Cafeteria Diet-Induced Obesity Worsens Experimental CKD
Abstract
:1. Introduction
2. Materials and Methods
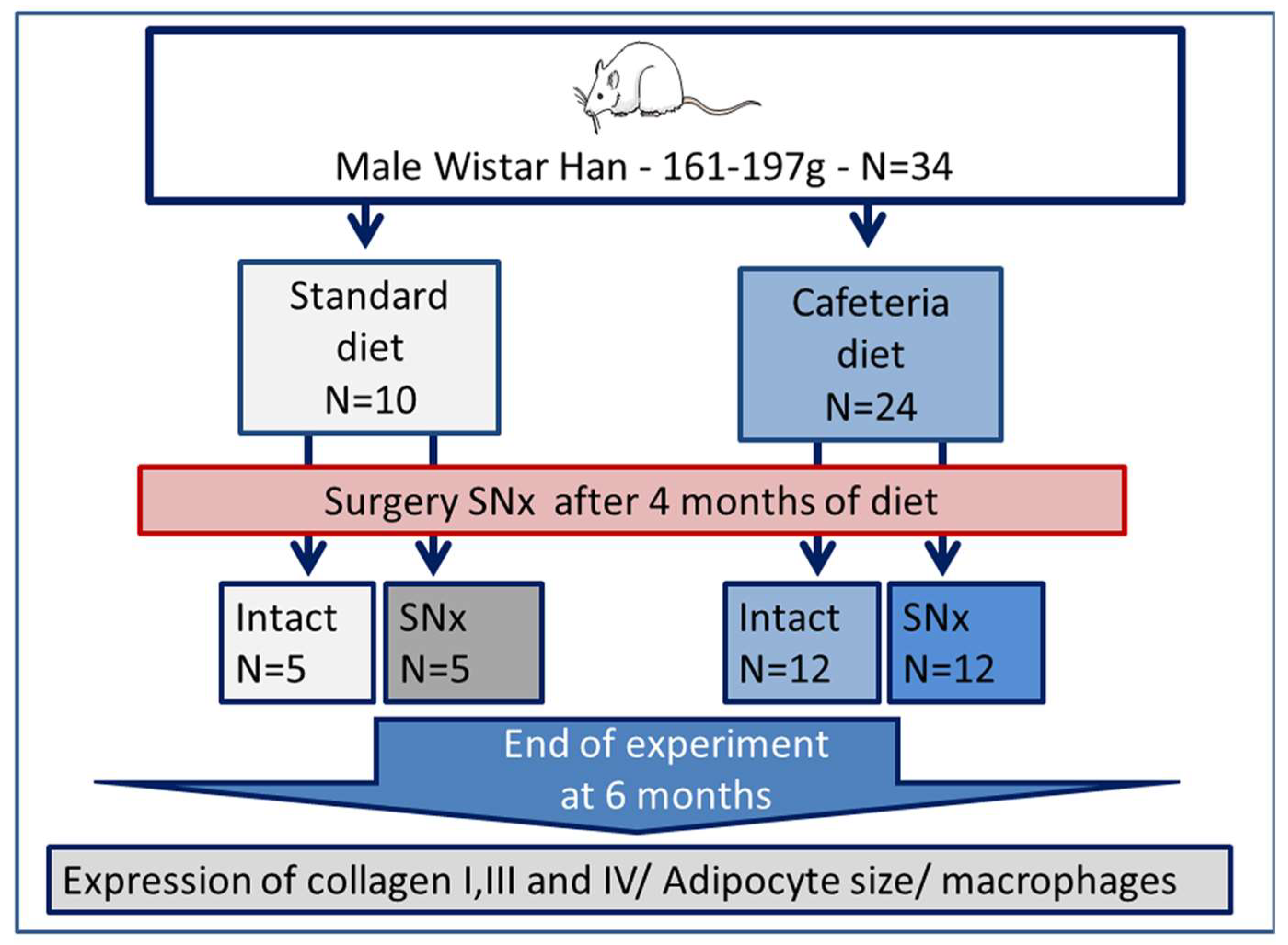
2.1. Animals
2.2. Blood Parameters
2.3. Histology
2.4. Immunochemistry
2.5. Adipokine Determination
2.6. Statistical Analysis
3. Results
3.1. Influence of Cafeteria Diet and SNx on Kidney Function and Insulin Resistance
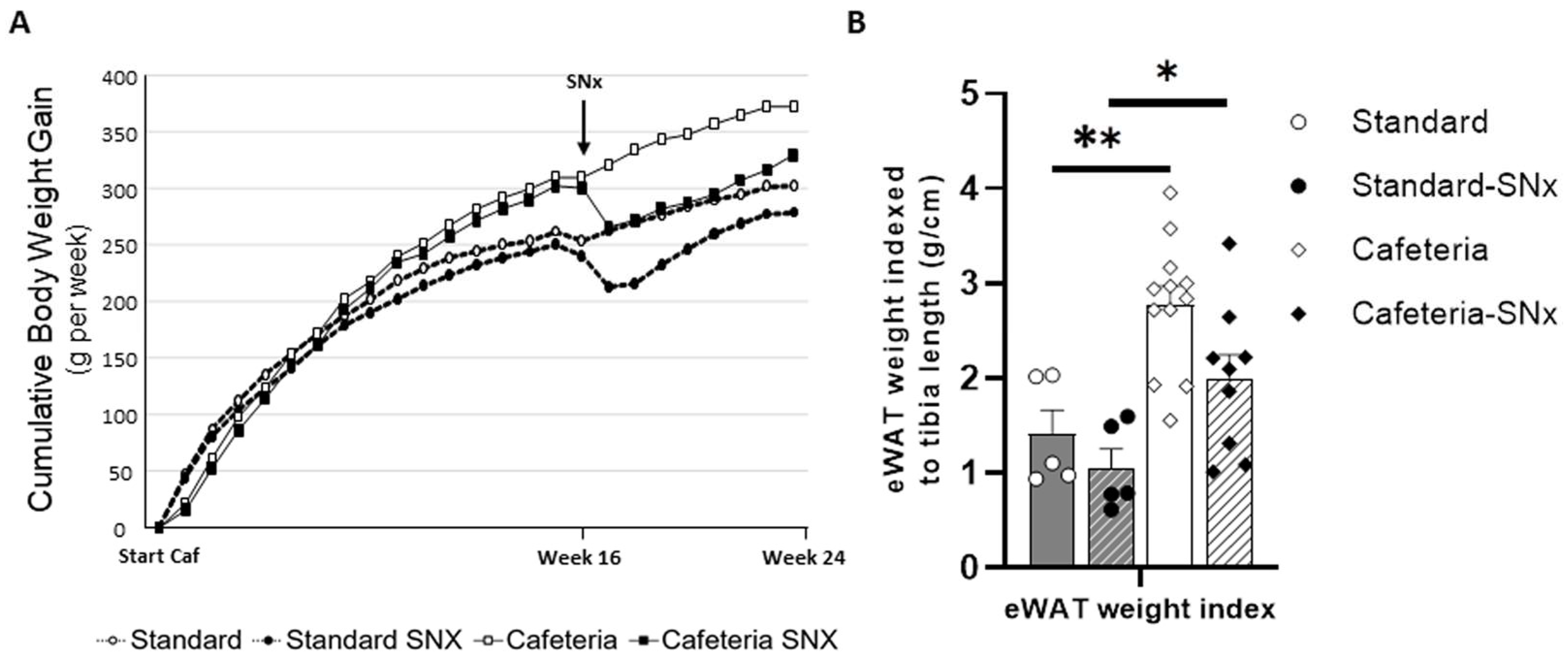
3.2. Effect of the Cafeteria Diet and SNX Surgery on Body Weight and Adipose Tissue
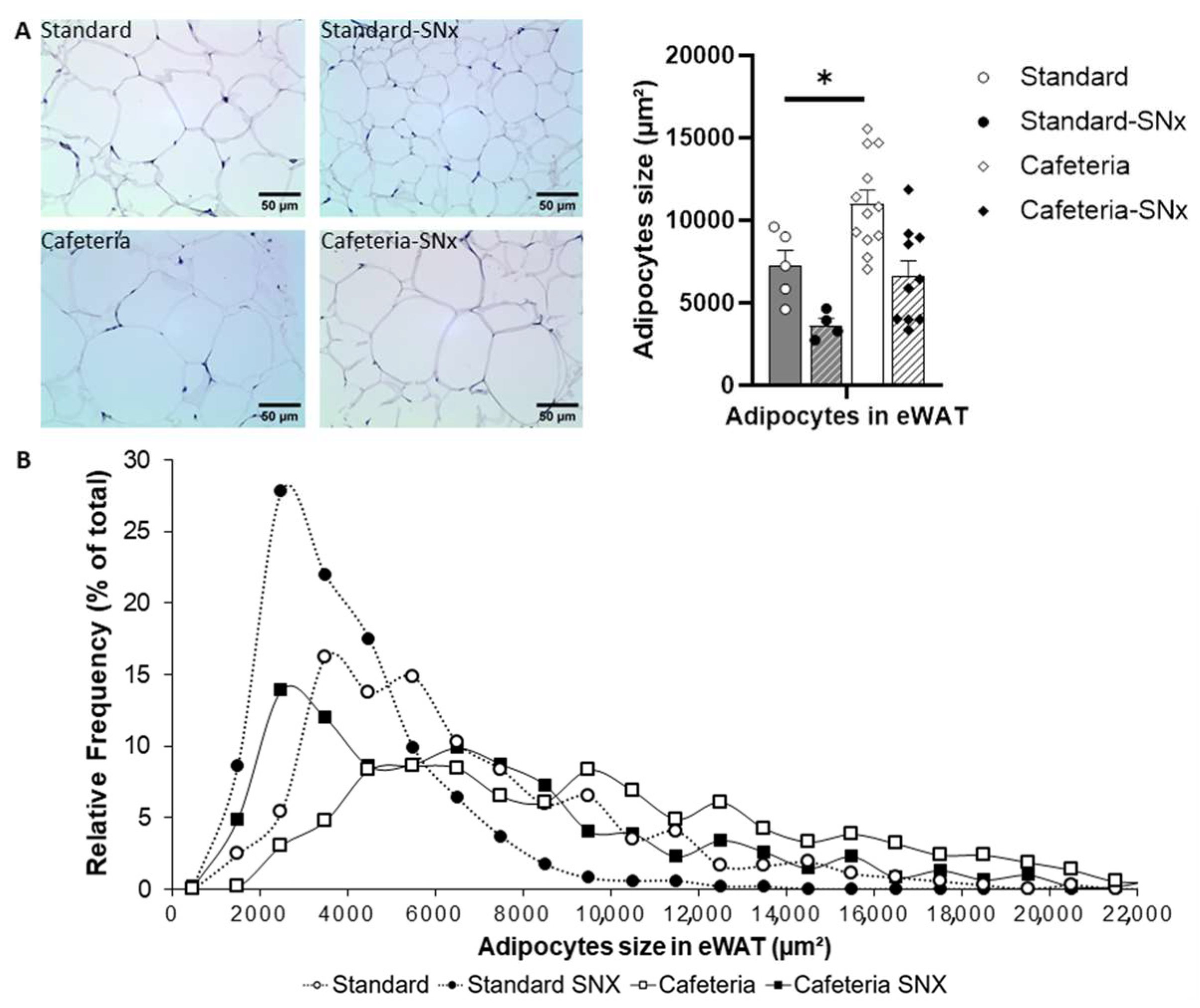
3.3. eWAT Examination: Adipocyte Sizes and Size Repartition
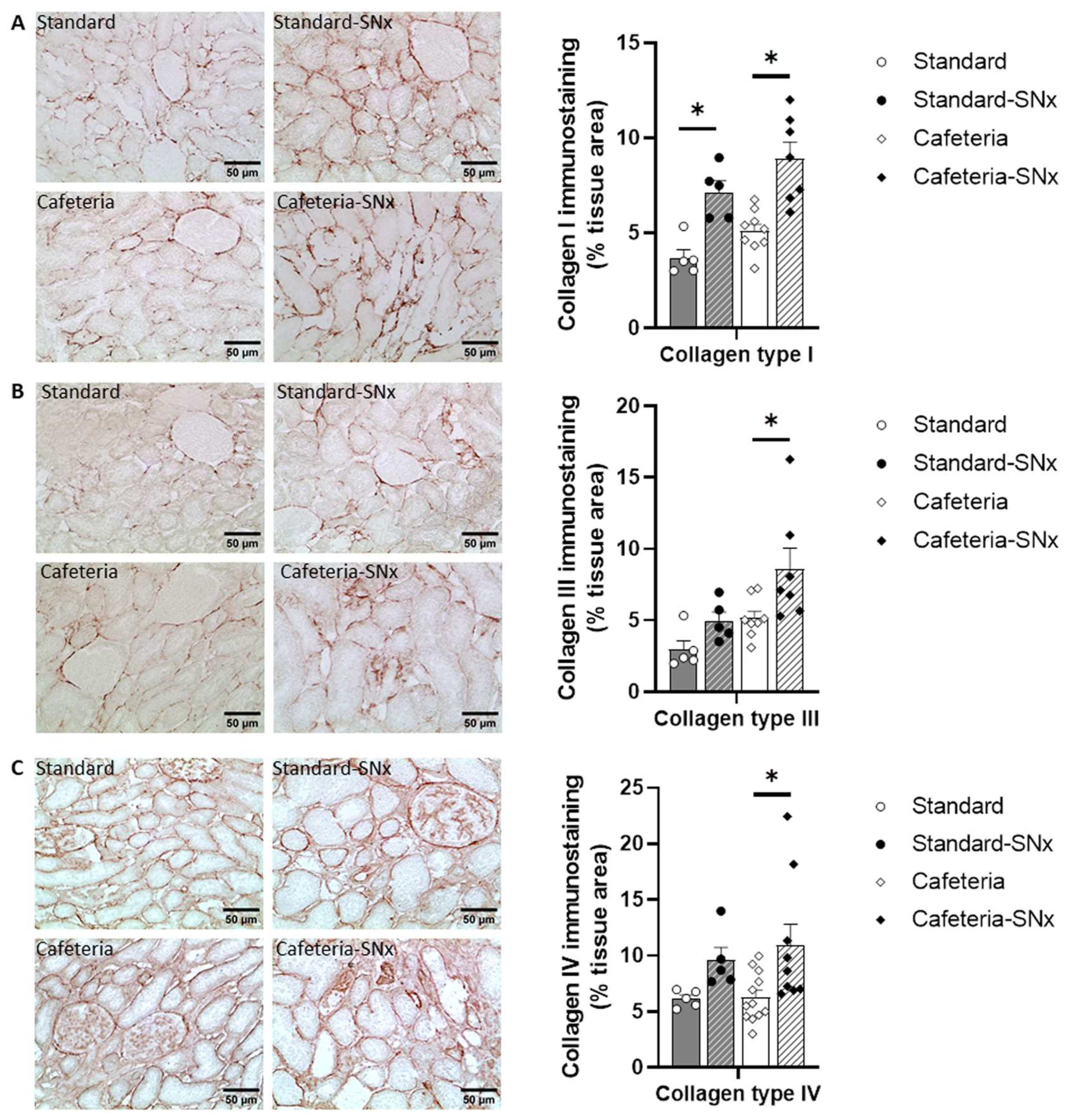
3.4. Kidney Fibrosis
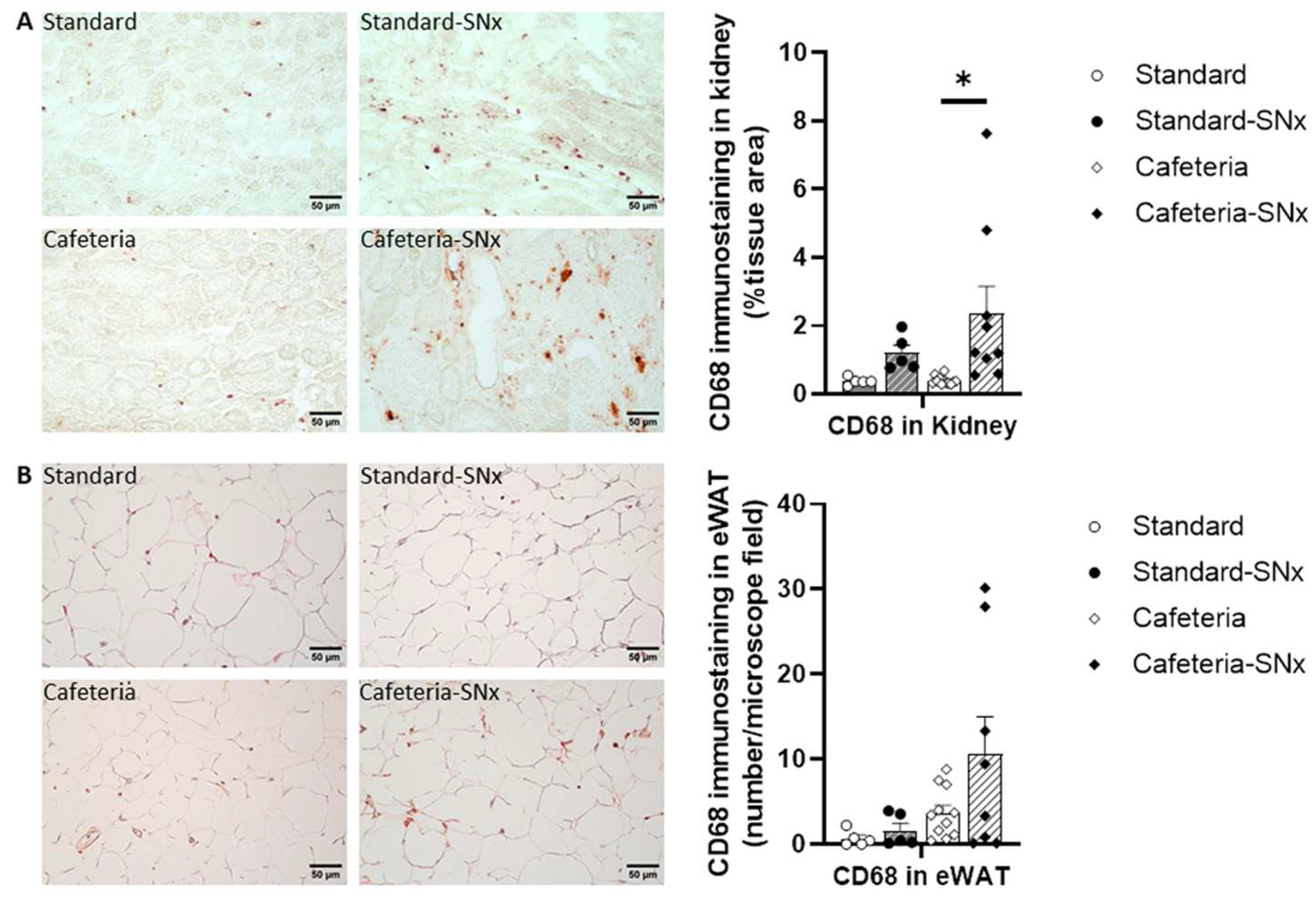
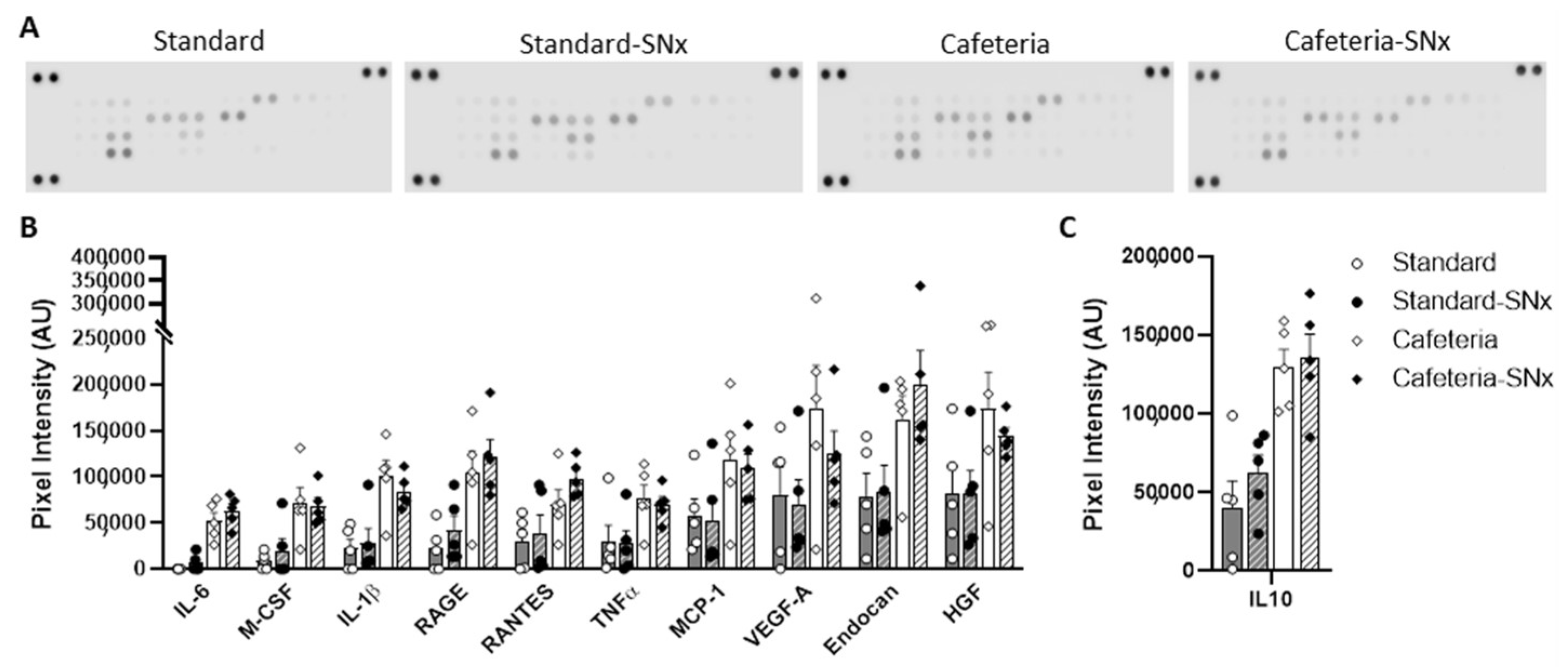
3.5. Inflammation in Kidney and eWAT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health Effects of Dietary Risks in 195 Countries, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128·9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryan, S.; Afful, J.; Carroll, M.; Te-Ching, C.; Orlando, D.; Fink, S.; Fryar, C. NHSR 158. National Health and Nutrition Examination Survey 2017–March 2020 Pre-Pandemic Data Files; National Center for Health Statistics: Hyattsville, MD, USA, 2021. [Google Scholar]
- Wang, M.; Wang, Z.; Chen, Y.; Dong, Y. Kidney Damage Caused by Obesity and Its Feasible Treatment Drugs. Int. J. Mol. Sci. 2022, 23, 747. [Google Scholar] [CrossRef] [PubMed]
- Bjørge, T.; Engeland, A.; Tverdal, A.; Smith, G.D. Body Mass Index in Adolescence in Relation to Cause-Specific Mortality: A Follow-up of 230,000 Norwegian Adolescents. Am. J. Epidemiol. 2008, 168, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meguid El Nahas, A.; Bello, A.K. Chronic Kidney Disease: The Global Challenge. Lancet 2005, 365, 331–340. [Google Scholar] [CrossRef]
- Kambham, N.; Markowitz, G.S.; Valeri, A.M.; Lin, J.; D’Agati, V.D. Obesity-Related Glomerulopathy: An Emerging Epidemic. Kidney Int. 2001, 59, 1498–1509. [Google Scholar] [CrossRef] [Green Version]
- Ejerblad, E.; Fored, C.M.; Lindblad, P.; Fryzek, J.; McLaughlin, J.K.; Nyrén, O. Obesity and Risk for Chronic Renal Failure. J. Am. Soc. Nephrol. 2006, 17, 1695–1702. [Google Scholar] [CrossRef] [Green Version]
- Kanbay, M.; Copur, S.; Siriopol, D.; Yildiz, A.B.; Berkkan, M.; Tuttle, K.R.; Zoccali, C. The Risk for Chronic Kidney Disease in Metabolically Healthy Obese Patients: A Systematic Review and Meta-analysis. Eur. J. Clin. Investig. 2023, 53, e13878. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory Links between Obesity and Metabolic Disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olefsky, J.M.; Glass, C.K. Macrophages, Inflammation, and Insulin Resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.M.; Saltiel, A.R. Adapting to Obesity with Adipose Tissue Inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Czaja-Stolc, S.; Potrykus, M.; Stankiewicz, M.; Kaska, Ł.; Małgorzewicz, S. Pro-Inflammatory Profile of Adipokines in Obesity Contributes to Pathogenesis, Nutritional Disorders, and Cardiovascular Risk in Chronic Kidney Disease. Nutrients 2022, 14, 1457. [Google Scholar] [CrossRef]
- Martin-Taboada, M.; Vila-Bedmar, R.; Medina-Gómez, G. From Obesity to Chronic Kidney Disease: How Can Adipose Tissue Affect Renal Function? Nephron 2021, 145, 609–613. [Google Scholar] [CrossRef]
- D’Agati, V.D.; Chagnac, A.; de Vries, A.P.J.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-Related Glomerulopathy: Clinical and Pathologic Characteristics and Pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Ikee, R.; Hamasaki, Y.; Oka, M.; Maesato, K.; Mano, T.; Moriya, H.; Ohtake, T.; Kobayashi, S. Glucose Metabolism, Insulin Resistance, and Renal Pathology in Non-Diabetic Chronic Kidney Disease. Nephron Clin. Pract. 2008, 108, c163–c168. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Kato, K.; Ohkido, I.; Yokoo, T. Role and Treatment of Insulin Resistance in Patients with Chronic Kidney Disease: A Review. Nutrients 2021, 13, 4349. [Google Scholar] [CrossRef]
- Câmara, N.O.S.; Iseki, K.; Kramer, H.; Liu, Z.-H.; Sharma, K. Kidney Disease and Obesity: Epidemiology, Mechanisms and Treatment. Nat. Rev. Nephrol. 2017, 13, 181–190. [Google Scholar] [CrossRef]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria Diet Is a Robust Model of Human Metabolic Syndrome With Liver and Adipose Inflammation: Comparison to High-Fat Diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef] [Green Version]
- Garber, J.C.; Barbeen, R.W.; Bielitzki, J.T.; Clayton, A.L.; Donovan, J.C.; Hendriksen, C.F.M.; Kohn, D.F.; Lipman, N.S.; Locke, P.A.; Melcher, J.; et al. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Research Council (USA), Institute for Laboratory Animal Research (USA), National Academies Press (USA), Eds.; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Plawecki, M.; Gayrard, N.; Jeanson, L.; Chauvin, A.; Lajoix, A.-D.; Cristol, J.-P.; Jover, B.; Raynaud, F. Cardiac Remodeling Associated with Chronic Kidney Disease Is Enhanced in a Rat Model of Metabolic Syndrome: Preparation of Mesenchymal Transition. Mol. Cell Biochem. 2023, 1–11. [Google Scholar] [CrossRef]
- Fujihara, C.K.; Kowala, M.C.; Breyer, M.D.; Sena, C.R.; Rodrigues, M.V.; Arias, S.C.A.; Fanelli, C.; Malheiros, D.M.; Jadhav, P.K.; Montrose-Rafizadeh, C.; et al. A Novel Aldosterone Antagonist Limits Renal Injury in 5/6 Nephrectomy. Sci. Rep. 2017, 7, 7899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacho, J.; Sevillano, J.; de Castro, J.; Herrera, E.; Ramos, M.P. Validation of Simple Indexes to Assess Insulin Sensitivity during Pregnancy in Wistar and Sprague-Dawley Rats. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1269–E1276. [Google Scholar] [CrossRef] [PubMed]
- Parlee, S.D.; Lentz, S.I.; Mori, H.; MacDougald, O.A. Quantifying Size and Number of Adipocytes in Adipose Tissue. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 537, pp. 93–122. ISBN 978-0-12-411619-1. [Google Scholar]
- Gayrard, N.; Muyor, K.; Notarnicola, C.; Duranton, F.; Jover, B.; Argilés, À. Optimisation of Cell and Ex Vivo Culture Conditions to Study Vascular Calcification. PLoS ONE 2020, 15, e0230201. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, K.-A.; Kim, J.-H.; Sul, H.S. Pref-1, a Preadipocyte Secreted Factor That Inhibits Adipogenesis. J. Nutr. 2006, 136, 2953–2956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spann, R.A.; Morrison, C.D.; den Hartigh, L.J. The Nuanced Metabolic Functions of Endogenous FGF21 Depend on the Nature of the Stimulus, Tissue Source, and Experimental Model. Front. Endocrinol. 2021, 12, 802541. [Google Scholar] [CrossRef]
- Arora, G.K.; Gupta, A.; Narayanan, S.; Guo, T.; Iyengar, P.; Infante, R.E. Cachexia-Associated Adipose Loss Induced by Tumor-Secreted Leukemia Inhibitory Factor Is Counterbalanced by Decreased Leptin. JCI Insight 2018, 3, e121221. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; McNutt, M.C.; Banfi, S.; Levin, M.G.; Holland, W.L.; Gusarova, V.; Gromada, J.; Cohen, J.C.; Hobbs, H.H. Hepatic ANGPTL3 Regulates Adipose Tissue Energy Homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 11630–11635. [Google Scholar] [CrossRef]
- Tenney, R.; Stansfield, K.; Pekala, P.H. Interleukin 11 Signaling in 3T3-L1 Adipocytes. J. Cell Physiol. 2005, 202, 160–166. [Google Scholar] [CrossRef]
- Dong, B.; Hiasa, M.; Higa, Y.; Ohnishi, Y.; Endo, I.; Kondo, T.; Takashi, Y.; Tsoumpra, M.; Kainuma, R.; Sawatsubashi, S.; et al. Osteoblast/Osteocyte-Derived Interleukin-11 Regulates Osteogenesis and Systemic Adipogenesis. Nat. Commun. 2022, 13, 7194. [Google Scholar] [CrossRef]
- Widjaja, A.A.; Viswanathan, S.; Shekeran, S.G.; Adami, E.; Lim, W.-W.; Chothani, S.; Tan, J.; Goh, J.W.T.; Chen, H.M.; Lim, S.Y.; et al. Targeting Endogenous Kidney Regeneration Using Anti-IL11 Therapy in Acute and Chronic Models of Kidney Disease. Nat. Commun. 2022, 13, 7497. [Google Scholar] [CrossRef]
- Kaji, K.; Yoshiji, H.; Ikenaka, Y.; Noguchi, R.; Aihara, Y.; Douhara, A.; Moriya, K.; Kawaratani, H.; Shirai, Y.; Yoshii, J.; et al. Dipeptidyl Peptidase-4 Inhibitor Attenuates Hepatic Fibrosis via Suppression of Activated Hepatic Stellate Cell in Rats. J. Gastroenterol. 2014, 49, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Min, H.S.; Kim, J.E.; Lee, M.H.; Song, H.K.; Kang, Y.S.; Lee, M.J.; Lee, J.E.; Kim, H.W.; Cha, J.J.; Chung, Y.Y.; et al. Dipeptidyl Peptidase IV Inhibitor Protects against Renal Interstitial Fibrosis in a Mouse Model of Ureteral Obstruction. Lab. Investig. 2014, 94, 598–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Akunuru, S.; Bierman, J.C.; Hodge, K.M.; Mitchell, M.C.; Foster, M.T.; Seeley, R.J.; Reizes, O. Diet-Induced Obese Mice Are Leptin Insufficient after Weight Reduction. Obesity 2009, 17, 1702–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.-J.; Kim, S.-S.; Lim, W.; Song, G.; Lee, C.-K. Leptin Is a Dose-Dependent Marker of Caloric Restriction in Adipose Tissues Located in Different Parts of the Mouse Body. Mol. Cell. Toxicol. 2018, 14, 53–59. [Google Scholar] [CrossRef]
- Gifford, C.C.; Lian, F.; Tang, J.; Costello, A.; Goldschmeding, R.; Samarakoon, R.; Higgins, P.J. PAI-1 Induction during Kidney Injury Promotes Fibrotic Epithelial Dysfunction via Deregulation of Klotho, P53, and TGF-Β1-Receptor Signaling. FASEB J. 2021, 35, e21725. [Google Scholar] [CrossRef]
- Ji, L.; Masuda, S.; Saito, H.; Inui, K. Down-Regulation of Rat Organic Cation Transporter ROCT2 by 5/6 Nephrectomy. Kidney Int. 2002, 62, 514–524. [Google Scholar] [CrossRef] [Green Version]
- Barata, K.; Yoshida, M.; Hokao, R.; Maekawa, A. Sequential Alterations in Clinical Biochemical Indicators of Renal Function in 5/6 Nephrectomized Rats--Basic Study for Renal Toxicity Using 5/6 Nephrectomized Rats. J. Toxicol. Sci. 1998, 23, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Li, Y.; Wang, F.; Jiang, Y.-F.; Jiang, Y.-S. Shenfushu Granule and Atropine Attenuate Microvasculature Loss in Rat Models with 5/6 Nephrectomy. Ren Fail 2012, 34, 600–609. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Gu, D.; Chen, C.-S.; Wu, X.; Hamm, L.L.; Muntner, P.; Batuman, V.; Lee, C.-H.; Whelton, P.K.; He, J. Association between the Metabolic Syndrome and Chronic Kidney Disease in Chinese Adults. Nephrol. Dialysis Transplant. 2007, 22, 1100–1106. [Google Scholar] [CrossRef] [Green Version]
- Zomorrodian, D.; Khajavi-Rad, A.; Avan, A.; Ebrahimi, M.; Nematy, M.; Azarpazhooh, M.R.; Emamian, M.; Sadeghzade, M.; Mirhafez, S.R.; Mohammadi, M.; et al. Metabolic Syndrome Components as Markers to Prognosticate the Risk of Developing Chronic Kidney Disease: Evidence-Based Study with 6492 Individuals. J. Epidemiol. Community Health 2015, 69, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.-C.; Wang, P.-H.; Lu, K.-Y.; Ko, C.-C.; She, Y.-H.; Lee, C.-C.; Wu, I.-W.; Sun, C.-Y.; Hsu, H.-J.; Pan, H.-C. Relationships Between Metabolic Body Composition Status and Rapid Kidney Function Decline in a Community-Based Population: A Prospective Observational Study. Front. Public Health 2022, 10, 895787. [Google Scholar] [CrossRef] [PubMed]
- Cantero-Navarro, E.; Rayego-Mateos, S.; Orejudo, M.; Tejedor-Santamaria, L.; Tejera-Muñoz, A.; Sanz, A.B.; Marquez-Exposito, L.; Marchant, V.; Santos-Sanchez, L.; Egido, J.; et al. Role of Macrophages and Related Cytokines in Kidney Disease. Front. Med. 2021, 8, 688060. [Google Scholar] [CrossRef] [PubMed]
- Laget, J.; Djohan, Y.F.; Jeanson, L.; Muyor, K.; Badia, E.; Cristol, J.P.; Coudray, C.; Feillet-Coudray, C.; Vigor, C.; Oger, C.; et al. Peripancreatic Adipose Tissue Remodeling and Inflammation during High Fat Intake of Palm Oils or Lard in Rats. Nutrients 2021, 13, 1134. [Google Scholar] [CrossRef] [PubMed]
- Vidé, J.; Bonafos, B.; Fouret, G.; Benlebna, M.; Poupon, J.; Jover, B.; Casas, F.; Jouy, N.; Feillet-Coudray, C.; Gaillet, S.; et al. Spirulina Platensis and Silicon-Enriched Spirulina Equally Improve Glucose Tolerance and Decrease the Enzymatic Activity of Hepatic NADPH Oxidase in Obesogenic Diet-Fed Rats. Food Funct. 2018, 9, 6165–6178. [Google Scholar] [CrossRef]
- Palau, N.; Rebuffat, S.A.; Altirriba, J.; Piquer, S.; Hanzu, F.A.; Gomis, R.; Barbera, A. Role of IGFBP-3 in the Regulation of β-Cell Mass during Obesity: Adipose Tissue/β-Cell Cross Talk. Endocrinology 2012, 153, 177–187. [Google Scholar] [CrossRef]
- Blüher, M. Adipokines—Removing Road Blocks to Obesity and Diabetes Therapy. Mol. Metab. 2014, 3, 230–240. [Google Scholar] [CrossRef]
- Ebert, T.; Gebhardt, C.; Scholz, M.; Wohland, T.; Schleinitz, D.; Fasshauer, M.; Blüher, M.; Stumvoll, M.; Kovacs, P.; Tönjes, A. Relationship Between 12 Adipocytokines and Distinct Components of the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2018, 103, 1015–1023. [Google Scholar] [CrossRef]
- Zhu, Q.; Scherer, P.E. Immunologic and Endocrine Functions of Adipose Tissue: Implications for Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 105–120. [Google Scholar] [CrossRef]
- Laget, J.; Vigor, C.; Nouvel, A.; Rocher, A.; Leroy, J.; Jeanson, L.; Reversat, G.; Oger, C.; Galano, J.-M.; Durand, T.; et al. Reduced Production of Isoprostanes by Peri-Pancreatic Adipose Tissue from Zucker Fa/Fa Rats as a New Mechanism for β-Cell Compensation in Insulin Resistance and Obesity. Free Radic Biol. Med. 2022, 182, 160–170. [Google Scholar] [CrossRef]
- Corden, B.; Adami, E.; Sweeney, M.; Schafer, S.; Cook, S.A. IL-11 in Cardiac and Renal Fibrosis: Late to the Party but a Central Player. Br. J. Pharmacol. 2020, 177, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Schafer, S.; Viswanathan, S.; Widjaja, A.A.; Lim, W.-W.; Moreno-Moral, A.; DeLaughter, D.M.; Ng, B.; Patone, G.; Chow, K.; Khin, E.; et al. IL-11 Is a Crucial Determinant of Cardiovascular Fibrosis. Nature 2017, 552, 110–115. [Google Scholar] [CrossRef]
- Strikoudis, A.; Cieślak, A.; Loffredo, L.; Chen, Y.-W.; Patel, N.; Saqi, A.; Lederer, D.J.; Snoeck, H.-W. Modeling of Fibrotic Lung Disease Using 3D Organoids Derived from Human Pluripotent Stem Cells. Cell Rep. 2019, 27, 3709–3723.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widjaja, A.A.; Viswanathan, S.; Wei Ting, J.G.; Tan, J.; Shekeran, S.G.; Carling, D.; Lim, W.-W.; Cook, S.A. IL11 Stimulates ERK/P90RSK to Inhibit LKB1/AMPK and Activate MTOR Initiating a Mesenchymal Program in Stromal, Epithelial, and Cancer Cells. iScience 2022, 25, 104806. [Google Scholar] [CrossRef]
- Lamers, D.; Famulla, S.; Wronkowitz, N.; Hartwig, S.; Lehr, S.; Ouwens, D.M.; Eckardt, K.; Kaufman, J.M.; Ryden, M.; Müller, S.; et al. Dipeptidyl Peptidase 4 Is a Novel Adipokine Potentially Linking Obesity to the Metabolic Syndrome. Diabetes 2011, 60, 1917–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sell, H.; Blüher, M.; Klöting, N.; Schlich, R.; Willems, M.; Ruppe, F.; Knoefel, W.T.; Dietrich, A.; Fielding, B.A.; Arner, P.; et al. Adipose Dipeptidyl Peptidase-4 and Obesity: Correlation with Insulin Resistance and Depot-Specific Release from Adipose Tissue in Vivo and in Vitro. Diabetes Care 2013, 36, 4083–4090. [Google Scholar] [CrossRef] [Green Version]
| Standard-Sham | Standard-SNx | Cafeteria-Sham | Cafeteria-SNx | |
|---|---|---|---|---|
| Creatinine µmol/L | 34.2 ± 5.1 | 54.0 ± 7.0 * | 31.7 ± 1.2 | 70.5 ± 10.3 * |
| Glycemia mg/dL | 90 ± 2 | 94 ± 2 | 95 ± 2 | 91 ± 3 |
| Insulin mU/L | 92 ± 17 | 66 ± 22 | 177 ± 13 † | 112 ± 19 *† |
| HOMA-IR | 3.3 ± 0.8 | 2.6 ± 1.0 | 6.9 ± 0.6 † | 4.4 ± 0.7 *† |
| Left kidney Wt mg | 1250 ± 34 | 1477 ± 82 | 1332 ± 34 | 1833 ± 160 *† |
| Mean Level ± Sem | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Standard-Sham | Standard-SNx | Cafeteria-Sham | Cafeteria-SNx | Surgery Effect (SNx vs. Sham) | Diet Effect (Cafeteria vs. Standard) | Diet Effect in SNx (Cafeteria-SNx vs. Standard-SNx) | |
| Pref1 | 13 ± 6 | 27 ± 14 | 83 ± 13 | 128 ± 29 | 0.12 | <0.001 | <0.01 |
| FGF21 | 30 ± 13 | 37 ± 6 | 126 ± 17 | 152 ± 22 | 0.28 | <0.001 | <0.001 |
| LIF | 62 ± 14 | 66 ± 20 | 131 ± 22 | 161 ± 10 | 0.33 | <0.001 | <0.01 |
| Angiopoietinlike3 | 107 ± 28 | 86 ± 18 | 115 ± 15 | 173 ± 8 | 0.35 | 0.02 | 0.02 |
| IL11 | 137 ± 22 | 59 ± 18 | 132 ± 12 | 185 ± 31 | 0.56 | 0.01 | <0.01 |
| DPPIV | 350 ± 45 | 248 ± 23 | 356 ± 27 | 419 ± 13 | 0.52 | <0.01 | <0.01 |
| Leptin | 824 ± 126 | 392 ± 58 | 802 ± 87 | 583 ± 96 | <0.01 | 0.39 | 0.50 |
| RANTES | 26 ± 11 | 33 ± 17 | 72 ± 15 | 105 ± 10 | 0.16 | <0.001 | <0.01 |
| Endocan | 74 ± 20 | 78 ± 16 | 166 ± 26 | 212 ± 35 | 0.33 | <0.001 | <0.01 |
| MCP1 | 57 ± 16 | 47 ± 16 | 114 ± 19 | 115 ± 15 | 0.77 | <0.01 | 0.04 |
| RAGE | 20 ± 10 | 40 ± 11 | 101 ± 15 | 127 ± 14 | 0.09 | <0.001 | <0.01 |
| IL6 | 0 ± 0 | 5 ± 3 | 54 ± 9 | 70 ± 13 | 0.22 | <0.001 | <0.001 |
| IL1b | 19 ± 9 | 24 ± 10 | 100 ± 12 | 91 ± 12 | 0.83 | <0.001 | <0.01 |
| MCSF | 8 ± 3 | 15 ± 10 | 69 ± 10 | 73 ± 10 | 0.52 | <0.001 | <0.01 |
| HGF | 77 ± 25 | 75 ± 15 | 170 ± 28 | 155 ± 14 | 0.70 | <0.001 | 0.07 |
| TNFa | 30 ± 17 | 26 ± 12 | 76 ± 11 | 76 ± 12 | 0.88 | <0.01 | 0.07 |
| VEGFA | 74 ± 28 | 64 ± 17 | 163 ± 33 | 127 ± 12 | 0.35 | <0.01 | 0.29 |
| IL10 | 37 ± 16 | 68 ± 18 | 138 ± 14 | 150 ± 28 | 0.30 | <0.001 | 0.049 |
| ICAM1 | 915 ± 85 | 781 ± 49 | 1077 ± 73 | 793 ± 36 | 0.00 | 0.19 | 0.99 |
| IGFBP1 | 51 ± 11 | 39 ± 11 | 94 ± 9 | 147 ± 31 | 0.28 | <0.001 | <0.01 |
| IGFBP2 | 234 ± 75 | 97 ± 34 | 235 ± 36 | 243 ± 33 | 0.20 | 0.14 | 0.18 |
| IGFBP3 | 1267 ± 159 | 989 ± 140 | 1115 ± 123 | 1368 ± 67 | 0.92 | 0.39 | 0.19 |
| IGFBP5 | 950 ± 60 | 804 ± 32 | 875 ± 85 | 804 ± 78 | 0.13 | 0.59 | 0.99 |
| IGFBP6 | 1716 ± 90 | 1420 ± 70 | 1784 ± 95 | 1439 ± 162 | 0.01 | 0.70 | 0.99 |
| IGFI | 312 ± 111 | 212 ± 30 | 273 ± 43 | 530 ± 203 | 0.52 | 0.26 | 0.27 |
| IGFII | 46 ± 16 | 67 ± 13 | 152 ± 19 | 162 ± 20 | 0.39 | <0.001 | <0.01 |
| TIMP1 | 134 ± 31 | 211 ± 66 | 349 ± 82 | 452 ± 101 | 0.24 | <0.01 | 0.14 |
| SerpinE1 | 30 ± 9 | 39 ± 10 | 94 ± 12 | 131 ± 22 | 0.13 | <0.001 | <0.01 |
| Resistin | 1833 ± 131 | 1382 ± 183 | 1374 ± 93 | 1546 ± 100 | 0.30 | 0.28 | 0.82 |
| Lipocalin2 | 1590 ± 563 | 1169 ± 233 | 1432 ± 368 | 2136 ± 418 | 0.74 | 0.34 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laget, J.; Cortijo, I.; Boukhaled, J.H.; Muyor, K.; Duranton, F.; Jover, B.; Raynaud, F.; Lajoix, A.-D.; Argilés, À.; Gayrard, N. Cafeteria Diet-Induced Obesity Worsens Experimental CKD. Nutrients 2023, 15, 3331. https://doi.org/10.3390/nu15153331
Laget J, Cortijo I, Boukhaled JH, Muyor K, Duranton F, Jover B, Raynaud F, Lajoix A-D, Argilés À, Gayrard N. Cafeteria Diet-Induced Obesity Worsens Experimental CKD. Nutrients. 2023; 15(15):3331. https://doi.org/10.3390/nu15153331
Chicago/Turabian StyleLaget, Jonas, Irene Cortijo, Juliana H. Boukhaled, Karen Muyor, Flore Duranton, Bernard Jover, Fabrice Raynaud, Anne-Dominique Lajoix, Àngel Argilés, and Nathalie Gayrard. 2023. "Cafeteria Diet-Induced Obesity Worsens Experimental CKD" Nutrients 15, no. 15: 3331. https://doi.org/10.3390/nu15153331
APA StyleLaget, J., Cortijo, I., Boukhaled, J. H., Muyor, K., Duranton, F., Jover, B., Raynaud, F., Lajoix, A.-D., Argilés, À., & Gayrard, N. (2023). Cafeteria Diet-Induced Obesity Worsens Experimental CKD. Nutrients, 15(15), 3331. https://doi.org/10.3390/nu15153331







