The Association between Macrosomia and Amino Acids’ Levels in Maternal and Cord Sera: A Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Study Participants
2.3. Data Collection
2.4. Blood Sample Analyses
2.5. Statistical Analyses
3. Results
3.1. Maternal and Neonatal Data
3.2. Comparative Analysis of Amino Acids’ Levels in Maternal and Cord Serum between the Two Groups
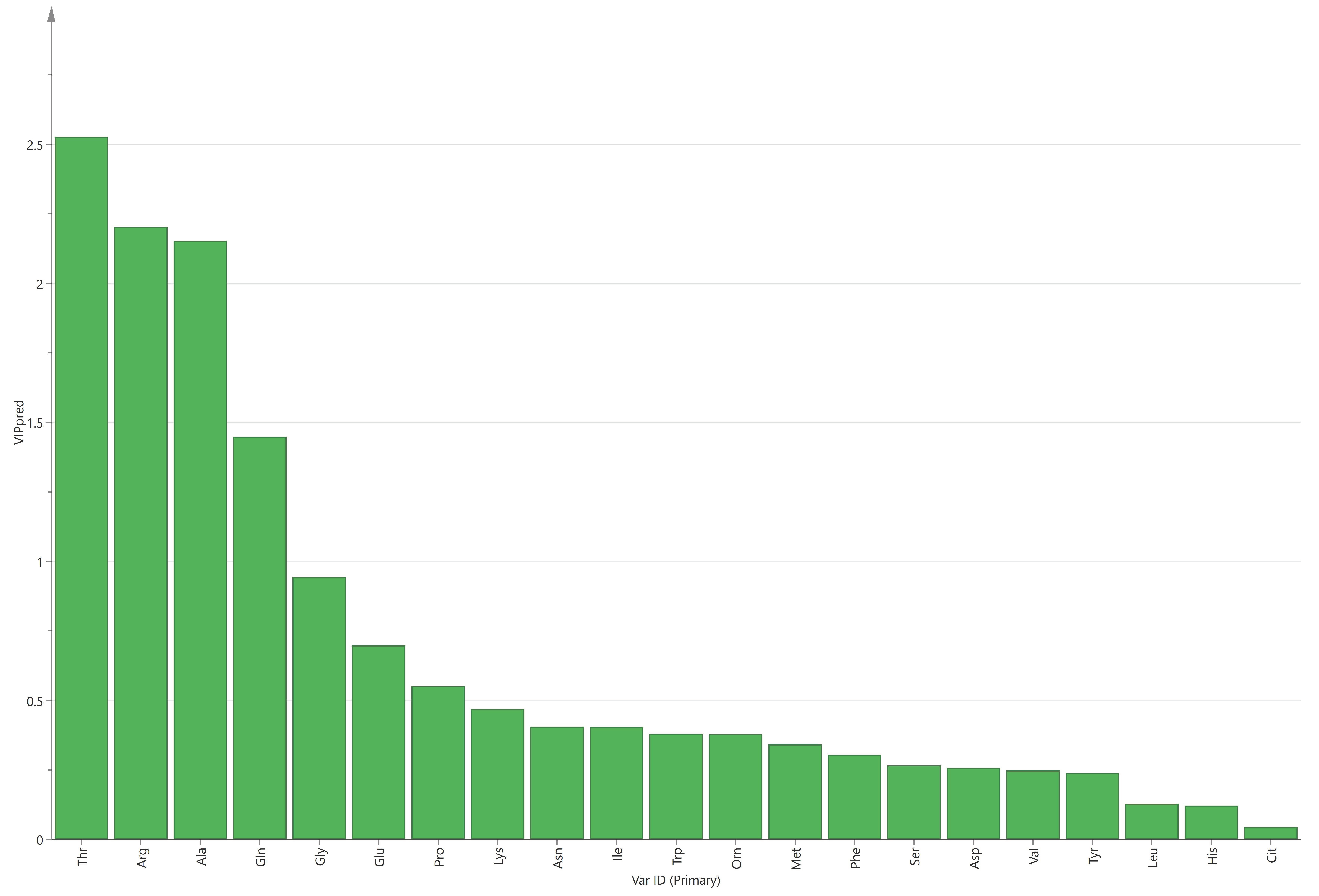
3.3. Relationship between Macrosomia and Amino Acids in Maternal and Cord Serum
4. Discussion
4.1. Macrosomia and Amino Acids in Maternal Serum
4.2. Macrosomia and Amino Acids in Cord Serum
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silasi, M. Fetal Macrosomia. In Obstetric Imaging: Fetal Diagnosis and Care, 2nd ed.; Copel, J.A., D’Alton, M.E., Feltovich, H., Gratacós, E., Krakow, D., Odibo, A.O., Platt, L.D., Tutschek, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 460–462.e461. [Google Scholar]
- Lu, Y.; Zhang, J.; Lu, X.; Xi, W.; Li, Z. Secular trends of macrosomia in southeast China, 1994-2005. BMC Public Health 2011, 11, 818. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Zhai, F.; Zhao, L.; Liu, A.; Yu, W.; Jia, F.; Zhang, J.; Li, J. Incidence of fetal macrosimia and influencing factors in China in 2006. Chin. J. Child Health Care 2008, 16, 11–13. [Google Scholar] [CrossRef]
- Li, G.; Kong, L.; Li, Z.; Zhang, L.; Fan, L.; Zou, L.; Chen, Y.; Ruan, Y.; Wang, X.; Zhang, W. Prevalence of macrosomia and its risk factors in china: A multicentre survey based on birth data involving 101,723 singleton term infants. Paediatr. Perinat. Epidemiol. 2014, 28, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hong, Y.; Zhu, L.; Wang, X.; Lv, Q.; Zhou, Q.; Ruan, M.; Chen, C. Risk factors and outcomes of macrosomia in China: A multicentric survey based on birth data. J. Matern-Fetal Neonatal Med. 2017, 30, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Cheyney, M.; Everson, C.L.; Bovbjerg, M.L. Fetal macrosomia in home and birth center births in the United States: Maternal, fetal, and newborn outcomes. Birth 2020, 47, 409–417. [Google Scholar] [CrossRef]
- Turkmen, S.; Johansson, S.; Dahmoun, M. Foetal Macrosomia and Foetal-Maternal Outcomes at Birth. J. Pregnancy 2018, 2018, 4790136. [Google Scholar] [CrossRef]
- Schellong, K.; Schulz, S.; Harder, T.; Plagemann, A. Birth weight and long-term overweight risk: Systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS ONE 2012, 7, e47776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collier, A.; Abraham, E.C.; Armstrong, J.; Godwin, J.; Monteath, K.; Lindsay, R. Reported prevalence of gestational diabetes in Scotland: The relationship with obesity, age, socioeconomic status, smoking and macrosomia, and how many are we missing? J. Diabetes Investig. 2017, 8, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Tan, J.; Liu, Z.H.; Liu, R.K.; Yang, Z. A cohort study on the correlation between birth weight, simple obesity, blood lipids, blood glucose and blood pressure from childhood to adolescenc. Chin. J. Intern. Med. 2007, 46, 923–925. [Google Scholar] [CrossRef]
- Van Lieshout, R.J.; Savoy, C.D.; Ferro, M.A.; Krzeczkowski, J.E.; Colman, I. Macrosomia and psychiatric risk in adolescence. Eur. Child. Adolesc. Psychiatry 2020, 29, 1537–1545. [Google Scholar] [CrossRef]
- Stotland, N.E.; Caughey, A.B.; Breed, E.M.; Escobar, G.J. Risk factors and obstetric complications associated with macrosomia. Int. J. Gynaecol. Obstet. 2004, 87, 220–226. [Google Scholar] [CrossRef]
- Wallace, S.; McEwan, A. Fetal macrosomia. Obstet. Gynaecol. Reprod. Med. 2007, 17, 58–61. [Google Scholar] [CrossRef]
- Bröer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef] [Green Version]
- King, J.C. Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr. 2000, 71, 1218S–1225S. [Google Scholar] [CrossRef] [Green Version]
- Shang, M.; Wen, Z. Increased placental IGF-1/mTOR activity in macrosomia born to women with gestational diabetes. Diabetes Res. Clin. Pract. 2018, 146, 211–219. [Google Scholar] [CrossRef]
- Kuruvilla, A.G.; D’Souza, S.W.; Glazier, J.D.; Mahendran, D.; Maresh, M.J.; Sibley, C.P. Altered activity of the system A amino acid transporter in microvillous membrane vesicles from placentas of macrosomic babies born to diabetic women. J. Clin. Investig. 1994, 94, 689–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, J.; Cheng, D.; Wang, Y.; Dong, W.; Sun, S.R.; Wang, D.P. Effects of amino acids and vitamin concentrations of pregnant women on birth weight of infants. Tianjin Sci. Tech. 2022, 49, 42–48. [Google Scholar] [CrossRef]
- Kalkhoff, R.K.; Kandaraki, E.; Morrow, P.G.; Mitchell, T.H.; Kelber, S.; Borkowf, H.I. Relationship between neonatal birth weight and maternal plasma amino acid profiles in lean and obese nondiabetic women and in type I diabetic pregnant women. Metabolism 1988, 37, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Tsyvian, P.B.; Bashmakova, N.V.; Kovtun, O.P.; Makarenko, L.V.; Pestryaeva, L.A. Maternal and newborn infants amino acid concentrations in obese women born themselves with normal and small for gestational age birth weight. J. Dev. Orig. Health Dis. 2015, 6, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Cetin, I.; Ronzoni, S.; Marconi, A.M.; Perugino, G.; Corbetta, C.; Battaglia, F.C.; Pardi, G. Maternal concentrations and fetal-maternal concentration differences of plasma amino acids in normal and intrauterine growth-restricted pregnancies. Am. J. Obstet. Gynecol. 1996, 174, 1575–1583. [Google Scholar] [CrossRef]
- Chen, X.; Liang, K.H.; Zhu, H.; Wang, J. Analysis method and application of free amino acids. J. Food Saf. Food Qual. 2021, 12, 7298–7304. [Google Scholar] [CrossRef]
- Wang, J.; Duan, Y.; Yang, J.; Li, J.; Li, F.; Zhou, P.; Liu, C.; Zhao, Y.; Gu, X.; Yuan, C.; et al. Cohort profile: The Taicang and Wuqiang mother-child cohort study (TAWS) in China. BMJ Open 2022, 12, e060868. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsson, O.; Barman, M.; Savolainen, O.; Ross, A.B.; Sandin, A.; Jacobsson, B.; Wold, A.E.; Sandberg, A.S.; Brunius, C. Differences between Arterial and Venous Umbilical Cord Plasma Metabolome and Association with Parity. Metabolites 2022, 12, 175. [Google Scholar] [CrossRef]
- Anand, N.S.; Ji, Y.; Wang, G.; Hong, X.; van der Rijn, M.; Riley, A.; Pearson, C.; Zuckerman, B.; Wang, X. Maternal and cord plasma branched-chain amino acids and child risk of attention-deficit hyperactivity disorder: A prospective birth cohort study. J. Child Psychol. Psychiatry Allied Discip. 2021, 62, 868–875. [Google Scholar] [CrossRef]
- Henriksen, T. The macrosomic fetus: A challenge in current obstetrics. Acta Obstet. Gynecol. Scand. 2008, 87, 134–145. [Google Scholar] [CrossRef]
- Segregur, J.; Buković, D.; Milinović, D.; Oresković, S.; Pavelić, J.; Zupić, T.; Persec, J.; Pavić, M. Fetal macrosomia in pregnant women with gestational diabetes. Coll. Antropol. 2009, 33, 1121–1127. [Google Scholar]
- Battaglia, F.C.; Meschia, G. Fetal nutrition. Annu. Rev. Nutr. 1988, 8, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Meininger, C.J. Arginine nutrition and cardiovascular function. J. Nutr. 2000, 130, 2626–2629. [Google Scholar] [CrossRef] [Green Version]
- Szlas, A.; Kurek, J.M.; Krejpcio, Z. The Potential of L-Arginine in Prevention and Treatment of Disturbed Carbohydrate and Lipid Metabolism—A Review. Nutrients 2022, 14, 961. [Google Scholar] [CrossRef]
- Tapiero, H.; Mathé, G.; Couvreur, P.; Tew, K.D., II. Glutamine and glutamate. Biomed. Pharmacother. 2002, 56, 446–457. [Google Scholar] [CrossRef]
- Moro, J.; Roisne-Hamelin, G.; Chaumontet, C.; Even, P.C.; Blais, A.; Cansell, C.; Piedcoq, J.; Gaudichon, C.; Tome, D.; Azzout-Marniche, D. Lysine or Threonine Deficiency Decreases Body Weight Gain in Growing Rats despite an Increase in Food Intake without Increasing Energy Expenditure in Response to FGF21. Nutrients 2022, 15, 197. [Google Scholar] [CrossRef] [PubMed]
- Felig, P. The glucose-alanine cycle. Metabolism 1973, 22, 179–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Hu, S.; Xie, D.Q.; Ling, Y.K.; Ma, W.W.; Li, L. Association between maternal-cord blood plasma amino acids concentrations and birth weight. Acta Nutr. Sin. 2019, 41, 30–35. [Google Scholar] [CrossRef]
- Avagliano, L.; Garò, C.; Marconi, A.M. Placental amino acids transport in intrauterine growth restriction. J. Pregnancy 2012, 2012, 972562. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, A.M.; Carelli, S.; Castoldi, R.E.; Gorio, A.; Taricco, E.; Cetin, I. Plasma amino acid concentrations throughout normal pregnancy and early stages of intrauterine growth restricted pregnancy. J. Matern. -Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2004, 15, 356–362. [Google Scholar] [CrossRef]
- Moghissi, K.S.; Churchill, J.A.; Kurrie, D. Relationship of maternal amino acids and proteins to fetal growth and mental development. Am. J. Obstet. Gynecol. 1975, 123, 398–410. [Google Scholar] [CrossRef]
- Cetin, I.; Corbetta, C.; Sereni, L.P.; Marconi, A.M.; Bozzetti, P.; Pardi, G.; Battaglia, F.C. Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. Am. J. Obstet. Gynecol. 1990, 162, 253–261. [Google Scholar] [CrossRef]
- Moros, G.; Boutsikou, T.; Fotakis, C.; Iliodromiti, Z.; Sokou, R.; Katsila, T.; Xanthos, T.; Iacovidou, N.; Zoumpoulakis, P. Insights into intrauterine growth restriction based on maternal and umbilical cord blood metabolomics. Sci. Rep. 2021, 11, 7824. [Google Scholar] [CrossRef]
- Mansell, T.; Vlahos, A.; Collier, F.; Ponsonby, A.L.; Vuillermin, P.; Ellul, S.; Tang, M.L.K.; Burgner, D.; Saffery, R. The newborn metabolome: Associations with gestational diabetes, sex, gestation, birth mode, and birth weight. Pediatr. Res. 2022, 91, 1864–1873. [Google Scholar] [CrossRef]
- Solomons, N.W. Developmental origins of health and disease: Concepts, caveats, and consequences for public health nutrition. Nutr. Rev. 2009, 67 (Suppl. 1), S12–S16. [Google Scholar] [CrossRef]
- Jansson, T.; Powell, T. Human Placental Transport in Altered Fetal Growth: Does the Placenta Function as a Nutrient Sensor?—A Review. Placenta 2006, 27, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, F.C.; Regnault, T.R. Placental transport and metabolism of amino acids. Placenta 2001, 22, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Lekva, T.; Roland, M.C.P.; Michelsen, A.E.; Friis, C.M.; Aukrust, P.; Bollerslev, J.; Henriksen, T.; Ueland, T. Large Reduction in Adiponectin During Pregnancy Is Associated With Large-for-Gestational-Age Newborns. J. Clin. Endocrinol. Metab. 2017, 102, 2552–2559. [Google Scholar] [CrossRef]
- Farley, D.M.; Choi, J.; Dudley, D.J.; Li, C.; Jenkins, S.L.; Myatt, L.; Nathanielsz, P.W. Placental amino acid transport and placental leptin resistance in pregnancies complicated by maternal obesity. Placenta 2010, 31, 718–724. [Google Scholar] [CrossRef]
- Parrott, M.S.; von Versen-Hoeynck, F.; Ness, R.B.; Markovic, N.; Roberts, J.M. System A amino acid transporter activity in term placenta is substrate specific and inversely related to amino acid concentration. Reprod. Sci. 2007, 14, 687–693. [Google Scholar] [CrossRef]
- Chassen, S.; Jansson, T. Complex, coordinated and highly regulated changes in placental signaling and nutrient transport capacity in IUGR. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165373. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Macrosomia (n = 39) | Control (n = 39) | p-Value |
|---|---|---|---|
| Maternal age (year) | 28.7 ± 3.7 | 27.8 ± 2.5 | 0.203 |
| Pre-pregnancy BMI (kg/m2) | 25.1 ± 4.1 | 23.8 ± 4.5 | 0.084 |
| GWG (kg) | 16.3 ± 3.1 | 15.0 ± 2.9 | 0.049 |
| Parity | 2.3 ± 0.7 | 2.1 ± 0.4 | 0.226 |
| Cesarean section (%) | 53.9 | 48.7 | 0.651 |
| Gestational age at delivery (week) | 40.1 ± 1.0 | 39.6 ± 1.2 | 0.071 |
| Birth weight (g) | 4239.7 ± 172.9 | 3402.6 ± 309.3 | <0.001 |
| Birth length (cm) | 51.9 ± 1.1 | 50.0 ± 0.2 | <0.001 |
| Gender (boy, %) | 51.3 | 51.3 | 1.000 |
| Amino Acid (μg/mL) | Macrosomia (n = 39) | Control (n = 39) | p-Value |
|---|---|---|---|
| Total AAs | 325.88 (302.99, 380.30) | 318.54 (289.43, 379.70) | 0.108 |
| EAA | 136.67 ± 23.09 | 127.73 ± 31.37 | 0.118 |
| BCAA | 44.48 ± 7.93 | 43.24 ± 11.87 | 0.560 |
| NEAA | 205.93 ± 30.24 | 200.43 ± 41.69 | 0.423 |
| Val | 21.97 ± 3.67 | 20.87 ± 5.52 | 0.303 |
| Leu | 14.59 ± 3.30 | 13.71 ± 4.17 | 0.308 |
| Ile | 7.92 ± 1.66 | 8.66 ± 2.60 | 0.139 |
| Thr | 27.10 (22.60, 28.90) | 19.30 (17.10, 21.80) | <0.001 |
| Lys | 19.90 (17.60, 26.80) | 18.60 (16.70, 23.50) | 0.356 |
| Met | 3.70 ± 0.80 | 3.17 ± 0.83 | 0.006 |
| His | 15.27 ± 3.23 | 15.35 ± 4.00 | 0.921 |
| Phe | 14.99 ± 3.21 | 15.08 ± 3.71 | 0.963 |
| Trp | 9.55 ± 1.34 | 9.70 ± 2.72 | 0.965 |
| Asn | 6.63 ± 1.57 | 5.88 ± 1.76 | 0.037 |
| Asp | 5.77 ± 1.88 | 6.02 ± 2.11 | 0.599 |
| Gln | 48.90 (44.60, 54.00) | 45.30 (39.80, 50.90) | 0.045 |
| Glu | 14.13 ± 5.99 | 16.01 ± 6.63 | 0.177 |
| Arg | 19.74 ± 5.36 | 25.28 ± 7.00 | <0.001 |
| Cit | 3.22 ± 0.78 | 3.31 ± 1.18 | 0.694 |
| Tyr | 9.99 ± 2.11 | 10.16 ± 2.82 | 0.760 |
| Orn | 6.29 (5.19, 8.03) | 5.74 (4.26, 7.24) | 0.148 |
| Gly | 17.30 (7.50, 19.70) | 15.80 (7.50, 18.30) | 0.103 |
| Ala | 36.40 (33.60, 39.50) | 31.70 (28.10, 34.80) | <0.001 |
| Ser | 15.50 (11.70, 19.00) | 16.00 (13.70, 18.10 | 0.500 |
| Pro | 18.80 (17.20, 23.90) | 18.70 (14.60, 21.70) | 0.335 |
| Amino Acid (μg/mL) | Macrosomia (n = 39) | Control (n = 39) | p-Value |
|---|---|---|---|
| Total AAs | 423.60 (391.89, 460.66) | 422.07 (375.95, 542.53) | 0.589 |
| EAA | 183.20 ± 25.55 | 200.97 ± 48.21 | 0.047 |
| BCAA | 56.60 ± 9.23 | 62.77 ± 15.66 | 0.037 |
| NEAA | 238.56 (225.49, 255.61) | 233.05 (210.77, 299.85) | 0.758 |
| Val | 29.15 ± 5.13 | 33.82 ± 8.81 | 0.006 |
| Leu | 17.80 (15.50, 19.00) | 15.30 (12.80, 19.00) | 0.133 |
| Ile | 10.32 ± 1.93 | 12.50 ± 3.14 | <0.001 |
| Thr | 31.52 ± 7.59 | 29.95 ± 8.90 | 0.253 |
| Lys | 43.00 ± 7.93 | 49.33 ± 14.43 | 0.020 |
| Met | 4.80 (4.44, 5.16) | 5.04 (4.17, 6.19) | 0.366 |
| His | 17.52 ± 2.59 | 19.24 ± 4.54 | 0.045 |
| Phe | 15.90 (14.20, 17.60) | 17.70 (14.80, 21.60) | 0.019 |
| Trp | 15.50 (14.40, 16.70) | 16.50 (14.50, 22.40) | 0.022 |
| Asn | 6.16 ± 1.57 | 5.96 ± 1.73 | 0.500 |
| Asp | 4.13 (3.47, 4.85) | 4.51 (3.41, 5.63) | 0.512 |
| Gln | 55.60 (37.90, 65.50) | 33.70 (20.60, 46.30) | <0.001 |
| Glu | 31.02 ± 17.48 | 45.38 ± 19.34 | <0.001 |
| Arg | 11.67 ± 4.31 | 13.85 ± 5.16 | 0.046 |
| Cit | 2.80 (2.49, 3.22) | 2.94 (2.48, 3.40) | 0.256 |
| Tyr | 12.50 (11.90, 15.30) | 14.40 (12.20, 18.40) | 0.048 |
| Orn | 18.74 ± 5.48 | 21.07 ± 8.61 | 0.255 |
| Gly | 22.60 (20.00, 25.40) | 22.70 (19.50, 29.10) | 0.601 |
| Ala | 40.30 ± 6.76 | 41.28 ± 10.27 | 0.727 |
| Ser | 18.66 ± 3.08 | 20.24 ± 5.38 | 0.182 |
| Pro | 20.50 (17.60, 22.30) | 19.70 (17.00, 23.90) | 0.948 |
| Maternal/Cord | Macrosomia (n = 39) | Control (n = 39) | p-Value |
|---|---|---|---|
| Total AAs | 0.78 (0.71, 0.93) | 0.73 (0.59, 0.90) | 0.194 |
| EAA | 0.72 (0.64, 0.83) | 0.62 (0.51, 0.77) | 0.027 |
| BCAA | 0.77 (0.66, 0.87) | 0.71(0.52, 0.87) | 0.088 |
| NEAA | 0.85 (0.75, 0.99) | 0.85(0.65, 1.00) | 0.596 |
| Val | 0.73 (0.65, 0.86) | 0.62 (0.47, 0.78) | 0.004 |
| Leu | 0.82 (0.69, 1.04) | 0.90(0.63, 1.15) | 0.826 |
| Ile | 0.75 (0.62, 0.85) | 0.72(0.56, 0.89) | 0.225 |
| Thr | 0.89 (0.76, 0.93) | 0.69 (0.59, 0.80) | <0.001 |
| Lys | 0.48 (0.38, 0.64) | 0.39 (0.30, 0.55) | 0.051 |
| Met | 0.73 (0.61, 0.98) | 0.57 (0.42, 0.85) | 0.008 |
| His | 0.89 (0.76, 0.98) | 0.78 (0.61, 1.08) | 0.191 |
| Phe | 0.95 ± 0.27 | 0.85 ± 0.29 | 0.107 |
| Trp | 0.60 (0.56, 0.68) | 0.51 (0.39, 0.68) | 0.032 |
| Asn | 1.03 (0.86, 1.39) | 1.06 (0.70, 1.29) | 0.285 |
| Asp | 1.36(0.98, 1.88) | 1.46 (0.84, 1.99) | 0.818 |
| Gln | 0.88 (0.78, 1.23) | 1.40(1.03, 2.18) | 0.004 |
| Glu | 0.44 (0.31, 0.77) | 0.37 (0.25, 0.49) | 0.077 |
| Arg | 1.77 (1.23, 2.27) | 1.81 (1.31, 2.73) | 0.772 |
| Cit | 1.18 ± 0.33 | 1.10 ± 0.43 | 0.375 |
| Tyr | 0.71 (0.65, 0.87) | 0.62 (0.51, 0.88) | 0.088 |
| Orn | 0.36 (0.28, 0.48) | 0.30 (0.19, 0.42) | 0.089 |
| Gly | 0.79 (0.53, 0.93) | 0.58 (0.35, 0.86) | 0.054 |
| Ala | 0.96 ± 0.24 | 0.81 ± 0.21 | 0.004 |
| Ser | 0.86 ± 0.27 | 0.85 ± 0.29 | 0.931 |
| Pro | 1.02 (0.81, 1.12) | 0.90 (0.78, 1.14) | 0.516 |
| Amino Acid | OR | 95% CI | p-Value | Adjusted OR # | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Maternal serum | ||||||
| Asn | 0.74 | 0.38–1.44 | 0.371 | 0.73 | 0.35–1.53 | 0.408 |
| Met | 2.55 | 0.70–9.22 | 0.154 | 3.84 | 0.95–15.62 | 0.060 |
| Arg | 0.76 | 0.65–0.87 | <0.001 | 0.72 | 0.60–0.86 | 0.000 |
| Gly | 1.04 | 0.90–1.20 | 0.610 | 1.02 | 0.84–1.23 | 0.859 |
| Ala | 1.05 | 0.90–1.22 | 0.561 | 1.04 | 0.87–1.25 | 0.637 |
| Thr | 1.31 | 1.11–1.55 | 0.002 | 1.43 | 1.15–1.78 | 0.001 |
| Cord serum | ||||||
| Lys | 1.00 | 0.91–1.11 | 0.999 | 1.02 | 0.90–1.15 | 0.786 |
| Glu | 1.14 | 1.04–1.25 | 0.004 | 1.12 | 1.02–1.23 | 0.015 |
| Gln | 1.19 | 1.09–1.31 | 0.000 | 1.16 | 1.06–1.28 | 0.002 |
| Met | 0.81 | 0.38–1.72 | 0.587 | 1.05 | 0.46–2.42 | 0.901 |
| His | 0.67 | 0.48–0.92 | 0.015 | 0.69 | 0.48–0.99 | 0.047 |
| Phe | 1.10 | 0.86–1.41 | 0.434 | 1.07 | 0.82–1.40 | 0.614 |
| Arg | 1.05 | 0.90–1.22 | 0.541 | 1.05 | 0.90–1.23 | 0.535 |
| Trp | 0.69 | 0.47–1.03 | 0.071 | 0.71 | 0.46–1.08 | 0.105 |
| Tyr | 1.23 | 0.81–1.87 | 0.342 | 1.12 | 0.71–1.77 | 0.613 |
| Val | 0.94 | 0.76–1.15 | 0.532 | 0.94 | 0.75–1.19 | 0.623 |
| Ile | 0.66 | 0.44–0.99 | 0.047 | 0.65 | 0.42–1.00 | 0.051 |
| Maternal/cord | ||||||
| Val | 2.78 | 0.02–326.30 | 0.674 | 4.76 | 0.04–637.65 | 0.532 |
| Thr | 36.99 | 0.50–999.99 | 0.100 | 54.72 | 0.39–999.99 | 0.114 |
| Met | 0.68 | 0.01–49.92 | 0.858 | 0.72 | 0.01–89.36 | 0.895 |
| Trp | 0.16 | 0.01–10.67 | 0.392 | 0.31 | 0.01–23.79 | 0.597 |
| Gln | 0.58 | 0.33–1.02 | 0.059 | 0.65 | 0.37–1.15 | 0.137 |
| Ala | 8.19 | 0.20–337.06 | 0.267 | 1.80 | 0.02–155.33 | 0.796 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, X.; Duan, Y.; Wang, Y.; Wang, J.; Yang, Z.; Shao, L.; Li, L.; Lai, J. The Association between Macrosomia and Amino Acids’ Levels in Maternal and Cord Sera: A Case-Control Study. Nutrients 2023, 15, 3440. https://doi.org/10.3390/nu15153440
Xing X, Duan Y, Wang Y, Wang J, Yang Z, Shao L, Li L, Lai J. The Association between Macrosomia and Amino Acids’ Levels in Maternal and Cord Sera: A Case-Control Study. Nutrients. 2023; 15(15):3440. https://doi.org/10.3390/nu15153440
Chicago/Turabian StyleXing, Xinxin, Yifan Duan, Ye Wang, Jie Wang, Zhenyu Yang, Lijun Shao, Lin Li, and Jianqiang Lai. 2023. "The Association between Macrosomia and Amino Acids’ Levels in Maternal and Cord Sera: A Case-Control Study" Nutrients 15, no. 15: 3440. https://doi.org/10.3390/nu15153440
APA StyleXing, X., Duan, Y., Wang, Y., Wang, J., Yang, Z., Shao, L., Li, L., & Lai, J. (2023). The Association between Macrosomia and Amino Acids’ Levels in Maternal and Cord Sera: A Case-Control Study. Nutrients, 15(15), 3440. https://doi.org/10.3390/nu15153440





