Effects of Dietary Sugar Reduction on Biomarkers of Cardiometabolic Health in Latino Youth: Secondary Analyses from a Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment and Enrollment
2.2. Study Design
2.3. Study Diets
2.4. Glucose Tolerance and Determinants of Glucose Tolerance
2.5. Fasting Serum Lipid Profile & Biomarkers
2.6. Anthropometrics
2.7. Dietary Intake and Physical Activity
2.8. Statistical Analysis
3. Results
3.1. Description of Participants and Adverse Events
3.2. Intervention Adherence and Dietary Intakes
3.3. Glucose Tolerance and Determinants of Glucose Tolerance
3.4. Blood Pressure and Fasting Serum Lipids
3.5. Inflammatory Markers & Adipokines
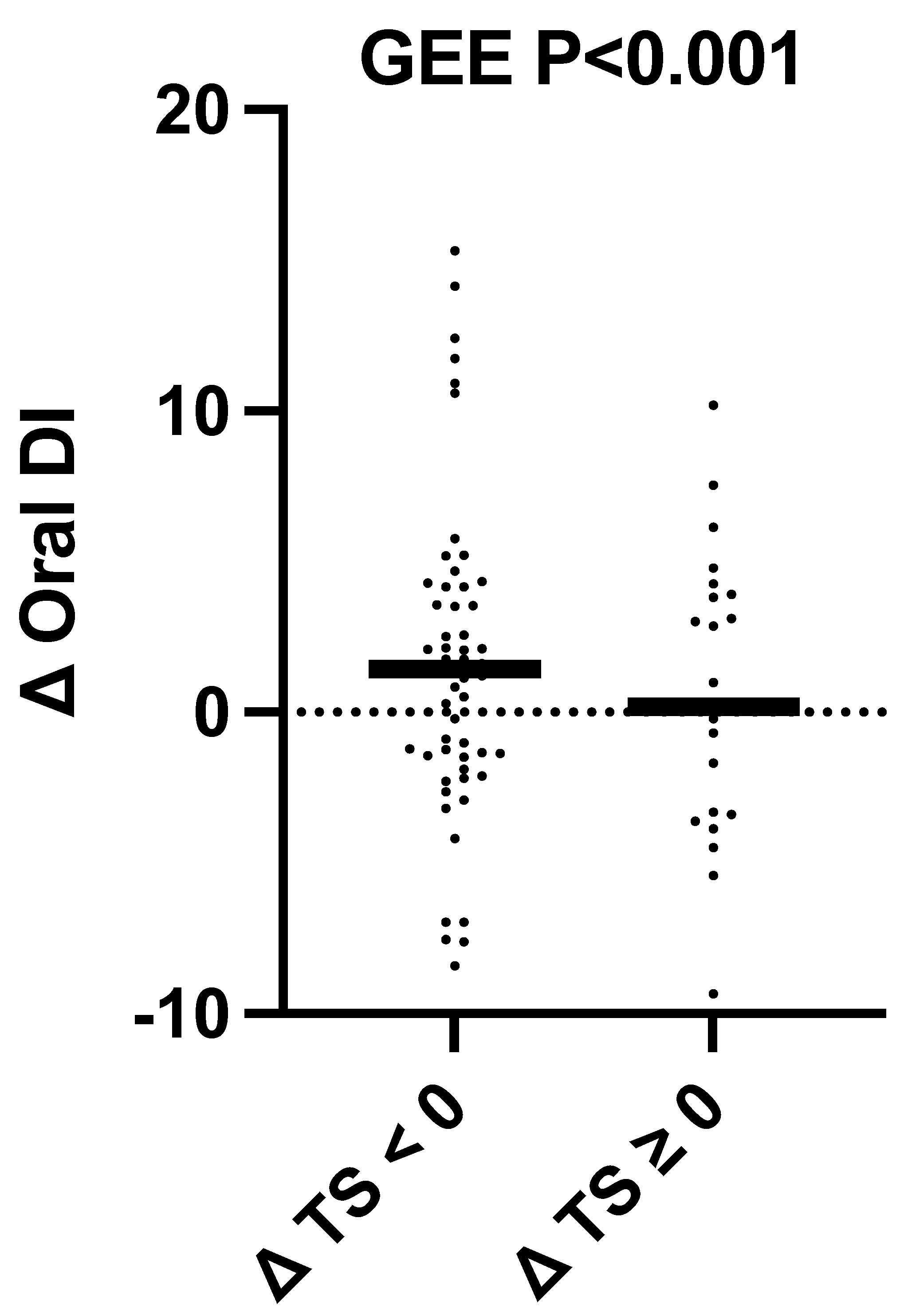
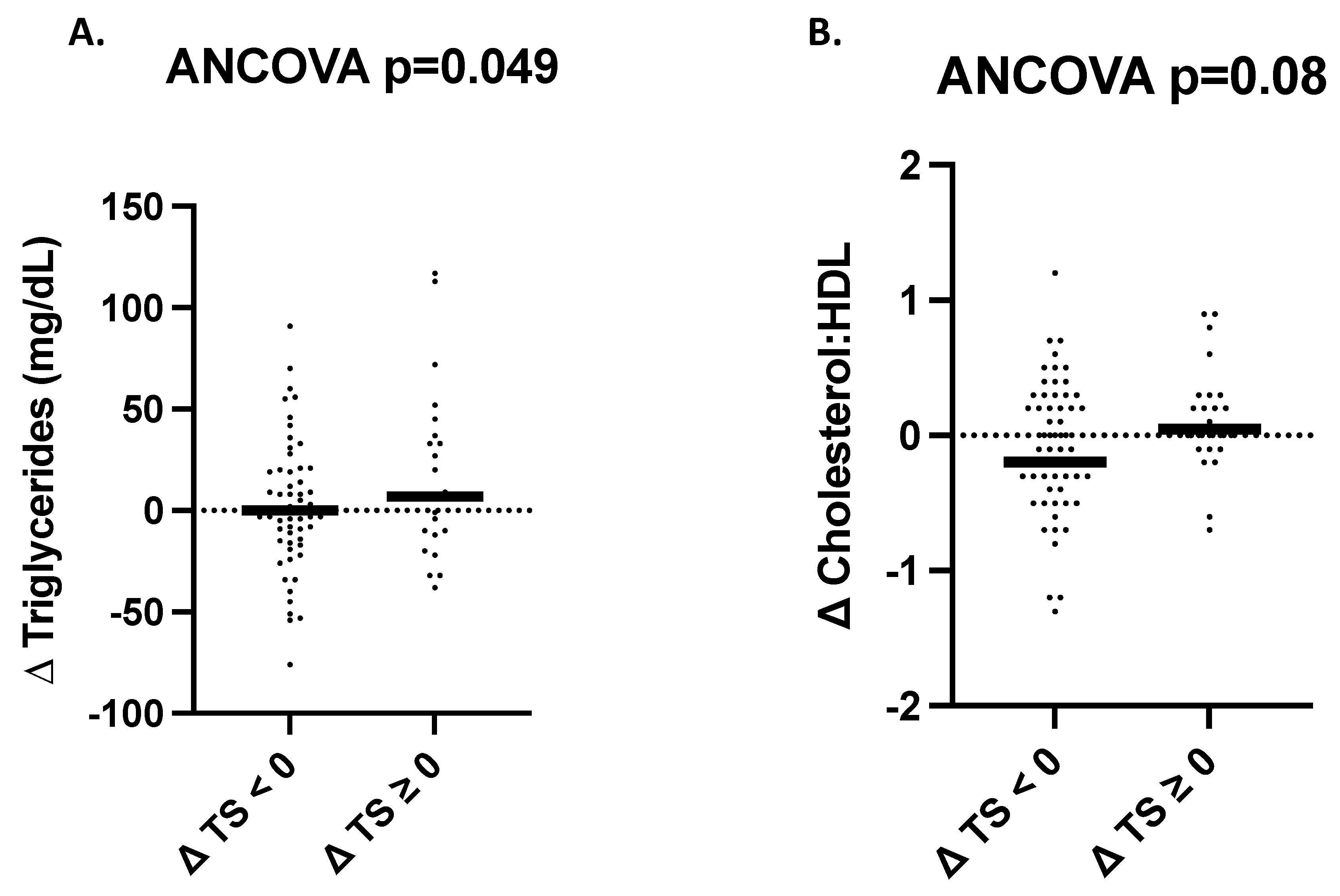
3.6. Changes in Cardiometabolic Health Outcomes as a Function of Change in Total Sugar Intake Regardless of Intervention Group Assignment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Skinner, A.C.; Ravanbakht, S.N.; Skelton, J.A.; Perrin, E.M.; Amrstrong, S.C. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics 2018, 141, e20173459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejavitte, R.A.S.; Enes, C.C.; Nucci, L.B. Prevalence of metabolic syndrome and its associated factors in overweight and obese adolescents. J. Pediatr. Endocrinol. Metab. 2020, 33, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Goran, M.I.; Bergman, R.N.; Cruz, M.L.; Watanabe, R. Insulin resistance and associated compensatory responses in african-american and Hispanic children. Diabetes Care 2002, 25, 2184–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Light, K.; Henderson, M.; O’Loughlin, J.; Mathieu, M.; Paradis, G.; Gray-Donald, K. Consumption of added sugars from liquid but not solid sources predicts impaired glucose homeostasis and insulin resistance among youth at risk of obesity. J. Nutr. 2014, 144, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Kynde, I.; Johnsen, N.F.; Wedderkopp, N.; Bygbjerg, I.B.C.; Helge, J.W.; Heitmann, B.L. Intake of total dietary sugar and fibre is associated with insulin resistance among Danish 8–10- and 14–16-year-old girls but not boys. European Youth Heart Studies I and II. Public Health Nutr. 2010, 13, 1669–1674. [Google Scholar]
- Wang, J.W.; Mark, S.; Henderson, M.; O’Loughlin, J.; Tremblay, A.; Wortman, J.; Paradi, G.; Gray-Donald, K. Adiposity and glucose intolerance exacerbate components of metabolic syndrome in children consuming sugar-sweetened beverages: QUALITY cohort study. Pediatr. Obes. 2013, 8, 284–293. [Google Scholar] [CrossRef]
- Sharma, S.; Roberts, L.S.; Lustig, R.H.; Fleming, S.E. Carbohydrate intake and cardiometabolic risk factors in high BMI African American children. Nutr. Metab. 2010, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Bremer, A.A.; Auinger, P.; Byrd, R.S. Relationship between insulin resistance-associated metabolic parameters and anthropometric measurements with sugar-sweetened beverage intake and physical activity levels in US adolescents: Findings from the 1999–2004 National Health and Nutrition Examination Survey. Arch. Pediatr. Adolesc. Med. 2009, 163, 328–335. [Google Scholar]
- Davis, J.N.; Ventura, E.E.; Weigensberg, M.J.; Ball, G.D.C.; Cruz, M.L.; Shaibi, G.Q.; Goran, M.I. The relation of sugar intake to beta cell function in overweight Latino children. Am. J. Clin. Nutr. 2005, 82, 1004–1010. [Google Scholar] [CrossRef] [Green Version]
- Vos, M.B.; Kaar, J.L.; Welsch, J.A.; Van Horn, L.V.; Feig, D.I.; Anderson, C.A.M.; Patel, M.J.; Munos, J.C.; Krebs, N.F.; Xanthakos, S.A.; et al. Added Sugars and Cardiovascular Disease Risk in Children: A Scientific Statement from the American Heart Association. Circulation 2017, 135, e1017–e1034. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, S.; Choi, H.K.; Lustig, R.H.; Hsu, C. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J. Pediatr. 2009, 154, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Kell, K.P.; Cardel, M.I.; Brown, M.M.B.; Fernandez, J.R. Added sugars in the diet are positively associated with diastolic blood pressure and triglycerides in children. Am. J. Clin. Nutr. 2014, 100, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aumueller, N.; Gruszfeld, D.; Gradowska, K.; Escribano, J.; Ferre, N.; Martin, F.; Poncelet, P.; ReDionigi, A.; Koletzko, B.; Grote, V.; et al. Influence of total sugar intake on metabolic blood markers at 8 years of age in the Childhood Obesity Project. Eur. J. Nutr. 2021, 60, 435–442. [Google Scholar] [CrossRef]
- de Moraes, M.M.; Mediano, M.F.F.; de Souza, R.A.G.; Moura, A.S.; da Veiga, G.V.; Sichieri, R. Discouraging soft drink consumption reduces blood glucose and cholesterol of Brazilian elementary students: Secondary analysis of a randomized controlled trial. Prev. Med. 2017, 100, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.N.; Ventura, E.E.; Shaibi, G.Q.; Weignesberg, M.J.; Spruijt-Metz, D.; Watanabe, R.M.; Goran, M.I. Reduction in added sugar intake and improvement in insulin secretion in overweight latina adolescents. Metab. Syndr. Relat. Disord. 2007, 5, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Ventura, E.; Davis, J.; Byrd-William, C.; Alexander, K.; McClain, A.; Lane, C.J.; Spruijt-Metz, D.; Weigensberg, M.; Goran, M.I. Reduction in risk factors for type 2 diabetes mellitus in response to a low-sugar, high-fiber dietary intervention in overweight Latino adolescents. Arch. Pediatr. Adolesc. Med. 2009, 163, 320–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.N.; Kelly, L.A.; Lane, C.J.; Ventura, E.E.; Byrd-William, C.E.; Alexandar, K.A.; Azen, S.P.; Chou, C.; Spruijt-Metz, D.; Weigensberg, M.J.; et al. Randomized control trial to improve adiposity and insulin resistance in overweight Latino adolescents. Obesity 2009, 17, 1542–1548. [Google Scholar] [CrossRef] [Green Version]
- Hasson, R.E.; Adam, T.C.; Davis, J.N.; Kelly, L.A.; Ventura, E.E.; Byrd-Williams, C.E.; Toledo-Corral, C.M.; Roberts, C.K.; Lane, C.J.; Azen, S.P.; et al. Randomized controlled trial to improve adiposity, inflammation, and insulin resistance in obese African-American and Latino youth. Obesity 2012, 20, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.A.; Jones, R.B.; Rios, C.; Corona, Y.; Berger, P.K.; Plows, J.F.; Alderete, T.L.; Fogel, J.; Hampson, H.; Hartiala, J.A.; et al. Clinical Intervention to Reduce Dietary Sugar Does Not Affect Liver Fat in Latino Youth, Regardless of PNPLA3 Genotype: A Randomized Controlled Trial. J. Nutr. 2022, 152, 1655–1665. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. 2015–2020 Dietary Guidelines for Americans, 8th ed. December 2015. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 18 June 2023).
- Center for Nutrition Policy and Promotion (U.S.). Getting Started with MyPlate: ChooseMyPlate.gov. 2010. Available online: https://www.myplate.gov/ (accessed on 18 June 2023).
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Herzberg-Schäfer, S.A.; Staiger, H.; Heni, M.; Ketterer, C.; Guthoff, M.; Kantartzis, K.; Machicao, F.; Stefan, N.; Haring, H.; Fritsche, A. Evaluation of fasting state-/oral glucose tolerance test-derived measures of insulin release for the detection of genetically impaired β-cell function. PLoS ONE 2010, 5, e14194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mari, A.; Schmitz, O.; Gastaldelli, A.; Oestergaard, T.; Nyholm, B.; Ferrannini, E. Meal and oral glucose tests for assessment of beta -cell function: Modeling analysis in normal subjects. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E1159–E1166. [Google Scholar] [CrossRef] [PubMed]
- National Health and Nutrition Examination Survey (U.S.); National Center for Health Statistics (U.S.); Westat. National Health and Nutrition Examination Survey III: Body Measurements (Anthropometry) Manual; Westat, Inc.: Rockville, MD, USA, 1988. Available online: https://stacks.cdc.gov/view/cdc/53134 (accessed on 18 June 2023).
- Lohman, G.T.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Chicago, IL, USA, 1988. [Google Scholar]
- Weston, A.T.; Petosa, R.; Pate, R.R. Validation of an Instrument for Measurement of Physical Activity in Youth. Med. Sci. Sports Exerc. 1997, 29, 138–143. [Google Scholar] [CrossRef]
- R Core Team, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna Austria, 2021. [Google Scholar]
- Team, R. RStudio: Integrated Development Environment for R; PBC: Boston, MA, USA, 2021. [Google Scholar]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gotzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utzschneider, K.M.; Prigeon, R.L.; Faulenbach, M.V.; Tong, J.; Carr, D.B.; Boyko, E.J.; Leonetti, D.L.; McNeely, M.J.; Fujimoto, W.Y.; Kahn, S.E. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009, 32, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, T.A.; Xiang, A.H.; Peters, R.K.; Kjos, S.L.; Marroquin, A.; Goico, J.; Ochoa, C.; Tan, S.; Berkowitz, K.; Hodis, H.N.; et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 2002, 51, 2796–2803. [Google Scholar] [CrossRef] [Green Version]
- Association, A.D. Type 2 diabetes in children and adolescents. American Diabetes Association. Pediatrics 2000, 105 Pt 1, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Welsh, J.A.; Sharma, A.; Cunningham, S.A.; Vos, M.B. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation 2011, 123, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Kosova, E.C.; Auinger, P.; Bremer, A.A. The relationships between sugar-sweetened beverage intake and cardiometabolic markers in young children. J. Acad. Nutr. Diet. 2013, 113, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Sawani, A.; Farhangi, M.; Aluganti, C.; Maul, T.M. Limiting Dietary Sugar Improves Pediatric Sinonasal Symptoms and Reduces Inflammation. J. Med. Food 2018, 21, 527–534. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Anderson, R.N. The Leading Causes of Death in the US for 2020. JAMA 2021, 325, 1829–1830. [Google Scholar] [CrossRef] [PubMed]
- Te Morenga, L.S.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012, 346, e7492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
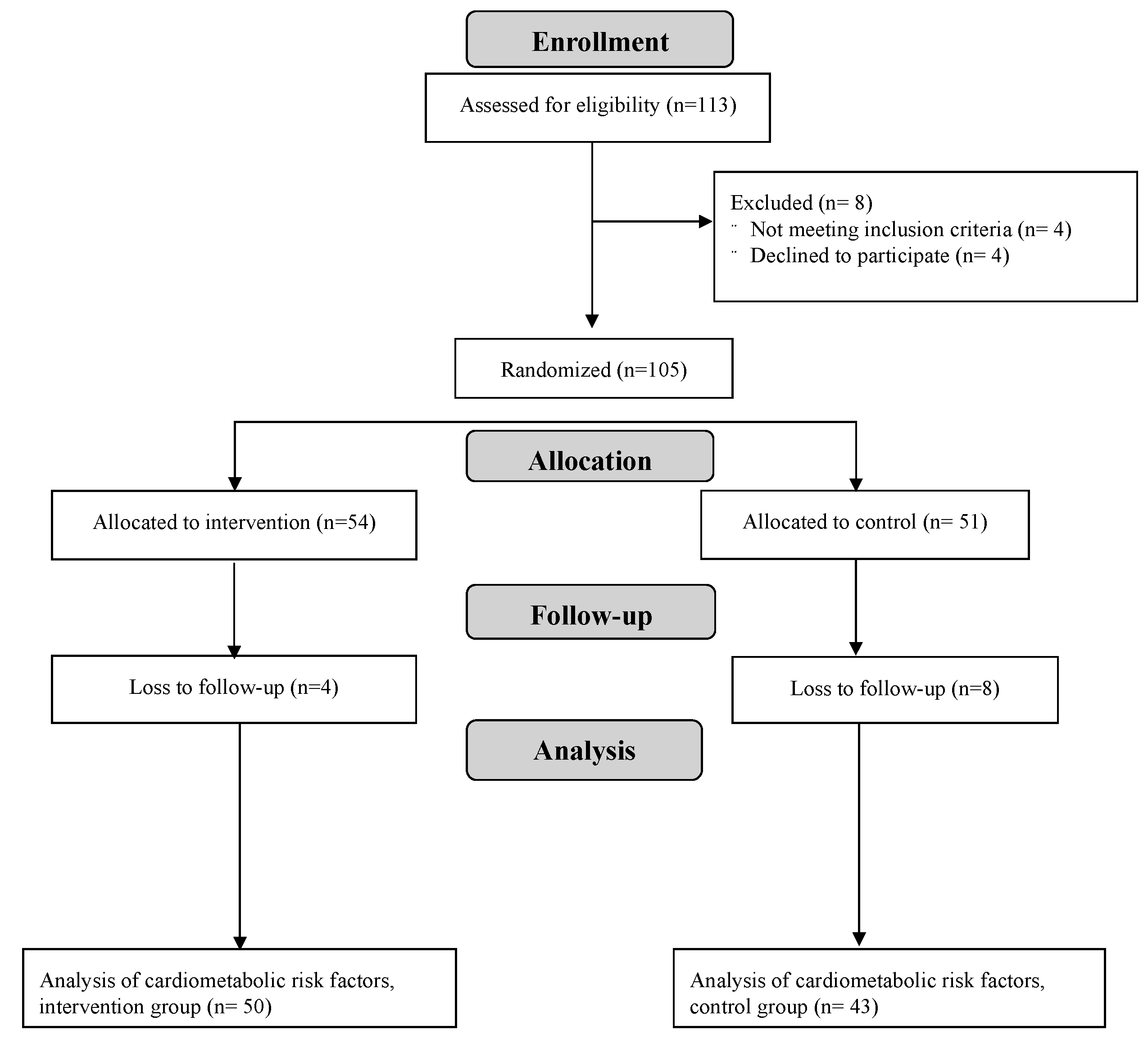
| Variable | Intervention (n = 50) | Control (n = 43) |
|---|---|---|
| Age (years) | 15.0 (13.0, 16.0) | 15.0 (13.0, 17.0) |
| Male sex (%) | 23 (46%) | 21 (49%) |
| Tanner stage ≥ 4 (%) 1 | 35 (70%) | 24 (57%) |
| Body weight (kg) | 89.3 ± 18.8 | 92.7 ± 21.4 |
| BMI (kg/m2) 2 | 32.1 (29.3, 37.1) | 34.1 (30.3, 37.6) |
| Fasting glucose (mg/dL) | 84.6 ± 7.7 | 86.3 ± 7.6 |
| U/mL) | 25.6 (14.1, 41.3) | 25.2 (14.2, 38.7) |
| 2-h glucose (mg/dL) | 115.4 ± 20.7 | 114.6 ± 20.5 |
| 2-hU/mL) | 287.5 (173.6, 519.4) | 206.0 (132.6, 386.0) |
| Glycated hemoglobin (%) | 5.5 ± 0.4 | 5.5 ± 0.3 |
| HOMA-IR | 5.0 (3.2, 8.7) | 5.4 (3.0, 9.0) |
| Matsuda ISI | 1.2 (0.7, 1.6) | 1.2 (0.8, 1.9) |
| Insulinogenic index | 5.7 (4.1, 8.4) | 6.2 (4.5, 9.3) |
| Oral DI | 6.8 (4.3, 9.0) | 8.7 (4.5, 11.6) |
| AUC glucose (mg/dL × min) | 14,796 ± 2126 | 14,462 ± 1959 |
| U/mL × min) | 23,959 (15,675, 34,554) | 19,986 (12,631, 283,559) |
| Cholesterol (mg/dL) 3 | 143 (132, 155) | 142 (125, 158) |
| Triglycerides (mg/dL) 4 | 101 (76, 134) | 97 (82, 134) |
| HDL-Cholesterol (mg/dL) 5 | 40.0 (35.0, 44.0) | 39.0 (35.0, 44.0) |
| LDL-Cholesterol (mg/dL) 6 | 79.5 (67.3, 93.8) | 77.0 (65.5, 93.0) |
| Cholesterol:HDL cholesterol ratio 3 | 3.7 ± 0.9 | 3.6 ± 1.0 |
| Systolic blood pressure (mmHg) | 115 ± 11 | 116 ± 12 |
| Diastolic blood pressure (mmHg) 1 | 69 ± 7 | 69 ± 7 |
| C-reactive protein (mg/L) 4 | 1.9 (0.9, 4.6) | 1.9 (0.7, 6.2) |
| Interleukin-6 (pg/mL) 5 | 8.5 (4.6, 44.6) | 6.1 (2.3, 19.9) |
| TNF- 7 | 2.8 ± 0.9 | 3.1 ± 1.1 |
| Leptin 7 | 6034 (3460, 10,191) | 8657 (4714, 10,658) |
| MCP-1 8 | 101 ± 31 | 107 ± 31 |
| Total energy intake (kcal) 1 | 1451 (1224, 1778) | 1488 (1227, 1727) |
| Total sugar intake (%E) 1 | 18.5 ± 6.6 | 20.2 ± 6.1 |
| Added sugar intake (%E) 1 | 11.1 ± 6.1 | 12.6 ± 5.7 |
| Free sugar intake (%E) 1 | 11.7 ± 6.1 | 13.6 ± 6.7 |
| Free sugar intake > 10% 1 | 31 (62%) | 29 (69%) |
| Physical activity (Met-h/week) | 59.1 (55.3, 65.9) | 56.8 (53.4, 72.3) |
| Intervention | Control | ANCOVA | GEE | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | Raw | Adjusted | Sex Interaction | ||
| Fasting Glucose (mg/dL) 1 | 84.6 ± 7.7 | 0.3 (−3.0, 3.3) | 86.3 ± 7.6 | 0.5 (−6.2, 5.5) | 0.43 | 0.21 | 0.91 | 0.19 |
| 2-h glucose (mg/dL) 2 | 115.0 ± 20.7 | 0.4 ± 19.3 | 115 ± 20.8 | 3.9 ± 23.7 | 0.42 | 0.25 | 0.38 | 0.25 |
| HbA1c (%) 3 | 5.4 ± 0.4 | 0.0 (−0.1, 0.1) | 5.5 ± 0.3 | 0.0 (−0.1, 0.2) | 0.46 | 0.16 | 0.02 | 0.15 |
| AUC glucose (mg/dL × min) 4 | 14,893 ± 2026 | −251 ± 1660 | 14,551 ± 1963 | 108 ± 1785 | 0.51 | 0.58 | 0.35 | 0.56 |
| U/mL) 5 | 23.3 (13.7, 36.9) | −2.1 (−8.7, 4.8) | 23.4 (14.1, 37.0) | −0.3 (−11.7, 27.4) | 0.39 | 0.36 | 0.18 | 0.25 |
| 2-hU/mL) 6 | 277.4 (173.5, 514.3) | −63.8 (−150.8, 46.4) | 190.5 (132.1, 346.9) | 47.9 (−98.7, 124.0) | 0.38 | 0.44 | 0.92 | 0.22 |
| HOMA-IR 7 | 5.1 (3.1, 8.8) | −0.4 (−2.2, 0.8) | 5.3 (3.1, 8.5) | −0.2 (−2.4, 2.8) | 0.28 | 0.23 | 0.03 | 0.27 |
| Matsuda ISI 8 | 1.1 (0.7, 1.6) | 0.2 ± 0.8 | 1.2 (0.7, 1.8) | 0.0 ± 0.7 | 0.11 | 0.20 | 0.90 | 0.14 |
| U/mL × min) 9 | 23,959 (16,285, 34,554) | −2417 (−10,819, 4864) | 19,406 (12,351, 25,221) | −131 (−5017, 5458) | 0.31 | 0.38 | 0.54 | 0.24 |
| Insulinogenic Index 10 | 5.6 (4.1, 8.3) | 0.3 (−1.4, 2.4) | 6.2 (4.5, 9.4) | 0.1 (−1.3, 1.2) | 0.34 | 0.34 | 0.28 | 0.38 |
| Oral DI 11 | 6.4 (4.1, 8.8) | 1.8 ± 5.0 | 7.6 (4.2, 11.2) | 0.1 ± 5.1 | 0.18 | 0.26 | 0.53 | 0.35 |
| Intervention (n = 46) | Control (n = 43) | ANCOVA | GEE * | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | Raw | Adjusted | Sex Interaction | ||
| Cholesterol (mg/dL) | 143.5 (134.2, 155.2) | 0.3 ± 13.1 | 142.0 (125.0, 157.5) | −1.8 ± 14.2 | 0.59 | 0.40 | 0.81 | 0.50 |
| Triglycerides (mg/dL) 1 | 101.0 (76.0, 134.0) | 5.0 (−10.0, 21.0) | 96.5 (82.2, 134.2) | 0.0 (−15.5, 25.5) | 0.77 | 0.64 | 0.44 | 0.63 |
| LDL cholesterol (mg/dL) | 80.7 ± 21.5 | −0.9 ± 10.8 | 80.2 ± 23.2 | −2.9 ± 12.4 | 0.39 | 0.30 | 0.65 | 0.36 |
| HDL cholesterol (mg/dL) 2 | 40.0 (35.0, 44.0) | 0.3 ± 4.4 | 39.0 (35.0, 44.0) | −0.6 ± 4.5 | 0.42 | 0.37 | 0.43 | 0.43 |
| Cholesterol:HDL 3 | 3.7 ± 0.9 | 0.0 ± 0.4 | 3.6 ± 1.0 | 0.0 ± 0.5 | 0.78 | 0.65 | 0.37 | 0.66 |
| Systolic BP (mmHg) 4 | 115.5 ± 10.7 | −1.6 ± 8.8 | 115.7 ± 11.9 | −2.9 ± 9.5 | 0.54 | 0.49 | <0.01 | 0.46 |
| Diastolic BP (mmHg) 5 | 68.7 ± 6.4 | −0.5 (−3.5, 3.0) | 68.9 ± 7.1 | −0.5 (−5.0, 4.9) | 0.42 | 0.46 | <0.05 | 0.44 |
| Intervention | Control | ANCOVA | GEE | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | Raw | Adjusted | Sex Interaction | Adjusted | |
| CRP 1 | 1.8 (0.7, 4.5) | −0.1 (−1.0, 1.1) | 1.5 (0.7, 5.7) | −0.1 (−0.8, 0.7) | 0.37 | 0.38 | 0.44 | 0.37 |
| IL-6 2 | 9.1 (5.6, 47.2) | 0.0 (−3.0, 5.5) | 6.6 (2.9, 21.1) | 1.0 (−2.3, 3.9) | 0.15 | 0.07 | 0.40 | 0.24 |
| TNF- 3 | 2.8 ± 0.9 | 0.2 (−0.2, 0.6) | 3.1 ± 1.1 | 0.0 (−0.3, 0.5) | 0.25 | 0.52 | 0.07 | 0.37 |
| MCP-1 4 | 99 ± 27 | 2.6 ± 18.9 | 107 ± 31 | 3.7 ± 26.2 | 0.60 | 0.38 | 0.64 | 0.66 |
| Leptin 5 | 6034 (3460, 10,191) | −76 ± 2419 | 8657 (4714, 10,658) | −283 ± 2779 | 0.96 | 0.70 | 0.81 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, K.A.; Mokhtari, P.; Holzhausen, E.A.; Alderete, T.L.; Allayee, H.; Nayak, K.S.; Sinatra, F.R.; Pickering, T.A.; Mack, W.; Kohli, R.; et al. Effects of Dietary Sugar Reduction on Biomarkers of Cardiometabolic Health in Latino Youth: Secondary Analyses from a Randomized Controlled Trial. Nutrients 2023, 15, 3338. https://doi.org/10.3390/nu15153338
Schmidt KA, Mokhtari P, Holzhausen EA, Alderete TL, Allayee H, Nayak KS, Sinatra FR, Pickering TA, Mack W, Kohli R, et al. Effects of Dietary Sugar Reduction on Biomarkers of Cardiometabolic Health in Latino Youth: Secondary Analyses from a Randomized Controlled Trial. Nutrients. 2023; 15(15):3338. https://doi.org/10.3390/nu15153338
Chicago/Turabian StyleSchmidt, Kelsey A., Pari Mokhtari, Elizabeth A. Holzhausen, Tanya L. Alderete, Hooman Allayee, Krishna S. Nayak, Frank R. Sinatra, Trevor A. Pickering, Wendy Mack, Rohit Kohli, and et al. 2023. "Effects of Dietary Sugar Reduction on Biomarkers of Cardiometabolic Health in Latino Youth: Secondary Analyses from a Randomized Controlled Trial" Nutrients 15, no. 15: 3338. https://doi.org/10.3390/nu15153338
APA StyleSchmidt, K. A., Mokhtari, P., Holzhausen, E. A., Alderete, T. L., Allayee, H., Nayak, K. S., Sinatra, F. R., Pickering, T. A., Mack, W., Kohli, R., & Goran, M. I. (2023). Effects of Dietary Sugar Reduction on Biomarkers of Cardiometabolic Health in Latino Youth: Secondary Analyses from a Randomized Controlled Trial. Nutrients, 15(15), 3338. https://doi.org/10.3390/nu15153338








