Research Progress of Maternal Metabolism on Cardiac Development and Function in Offspring
Abstract
:1. Introduction
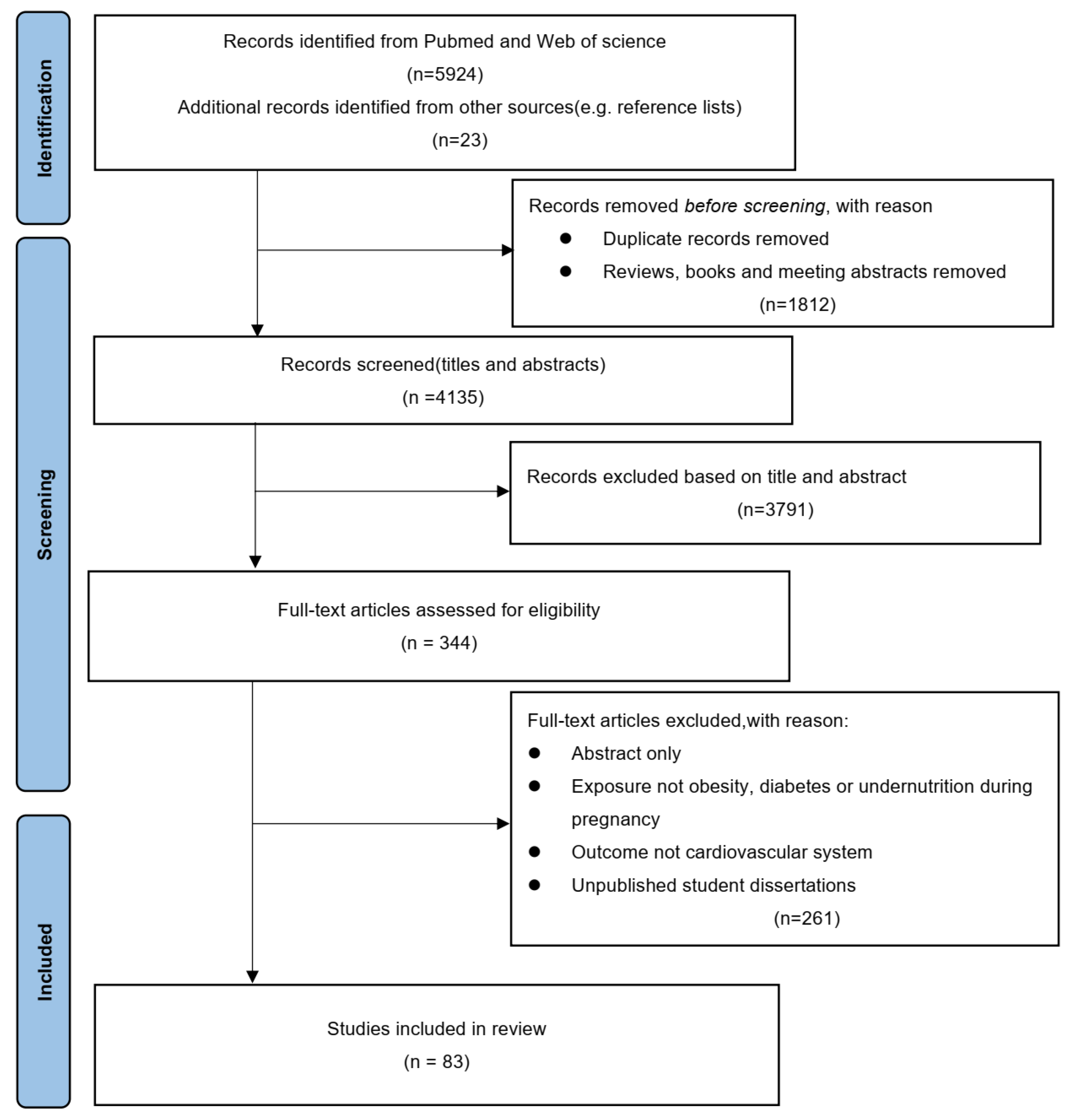
2. Methods
2.1. Search Strategy
2.2. Inclusion/Exclusion Criteria
3. Results
3.1. Human Studies of the Effect of Maternal Metabolism on Cardiac Development and Function in Offspring
3.1.1. Maternal Diabetes
3.1.2. Maternal Obesity
3.1.3. Maternal Undernutrition
3.2. Animal Models of the Effect of Maternal Metabolism on Cardiovascular Disease in Offspring
3.2.1. Maternal Metabolism and the Risk of Congenital Heart Diseases in Offspring
3.2.2. Maternal Metabolism and Long-Term Cardiac Diseases in Offspring
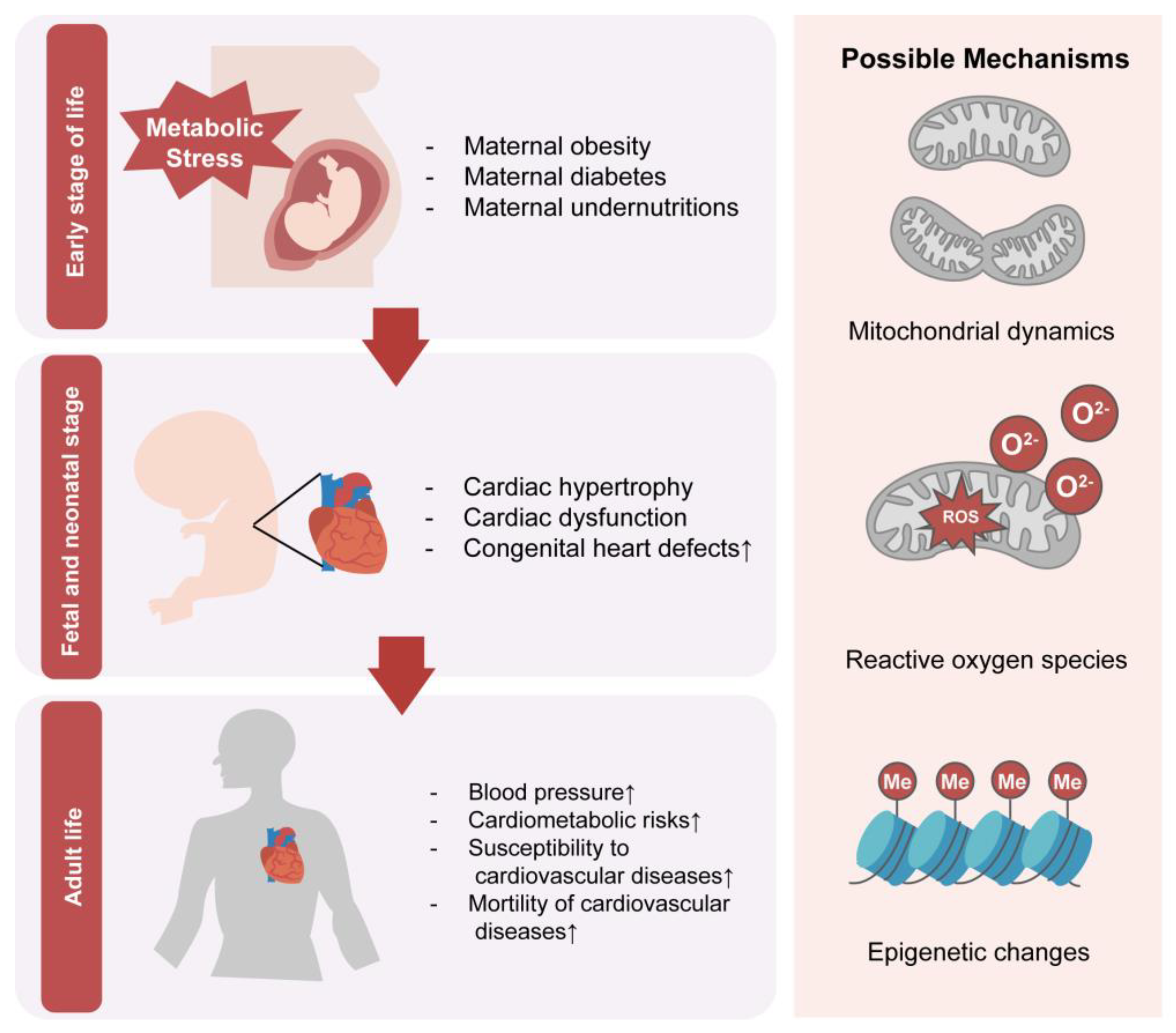
3.3. Mechanisms of the Effect of Maternal Metabolism on Cardiac Development and Function in Offspring
3.3.1. Maternal Metabolism Influences Cardiac Mitochondria in Offspring
3.3.2. Nutritional Molecular Signals Can Directly Affect Cardiac Gene Expression
3.3.3. Reactive Oxygen Species Could Mediate Modulating Reactions towards Environmental Changes
3.3.4. Epigenetic Regulation Plays a Vital Role in Cardiac Changes Induced by Maternal Nutritional Disorders
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Houyel, L.; Meilhac, S.M. Heart Development and Congenital Structural Heart Defects. Annu. Rev. Genom. Hum. Genet. 2021, 22, 257–284. [Google Scholar] [CrossRef] [PubMed]
- Sizarov, A.; Ya, J.; de Boer, B.A.; Lamers, W.H.; Christoffels, V.M.; Moorman, A.F. Formation of the building plan of the human heart: Morphogenesis, growth, and differentiation. Circulation 2011, 123, 1125–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLaughter, D.M.; Bick, A.G.; Wakimoto, H.; McKean, D.; Gorham, J.M.; Kathiriya, I.S.; Hinson, J.T.; Homsy, J.; Gray, J.; Pu, W.; et al. Single-Cell Resolution of Temporal Gene Expression during Heart Development. Dev. Cell 2016, 39, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Knutson, A.K.; Williams, A.L.; Boisvert, W.A.; Shohet, R.V. HIF in the heart: Development, metabolism, ischemia, and atherosclerosis. J. Clin. Investig. 2021, 131, e137557. [Google Scholar] [CrossRef]
- MacGrogan, D.; Münch, J.; de la Pompa, J.L. Notch and interacting signalling pathways in cardiac development, disease, and regeneration. Nat. Rev. Cardiol. 2018, 15, 685–704. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, L.; Zhao, B.; Guan, K.L. The hippo pathway in heart development, regeneration, and diseases. Circ. Res. 2015, 116, 1431–1447. [Google Scholar] [CrossRef] [PubMed]
- Kolwicz, S.C., Jr.; Purohit, S.; Tian, R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ. Res. 2013, 113, 603–616. [Google Scholar] [CrossRef]
- Gibb, A.A.; Hill, B.G. Metabolic Coordination of Physiological and Pathological Cardiac Remodeling. Circ. Res. 2018, 123, 107–128. [Google Scholar] [CrossRef]
- Maréchal, L.; Sicotte, B.; Caron, V.; Brochu, M.; Tremblay, A. Fetal Cardiac Lipid Sensing Triggers an Early and Sex-related Metabolic Energy Switch in Intrauterine Growth Restriction. J. Clin. Endocrinol. Metab. 2021, 106, 3295–3311. [Google Scholar] [CrossRef]
- Yu, Y.; Arah, O.A.; Liew, Z.; Cnattingius, S.; Olsen, J.; Sørensen, H.T.; Qin, G.; Li, J. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: Population based cohort study with 40 years of follow-up. BMJ 2019, 367, l6398. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.N.; Huang, X.M.; Zhao, X.Y.; Wang, W.; Wen, R.; Gao, S.Y. Risks of specific congenital anomalies in offspring of women with diabetes: A systematic review and meta-analysis of population-based studies including over 80 million births. PLoS Med. 2022, 19, e1003900. [Google Scholar] [CrossRef]
- Ghaderian, M.; Hemmat, M.; Behdad, S.; Saeedi, M.; Shahsanaei, F. Fetal Cardiac Functional Abnormalities Assessed by Echocardiography in Mothers Suffering Gestational Diabetes Mellitus: A Systematic Review and Meta-analysis. Curr. Probl. Cardiol. 2021, 46, 100658. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, B.; Sun, Y.; Du, Y.; Santillan, M.K.; Santillan, D.A.; Snetselaar, L.G.; Bao, W. Association of Maternal Prepregnancy Diabetes and Gestational Diabetes Mellitus With Congenital Anomalies of the Newborn. Diabetes Care 2020, 43, 2983–2990. [Google Scholar] [CrossRef] [PubMed]
- Øyen, N.; Diaz, L.J.; Leirgul, E.; Boyd, H.A.; Priest, J.; Mathiesen, E.R.; Quertermous, T.; Wohlfahrt, J.; Melbye, M. Prepregnancy Diabetes and Offspring Risk of Congenital Heart Disease: A Nationwide Cohort Study. Circulation 2016, 133, 2243–2253. [Google Scholar] [CrossRef]
- Priest, J.R.; Yang, W.; Reaven, G.; Knowles, J.W.; Shaw, G.M. Maternal Midpregnancy Glucose Levels and Risk of Congenital Heart Disease in Offspring. JAMA Pediatr. 2015, 169, 1112–1116. [Google Scholar] [CrossRef]
- Zhao, M.; Diao, J.; Huang, P.; Li, J.; Li, Y.; Yang, Y.; Luo, L.; Zhang, S.; Chen, L.; Wang, T.; et al. Association of Maternal Diabetes Mellitus and Polymorphisms of the NKX2.5 Gene in Children with Congenital Heart Disease: A Single Centre-Based Case-Control Study. J. Diabetes Res. 2020, 2020, 3854630. [Google Scholar] [CrossRef]
- Tinker, S.C.; Gilboa, S.M.; Moore, C.A.; Waller, D.K.; Simeone, R.M.; Kim, S.Y.; Jamieson, D.J.; Botto, L.D.; Reefhuis, J. Specific birth defects in pregnancies of women with diabetes: National Birth Defects Prevention Study, 1997–2011. Am. J. Obstet. Gynecol. 2020, 222, 176.e1–176.e11. [Google Scholar] [CrossRef]
- Leirgul, E.; Brodwall, K.; Greve, G.; Vollset, S.E.; Holmstrøm, H.; Tell, G.S.; Øyen, N. Maternal Diabetes, Birth Weight, and Neonatal Risk of Congenital Heart Defects in Norway, 1994–2009. Obstet. Gynecol. 2016, 128, 1116–1125. [Google Scholar] [CrossRef]
- Tam, W.H.; Ma, R.C.W.; Ozaki, R.; Li, A.M.; Chan, M.H.M.; Yuen, L.Y.; Lao, T.T.H.; Yang, X.; Ho, C.S.; Tutino, G.E.; et al. In Utero Exposure to Maternal Hyperglycemia Increases Childhood Cardiometabolic Risk in Offspring. Diabetes Care 2017, 40, 679–686. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wu, Y.; Du, B.; Yu, X.; Wang, H.; Niu, Y.; Wang, J.; Chen, S.; Sun, K. Associations of maternal gestational diabetes mellitus with alterations in cardiovascular system in early childhood. Diabetes Metab. Res. Rev. 2022, 38, e3551. [Google Scholar] [CrossRef]
- Kaseva, N.; Vääräsmäki, M.; Sundvall, J.; Matinolli, H.M.; Sipola, M.; Tikanmäki, M.; Heinonen, K.; Lano, A.; Wehkalampi, K.; Wolke, D.; et al. Gestational Diabetes But Not Prepregnancy Overweight Predicts for Cardiometabolic Markers in Offspring Twenty Years Later. J. Clin. Endocrinol. Metab. 2019, 104, 2785–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillemette, L.; Wicklow, B.; Sellers, E.A.C.; Dart, A.; Shen, G.X.; Dolinsky, V.W.; Gordon, J.W.; Jassal, D.S.; Nickel, N.; Duhamel, T.A.; et al. Intrauterine exposure to diabetes and risk of cardiovascular disease in adolescence and early adulthood: A population-based birth cohort study. Can. Med. Assoc. J. 2020, 192, E1104–E1113. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.; Eriksson, J.G.; Broekman, B.F. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, M.; Razaz, N.; Edstedt Bonamy, A.K.; Villamor, E.; Cnattingius, S. Maternal Overweight and Obesity and Risk of Congenital Heart Defects. J. Am. Coll. Cardiol. 2019, 73, 44–53. [Google Scholar] [CrossRef]
- Cai, G.J.; Sun, X.X.; Zhang, L.; Hong, Q. Association between maternal body mass index and congenital heart defects in offspring: A systematic review. Am. J. Obstet. Gynecol. 2014, 211, 91–117. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.; Wootton, R.E.; Yang, Q.; Oddie, S.; Wright, J.; Yang, T.C.; Magnus, M.; Andreassen, O.A.; Borges, M.C.; Caputo, M.; et al. The effect of maternal BMI, smoking and alcohol on congenital heart diseases: A Mendelian randomisation study. BMC Med. 2023, 21, 35. [Google Scholar] [CrossRef]
- Persson, M.; Cnattingius, S.; Villamor, E.; Söderling, J.; Pasternak, B.; Stephansson, O.; Neovius, M. Risk of major congenital malformations in relation to maternal overweight and obesity severity: Cohort study of 1.2 million singletons. BMJ 2017, 357, j2563. [Google Scholar] [CrossRef] [Green Version]
- Groves, A.M.; Price, A.N.; Russell-Webster, T.; Jhaveri, S.; Yang, Y.; Battersby, E.E.; Shahid, S.; Costa Vieira, M.; Hughes, E.; Miller, F.; et al. Impact of maternal obesity on neonatal heart rate and cardiac size. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 481–487. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; Liistro, T.; Gargani, L.; Ait Ali, L.; D’Angelo, G.; Rocchiccioli, S.; La Rosa, F.; Kemeny, A.; Sanguinetti, E.; Ucciferri, N.; et al. Maternal Obesity and Cardiac Development in the Offspring: Study in Human Neonates and Minipigs. JACC Cardiovasc. Imaging 2018, 11, 1750–1755. [Google Scholar] [CrossRef]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Yang, Z.; Zou, Z.; Ma, J. Infant exposure to Chinese famine increased the risk of hypertension in adulthood: Results from the China Health and Retirement Longitudinal Study. BMC Public Health 2016, 16, 435. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Lumey, L.H. Exposure to the Chinese famine of 1959-61 in early life and long-term health conditions: A systematic review and meta-analysis. Int. J. Epidemiol. 2017, 46, 1157–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Painter, R.C.; Roseboom, T.J.; Bleker, O.P. Prenatal exposure to the Dutch famine and disease in later life: An overview. Reprod. Toxicol. 2005, 20, 345–352. [Google Scholar] [CrossRef]
- Roseboom, T.J.; Painter, R.C.; van Abeelen, A.F.; Veenendaal, M.V.; de Rooij, S.R. Hungry in the womb: What are the consequences? Lessons from the Dutch famine. Maturitas 2011, 70, 141–145. [Google Scholar] [CrossRef]
- Painter, R.C.; de Rooij, S.R.; Bossuyt, P.M.; Phillips, D.I.; Osmond, C.; Barker, D.J.; Bleker, O.P.; Roseboom, T.J. Blood pressure response to psychological stressors in adults after prenatal exposure to the Dutch famine. J. Hypertens. 2006, 24, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, T.J.; van der Meulen, J.H.; Osmond, C.; Barker, D.J.; Ravelli, A.C.; Schroeder-Tanka, J.M.; van Montfrans, G.A.; Michels, R.P.; Bleker, O.P. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–1945. Heart 2000, 84, 595–598. [Google Scholar] [CrossRef] [Green Version]
- Painter, R.C.; de Rooij, S.R.; Bossuyt, P.M.; Simmers, T.A.; Osmond, C.; Barker, D.J.; Bleker, O.P.; Roseboom, T.J. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am. J. Clin. Nutr. 2006, 84, 322–327; quiz 466–467. [Google Scholar] [CrossRef]
- Crispi, F.; Bijnens, B.; Figueras, F.; Bartrons, J.; Eixarch, E.; Le Noble, F.; Ahmed, A.; Gratacós, E. Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation 2010, 121, 2427–2436. [Google Scholar] [CrossRef] [Green Version]
- Lumey, L.H.; Martini, L.H.; Myerson, M.; Stein, A.D.; Prineas, R.J. No relation between coronary artery disease or electrocardiographic markers of disease in middle age and prenatal exposure to the Dutch famine of 1944–5. Heart 2012, 98, 1653–1659. [Google Scholar] [CrossRef]
- Ekamper, P.; van Poppel, F.; Stein, A.D.; Bijwaard, G.E.; Lumey, L.H. Prenatal famine exposure and adult mortality from cancer, cardiovascular disease, and other causes through age 63 years. Am. J. Epidemiol. 2015, 181, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Manivannan, S.; Mansfield, C.; Zhang, X.; Kodigepalli, K.M.; Majumdar, U.; Garg, V.; Basu, M. Single-cell transcriptomic profiling unveils dysregulation of cardiac progenitor cells and cardiomyocytes in a mouse model of maternal hyperglycemia. Commun. Biol. 2022, 5, 820. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Li, Y.; Guan, L.; Li, Q.; Shi, C.; Ma, X.; Song, Y. Elevated MST1 leads to apoptosis via depletion of YAP1 in cardiomyocytes exposed to high glucose. Mol. Med. 2021, 27, 13. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Xu, L.; Lu, J.; Shen, Y.; Tang, Y.; Wang, X.; Wu, Y.; Sun, H.; Guo, T. Down-regulation of the insulin signaling pathway by SHC may correlate with congenital heart disease in Chinese populations. Clin. Sci. 2020, 134, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Saiyin, T.; Engineer, A.; Greco, E.R.; Kim, M.Y.; Lu, X.; Jones, D.L.; Feng, Q. Maternal voluntary exercise mitigates oxidative stress and incidence of congenital heart defects in pre-gestational diabetes. J. Cell. Mol. Med. 2019, 23, 5553–5565. [Google Scholar] [CrossRef]
- Engineer, A.; Saiyin, T.; Lu, X.; Kucey, A.S.; Urquhart, B.L.; Drysdale, T.A.; Norozi, K.; Feng, Q. Sapropterin Treatment Prevents Congenital Heart Defects Induced by Pregestational Diabetes Mellitus in Mice. J. Am. Heart Assoc. 2018, 7, e009624. [Google Scholar] [CrossRef] [Green Version]
- Basu, M.; Zhu, J.Y.; LaHaye, S.; Majumdar, U.; Jiao, K.; Han, Z.; Garg, V. Epigenetic mechanisms underlying maternal diabetes-associated risk of congenital heart disease. JCI Insight 2017, 2, e95085. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Reece, E.A.; Zhong, J.; Dong, D.; Shen, W.B.; Harman, C.R.; Yang, P. Type 2 diabetes mellitus induces congenital heart defects in murine embryos by increasing oxidative stress, endoplasmic reticulum stress, and apoptosis. Am. J. Obstet. Gynecol. 2016, 215, 366.e1–366.e10. [Google Scholar] [CrossRef] [Green Version]
- Dong, D.; Zhang, Y.; Reece, E.A.; Wang, L.; Harman, C.R.; Yang, P. microRNA expression profiling and functional annotation analysis of their targets modulated by oxidative stress during embryonic heart development in diabetic mice. Reprod. Toxicol. 2016, 65, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wu, Y.; Quon, M.J.; Li, X.; Yang, P. ASK1 mediates the teratogenicity of diabetes in the developing heart by inducing ER stress and inhibiting critical factors essential for cardiac development. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E487–E499. [Google Scholar] [CrossRef] [Green Version]
- Moazzen, H.; Lu, X.; Ma, N.L.; Velenosi, T.J.; Urquhart, B.L.; Wisse, L.J.; Gittenberger-de Groot, A.C.; Feng, Q. N-Acetylcysteine prevents congenital heart defects induced by pregestational diabetes. Cardiovasc. Diabetol. 2014, 13, 46. [Google Scholar] [CrossRef] [Green Version]
- Vijaya, M.; Manikandan, J.; Parakalan, R.; Dheen, S.T.; Kumar, S.D.; Tay, S.S. Differential gene expression profiles during embryonic heart development in diabetic mice pregnancy. Gene 2013, 516, 218–227. [Google Scholar] [CrossRef]
- Scott-Drechsel, D.E.; Rugonyi, S.; Marks, D.L.; Thornburg, K.L.; Hinds, M.T. Hyperglycemia slows embryonic growth and suppresses cell cycle via cyclin D1 and p21. Diabetes 2013, 62, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.D.; Vijaya, M.; Samy, R.P.; Dheen, S.T.; Ren, M.; Watt, F.; Kang, Y.J.; Bay, B.H.; Tay, S.S. Zinc supplementation prevents cardiomyocyte apoptosis and congenital heart defects in embryos of diabetic mice. Free Radic. Biol. Med. 2012, 53, 1595–1606. [Google Scholar] [CrossRef]
- Ejdesjö, A.; Wentzel, P.; Eriksson, U.J. Influence of maternal metabolism and parental genetics on fetal maldevelopment in diabetic rat pregnancy. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1198–E1209. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.D.; Dheen, S.T.; Tay, S.S. Maternal diabetes induces congenital heart defects in mice by altering the expression of genes involved in cardiovascular development. Cardiovasc. Diabetol. 2007, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Mdaki, K.S.; Larsen, T.D.; Wachal, A.L.; Schimelpfenig, M.D.; Weaver, L.J.; Dooyema, S.D.; Louwagie, E.J.; Baack, M.L. Maternal high-fat diet impairs cardiac function in offspring of diabetic pregnancy through metabolic stress and mitochondrial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H681–H692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zhu, C.; Sun, M.; Maimaiti, R.; Ford, S.P.; Nathanielsz, P.W.; Ren, J.; Guo, W. Maternal obesity impairs fetal cardiomyocyte contractile function in sheep. FASEB J. 2019, 33, 2587–2598. [Google Scholar] [CrossRef] [Green Version]
- Loche, E.; Blackmore, H.L.; Carpenter, A.A.; Beeson, J.H.; Pinnock, A.; Ashmore, T.J.; Aiken, C.E.; de Almeida-Faria, J.; Schoonejans, J.M.; Giussani, D.A.; et al. Maternal diet-induced obesity programmes cardiac dysfunction in male mice independently of post-weaning diet. Cardiovasc. Res. 2018, 114, 1372–1384. [Google Scholar] [CrossRef] [Green Version]
- Kwong, W.Y.; Wild, A.E.; Roberts, P.; Willis, A.C.; Fleming, T.P. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 2000, 127, 4195–4202. [Google Scholar] [CrossRef]
- Darby, J.R.T.; McMillen, I.C.; Morrison, J.L. Maternal undernutrition in late gestation increases IGF2 signalling molecules and collagen deposition in the right ventricle of the fetal sheep heart. J. Physiol. 2018, 596, 2345–2358. [Google Scholar] [CrossRef] [Green Version]
- Jonker, S.S.; Kamna, D.; LoTurco, D.; Kailey, J.; Brown, L.D. IUGR impairs cardiomyocyte growth and maturation in fetal sheep. J. Endocrinol. 2018, 239, 253–265. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.P.; Tavares, L.C.; Duarte, A.I.; Baldeiras, I.; Cunha-Oliveira, T.; Martins, J.D.; Santos, M.S.; Maloyan, A.; Moreno, A.J.; Cox, L.A.; et al. Sex-dependent vulnerability of fetal nonhuman primate cardiac mitochondria to moderate maternal nutrient reduction. Clin. Sci. 2021, 135, 1103–1126. [Google Scholar] [CrossRef]
- Louwagie, E.J.; Larsen, T.D.; Wachal, A.L.; Gandy, T.C.T.; Eclov, J.A.; Rideout, T.C.; Kern, K.A.; Cain, J.T.; Anderson, R.H.; Mdaki, K.S.; et al. Age and Sex Influence Mitochondria and Cardiac Health in Offspring Exposed to Maternal Glucolipotoxicity. iScience 2020, 23, 101746. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, S.; Wang, X.; Wu, G.; Zhang, Y.; Fu, C.; Hu, C.; Liu, Z.; Luo, X.; Wang, J.; et al. Exposure to maternal diabetes induces endothelial dysfunction and hypertension in adult male rat offspring. Microvasc. Res. 2021, 133, 104076. [Google Scholar] [CrossRef]
- Schütte, T.; Kedziora, S.M.; Haase, N.; Herse, F.; Alenina, N.; Müller, D.N.; Bader, M.; Schupp, M.; Dechend, R.; Golic, M.; et al. Diabetic pregnancy as a novel risk factor for cardiac dysfunction in the offspring-the heart as a target for fetal programming in rats. Diabetologia 2021, 64, 2829–2842. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, Y.C.; Liang, Y.; Lin, X.H.; Tan, Y.J.; Wu, D.D.; Li, X.Z.; Ye, B.Z.; Kong, F.Q.; Sheng, J.Z.; et al. The impaired myocardial ischemic tolerance in adult offspring of diabetic pregnancy is restored by maternal melatonin treatment. J. Pineal Res. 2016, 61, 340–352. [Google Scholar] [CrossRef]
- Chen, Z.; Gong, L.; Zhang, P.; Li, Y.; Liu, B.; Zhang, L.; Zhuang, J.; Xiao, D. Epigenetic Down-Regulation of Sirt 1 via DNA Methylation and Oxidative Stress Signaling Contributes to the Gestational Diabetes Mellitus-Induced Fetal Programming of Heart Ischemia-Sensitive Phenotype in Late Life. Int. J. Biol. Sci. 2019, 15, 1240–1251. [Google Scholar] [CrossRef]
- Vaughan, O.R.; Rosario, F.J.; Chan, J.; Cox, L.A.; Ferchaud-Roucher, V.; Zemski-Berry, K.A.; Reusch, J.E.B.; Keller, A.C.; Powell, T.L.; Jansson, T. Maternal obesity causes fetal cardiac hypertrophy and alters adult offspring myocardial metabolism in mice. J. Physiol. 2022, 600, 3169–3191. [Google Scholar] [CrossRef]
- Ahmed, A.; Liang, M.; Chi, L.; Zhou, Y.Q.; Sled, J.G.; Wilson, M.D.; Delgado-Olguín, P. Maternal obesity persistently alters cardiac progenitor gene expression and programs adult-onset heart disease susceptibility. Mol. Metab. 2021, 43, 101116. [Google Scholar] [CrossRef]
- Xue, Q.; Chen, P.; Li, X.; Zhang, G.; Patterson, A.J.; Luo, J. Maternal High-Fat Diet Causes a Sex-Dependent Increase in AGTR2 Expression and Cardiac Dysfunction in Adult Male Rat Offspring. Biol. Reprod. 2015, 93, 49. [Google Scholar] [CrossRef]
- Watkins, A.J.; Wilkins, A.; Cunningham, C.; Perry, V.H.; Seet, M.J.; Osmond, C.; Eckert, J.J.; Torrens, C.; Cagampang, F.R.; Cleal, J.; et al. Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. J. Physiol. 2008, 586, 2231–2244. [Google Scholar] [CrossRef]
- Larsen, T.D.; Sabey, K.H.; Knutson, A.J.; Gandy, T.C.T.; Louwagie, E.J.; Lauterboeck, L.; Mdaki, K.S.; Baack, M.L. Diabetic Pregnancy and Maternal High-Fat Diet Impair Mitochondrial Dynamism in the Developing Fetal Rat Heart by Sex-Specific Mechanisms. Int. J. Mol. Sci. 2019, 20, 3090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, C.C.; Larsen, T.D.; Eclov, J.A.; Louwagie, E.J.; Gandy, T.C.T.; Faustino, R.S.; Baack, M.L. Maternal High Fat Diet and Diabetes Disrupts Transcriptomic Pathways That Regulate Cardiac Metabolism and Cell Fate in Newborn Rat Hearts. Front. Endocrinol. 2020, 11, 570846. [Google Scholar] [CrossRef]
- Raji, S.R.; Nandini, R.J.; Ashok, S.; Anand, R.C.; Vivek, P.V.; Karunakaran, J.; Sreelatha, H.V.; Manjunatha, S.; Gopala, S. Diminished substrate-mediated cardiac mitochondrial respiration and elevated autophagy in adult male offspring of gestational diabetic rats. IUBMB Life 2021, 73, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Porter, D.F.; Lopez-Pajares, V.; Siprashvili, Z.; Meyers, R.M.; Bai, Y.; Nguyen, D.T.; Ko, L.A.; Zarnegar, B.J.; Ferguson, I.D.; et al. Glucose dissociates DDX21 dimers to regulate mRNA splicing and tissue differentiation. Cell 2023, 186, 80–97.e26. [Google Scholar] [CrossRef]
- Su, D.; Zhou, Y.; Hu, S.; Guan, L.; Shi, C.; Wang, Q.; Chen, Y.; Lu, C.; Li, Q.; Ma, X. Role of GAB1/PI3K/AKT signaling high glucose-induced cardiomyocyte apoptosis. Biomed. Pharmacother. 2017, 93, 1197–1204. [Google Scholar] [CrossRef]
- Lindegaard, M.L.; Nielsen, L.B. Maternal diabetes causes coordinated down-regulation of genes involved with lipid metabolism in the murine fetal heart. Metabolism 2008, 57, 766–773. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Lam, N.T.; Savla, J.J.; Nakada, Y.; Pereira, A.H.M.; Elnwasany, A.; Menendez-Montes, I.; Ensley, E.L.; Petric, U.B.; Sharma, G.; et al. Mitochondrial Substrate Utilization Regulates Cardiomyocyte Cell Cycle Progression. Nat. Metab. 2020, 2, 167–178. [Google Scholar] [CrossRef]
- Luo, H.; Lan, C.; Fan, C.; Gong, X.; Chen, C.; Yu, C.; Wang, J.; Luo, X.; Hu, C.; Jose, P.A.; et al. Down-regulation of AMPK/PPARδ signalling promotes endoplasmic reticulum stress-induced endothelial dysfunction in adult rat offspring exposed to maternal diabetes. Cardiovasc. Res. 2022, 118, 2304–2316. [Google Scholar] [CrossRef]
- Sun, C.; Velazquez, M.A.; Marfy-Smith, S.; Sheth, B.; Cox, A.; Johnston, D.A.; Smyth, N.; Fleming, T.P. Mouse early extra-embryonic lineages activate compensatory endocytosis in response to poor maternal nutrition. Development 2014, 141, 1140–1150. [Google Scholar] [CrossRef] [Green Version]
- Watkins, A.J.; Ursell, E.; Panton, R.; Papenbrock, T.; Hollis, L.; Cunningham, C.; Wilkins, A.; Perry, V.H.; Sheth, B.; Kwong, W.Y.; et al. Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol. Reprod. 2008, 78, 299–306. [Google Scholar] [CrossRef]
- Botting, K.J.; Loke, X.Y.; Zhang, S.; Andersen, J.B.; Nyengaard, J.R.; Morrison, J.L. IUGR decreases cardiomyocyte endowment and alters cardiac metabolism in a sex- and cause-of-IUGR-specific manner. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R48–R67. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Torres-Cuevas, I.; Parra-Llorca, A.; Sánchez-Illana, A.; Nuñez-Ramiro, A.; Kuligowski, J.; Cháfer-Pericás, C.; Cernada, M.; Escobar, J.; Vento, M. Oxygen and oxidative stress in the perinatal period. Redox Biol. 2017, 12, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Block, T.; El-Osta, A. Epigenetic programming, early life nutrition and the risk of metabolic disease. Atherosclerosis 2017, 266, 31–40. [Google Scholar] [CrossRef]
- Robinson, E.L.; Anene-Nzelu, C.G.; Rosa-Garrido, M.; Foo, R.S.Y. Cardiac epigenetics: Driving signals to the cardiac epigenome in development and disease. J. Mol. Cell. Cardiol. 2021, 151, 88. [Google Scholar] [CrossRef]
- Agarwal, P.; Morriseau, T.S.; Kereliuk, S.M.; Doucette, C.A.; Wicklow, B.A.; Dolinsky, V.W. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit. Rev. Clin. Lab. Sci. 2018, 55, 71–101. [Google Scholar] [CrossRef]
- Canouil, M.; Khamis, A.; Keikkala, E.; Hummel, S.; Lobbens, S.; Bonnefond, A.; Delahaye, F.; Tzala, E.; Mustaniemi, S.; Vääräsmäki, M.; et al. Epigenome-Wide Association Study Reveals Methylation Loci Associated With Offspring Gestational Diabetes Mellitus Exposure and Maternal Methylome. Diabetes Care 2021, 44, 1992–1999. [Google Scholar] [CrossRef]
- Weng, X.; Liu, F.; Zhang, H.; Kan, M.; Wang, T.; Dong, M.; Liu, Y. Genome-wide DNA methylation profiling in infants born to gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2018, 142, 10–18. [Google Scholar] [CrossRef]
- Reichetzeder, C.; Dwi Putra, S.E.; Pfab, T.; Slowinski, T.; Neuber, C.; Kleuser, B.; Hocher, B. Increased global placental DNA methylation levels are associated with gestational diabetes. Clin. Epigenet. 2016, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Piaggi, P.; Traurig, M.; Bogardus, C.; Knowler, W.C.; Baier, L.J.; Hanson, R.L. Differential methylation of genes in individuals exposed to maternal diabetes in utero. Diabetologia 2017, 60, 645–655. [Google Scholar] [CrossRef] [Green Version]
- Tobi, E.W.; Goeman, J.J.; Monajemi, R.; Gu, H.; Putter, H.; Zhang, Y.; Slieker, R.C.; Stok, A.P.; Thijssen, P.E.; Müller, F.; et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat. Commun. 2014, 5, 5592. [Google Scholar] [CrossRef] [Green Version]
- Bošković, A.; Rando, O.J. Transgenerational Epigenetic Inheritance. Annu. Rev. Genet. 2018, 52, 21–41. [Google Scholar] [CrossRef]
- Blin, G.; Liand, M.; Mauduit, C.; Chehade, H.; Benahmed, M.; Simeoni, U.; Siddeek, B. Maternal Exposure to High-Fat Diet Induces Long-Term Derepressive Chromatin Marks in the Heart. Nutrients 2020, 12, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyaya, B.; Larsen, T.; Barwari, S.; Louwagie, E.J.; Baack, M.L.; Dey, M. Prenatal Exposure to a Maternal High-Fat Diet Affects Histone Modification of Cardiometabolic Genes in Newborn Rats. Nutrients 2017, 9, 407. [Google Scholar] [CrossRef] [Green Version]
- Barwari, T.; Joshi, A.; Mayr, M. MicroRNAs in Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 68, 2577–2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lock, M.C.; Botting, K.J.; Tellam, R.L.; Brooks, D.; Morrison, J.L. Adverse Intrauterine Environment and Cardiac miRNA Expression. Int. J. Mol. Sci. 2017, 18, 2628. [Google Scholar] [CrossRef] [Green Version]
- Siddeek, B.; Mauduit, C.; Chehade, H.; Blin, G.; Liand, M.; Chindamo, M.; Benahmed, M.; Simeoni, U. Long-term impact of maternal high-fat diet on offspring cardiac health: Role of micro-RNA biogenesis. Cell Death Discov. 2019, 5, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Twinn, D.S.; Blackmore, H.L.; Siggens, L.; Giussani, D.A.; Cross, C.M.; Foo, R.; Ozanne, S.E. The programming of cardiac hypertrophy in the offspring by maternal obesity is associated with hyperinsulinemia, AKT, ERK, and mTOR activation. Endocrinology 2012, 153, 5961–5971. [Google Scholar] [CrossRef] [Green Version]
- Muralimanoharan, S.; Li, C.; Nakayasu, E.S.; Casey, C.P.; Metz, T.O.; Nathanielsz, P.W.; Maloyan, A. Sexual dimorphism in the fetal cardiac response to maternal nutrient restriction. J. Mol. Cell. Cardiol. 2017, 108, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Moore-Morris, T.; van Vliet, P.P.; Andelfinger, G.; Puceat, M. Role of Epigenetics in Cardiac Development and Congenital Diseases. Physiol. Rev. 2018, 98, 2453–2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study | Study Population | Exposures | Following Years | Outcomes | Major Findings |
|---|---|---|---|---|---|
| Øyen et al. [14] | 2,025,727 persons born alive in Denmark between 1978 and 2011 |
| Birth defects | All types of congenital heart diseases |
|
| Priest et al. [15] | 277 pregnant women in southern and central California |
| Birth defects | Cardiac malformations, including ToF, d-transposition of the great arteries (dTGA) |
|
| Tam et al. [19] | 970 mothers who joined the Hyperglycemia and Adverse Pregnancy Outcome study and their children |
| 7 years | Cardiometabolic risk |
|
| Kaseva et al. [21] | 906 pregnant women and their offspring from Uusimaa and northern Finland |
| 24.1 ± 1.3 years | Cardiometabolic risk |
|
| Guillemette et al. [22] | 293,546 people born between 1979 and 2005 in Manitoba, Canada |
| Up to 35 years | Cardiovascular disease, including cardiac arrest, myocardial infarction, ischemic heart disease, and cerebral infarction |
|
| Yu et al. [10] | All 2,432,000 liveborn children without congenital heart disease in Denmark during 1977–2016 |
| 40 years | Early onset CVD including ischemic heart disease, cerebrovascular disease, stroke, heart failure, atrial fibrillation, hypertensive disease, deep vein thrombosis, pulmonary embolism, other CVDs |
|
| Persson et al. [24] | 2,050,491 live singleton infants born between 1992 and 2012 in Sweden |
| Birth defects | Congenital heart defect |
|
| Groves et al. [28] | 87 neonates in UK |
| Newborns | Heart rates Heart variability Cardiac function |
|
| Guzzardi et al. [29] | 91 pregnant women in Italy |
| 12 months | Cardiac function |
|
| Wang et al. [31] | 1966 adults born between 1956 and 1964 in China |
| 45 years | Hypertension |
|
| Painter et al. [35] | 721 men and women born as term singletons in Amsterdam at about the time of the Dutch 1944–1945 famine |
| 58 years | Hypertension |
|
| Roseboom et al. [36] | 912 singletons born in Amsterdam at about the time of the Dutch 1944–1945 famine |
| 50 years | Coronary artery disease (CAD) |
|
| Painter et al. [37] | 837 singletons born in Amsterdam at about the time of the Dutch 1944–1945 famine |
| 50 years | CAD |
|
| Lumey et al. [39] | 1075 men and women born around the Dutch 1944–1945 |
| 58 years | CAD |
|
| Ekamper et al. [40] | 41,096 men born in 1944–1947 in the Netherlands |
| 63 years | Mortality of heart diseases |
|
| Study | Species | Gender | Age | Early Exposure | Second Hit | Major Findings |
|---|---|---|---|---|---|---|
| Pereia et al. [63] | Rat (Sprague Dawley) | M | P1, 3 W, 10 W, 6 W, 12 W | HF group: Gestational diabetes group: | - | Declined cardiac function in aged offspring |
| Louwagie et al. [64] | Rat (Sprague Dawley) | M | 12 W, 16 W, 20 W, 24 W | Streptozotocin, 35 mg/kg, IP, at E0 | - | Higher blood pressure |
| Yu et al. [65] | Rat (Sprague Dawley) | M | 8 W, 29 W–36 W | Transgenic Tet29 female dams, 1.5 mg/kg DOX in drinking water | High-fat diet challenge for 28 weeks | Cardiac dysfunction LV hypertrophy Altered proinflammatory status |
| Schütte et al. [66] | Mouse (C57BL/6) | M | 8–10 W | STZ, IP, 80 mg/kg for 3 days at 8 weeks old | Myocardial ischemia/reperfusion injury at 8–10 weeks old | Larger infarct size Augmented cardiac dysfunction Augmented myocardial apoptosis |
| Gao et al. [67] | Rat (Sprague Dawley) | M and F | 6 W | STZ, SC, 50 mg/kg at E12 | Heart ischemia for 24 h at 6 weeks-old | Larger infarct size LV dysfunction in male offspring |
| Chen et al. [68] | Mouse (C57BL/6) | M and F | E18.5, 3 W, 6 W, 9 W, 24 W | Obesogenic diet until body weight increased for 25% | - | Altered embryonic metabolic genes transcription Diminished cardiac diastolic function Female cardiac function worsens with age |
| Vaughan et al. [69] | Mouse (Chimeric) | M | E18.5, 16 W, 32 W | Obesogenic diet from 4 weeks old through whole life | Isoproterenol (60 mg/kg/day) in 8-week-old mice for 14 days | Exacerbated cardiac remodeling Altered gene expression of cardiac progenitors |
| Ahmed et al. [70] | Rat (Sprague Dawley) | M and F | 3 W | High-fat diet from E0 to E21 | Myocardial ischemia/reperfusion injury | Cardiac hypertrophy in male Increased infract size in male |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Luo, S.; Cui, J.; Tang, Y.; Huang, H.; Ding, G. Research Progress of Maternal Metabolism on Cardiac Development and Function in Offspring. Nutrients 2023, 15, 3388. https://doi.org/10.3390/nu15153388
Ren Z, Luo S, Cui J, Tang Y, Huang H, Ding G. Research Progress of Maternal Metabolism on Cardiac Development and Function in Offspring. Nutrients. 2023; 15(15):3388. https://doi.org/10.3390/nu15153388
Chicago/Turabian StyleRen, Zhuoran, Sisi Luo, Jiajun Cui, Yunhui Tang, Hefeng Huang, and Guolian Ding. 2023. "Research Progress of Maternal Metabolism on Cardiac Development and Function in Offspring" Nutrients 15, no. 15: 3388. https://doi.org/10.3390/nu15153388
APA StyleRen, Z., Luo, S., Cui, J., Tang, Y., Huang, H., & Ding, G. (2023). Research Progress of Maternal Metabolism on Cardiac Development and Function in Offspring. Nutrients, 15(15), 3388. https://doi.org/10.3390/nu15153388







