrolysate on the Subjective Negative Mood Status in Healthy Humans: A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 3491. https://doi.org/10.3390/nu15153491
Nakayama R, Nishi D, Sato M, Ito A, Uchiyama K, Higuchi Y, Takahashi H, Ohinata K. The Effect of the Rice Endosperm Protein Hydrolysate on the Subjective Negative Mood Status in Healthy Humans: A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Nutrients. 2023; 15(15):3491. https://doi.org/10.3390/nu15153491
Chicago/Turabian StyleNakayama, Ryoko, Daisuke Nishi, Masaru Sato, Akira Ito, Kimiko Uchiyama, Yuki Higuchi, Hajime Takahashi, and Kousaku Ohinata. 2023. "The Effect of the Rice Endosperm Protein Hydrolysate on the Subjective Negative Mood Status in Healthy Humans: A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial" Nutrients 15, no. 15: 3491. https://doi.org/10.3390/nu15153491
APA StyleNakayama, R., Nishi, D., Sato, M., Ito, A., Uchiyama, K., Higuchi, Y., Takahashi, H., & Ohinata, K. (2023). The Effect of the Rice Endosperm Protein Hydrolysate on the Subjective Negative Mood Status in Healthy Humans: A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Nutrients, 15(15), 3491. https://doi.org/10.3390/nu15153491







