Intervention Approaches in Studying the Response to Vitamin D3 Supplementation
Abstract
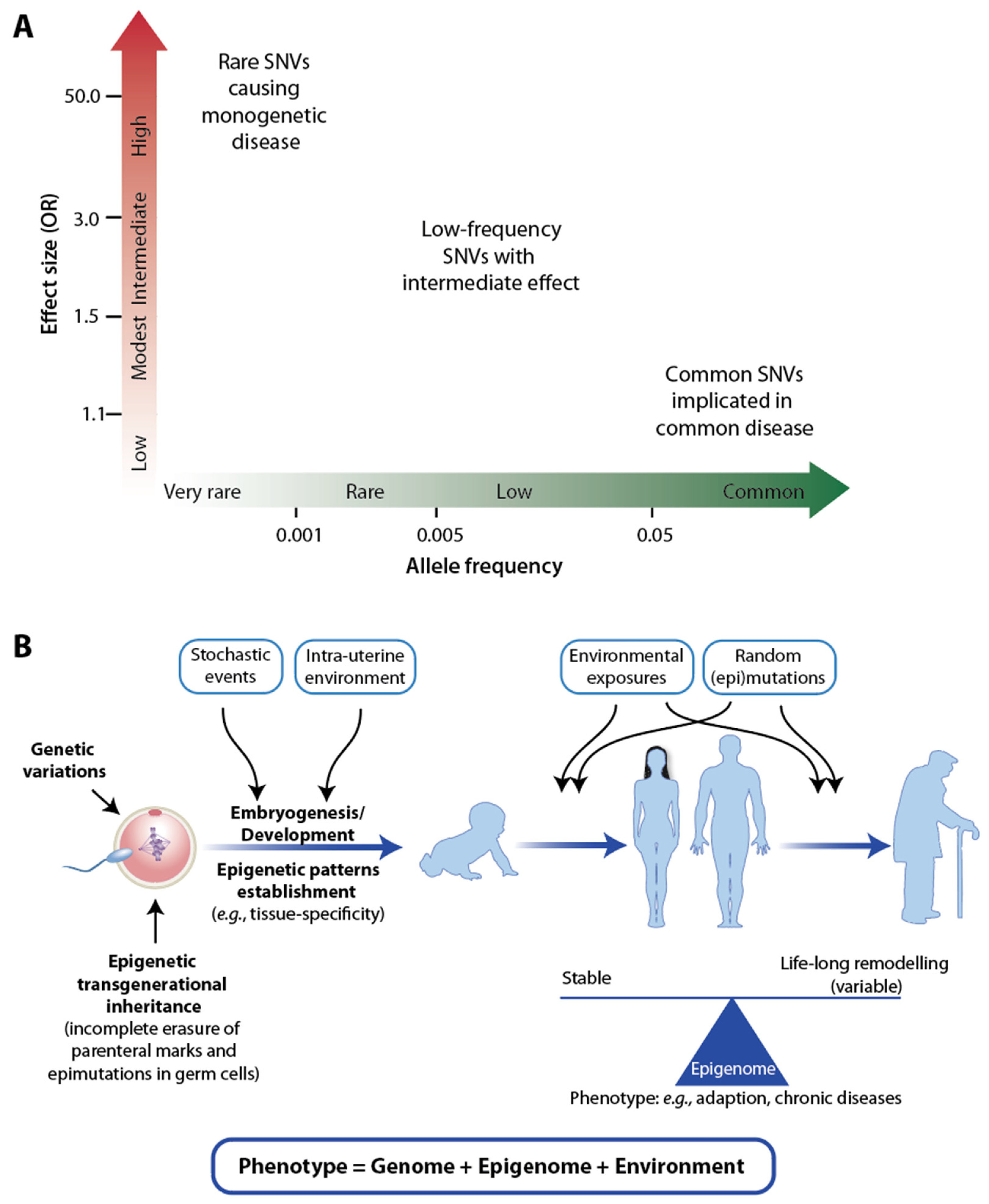
:1. Introduction
2. Large-Scale Vitamin D Intervention Studies
3. Genome-Wide Analyses
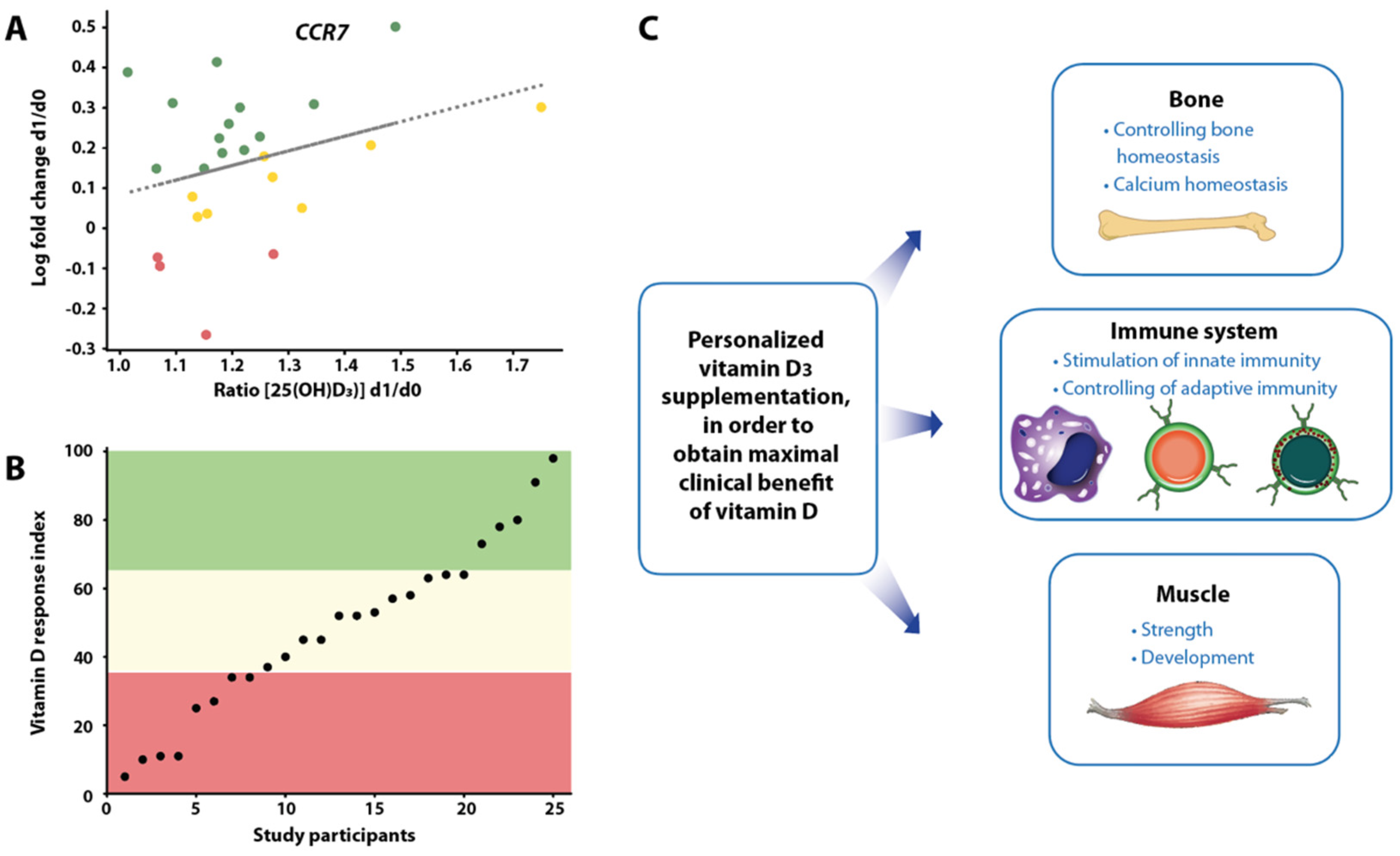
4. Segregation of Participants of Vitamin D Intervention Studies
5. Future View on Vitamin D Intervention Studies
6. Conclusions
Funding
Conflicts of Interest
References
- McMollum, E.V.; Simmonds, N.; Becker, J.E.; Shipley, P.G. Studies on experimental rickets: An experimental demonstration of the existence of a vitamin which promotes calcium deposition. J. Biol. Chem. 1922, 52, 293–298. [Google Scholar] [CrossRef]
- Bendik, I.; Friedel, A.; Roos, F.F.; Weber, P.; Eggersdorfer, M. Vitamin D: A critical and essential micronutrient for human health. Front. Physiol. 2014, 5, 248. [Google Scholar] [CrossRef]
- Holick, M.F. Photobiology of vitamin D. Vitam. D 2011, 3, 13–22. [Google Scholar] [CrossRef]
- Hanel, A.; Carlberg, C. Vitamin D and evolution: Pharmacologic implications. Biochem. Pharmacol. 2020, 173, 113595. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.V.; Luu, W.; Li, D.; Sharpe, L.J.; Brown, A.J. DHCR7: A vital enzyme switch between cholesterol and vitamin D production. Prog. Lipid Res. 2016, 64, 138–151. [Google Scholar] [CrossRef]
- Kuan, V.; Martineau, A.R.; Griffiths, C.J.; Hypponen, E.; Walton, R. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol. Biol. 2013, 13, 144. [Google Scholar] [CrossRef] [Green Version]
- Mathieson, I.; Lazaridis, I.; Rohland, N.; Mallick, S.; Patterson, N.; Roodenberg, S.A.; Harney, E.; Stewardson, K.; Fernandes, D.; Novak, M.; et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature 2015, 528, 499–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, D.; Mathieson, I. The evolution of skin pigmentation associated variation in West Eurasia. Proc. Nat. Acad. Sci. USA 2021, 118, e2009227118. [Google Scholar] [CrossRef]
- Hanel, A.; Carlberg, C. Skin color and vitamin D: An update. Exp. Dermatol. 2020, 29, 864–875. [Google Scholar] [CrossRef]
- Jablonski, N.G.; Chaplin, G. The evolution of human skin coloration. J. Hum. Evol. 2000, 39, 57–106. [Google Scholar] [CrossRef] [Green Version]
- Gunther, T.; Malmstrom, H.; Svensson, E.M.; Omrak, A.; Sanchez-Quinto, F.; Kilinc, G.M.; Krzewinska, M.; Eriksson, G.; Fraser, M.; Edlund, H.; et al. Population genomics of Mesolithic Scandinavia: Investigating early postglacial migration routes and high-latitude adaptation. PLoS Biol. 2018, 16, e2003703. [Google Scholar] [CrossRef] [PubMed]
- Lockau, L.; Atkinson, S.; Mays, S.; Prowse, T.; George, M.; Sperduti, A.; Bondioli, L.; Wood, C.; Ledger, M.; Brickley, M.B. Vitamin D deficiency and the ancient city: Skeletal evidence across the life course from the Roman period site of Isola Sacra, Italy. J. Anthropol. Archaeol. 2019, 55, 101069. [Google Scholar] [CrossRef]
- Mays, S.; Prowse, T.; George, M.; Brickley, M. Latitude, urbanization, age, and sex as risk factors for vitamin D deficiency disease in the Roman Empire. Am. J. Phys. Anthr. 2018, 167, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Hardy, A. Commentary: Bread and alum, syphilis and sunlight: Rickets in the nineteenth century. Int. J. Epidemiol. 2003, 32, 337–340. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Shen, F.; Petryk, A.; Tang, J.; Chen, X.; Sergi, C. “English Disease”: Historical notes on rickets, the bone-lung link and child neglect issues. Nutrients 2016, 8, 722. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Zerwekh, J.E. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008, 87, 1087S–1091S. [Google Scholar] [CrossRef] [Green Version]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Luxwolda, M.F.; Kuipers, R.S.; Kema, I.P.; Dijck-Brouwer, D.A.; Muskiet, F.A. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/L. Br. J. Nutr. 2012, 108, 1557–1561. [Google Scholar] [CrossRef] [Green Version]
- Institute-of-Medicine. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Ricordi, C.; Sanchez, J.; Clare-Salzler, M.J.; Padilla, N.; Fuenmayor, V.; Chavez, C.; Alvarez, A.; Baidal, D.; Alejandro, R.; et al. Influence of vitamin D on islet autoimmunity and beta-cell function in type 1 diabetes. Nutrients 2019, 11, 2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and multiple sclerosis: A comprehensive review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramagopalan, S.V.; Maugeri, N.J.; Handunnetthi, L.; Lincoln, M.R.; Orton, S.M.; Dyment, D.A.; Deluca, G.C.; Herrera, B.M.; Chao, M.J.; Sadovnick, A.D.; et al. Expression of the multiple sclerosis-associated MHC class II allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet. 2009, 5, e1000369. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The role of vitamin D in Inflammatory bowel disease: Mechanism to management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D and Its potential benefit for the COVID-19 pandemic. Endocr. Pract. 2021, 27, 484–493. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sahraian, M.A.; Ebrahimi, M.; Pazoki, M.; Kafan, S.; Tabriz, H.M.; Hadadi, A.; Montazeri, M.; Nasiri, M.; Shirvani, A.; et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS ONE 2020, 15, e0239799. [Google Scholar] [CrossRef]
- Huang, S.J.; Wang, X.H.; Liu, Z.D.; Cao, W.L.; Han, Y.; Ma, A.G.; Xu, S.F. Vitamin D deficiency and the risk of tuberculosis: A meta-analysis. Drug Des. Devel. Ther. 2017, 11, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Rook, G.A. The role of vitamin D in tuberculosis. Am. Rev. Respir Dis. 1988, 138, 768–770. [Google Scholar] [CrossRef]
- Munoz, A.; Grant, W.B. Vitamin D and cancer: An historical overview of the epidemiology and mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef] [PubMed]
- Sofianopoulou, E.; Kaptoge, S.K.; Afzal, S.; Jiang, T.; Gill, D.; Gundersen, T.E.; Bolton, T.R.; Allara, E.; Arnold, M.G.; Mason, A.M.; et al. Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: Observational and Mendelian randomisation analyses. Lancet Diabetes Endocrinol. 2021, 9, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Munoz, A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2022, 79, 217–230. [Google Scholar] [CrossRef]
- Carlberg, C.; Raczyk, M.; Zawrotna, N. Vitamin D: A master example of nutrigenomics. Redox Biol. 2023, 62, 102695. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D and is target genes. Nutrients 2022, 14, 1354. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, S.; Väisänen, S.; Pehkonen, P.; Seuter, S.; Benes, V.; Carlberg, C. Nuclear hormone 1α,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy. Nucleic Acids Res. 2011, 39, 9181–9193. [Google Scholar] [CrossRef] [Green Version]
- Seuter, S.; Neme, A.; Carlberg, C. Epigenomic PU.1-VDR crosstalk modulates vitamin D signaling. Biochim. Biophys. Acta 2017, 1860, 405–415. [Google Scholar] [CrossRef]
- Nurminen, V.; Neme, A.; Seuter, S.; Carlberg, C. The impact of the vitamin D-modulated epigenome on VDR target gene regulation. Biochim. Biophys. Acta 2018, 1861, 697–705. [Google Scholar] [CrossRef]
- Seuter, S.; Neme, A.; Carlberg, C. Epigenome-wide effects of vitamin D and their impact on the transcriptome of human monocytes involve CTCF. Nucleic Acids Res. 2016, 44, 4090–4104. [Google Scholar] [CrossRef] [Green Version]
- Carlberg, C.; Seuter, S.; Nurmi, T.; Tuomainen, T.P.; Virtanen, J.K.; Neme, A. In vivo response of the human epigenome to vitamin D: A proof-of-principle study. J. Steroid Biochem. Mol. Biol. 2018, 180, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Neme, A.; Seuter, S.; Malinen, M.; Nurmi, T.; Tuomainen, T.P.; Virtanen, J.K.; Carlberg, C. In vivo transcriptome changes of human white blood cells in response to vitamin D. J. Steroid Biochem. Mol. Biol. 2019, 188, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Scragg, R.; Khaw, K.T.; Toop, L.; Sluyter, J.; Lawes, C.M.M.; Waayer, D.; Giovannucci, E.; Camargo, C.A., Jr. Monthly High-Dose Vitamin D Supplementation and Cancer Risk: A Post Hoc Analysis of the Vitamin D Assessment Randomized Clinical Trial. JAMA Oncol. 2018, 4, e182178. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, J.K.; Nurmi, T.; Aro, A.; Bertone-Johnson, E.R.; Hypponen, E.; Kroger, H.; Lamberg-Allardt, C.; Manson, J.E.; Mursu, J.; Mantyselka, P.; et al. Vitamin D supplementation and prevention of cardiovascular disease and cancer in the Finnish Vitamin D Trial: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 115, 1300–1310. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef] [Green Version]
- Bischoff-Ferrari, H.A.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; da Silva, J.A.P.; Blauth, M.; Felson, D.T.; McCloskey, E.V.; Watzl, B.; Hofbauer, L.C.; et al. Effect of Vitamin D Supplementation, Omega-3 Fatty Acid Supplementation, or a Strength-Training Exercise Program on Clinical Outcomes in Older Adults: The DO-HEALTH Randomized Clinical Trial. JAMA 2020, 324, 1855–1868. [Google Scholar] [CrossRef]
- Bouillon, R. Vitamin D status in Africa is worse than in other continents. Lancet Glob. Health 2020, 8, e20–e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlberg, C. Vitamin D in the context of evolution. Nutrients 2022, 14, 3018. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.M.; Giovannucci, E.L.; Willett, W.; Buring, J.E.; et al. Effect of vitamin D3 supplements on development of advanced cancer: A secondary analysis of the VITAL randomized cinical trial. JAMA Netw. Open 2020, 3, e2025850. [Google Scholar] [CrossRef]
- The 1000 Genomes Project Consortium; Abecasis, G.R.; Auton, A.; Brooks, L.D.; DePristo, M.A.; Durbin, R.M.; Handsaker, R.E.; Kang, H.M.; Marth, G.T.; McVean, G.A. An integrated map of genetic variation from 1092 human genomes. Nature 2012, 491, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Cano-Gamez, E.; Trynka, G. From GWAS to function: Using functional genomics to identify the mechanisms underlying complex diseases. Front. Genet. 2020, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; O’Reilly, P.F.; Aschard, H.; Hsu, Y.H.; Richards, J.B.; Dupuis, J.; Ingelsson, E.; Karasik, D.; Pilz, S.; Berry, D.; et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat. Commun. 2018, 9, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revez, J.A.; Lin, T.; Qiao, Z.; Xue, A.; Holtz, Y.; Zhu, Z.; Zeng, J.; Wang, H.; Sidorenko, J.; Kemper, K.E.; et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat. Commun. 2020, 11, 1647. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, B.L.; Zhu, G.; Medland, S.E.; Renteria, M.E.; Eyles, D.W.; Grasby, K.L.; McGrath, J.J.; Martin, N.G. Half the Genetic Variance in Vitamin D Concentration is Shared with Skin Colour and Sun Exposure Genes. Behav. Genet. 2019, 49, 386–398. [Google Scholar] [CrossRef]
- Carlberg, C.; Seuter, S.; de Mello, V.D.; Schwab, U.; Voutilainen, S.; Pulkki, K.; Nurmi, T.; Virtanen, J.; Tuomainen, T.P.; Uusitupa, M. Primary vitamin D target genes allow a categorization of possible benefits of vitamin D3 supplementation. PLoS ONE 2013, 8, e71042. [Google Scholar] [CrossRef] [Green Version]
- Wilfinger, J.; Seuter, S.; Tuomainen, T.-P.; Virtanen, J.K.; Voutilainen, S.; Nurmi, T.; de Mello, V.D.F.; Uusitupa, M.; Carlberg, C. Primary vitamin D receptor target genes as biomarkers for the vitamin D3 status in the hematopoietic system. J. Nutr. Biochem. 2014, 25, 875–884. [Google Scholar] [CrossRef]
- Ryynänen, J.; Neme, A.; Tuomainen, T.P.; Virtanen, J.K.; Voutilainen, S.; Nurmi, T.; de Mello, V.D.; Uusitupa, M.; Carlberg, C. Changes in vitamin D target gene expression in adipose tissue monitor the vitamin D response of human individuals. Mol. Nutr. Food Res. 2014, 58, 2036–2045. [Google Scholar] [CrossRef]
- Saksa, N.; Neme, A.; Ryynänen, J.; Uusitupa, M.; de Mello, V.D.; Voutilainen, S.; Nurmi, T.; Virtanen, J.K.; Tuomainen, T.P.; Carlberg, C. Dissecting high from low responders in a vitamin D3 intervention study. J. Steroid Biochem. Mol. Biol. 2015, 148, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Vukic, M.; Neme, A.; Seuter, S.; Saksa, N.; de Mello, V.D.; Nurmi, T.; Uusitupa, M.; Tuomainen, T.P.; Virtanen, J.K.; Carlberg, C. Relevance of vitamin D receptor target genes for monitoring the vitamin D responsiveness of primary human cells. PLoS ONE 2015, 10, e0124339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seuter, S.; Virtanen, J.K.; Nurmi, T.; Pihlajamäki, J.; Mursu, J.; Voutilainen, S.; Tuomainen, T.P.; Neme, A.; Carlberg, C. Molecular evaluation of vitamin D responsiveness of healthy young adults. J. Steroid Biochem. Mol. Biol. 2017, 174, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Hanel, A.; Carlberg, C. Time-resolved gene expression analysis monitors the regulation of inflammatory mediators and attenuation of adaptive immune response by vitamin D. Int. J. Mol. Sci. 2022, 23, 911. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Taylor, A.J.; Plant, K.; Dahl, A.; Lau, E.; Hill, M.; Sims, D.; Heger, A.; Emberson, J.; Armitage, J.; Clarke, R.; et al. Genomic Response to Vitamin D Supplementation in the Setting of a Randomized, Placebo-Controlled Trial. EBioMedicine 2018, 31, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Carlberg, C. Molecular approaches for optimizing vitamin D supplementation. Vitam. Horm. 2016, 100, 255–271. [Google Scholar] [CrossRef]
- Carlberg, C.; Haq, A. The concept of the personal vitamin D response index. J. Steroid Biochem. Mol. Biol. 2018, 175, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Hanel, A.; Neme, A.; Malinen, M.; Hamalainen, E.; Malmberg, H.R.; Etheve, S.; Tuomainen, T.P.; Virtanen, J.K.; Bendik, I.; Carlberg, C. Common and personal target genes of the micronutrient vitamin D in primary immune cells from human peripheral blood. Sci. Rep. 2020, 10, 21051. [Google Scholar] [CrossRef] [PubMed]
- Hanel, A.; Veldhuizen, C.; Carlberg, C. Gene-regulatory potential of 25-hydroxyvitamin D3 and D2. Front. Nutr. 2022, 9, 910601. [Google Scholar] [CrossRef]
- Rieder, M.J.; Reiner, A.P.; Gage, B.F.; Nickerson, D.A.; Eby, C.S.; McLeod, H.L.; Blough, D.K.; Thummel, K.E.; Veenstra, D.L.; Rettie, A.E. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N. Engl. J. Med. 2005, 352, 2285–2293. [Google Scholar] [CrossRef] [Green Version]
- Pierrot-Deseilligny, C.; Souberbielle, J.C. Is hypovitaminosis D one of the environmental risk factors for multiple sclerosis? Brain 2010, 133, 1869–1888. [Google Scholar] [CrossRef] [Green Version]
- Simpson, S., Jr.; Blizzard, L.; Otahal, P.; Van der Mei, I.; Taylor, B. Latitude is significantly associated with the prevalence of multiple sclerosis: A meta-analysis. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1132–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salzer, J.; Hallmans, G.; Nystrom, M.; Stenlund, H.; Wadell, G.; Sundstrom, P. Vitamin D as a protective factor in multiple sclerosis. Neurology 2012, 79, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Mangin, M.; Sinha, R.; Fincher, K. Inflammation and vitamin D: The infection connection. Inflamm. Res. 2014, 63, 803–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleet, J.C.; DeSmet, M.; Johnson, R.; Li, Y. Vitamin D and cancer: A review of molecular mechanisms. Biochem. J. 2012, 441, 61–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skoglund, P.; Mathieson, I. Ancient genomics of modern humans: The first decade. Annu. Rev. Genom. Hum. Genet. 2018, 19, 381–404. [Google Scholar] [CrossRef]
- Lazaridis, I. The evolutionary history of human populations in Europe. Curr. Opin. Genet. Dev. 2018, 53, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Akobeng, A.K. Principles of evidence based medicine. Arch. Dis. Child. 2005, 90, 837–840. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, V. The next generation of evidence-based medicine. Nat. Med. 2023, 29, 49–58. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [Green Version]
- Pico, A.R.; Kelder, T.; van Iersel, M.P.; Hanspers, K.; Conklin, B.R.; Evelo, C. WikiPathways: Pathway editing for the people. PLoS Biol. 2008, 6, e184. [Google Scholar] [CrossRef] [Green Version]
- Himmelstein, D.S.; Baranzini, S.E. Heterogeneous network edge prediction: A data integration approach to prioritize disease-associated genes. PLoS Comput. Biol. 2015, 11, e1004259. [Google Scholar] [CrossRef] [Green Version]
- Niarakis, A.; Helikar, T. A practical guide to mechanistic systems modeling in biology using a logic-based approach. Brief Bioinform. 2021, 22, bbaa236. [Google Scholar] [CrossRef]
- Harris, L.A.; Beik, S.; Ozawa, P.M.M.; Jimenez, L.; Weaver, A.M. Modeling heterogeneous tumor growth dynamics and cell-cell interactions at single-cell and cell-population resolution. Curr. Opin. Syst. Biol. 2019, 17, 24–34. [Google Scholar] [CrossRef]
- AlGhamdi, S.A.; Enaibsi, N.N.; Alsufiani, H.M.; Alshaibi, H.F.; Khoja, S.O.; Carlberg, C. A single oral vitamin D3 bolus reduces inflammatory markers in healthy Saudi males. Int. J. Mol. Sci. 2022, 23, 11992. [Google Scholar] [CrossRef] [PubMed]
- Alsufiani, H.M.; AlGhamdi, S.A.; AlShaibi, H.F.; Khoja, S.O.; Saif, S.F.; Carlberg, C. A single vtamin D3 bolus supplementation improves vitamin D status and reduces proinflammatory cytokines in healthy females. Nutrients 2022, 14, 3963. [Google Scholar] [CrossRef]
- Cipriani, C.; Romagnoli, E.; Pepe, J.; Russo, S.; Carlucci, L.; Piemonte, S.; Nieddu, L.; McMahon, D.J.; Singh, R.; Minisola, S. Long-term bioavailability after a single oral or intramuscular administration of 600,000 IU of ergocalciferol or cholecalciferol: Implications for treatment and prophylaxis. J. Clin. Endocrinol. Metab. 2013, 98, 2709–2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fassio, A.; Adami, G.; Rossini, M.; Giollo, A.; Caimmi, C.; Bixio, R.; Viapiana, O.; Milleri, S.; Gatti, M.; Gatti, D. Pharmacokinetics of Oral Cholecalciferol in Healthy Subjects with Vitamin D Deficiency: A Randomized Open-Label Study. Nutrients 2020, 12, 1553. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.K.; Manoharan, M.S.; Lee, G.C.; McKinnon, L.R.; Meunier, J.A.; Steri, M.; Harper, N.; Fiorillo, E.; Smith, A.M.; Restrepo, M.I.; et al. Immune resilience despite inflammatory stress promotes longevity and favorable health outcomes including resistance to infection. Nat. Commun. 2023, 14, 3286. [Google Scholar] [CrossRef]

| Study | Number of Participants | Age (Mean) | 25(OH)D3 Level Baseline Final | Duration | Intervention (Vitamin D3 Dose) | |
|---|---|---|---|---|---|---|
| VITAL (USA) | 25,871 | 67 y | 77 nM | 105 nM | 5.3 y | 50 µg/day |
| ViDA (New Zealand) | 5108 | 66 y | 66 nM | 135 nM | 3.3 y | 5000 µg once + 2500 µg/month |
| FIND (Finland) | 2495 | 68 y | 75 nM | 110 nM | 5 y | 40 and 80 µg/day |
| D2d (USA) | 2423 | 60 y | 70 nM | 135 nM | 2.5 y | 100 µg/day |
| DO-HEALTH (Europe) | 2157 | 75 y | 56 nM | 94 nM | 3 y | 50 µg /day |
| Study | Number of Participants | Age (Mean) | 25(OH)D3 Level Baseline Final | Duration | Intervention (Vitamin D3 Dose) | |
|---|---|---|---|---|---|---|
| VitDmet (Finland) | 71 | 67 y | 59 nM | 83 nM | 5 months | 0, 40 or 80 µg/day |
| VitDbol (Finland) | 35 | 26 y | 65 nM | 80 nM | 30 days | 2000 µg bolus once |
| VitDHiD (Finland) | 40 | 27 y | 73 nM | 98 nM | 1 day | 2000 µg bolus once |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gospodarska, E.; Ghosh Dastidar, R.; Carlberg, C. Intervention Approaches in Studying the Response to Vitamin D3 Supplementation. Nutrients 2023, 15, 3382. https://doi.org/10.3390/nu15153382
Gospodarska E, Ghosh Dastidar R, Carlberg C. Intervention Approaches in Studying the Response to Vitamin D3 Supplementation. Nutrients. 2023; 15(15):3382. https://doi.org/10.3390/nu15153382
Chicago/Turabian StyleGospodarska, Emilia, Ranjini Ghosh Dastidar, and Carsten Carlberg. 2023. "Intervention Approaches in Studying the Response to Vitamin D3 Supplementation" Nutrients 15, no. 15: 3382. https://doi.org/10.3390/nu15153382
APA StyleGospodarska, E., Ghosh Dastidar, R., & Carlberg, C. (2023). Intervention Approaches in Studying the Response to Vitamin D3 Supplementation. Nutrients, 15(15), 3382. https://doi.org/10.3390/nu15153382






