Compliance with Nutritional Recommendations and Gut Microbiota Profile in Galician Overweight/Obese and Normal-Weight Individuals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population and Sample Size
2.2. Anthropometric Measures
2.3. Dietary Information
2.4. Fecal Sample Collection and DNA Extraction
2.5. 16S rRNA Amplicon Sequencing
2.6. Statistical and Bioinformatic Analysis
3. Results
3.1. Nutritional Analysis and Adequation to the Objectives and the Recommended Daily Intakes for the Spanish Population
3.2. Analysis of the Gut Microbiota Composition
3.2.1. Alpha- and Beta-Diversity
3.2.2. Relative Abundance of Bacteria
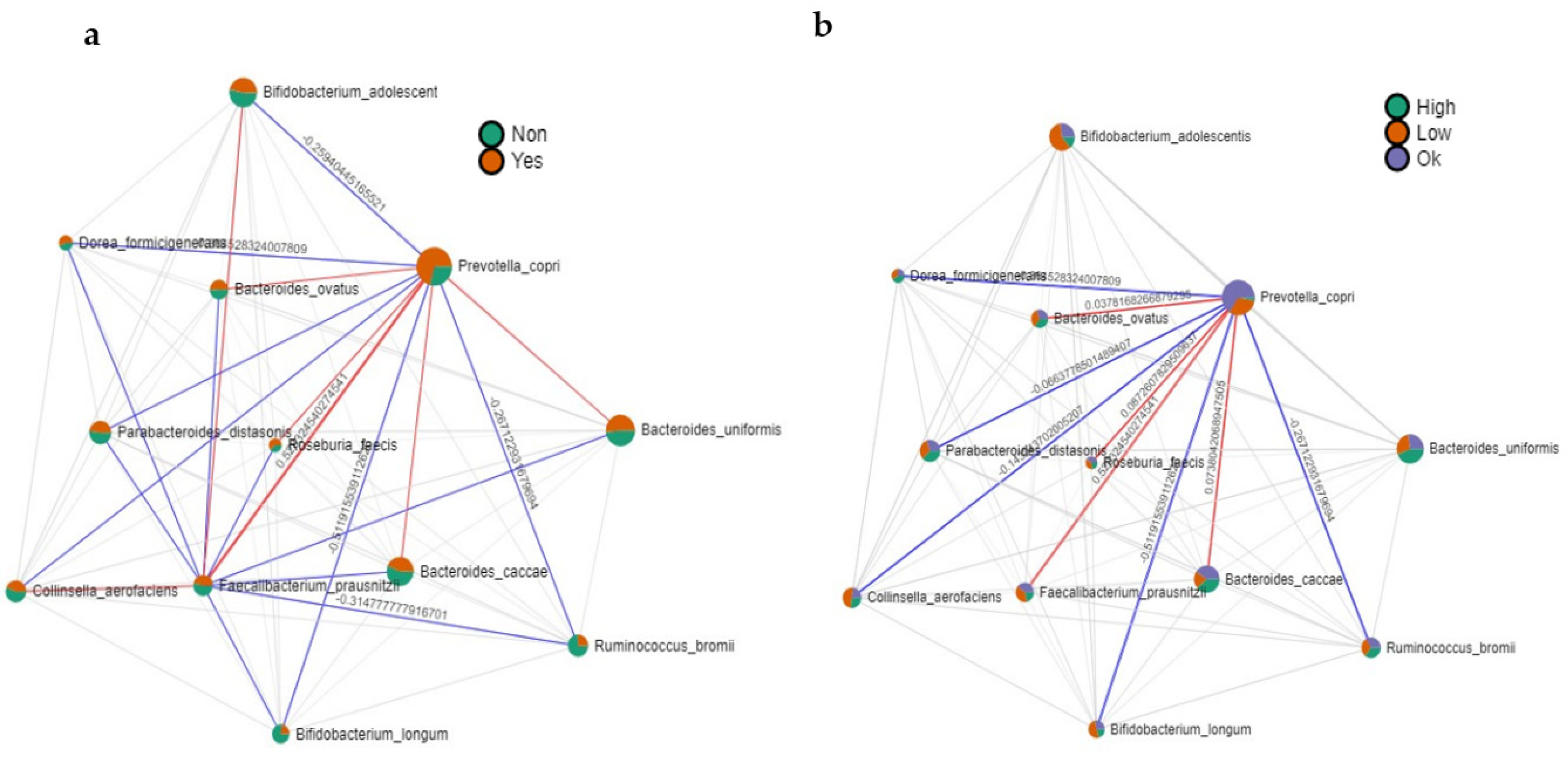
3.2.3. Correlation Analysis: Microbiota-Fiber, Microbiota-MUFAs
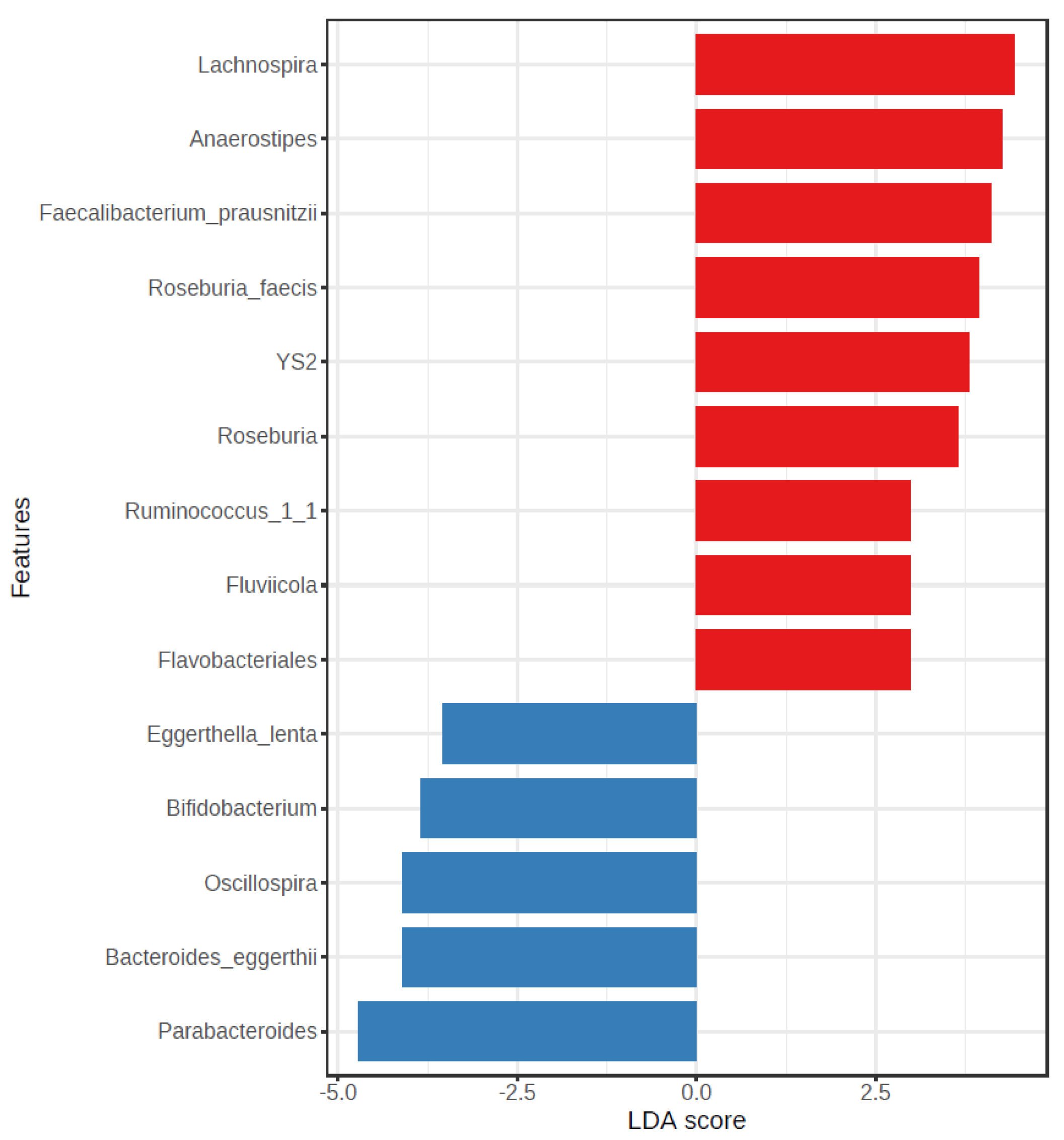
3.2.4. LEfSe Analysis
4. Discussion
4.1. Nutritional Analysis
4.2. Analysis of the Gut Microbiota Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Obesity and Overweight; World Health Organization: Copenhagen, Denmark, 2021; Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 October 2022).
- Guyenet, S.J.; Schwartz, M.W. Regulation of food intake, energy balance, and body fat mass: Implications for the pathogenesis and treatment of obesity. J. Clin. Endocrinol. Metab. 2012, 97, 745–755. [Google Scholar] [PubMed] [Green Version]
- Van Dijk, S.J.; Tellam, R.L.; Morrison, J.L.; Muhlhausler, B.S.; Molloy, P.L. Recent developments on the role of epigenetics in obesity and metabolic disease. Clin. Epigenetics 2015, 7, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Instituto Nacional de Estadística (INE). Encuesta Europea de Salud en España (EESE) 2020. Ministerio de Sanidad. 2020, pp. 1–31. Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/EncuestaEuropea/EncuestaEuropea2020/EESE2020_inf_evol_princip_result.pdf (accessed on 31 October 2022).
- Pérez-Rodrigo, C.; Hervás-Bárbara, G.; Gianzo-Citores, M.; Aranceta-Bartrina, J. Prevalencia de obesidad y factores de riesgo cardiovascular asociados en la población general española: Estudio ENPE. Rev. Esp. Cardiol. 2021, 75, 232–241. [Google Scholar] [CrossRef]
- Sinisterra-Loaiza, L.I.; Vazquez, B.I.; Miranda, J.M.; Cepeda, A.; Cardelle-Cobas, A. Food habits in the Galician population during confinement by COVID-19. Nutr. Hosp. 2020, 37, 1190–1196. [Google Scholar]
- Leis-Trabazo, R.; Perez, C.L.; Castro Perez, X.; Solla, P. Atlantic Diet. Nutrion and gastronomy in Galicia. Nutr. Hosp. 2019, 36, 7–13. [Google Scholar]
- Vaz Velho, M.; Pinheiro, R.; Rodriguez, A.S. The Atlantic Diet—Origin and features. Int. J. Food Stud. 2016, 5, 106–119. [Google Scholar] [CrossRef]
- Wolfe, B.; Kvach, E.; Eckel, R. Treatment of obesity: Weight loss and bariatric surgery. Circ. Res. 2016, 118, 1844–1855. [Google Scholar]
- Cheng, S.; Munukka, E.; Wiklund, P.; Pekkala, S.; Völgyi, E.; Xu, L.; Cheng, S.; Lyytikäinen, A.; Marjomäki, V.; Alen, M.; et al. Women with and without metabolic disorder differ in their gut microbiota composition. Obesity 2012, 20, 1082–1087. [Google Scholar]
- Castaner, O.; Goday, A.; Park, Y.M.; Lee, S.H.; Magkos, F.; Shiow, S.A.; Schröder, H. The gut microbiome profile in obesity: A systematic review. Int. J. Endocrinol. 2018, 2018, 4095789. [Google Scholar] [CrossRef]
- Thingholm, L.B.; Rühlemann, M.C.; Koch, M.; Fuqua, B.; Laucke, G.; Boehm, R.; Bang, C.; Franzosa, E.A.; Hübenthal, M.; Rahnavard, G.; et al. Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition. Cell Host Microbe 2019, 26, 252–264e10. [Google Scholar] [CrossRef]
- Collado, M.C.; Isolauri, E.; Laitinen, K.Ç.; Salminen, S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: A prospective follow-up study initiated in early pregnancy. Am. J. Clin. Nutr. 2010, 92, 1023–1030. [Google Scholar] [CrossRef] [Green Version]
- Damms-Machado, A.; Mitra, S.; Schollenberger, A.E.; Kramer, K.M.; Meile, T.; Königsrainer, A.; Huson, D.H.; Bischoff, S.C. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. BioMed Res. Int. 2015, 2015, 806248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remely, M.; Tesar, I.; Hippe, B.; Gnauer, S.; Rust, P.; Haslberger, A.G. Gut microbiota composition correlates with changes in body fat content due to weight loss. Benef. Microbes 2015, 6, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, A.; Collado, M.C.; García-Valdés, L.; Segura, M.T.; Martín-Lagos, J.A.; Anjos, T.; Martí-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, J.; Real, J.M.F.; Guarner, F.; Gueimonde, M.; Rodríguez, J.M.; de Pipaon, M.S.; Sanz, Y. Microbiota intestinal y salud. Gastroenterol. Hepatol. 2021, 44, 519–535. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet–microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Bervoets, L.; Van, H.K.; Kortleven, I.; Van, N.C.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.; Vankerckhoven, V. Differences in gut microbiota composition between obese and lean children: A cross-sectional study. Gut Pathog. 2013, 5, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, L.M.; Vázquez, R.G.; Arroyo, S.M.C.; Avalos, A.M.; Castillo, P.A.R.; Pérez, D.A.C.; Terrones, I.R.; Ibáñez, N.R.; Magallanes, M.M.R.; Langella, P.; et al. Correlation between diet and gut bacteria in a population of young adults. Int. J. Food Sci. Nutr. 2016, 67, 470–478. [Google Scholar] [CrossRef]
- Fernández-Navarro, T.; Salazar, N.; Gutiérrez-Díaz, I.; De los Reyes-Gavilán, C.G.; Gueimonde, M.; González, S. Different intestinal microbial profile in over-weight and obese subjects consuming a diet with low content of fiber and antioxidants. Nutrients 2017, 9, 551. [Google Scholar] [CrossRef] [Green Version]
- Sociedad Española para el Estudio de la Obesidad [SEEDO]. Consenso SEEDO’2000 para la Evaluación del Sobrepeso y la Obesidad y el Establecimiento de Criterios de Intervención Terapéutica. Med. Clín. 2000, 115, 587–597. [Google Scholar]
- Centro de Investigación de Endocrinología y Nutrición Clínica (IENVA). Calculadora de Dietas-Calibración de Dietas. Available online: https://calcdieta.ienva.org/tu_menu.php (accessed on 14 March 2022).
- Federación Española de Sociedades de Nutrición, Alimentación, y Dietética (FESNAD). Ingestas dietéticas de referencia (IDR) para la población Española, 2010. Act. Diet. 2010, 14, 196–197. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive statistical, functional and integrative analysis of microbiome data. Nucleic Acids Res. 2023, 1, gkad407. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Dietary Reference Values for the EU. DRV Finder. Available online: https://multimedia.efsa.europa.eu/drvs/index.htm (accessed on 28 July 2023).
- Muñiz Garcia, J.; Pérez Castro, T.; Hervada Vidal, X.; Gómez Amorín, A.; Amigo Quintana, M.; Daporta Padín, P.; Seoane Díaz, B.; Lado Lema, M.E.; Martínez Lorente, A.M.; Blanco Iglesias, O.; et al. Xunta de Galicia, Consellería de Sanidad. 2008, pp. 1–88. Available online: https://www.sergas.es/cas/Publicaciones/Docs/SaludPublica/PDF-2153-es.pdf (accessed on 27 July 2023).
- Bartrina, J.A.; Majem, L.S. Consenso de la Sociedad Españolade Nutrición Comunitaria 2011. Objetivos nutricionales para la población Española. Rev. Española Nutr. Comunitaria 2011, 17, 178–199. [Google Scholar]
- Agostoni, C.; Bresson, J.L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Puga, E.; Lendoiro, R. Requerimientos nutricionales de energía y macronutrientes. In Fisiología y Fisiopatología de la Nutrición: I Curso de Especialización en Nutrición; Cordido Carballido, F., Ed.; Universidade de A Coruña: A Coruña, Spain, 2005; pp. 53–72. [Google Scholar]
- Hoffmann Sarda, F.A.; Giuntini, E.B. Carbohydrates for glycemic control: Functional and microbiome aspects. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The health benefits of dietary fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef]
- Hervik, A.K.; Svihus, B. The Role of Fiber in Energy Balance. J. Nutr. Metab. 2019, 2019, 4983657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partearroyo, T.; Laja, A.; Varela-Moreiras, G. Fortalezas y debilidades de la alimentación en la población española del siglo XXI. Nutr. Hosp. 2019, 36, 3–6. [Google Scholar] [PubMed] [Green Version]
- Álvarez Escudero, E.; Sánchez González, P. La fibra dietética. Nutr. Hosp. 2006, 21, 61–72. [Google Scholar]
- Abreuy Abreu, A.T.; Milke-García, M.P.; Argüello-Arévalo, G.A.; Calderón-de la Barca, A.M.; Carmona-Sánchez, R.I.; Consuelo-Sánchez, A.; Coss-Adame, E.; García-Cedillo, M.F.; Hernández-Rosiles, V.; Icaza-Chávez, M.E.; et al. Dietary fiber and the microbiota: A narrative review by a group of experts from the Asociación Mexicana de Gastroenterología. Rev. Gastroenterol. México (Engl. Ed.) 2021, 86, 287–304. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [Green Version]
- García, S.M.; Fantuzzi, G.; Angelini, J.M.; Bourgeois, M.J.; Elgart, J.F.; Etchegoyen, G.; Giampieri, C.; González, L.; Kronsbein, P.; Martínez, C.; et al. Ingesta alimentaria en la población adulta de dos ciudades de la provincia de Buenos Aires: Su adecuación a las recomendaciones nutricionales. Actual. Nutr. 2018, 19, 38–43. [Google Scholar]
- Companys, J.; Gosalbes, M.J.; Pla-Pagà, L.; Calderón-Pérez, L.; Llauradó, E.; Pedret, A.; Valls, R.M.; Jiménez-Hernández, N.; Sandoval-Ramirez, B.A.; del Bas, J.M.; et al. Gut microbiota profile and its association with clinical variables and dietary intake in overweight/obese and lean subjects: A cross-sectional study. Nutrients 2021, 13, 2032. [Google Scholar] [CrossRef]
- Carrillo Fernández, L.; Dalmau Serra, J.; Martínez Álvarez, J.R.; Solà Alberich, R.; Pérez Jiménez, F. Grasas de la dieta y salud cardiovascular. Atención Primaria 2011, 43, 157.e1–157.e16. [Google Scholar] [CrossRef]
- Weggemans, R.M.; Zock, P.L.; Tai, E.S.; Ordovas, J.M.; Molhuizen, H.O.F.; Katan, M.B. ATP binding cassette G5 C1950G polymorphism may affect blood cholesterol concentrations in humans. Clin. Genet. 2002, 62, 226–229. [Google Scholar] [CrossRef]
- Brouns, F. Overweight and diabetes prevention: Is a low-carbohydrate-high-fat diet recommendable? Eur. J. Nutr. 2018, 57, 1301–1312. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, P.; Leire, U.; Bergera, M.; Rodríguez, P.; Teresa, A.; Gaspar, V.; Moreno, E.R.; Manuel, J.; Torres, Á.; Moreiras, G.V. Frutas y Hortalizas: Nutrición y Salud en la España del S. XXI. Available online: www.fen.org.es/storage/app/media/imgPublicaciones/INFORME_FRUTAS_Y_HORTALIZAS_FEN_2018-v1.pdf (accessed on 12 June 2023).
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cena, H.; Calder, P.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). Publicación de las Opiniones Científicas de EFSA Sobre Ingestas Diarias de Referencia de Sodio y Cloruro. Ministerio de Consumo. 4 September 2019. Available online: https://www.aesan.gob.es/AECOSAN/web/noticias_y_actualizaciones/noticias/2019/sodio.htm. (accessed on 25 April 2023).
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J. Global sodium consumption and death from cardiovascular causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef] [Green Version]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium intake and hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef] [Green Version]
- Golloso-Gubat, M.J.; Ducarmon, Q.R.; Tan, R.C.A.; Zwittink, R.D.; Kuijper, E.J.; Nacis, J.S.; Santos, N.L.C. Gut microbiota and dietary intake of normal-weight and overweight Filipino children. Microorganisms 2020, 8, 1015. [Google Scholar] [CrossRef] [PubMed]
- Duca, F.A.; Sakar, Y.; Lepage, P.; Devime, F.; Langelier, B.; Dore, J.; Covasa, M. Replication of obesity and associated signaling pathways through transfer of microbiota from obese-prone rats. Diabetes 2014, 63, 1624–1636. [Google Scholar] [CrossRef] [Green Version]
- Hartstra, A.V.; Bouter, K.E.; Backhed, F.; Nieuwdor, M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care 2015, 38, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Devaraj, S.; Hemarajata, P.; Versalovic, J. Metabolismo corporal: Implicaciones con la obesidad y la diabetes. Acta Bioquim. Clin. Latinoam. 2013, 47, 421–434. [Google Scholar]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid. Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- WHO. Physical Activity. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 27 July 2023).
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of gut microbiota in people with obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human colonic microbiota associated with diet, obesity and weight loss. Int. J. Obes. 2008, 32, 1720–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Effendi, R.M.R.A.; Anshory, M.; Kalim, H.; Dwiyana, R.F.; Suwarsa, O.; Pardo, L.M.; Nijsten, T.E.C.; Thio, H.B. Akkermansia muciniphila and Faecalibacterium prausnitzii in Immune-Related Diseases. Microorganisms 2022, 10, 2382. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, W.; Huang, W.; Lin, Y.; Chan, F.K.; Ng, S.C. Gut microbiota in patients with obesity and metabolic disorders—A systematic review. Genes Nutr. 2022, 17, 2. [Google Scholar] [CrossRef]
- Aranaz, P.; Ramos-Lopez, O.; Cuevas-Sierra, A. A predictive regression model of the obesity-related inflammatory status based on gut microbiota composition. Int. J. Obes. 2021, 45, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Caso, J.R.; MacDowell, K.S.; González-Pinto, A. Gut microbiota, innate immune pathways, and inflammatory control mechanisms in patients with major depressive disorder. Transl. Psychiatry 2021, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A marker of health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira—A candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef]
- Chen, Y.R.; Zheng, H.M.; Zhang, G.X.; Chen, F.I.; Yang, Z.C. High Oscillospira abundance indicates constipation and low BMI in the Guangdong Gut Microbiome Project. Sci. Rep. 2020, 10, 9364. [Google Scholar] [CrossRef]
- Cai, H.; Wen, Z.; Zhao, L.; Yu, D.; Meng, K.; Yang, P. Lactobacillus plantarum FRT4 alleviated obesity by modulating gut microbiota and liver metabolome in high-fat diet-induced obese mice. Food Nutr. Res. 2022, 9, 66. [Google Scholar] [CrossRef]
- López-Contreras, B.E.; Morán-Ramos, S.; Villarruel-Vázquez, R.; Macías-Kauffer, L.; Villamil-Ramírez, H.; León-Mimila, P.; Vega-Badillo, J.; Sánchez-Muñoz, F.; Llanos-Moreno, L.E.; Canizalez-Román, A.; et al. Composition of gut microbiota in obese and normal-weight Mexican school-age children and its association with metabolic traits. Pediatr. Obes. 2018, 13, 381–388. [Google Scholar] [CrossRef]
- Million, M.; Maraninchi, M.; Henry, M. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes. 2012, 36, 817–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Peters, B.A.; Friedlander, C.; Freiman, H.J.; Goedert, J.J.; Sinha, R.; Miller, G.; Bernstein, M.A.; Hayes, R.B.; Ahn, J. Association of dietary fibre intake and gut microbiota in adults. Br. J. Nutr. 2018, 120, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Abreu, M.T. Low-fat, high-fiber diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Rosés, C.; Cuevas-Sierra, A.; Quintana, S.; Riezu-Boj, J.I.; Martínez, J.A.; Milagro, F.I.; Barceló, A. Gut microbiota bacterial species associated with Mediterranean Diet-related food groups in a Northern Spanish population. Nutrients 2021, 13, 636. [Google Scholar] [CrossRef]
- Benus, R.F.; van der Werf, T.S.; Welling, G.W.; Judd, P.A.; Taylor, M.A.; Harmsen, H.J.; Whelan, K. Association between Faecalibacterium prausnitzii and dietary fibre in colonic fermentation in healthy human subjects. Br. J. Nutr. 2010, 104, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Fava, F.R.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int. J. Obes. 2013, 37, 216–223. [Google Scholar] [CrossRef] [Green Version]



| DIET ADEQUACY | |||||
|---|---|---|---|---|---|
| CALORIC PROFILE | NUTRITIONAL OBJECTIVES | VALUES OF PARTICIPANTS’ DIET | DÉFICIT | ADEQUATE | EXCESS |
| Carbohydrates (% Energy) | 50–55 | 38.9 ± 7.8 | 96 (88.1%) | 11 (10.1%) | 1 (0.9%) |
| Fiber (g/1000 kcal) | >14 | 11.7 ± 7.7 | 91 (84.3%) | 15 (13.9%) | 2 (1.9%) |
| Lipids (% Energy) | 30–35 | 41.8 ± 8.0 | 7 (6.4%) | 15 (13.9%) | 86 (79.6%) |
| LIPID PROFILE | |||||
| SFA (% Energy) | ≤7–8 | 12.0 ± 3.2 | - | 13 (0.0%) | 95 (88%) |
| MUFA (% Energy) | 20 | 20.8 ± 16.1 | 36 (33.3%) | 45 (41.7%) | 27 (25.0%) |
| PUFA (% Energy) | 5 | 5.9 ± 3.6 | 39 (38.0%) | 23 (21.2%) | 62 (57.4%) |
| Cholesterol (mg) | <300 | 347.0 ± 138.6 | 0 (0.0%) | 46 (42.6%) | 62 (57.4%) |
| OTHERS | |||||
| Water (mL) | 2000 | 2063.3 ± 627.9 | 49 (45.4%) | 59 (54.6%) | 0 (0.0%) |
| Fruits and vegetables | 5 portions | 1.8 ± 1.5 | 102 (94.4%) | 5 (4.6%) | 1 (0.9%) |
| Sugar (% Energy) | 6–10 | 18.1 ± 7.4 | - | 13 (12.0%) | 95 (88.0%) |
| Alcohol (g) | ≤1 SDU women ≤2 SDU men | 10.84 ± 44.43 | - | 100 (92.6%) | 8 (7.4%) |
| NW (n = 40) | OW (n = 35) | OB (n = 33) | ||||||
|---|---|---|---|---|---|---|---|---|
| CALORIC PROFILE | Nutritional Objectives | Mean ± SD | Intake Adequacy: n (%) | Mean ± SD | Intake Adequacy: n (%) | Mean ± SD | Intake Adequacy: n (%) | p Value |
| Carbohydrates (% Energy) | 50–55% | 40.3 ± 7.1 | 5 (12.5%) | 37.4 ± 7.7 | 1 (3.0%) | 38.8 ± 8.6 | 4(11.4%) | 0.274 |
| Lipids (% Energy) | 30–35% | 40.6 ± 6.3 | 6 (15.0%) | 43.6 ± 9.6 | 5 (15.2%) | 41.9 ± 7.5 | 5 (14.3%) | 0.280 |
| Fiber (g/1000 Kcal) | >14 | 14.2 ± 9.5 | 13 (32.5%) | 12.5 ± 10.14 | 4 (12.0%) | 9.1 ± 2.9 | 5 (14.3%) | 0.038 |
| LIPID PROFILE | ||||||||
| SFA (% Energy) | ≤7–8 | 11.8 ± 2.8 | 3 (7.5%) | 11.9 ± 3.0 | 4 (12.1%) | 15.5 ± 8.1 | 0 (0.0%) | 0.559 |
| MUFA (% Energy) | 20 | 20.3 ± 5.7 | 10 (25.0%) | 19.4 ± 5.7 | 12 (36.4%) | 6.5 ± 5.3 | 5 (14.3%) | <0.001 |
| PUFA (% Energy) | 5 | 5.8 ± 2.1 | 12 (30.0%) | 6.5 ± 5.3 | 19 (57.6%) | 5.4 ± 2.7 | 6 (17.4%) | 0.874 |
| Cholesterol (mg) | <300 mg | 340.3 ± 137.7 | 19 (47.5%) | 348.0 ± 129.8 | 11 (33.0%) | 354.1 ± 152.1 | 10 (28.6%) | 0.915 |
| OTHERS | ||||||||
| Water (mL) | 2000 mL | 2305.36 ± 487.6 | 23 (57.5%) | 2128.0 ± 682.2 | 20 (57.4%) | 2098.1 ± 736.4 | 16 (48.5%) | 0.751 |
| Fruits and vegetables | 5 portions | 1.9 ± 1.5 | 3 (7.5%) | 1.3 ± 1.4 | 1 (2.8%) | 2.2 ± 1.5 | 1 (3.0%) | 0.785 |
| Sugar | 6–10 | 20.0 ± 9.0 | 1 (2.5%) | 19.0 ± 7.1 | 5 (14.3%) | 16.0 ± 5.1 | 7 (21.2%) | 0.632 |
| Alcohol (g) | ≤1 SDU women ≤2 SDU men | 3.8 ± 9.8 | 39 (97.5%) | 4.0 ± 8.7 | 32 (97.0%) | 3.9 ± 8.8 | 31 (88.6%) | 0.298 |
| NW (n = 40) | OW (n = 35) | OB (n = 33) | |||||
|---|---|---|---|---|---|---|---|
| Vitamins | Average ± SD | Intake Adequacy n (%) | Average ± SD | Intake Adequacy n (%) | Average ± SD | Intake Adequacy n (%) | p Value |
| Thiamine (mg) | 1.5 ± 0.8 | 32 (80.0%) | 1.9 ± 0.9 | 31 (88.6%) | 1.4 ± 0.3 | 29 (87.9%) | 0.009 |
| Riboflavin (mg) | 1.7 ± 0.5 | 30 (75.0%) | 2.1 ± 0.6 | 31 (88.6%) | 1.7 ± 0.4 | 27 (81.9%) | 0.007 |
| Niacin (mg) | 30.5 ± 10.7 | 30 (75.0%) | 40.4 ±14.5 | 16 (45.7%) | 31.6 ± 7.7 | 25 (75.8%) | 0.0005 |
| Vitamin B6 (µg) | 2.0 ± 0.8 | 35 (87.5%) | 2.3 ± 1.1 | 33 (94.3%) | 2.0 ± 0.6 | 30 (90.9%) | 0.159 |
| Folic acid (µg) | 281.3 ± 126.3 | 13 (32.5%) | 278.3 ± 105.2 | 10 (28.6%) | 255.3 ± 74.6 | 8 (24.2%) | 0.537 |
| Vitamin B12 (µg) | 6.1 ± 6.0 | 40 (100%) | 6.4 ± 2.5 | 35 (100%) | 5.6 ± 2.8 | 33 (100%) | 0.697 |
| Vitamin C (mg) | 151.2 ± 87.7 | 35 (87.5%) | 166.2 ± 79.6 | 33 (94.3%) | 160.7 ± 68.5 | 33 (100%) | 0.738 |
| Vitamin A (µg) | 798.4 ± 603.1 | 24 (60.0%) | 750.5 ± 452.7 | 19 (54.3%) | 745.0 ± 406.8 | 17 (51.5%) | 0.868 |
| Vitamin D (µg) | 2.9 ± 3.2 | 8 (20.0%) | 4.6 ± 6.0 | 11 (31.4%) | 4.5 ± 6.5 | 11 (33.3%) | 0.321 |
| Vitamin E (mg) | 8.3 ± 8.1 | 5 (12.5%) | 7.8 ± 9.9 | 4 (11.4%) | 5.9 ± 5.6 | 2 (6.1%) | 0.416 |
| Minerals | |||||||
| Calcium (mg) | 719.8 ± 297.1 | 7 (17.5%) | 841.2 ± 415.0 | 12 (34.3%) | 792.9 ± 348.4 | 10 (30.3%) | 0.331 |
| Magnesium (mg) | 325.7 ± 134.9 | 23 (57.5%) | 368.9 ± 95.8 | 23 (65.7%) | 308.8 ± 81.0 | 15 (45.5%) | 0.063 |
| Potassium (mg) | 3086.7 ± 904.1 | 22 (55.0%) | 4044.5 ± 1135.9 | 26 (74.3%) | 3511.8 ± 745.6 | 24 (72.7%) | 0.0001 |
| Phosporus (mg) | 1415.5 ± 500.5 | 38 (95.0%) | 1716.9 ± 472.9 | 34 (97.1%) | 1436.8 ± 380.6 | 32 (97.0%) | 0.009 |
| Iron (mg) | 16.7 ± 9.0 | 23 (57.5%) | 18.3 ± 5.8 | 29 (82.9%) | 14.0 ± 2.8 | 21 (63.6%) | 0.035 |
| Iodine (µg) | 184.4 ± 150.3 | 16 (40.0%) | 347.5 ± 197.4 | 25 (71.4%) | 292.5 ± 153.6 | 25 (75.8%) | 0.0002 |
| Zinc (mg) | 24.4 ± 78.7 | 29 (72.5%) | 14.1 ± 3.5 | 34 (97.1%) | 10.8 ± 2.9 | 27 (81.8%) | 0.0445 |
| Sodium (mg) | 2610.9 ± 677.5 | 6 (15.0%) | 5609.5 ± 1219.7 | 0 (0.0%) | 3877.1 ± 346.0 | 0 (0.0%) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinisterra-Loaiza, L.; Alonso-Lovera, P.; Cardelle-Cobas, A.; Miranda, J.M.; Vázquez, B.I.; Cepeda, A. Compliance with Nutritional Recommendations and Gut Microbiota Profile in Galician Overweight/Obese and Normal-Weight Individuals. Nutrients 2023, 15, 3418. https://doi.org/10.3390/nu15153418
Sinisterra-Loaiza L, Alonso-Lovera P, Cardelle-Cobas A, Miranda JM, Vázquez BI, Cepeda A. Compliance with Nutritional Recommendations and Gut Microbiota Profile in Galician Overweight/Obese and Normal-Weight Individuals. Nutrients. 2023; 15(15):3418. https://doi.org/10.3390/nu15153418
Chicago/Turabian StyleSinisterra-Loaiza, Laura, Patricia Alonso-Lovera, Alejandra Cardelle-Cobas, Jose Manuel Miranda, Beatriz I. Vázquez, and Alberto Cepeda. 2023. "Compliance with Nutritional Recommendations and Gut Microbiota Profile in Galician Overweight/Obese and Normal-Weight Individuals" Nutrients 15, no. 15: 3418. https://doi.org/10.3390/nu15153418
APA StyleSinisterra-Loaiza, L., Alonso-Lovera, P., Cardelle-Cobas, A., Miranda, J. M., Vázquez, B. I., & Cepeda, A. (2023). Compliance with Nutritional Recommendations and Gut Microbiota Profile in Galician Overweight/Obese and Normal-Weight Individuals. Nutrients, 15(15), 3418. https://doi.org/10.3390/nu15153418










