Higher Dietary Acid Load Might Be a Potent Derivative Factor for Multiple Sclerosis: The Results from a Case–Control Study
Abstract
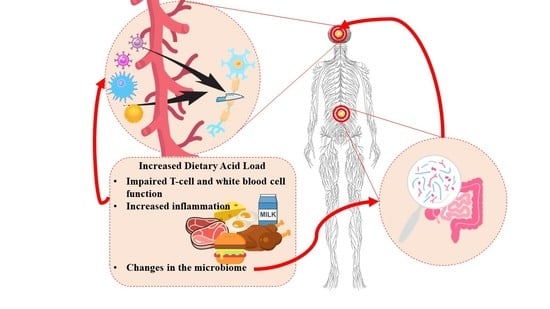
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Participants
2.3. Demographic and Anthropometric Data
2.4. Dietary Assessments
2.5. Dietary Acid Load
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Milo, R.; Kahana, E. Multiple sclerosis: Geoepidemiology, genetics and the environment. Autoimmun. Rev. 2010, 9, A387–A394. [Google Scholar] [CrossRef]
- Oh, J.; Vidal-Jordana, A.; Montalban, X. Multiple sclerosis: Clinical aspects. Curr. Opin. Neurol. 2018, 31, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.J.; Watson, C. Characteristics, burden of illness, and physical functioning of patients with relapsing-remitting and secondary progressive multiple sclerosis: A cross-sectional US survey. Neuropsychiatr. Dis. Treat. 2017, 13, 1349. [Google Scholar] [CrossRef] [Green Version]
- Jahromi, S.R.; Toghae, M.; Jahromi, M.J.R.; Aloosh, M. Dietary pattern and risk of multiple sclerosis. Iran. J. Neurol. 2012, 11, 47–53. [Google Scholar]
- Sedaghat, F.; Jessri, M.; Behrooz, M.; Mirghotbi, M.; Rashidkhani, B. Mediterranean diet adherence and risk of multiple sclerosis: A case-control study. Asia Pac. J. Clin. Nutr. 2016, 25, 377–384. [Google Scholar] [CrossRef]
- Alfredsson, L.; Olsson, T.; Hedström, A.K. Inverse association between Mediterranean diet and risk of multiple sclerosis. Mult. Scler. J. 2023. [Google Scholar] [CrossRef]
- Stoiloudis, P.; Kesidou, E.; Bakirtzis, C.; Sintila, S.-A.; Konstantinidou, N.; Boziki, M.; Grigoriadis, N. The role of diet and interventions on multiple sclerosis: A review. Nutrients 2022, 14, 1150. [Google Scholar] [CrossRef]
- Bühlmeier, J.; Harris, C.; Koletzko, S.; Lehmann, I.; Bauer, C.-P.; Schikowski, T.; Berg, A.v.; Berdel, D.; Heinrich, J.; Hebebrand, J. Dietary acid load and mental health outcomes in children and adolescents: Results from the GINIplus and LISA birth cohort studies. Nutrients 2018, 10, 582. [Google Scholar] [CrossRef] [Green Version]
- Bland, J.S. Age-related Disease: A Revolution is Coming, Part 2—Dietary Acid Load, Hypertension, and Cardiovascular Disease. Integr. Med. 2018, 17, 12–15. [Google Scholar]
- Remer, T.; Dimitriou, T.; Manz, F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am. J. Clin. Nutr. 2003, 77, 1255–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frassetto, L.A.; Todd, K.M.; Morris, R.C., Jr.; Sebastian, A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am. J. Clin. Nutr. 1998, 68, 576–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noormohammadi, M.; Ghorbani, Z.; Naser Moghadasi, A.; Saeedirad, Z.; Shahemi, S.; Ghanaatgar, M.; Rezaeimanesh, N.; Hekmatdoost, A.; Ghaemi, A.; Razeghi Jahromi, S. MIND Diet Adherence Might be Associated with a Reduced Odds of Multiple Sclerosis: Results from a Case–Control Study. Neurol. Ther. 2022, 11, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Asghari, G.; Rezazadeh, A.; Hosseini-Esfahani, F.; Mehrabi, Y.; Mirmiran, P.; Azizi, F. Reliability, comparative validity and stability of dietary patterns derived from an FFQ in the Tehran Lipid and Glucose Study. Br. J. Nutr. 2012, 108, 1109–1117. [Google Scholar] [CrossRef]
- Esfahani, F.H.; Asghari, G.; Mirmiran, P.; Azizi, F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J. Epidemiol. 2010, 20, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Mirmiran, P.; Esfahani, F.H.; Mehrabi, Y.; Hedayati, M.; Azizi, F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010, 13, 654–662. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.; Hu, F. Anthropometric measures and body composition. Nutr. Epidemiol. 2013, 15, 213–240. [Google Scholar]
- Azar, M.; Sarkisian, E. Food Composition Table of Iran; National Nutrition and Food Research Institute, Shaheed Beheshti University: Tehran, Iran, 1980; p. 65. [Google Scholar]
- USDA National Nutrient Database For Standard Reference. 2019. Available online: http://www.ars.usda.gov/,r.c.a.a.andnews/docs.htm?docid=18880 (accessed on 15 January 2021).
- Zwart, S.R.; Hargens, A.R.; Smith, S.M. The ratio of animal protein intake to potassium intake is a predictor of bone resorption in space flight analogues and in ambulatory subjects. Am. J. Clin. Nutr. 2004, 80, 1058–1065. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, M.; Jahromi, S.R.; Togha, M.; Ghorbani, Z.; Hekmatdoost, A.; Rafiee, P.; Torkan, B.; Shirani, P.; Ansari, H.; Karami, A.; et al. The Association Between Dietary Acid Load and Odds of Migraine: A Case–Control Survey. Neurol. Ther. 2021, 10, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Katz Sand, I.; Benn, E.K.T.; Fabian, M.; Fitzgerald, K.C.; Digga, E.; Deshpande, R.; Miller, A.; Gallo, S.; Arab, L. Randomized-controlled trial of a modified Mediterranean dietary program for multiple sclerosis: A pilot study. Mult. Scler. Relat. Disord. 2019, 36, 101403. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Tyry, T.; Salter, A.; Cofield, S.S.; Cutter, G.; Fox, R.; Marrie, R.A. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 2018, 90, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Rezazadegan, M.; Mirzaei, S.; Asadi, A.; Akhlaghi, M.; Saneei, P. Association between dietary acid load and metabolic health status in overweight and obese adolescents. Sci. Rep. 2022, 12, 10799. [Google Scholar] [CrossRef]
- Lee, K.W.; Shin, D. Positive association between dietary acid load and future insulin resistance risk: Findings from the Korean Genome and Epidemiology Study. Nutr. J. 2020, 19, 137. [Google Scholar] [CrossRef]
- Soliman, R.H.; Farhan, H.M.; Hegazy, M.; Oraby, M.I.; Kamel, S.H.; Hassan, A. Impact of insulin resistance and metabolic syndrome on disability in patients with multiple sclerosis. Egypt J. Neurol. Psychiatry Neurosurg. 2020, 56, 18. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, S.R.; Simão, A.N.C.; Kallaur, A.P.; de Almeida, E.R.D.; Morimoto, H.K.; Lopes, J.; Dichi, I.; Kaimen-Maciel, D.R.; Reiche, E.M.V. Disability in patients with multiple sclerosis: Influence of insulin resistance, adiposity, and oxidative stress. Nutrition 2014, 30, 268–273. [Google Scholar] [CrossRef]
- Ruiz-Argüelles, A.; Méndez-Huerta, M.A.; Lozano, C.D.; Ruiz-Argüelles, G.J. Metabolomic profile of insulin resistance in patients with multiple sclerosis is associated to the severity of the disease. Mult. Scler. Relat. Disord. 2018, 25, 316–321. [Google Scholar] [CrossRef]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef]
- Turner, M.P.; Hubbard, N.A.; Sivakolundu, D.K.; Himes, L.M.; Hutchison, J.L.; Hart, J., Jr.; Spence, J.S.; Frohman, E.M.; Frohman, T.C.; Okuda, D.T. Preserved canonicality of the BOLD hemodynamic response reflects healthy cognition: Insights into the healthy brain through the window of multiple sclerosis. NeuroImage 2019, 190, 46–55. [Google Scholar] [CrossRef]
- Tarlinton, R.E.; Khaibullin, T.; Granatov, E.; Martynova, E.; Rizvanov, A.; Khaiboullina, S. The interaction between viral and environmental risk factors in the pathogenesis of multiple sclerosis. Int. J. Mol. Sci. 2019, 20, 303. [Google Scholar] [CrossRef] [Green Version]
- Riccio, P.; Rossano, R. Nutrition facts in multiple sclerosis. ASN Neuro 2015, 7, 1759091414568185. [Google Scholar] [CrossRef] [Green Version]
- Katz Sand, I. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Curr. Nutr. Rep. 2018, 7, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. 2019, 24, 810–834. [Google Scholar] [CrossRef] [PubMed]
- Altowaijri, G.; Fryman, A.; Yadav, V. Dietary Interventions and Multiple Sclerosis. Curr. Neurol. Neurosci. Rep. 2017, 17, 28. [Google Scholar] [CrossRef]
- Abbasalizad Farhangi, M.; Nikniaz, L.; Nikniaz, Z. Higher dietary acid load potentially increases serum triglyceride and obesity prevalence in adults: An updated systematic review and meta-analysis. PLoS ONE 2019, 14, e0216547. [Google Scholar] [CrossRef] [Green Version]
- Mokry, L.E.; Ross, S.; Timpson, N.J.; Sawcer, S.; Smith, G.D.; Richards, J.B. Obesity and multiple sclerosis: A mendelian randomization study. PLoS Med 2016, 13, e1002053. [Google Scholar] [CrossRef] [Green Version]
- Russell, R.; Langer-Gould, A.; Gonzales, E.; Smith, J.; Brennan, V.; Pereira, G.; Lucas, R.; Begley, A.; Black, L. Obesity, dieting, and multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 39, 101889. [Google Scholar] [CrossRef]
- Vucenik, I.; Stains, J.P. Obesity and cancer risk: Evidence, mechanisms, and recommendations. Ann. N. Y. Acad. Sci. 2012, 1271, 37. [Google Scholar] [CrossRef] [PubMed]
- Bisson, E.J.; Finlayson, M.L.; Ekuma, O.; Leslie, W.D.; Marrie, R.A. Multiple sclerosis is associated with low bone mineral density and osteoporosis. Neurol. Clin. Pract. 2019, 9, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Mangano, K.M.; Walsh, S.J.; Kenny, A.M.; Insogna, K.L.; Kerstetter, J.E. Dietary acid load is associated with lower bone mineral density in men with low intake of dietary calcium. J. Bone Miner Res. 2014, 29, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Gholami, F.; Naghshi, S.; Samadi, M.; Rasaei, N.; Mirzaei, K. Dietary Acid Load and Bone Health: A Systematic Review and Meta-Analysis of Observational Studies. Front. Nutr. 2022, 9, 869132. [Google Scholar] [CrossRef]
- Boeschoten, R.E.; Braamse, A.M.J.; Beekman, A.T.F.; Cuijpers, P.; van Oppen, P.; Dekker, J.; Uitdehaag, B.M.J. Prevalence of depression and anxiety in Multiple Sclerosis: A systematic review and meta-analysis. J. Neurol. Sci. 2017, 372, 331–341. [Google Scholar] [CrossRef]
- Hemmati, A.; Ghoreishy, S.M.; Karami, K.; Imani, H.; Farsani, G.M.; Mousavi, S.E.; Asoudeh, F.; Shariati-Bafghi, S.E.; Karamati, M. The association between dietary patterns and depression in adolescents: A cross-sectional study. Clin. Nutr. ESPEN 2021, 46, 271–275. [Google Scholar] [CrossRef]
- Yin, W.; Löf, M.; Chen, R.; Hultman, C.M.; Fang, F.; Sandin, S. Mediterranean diet and depression: A population-based cohort study. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 153. [Google Scholar] [CrossRef]
- Milajerdi, A.; Hassanzadeh Keshteli, A.; Haghighatdoost, F.; Azadbakht, L.; Esmaillzadeh, A.; Adibi, P. Dietary acid load in relation to depression and anxiety in adults. J. Hum. Nutr. Diet 2020, 33, 48–55. [Google Scholar] [CrossRef]
| Variables 2 | Healthy Controls (n = 130) | Patients with Multiple Sclerosis (n = 109) | p-Value |
|---|---|---|---|
| Age (years) | 35 (29, 42) | 33 (29, 38) | 0.040 |
| Sex, female, frequency (percentages) | 92 (70.8) | 84 (77.1) | 0.271 |
| Body mass index, Kg/m2, Mean (SD) | 26.92 ± 3.52 | 25.18 ± 3.86 | <0.001 |
| Total calorie intake (Kcal/day) | 2303.04 (1857.29, 2663.53) | 2542.49 (2143.03, 3109.57) | <0.001 |
| Protein (gr/day) | 82.14 ± 25.33 | 85.65 ± 26.59 | 0.298 |
| Carbohydrates (gr/day) | 289.49 (225.25, 350.44) | 331.06 (281.07, 426.16) | <0.001 |
| Fat (gr/day) | 88.53 (72.64, 112.04) | 100.07 (83.44, 128.11) | 0.002 |
| Cholesterol (mg/day) | 270.78 (202.89, 377.31) | 319.59 (243.18, 397.73) | 0.055 |
| Saturated fatty acids (gr/day) | 30.08 (23.63, 36.64) | 35.89 (27.12, 48.39) | <0.001 |
| Sodium (mg/day) | 3468.27 (2724.48, 4414.04) | 7764.76 (5058.46, 9057.54) | <0.001 |
| Fiber (gr/day) | 23.18 (19.10, 31.23) | 19.61 (15.80, 26.79) | 0.001 |
| Trans fatty acids (gr/day) | 2.31 (1.45, 7.68) | 2.94 (1.93, 5.23) | 0.555 |
| Poly unsaturated fatty acids (gr/day) | 28.38 (22.13, 35.71) | 27.55 (22.62, 32.83) | 0.227 |
| Iron (mg/day) | 17.02 (13.35, 22.20) | 15.71 (12.86, 19.13) | 0.053 |
| Calcium (mg/day) | 1002.45 ± 380.51 | 952.22 ± 330.81 | 0.282 |
| Folate (mcg/day) | 416.04 (327.31, 531.16) | 300.90 (242.53, 361.99) | <0.001 |
| Sugar (gr/day) | 83.06 (65.56, 110.00) | 105.98 (80.30, 140.59) | <0.001 |
| Glucose (gr/day) | 10.95 (7.91, 16.16) | 19.02 (12.35, 29.14) | <0.001 |
| Vitamin A (RAE/day) | 1287.93 (915.67, 2212.71) | 879.30 (625.63, 1301.77) | <0.001 |
| Vitamin D (mcg/day) | 1.12 (0.40, 2.27) | 1.05 (0.40, 1.63) | 0.346 |
| Vitamin C (mg/day) | 141.89 (97.06, 202.01) | 117.90 (88.96, 162.63) | 0.034 |
| Vitamin E (mg/day) | 3.92 (3.02, 5.60) | 5.02 (3.88, 6.21) | <0.001 |
| Beta carotene (mg/day) | 649.17 (368.51, 1325.09) | 250.40 (127.84, 403.39) | <0.001 |
| Mono unsaturated fatty acids (gr/day) | 32.84 (26.11, 40.35) | 39.48 (31.24, 49.20) | <0.001 |
| Phosphorus (mg/day) | 1201.42 (912.80, 1567.49) | 1101.12 (901.42, 1362.15) | 0.028 |
| Magnesium (mg/day) | 298.85 (233.58, 376.01) | 233.17 (198.20, 274.09) | <0.001 |
| Potassium (mg/day) | 3770.32 (2956.91, 4581.18) | 2976.51 (2500.05, 3593.60) | <0.001 |
| Zinc (mg/day) | 9.99 (7.68, 12.17) | 9.82(7.90, 11.92) | 0.885 |
| Copper (mg/day) | 1.63 (1.13, 2.36) | 1.38 (1.08, 1.81) | 0.025 |
| Manganese (mg/day) | 3.52 (2.84, 4.48) | 2.66 (2.17, 3.43) | <0.001 |
| Selenium (µmol/day) | 0.58 (0.34, 1.09) | 1.12 (0.63, 1.19) | <0.001 |
| Vitamin B1 (mg/day) | 10.29 (8.14, 13.25) | 8.74 (6.91, 11.01) | <0.001 |
| Vitamin B2 (mg/day) | 1.72 (1.31, 2.18) | 1.54 (1.27, 1.82) | 0.015 |
| Vitamin B3 (mg/day) | 1.61 (1.09, 2.06) | 1.46 (1.12, 1.81) | 0.111 |
| Vitamin B6 (mg/day) | 18.82 (14.03, 23.65) | 20.65 (16.73, 25.70) | 0.016 |
| Vitamin B12 (mcg/day) | 1.32 (1.03, 1.79) | 1.19 (1.00, 1.52) | 0.027 |
| Vitamin B5 (mg/day) | 5.28 (3.42, 7.61) | 5.81 (3.87, 9.03) | 0.152 |
| Vitamin B8 (mcg/day) | 5.50 (4.13, 6.94) | 4.82 (4.10, 5.60) | 0.004 |
| Vitamin K (mcg/day) | 21.05 (16.53, 28.10) | 20.01 (16.28, 25.93) | 0.271 |
| Caffeine (mg/day) | 173.64 (101.00, 252.93) | 55.71 (42.25, 82.16) | <0.001 |
| PRAL | −13.62 (−25.37, −4.60) | −0.01 (−14.92, 9.29) | <0.001 |
| NEAP | 35.59 (30.23, 42.73) | 48.09 (37.16, 63.15) | <0.001 |
| Protein/potassium | 0.021 (0.019, 0.024) | 0.027 (0.022, 0.034) | <0.001 |
| Tertiles of PRAL | T1 (n = 57; Cases = 14) | T2 (n = 68; Cases = 24) | T3 (n = 114; Cases = 71) | p-Value |
|---|---|---|---|---|
| Age (years) | 35 (29.5, 42) | 35 (29, 43.75) | 32 (29, 39) | 0.102 |
| Body mass index | 27.83 (24.79, 30.49) | 26.52 (23.03, 29.61) | 24.68 (22.84, 27.36) | <0.001 |
| Total calorie intake (Kcal/day) | 2455.61 (1923.21, 2764.37) | 2179.55 (1787.31, 2649.86) | 2489.45 (2115.30, 3064.67) | 0.009 |
| Protein (gr/day) | 84.48 (65.51, 100.43) | 71.81 (57.18, 89.03) | 90.69 (72.08, 106.32) | <0.001 |
| Carbohydrates (gr/day) | 334.05 (234.74, 413.91) | 298.32 (232.52, 361.86) | 317.73 (256.07, 395.17) | 0.283 |
| Fat (gr/day) | 90.87 (74.19, 108.71) | 88.45 (71.73, 106.73) | 103.95 (81.39, 126.31) | 0.002 |
| Cholesterol (mg/day) | 253.75 (195.76, 361.65) | 270.84 (196.16, 354.20) | 322.65 (249.41, 425.16) | 0.002 |
| Saturated fatty acids (gr/day) | 29.27 (23.45, 34.97) | 28.92 (22.72, 36.70) | 36.67 (27.43, 44.75) | <0.001 |
| Sodium (mg/day) | 4404.13 (3018.33, 6912.04) | 4291.07 (3387.02, 7211.30) | 5187.42 (3361.90, 8632.02) | 0.126 |
| Fiber (gr/day) | 31.44 (25.64, 38.09) | 21.71 (18.87, 26.45) | 18.70 (15.03, 23.16) | <0.001 |
| Trans fatty acids (gr/day) | 2.05 (1.33, 5.49) | 2.31 (1.34, 6.14) | 3.36 (1.96, 6.82) | 0.026 |
| Poly unsaturated fatty acids (gr/day) | 29.61 (22.26, 36.67) | 28.51 (20.49, 33.25) | 27.77 (23.34, 33.67) | 0.517 |
| Iron (mg/day) | 19.08 (13.89, 23.93) | 14.96 (12.22, 18.86) | 16.00 (12.98, 19.98) | 0.060 |
| Calcium (mg/day) | 1129.71 (932.33, 1386.36) | 854.27 (714.04, 1032.45) | 956.18 (712.14, 1165.62) | <0.001 |
| Folate (mcg/day) | 448.29 (376.26, 595.81) | 353.07 (294.81, 411.45) | 305.94 (234.99, 385.30) | <0.001 |
| Sugar (gr/day) | 110.89 (85.48, 143.11) | 83.61 (69.34, 122.10) | 91.28 (67.52, 111.28) | 0.003 |
| Glucose (gr/day) | 16.13 (11.82, 25.24) | 12.21 (8.72, 20.43) | 14.41 (9.27, 23.50) | 0.085 |
| Vitamin A (RAE/day) | 1691.52 (1132.61, 2498.88) | 1039.54 (745.08, 1617.16) | 943.72 (600.93, 1334.02) | <0.001 |
| Vitamin D (mcg/day) | 1.65 (0.67, 2.40) | 0.75 (0.36, 1.36) | 0.95 (0.40, 1.71) | 0.001 |
| Vitamin C (mg/day) | 214.97 (169.30, 263.73) | 144.07 (109.42, 176.59) | 101.28 (74.09, 130.19) | <0.001 |
| Vitamin E (mg/day) | 5.10 (3.62, 6.43) | 3.83 (3.06, 5.68) | 4.51 (3.65, 5.59) | 0.008 |
| Beta carotene (mg/day) | 967.67 (451.67, 1637.04) | 466.47 (308.46, 726.69) | 270.82 (130.35, 497.51) | <0.001 |
| Mono unsaturated fatty acids (gr/day) | 34.77 (26.96, 41.78) | 35.60 (25.38, 41.59) | 38.38 (30.06, 47.03) | 0.019 |
| Phosphorus (mg/day) | 1397.21 (1024.42, 1646.48) | 981.50 (846.87, 1215.95) | 1161.92 (897.13, 1460.60) | <0.001 |
| Magnesium (mg/day) | 358.06 (291.29, 434.20) | 248.59 (206.39, 298.06) | 234.08 (192.43, 296.33) | <0.001 |
| Potassium (mg/day) | 4828.35 (3851.84, 5584.11) | 3199.99 (2780.45, 3915.69) | 2922.91 (2371.45, 3606.47) | <0.001 |
| Zinc (mg/day) | 10.62 (8.70, 12.44) | 8.44 (7.14, 10.37) | 10.68 (8.05, 12.85) | 0.001 |
| Copper (mg/day) | 1.78 (1.35, 2.67) | 1.30 (1.03, 1.80) | 1.42 (1.08, 1.96) | <0.001 |
| Manganese (mg/day) | 3.95 (3.12, 4.92) | 3.13 (2.40, 3.64) | 2.91 (2.13, 3.56) | <0.001 |
| Selenium (µmol/day) | 1.08 (0.37, 1.14) | 0.67 (0.36, 1.14) | 0.69 (0.49, 1.14) | 0.664 |
| Vitamin B1 (mg/day) | 1.74 (1.38, 2.21) | 1.53 (1.23, 1.90) | 1.59 (1.29, 2.02) | 0.084 |
| Vitamin B2 (mg/day) | 1.83 (1.33, 2.20) | 1.28 (1.03, 1.69) | 1.53 (1.15, 1.98) | 0.001 |
| Vitamin B3 (mg/day) | 18.71 (14.54, 23.49) | 17.55 (13.43, 22.75) | 21.39 (17.31, 26.12) | <0.001 |
| Vitamin B6 (mg/day) | 1.63 (1.29, 1.99) | 1.20 (1.00, 1.62) | 1.15 (0.94, 1.41) | <0.001 |
| Vitamin B12 (mcg/day) | 4.63 (2.90, 7.16) | 4.11 (3.20, 6.00) | 6.26 (4.67, 9.40) | <0.001 |
| Vitamin B5 (mg/day) | 6.25 (4.99, 7.21) | 4.59 (3.87, 5.77) | 5.06 (4.09, 5.97) | <0.001 |
| Vitamin B8 (mcg/day) | 24.56 (19.77, 33.70) | 18.08 (15.12, 23.65) | 20.64 (16.46, 25.43) | <0.001 |
| Vitamin K (mcg/day) | 233.58 (114.81, 315.09) | 118.22 (71.45, 166.93) | 60.84 (44.64, 94.09) | <0.001 |
| Caffeine (mg/day) | 148.11 (98.80, 251.17) | 126.20 (82.35, 199.95) | 105.22 (62.22, 160.64) | 0.011 |
| Tertiles of NEAP | T1 (n = 58; Cases = 15) | T2 (n = 64; Cases = 20) | T3 (n = 117; Cases = 74) | p-Value |
|---|---|---|---|---|
| Age (years) | 35.5 (28, 42) | 34.5 (29, 43.75) | 32 (29, 38.5) | 0.165 |
| Body mass index | 27.83 (25.37, 30.81) | 25.55 (22.95, 29.19) | 24.84 (23.03, 27.62) | 0.001 |
| Total calorie intake (Kcal/day) | 2151.90 (1704.43, 2662.15) | 2351.30 (1908.86, 2741.66) | 2501.55 (2184.21, 3062.24) | 0.001 |
| Protein (gr/day) | 70.66 (54.13, 89.85) | 74.88 (58.50, 98.22) | 90.82 (74.10, 106.63) | <0.001 |
| Carbohydrates (gr/day) | 290.18 (223.21, 367.43) | 309.01 (249.65, 374.67) | 324.74 (261.85, 402.44) | 0.082 |
| Fat (gr/day) | 83.20 (67.58, 107.73) | 86.68 (74.19, 113.26) | 101.76 (86.07, 126.64) | <0.001 |
| Cholesterol (mg/day) | 235.58 (173.85, 319.09) | 269.32 (205.38, 368.94) | 333.99 (255.98, 425.46) | <0.001 |
| Saturated fatty acids (gr/day) | 27.42 (21.21, 33.39) | 30.03 (23.73, 38.38) | 35.76 (28.62, 44.97) | <0.001 |
| Sodium (mg/day) | 4293.05 (2913.74, 6711.94) | 4238.50 (3424.67, 6948.42) | 5639.32 (3575.15, 8714.47) | 0.006 |
| Fiber (gr/day) | 28.38 (20.44, 35.21) | 26.33 (20.99, 33.25) | 28.20 (24.42, 33.89) | 0.453 |
| Trans fatty acids (gr/day) | 14.75 (11.96, 20.49) | 16.22 (12.81, 21.78) | 16.78 (13.23, 20.88) | 0.429 |
| Poly unsaturated fatty acids (gr/day) | 1019.86 (712.88, 1228.20) | 920.18 (736.53, 1094.66) | 970.13 (735.62, 1174.99) | 0.603 |
| Iron (mg/day) | 413.44 (318.10, 569.96) | 385.24 (297.55, 462.05) | 318.03 (253.99, 387.57) | <0.001 |
| Calcium (mg/day) | 27.37 (21.69, 37.11) | 22.68 (18.41, 31.73) | 19.41 (15.62, 24.58) | <0.001 |
| Folate (mcg/day) | 2.21 (1.22, 7.64) | 2.31 (1.44, 5.44) | 3.30 (1.92, 6.43) | 0.052 |
| Sugar (gr/day) | 105.89 (70.07, 135.07) | 93.65 (71.05, 123.20) | 90.94 (69.54, 115.28) | 0.254 |
| Glucose (gr/day) | 14.33 (10.46, 22.88) | 12.32 (8.84, 20.43) | 14.46 (9.30, 23.61) | 0.415 |
| Vitamin A (RAE/day) | 1368.27 (942.98, 2384.53) | 1192.73 (745.08, 1751.19) | 958.90 (668.98, 1342.54) | 0.001 |
| Vitamin D (mcg/day) | 1.36 (0.22, 2.32) | 0.96 (0.39, 1.59) | 1.16 (0.47, 1.84) | 0.668 |
| Vitamin C (mg/day) | 198.96 (135.44, 261.79) | 152.12 (102.24, 193.02) | 104.49 (79.05, 136.63) | <0.001 |
| Vitamin E (mg/day) | 4.00 (3.26, 6.35) | 4.45 (3.63, 5.82) | 4.52 (3.51, 5.60) | 0.899 |
| Beta carotene (mg/day) | 794.79 (362.98, 1573.57) | 486.78 (283.64, 822.95) | 296.63 (131.24, 529.22) | <0.001 |
| Mono unsaturated fatty acids (gr/day) | 31.31 (25.92, 41.61) | 35.90 (27.31, 40.17) | 38.30 (30.27, 47.07) | 0.009 |
| Phosphorus (mg/day) | 1195.65 (913.93, 1600.80) | 1048.96 (831.67, 1408.12) | 1168.35 (922.96, 1438.67) | 0.494 |
| Magnesium (mg/day) | 298.61 (241.57, 427.25) | 264.88 (212.73, 345.52) | 244.25 (199.62, 296.85) | <0.001 |
| Potassium (mg/day) | 4012.29 (3151.91, 5448.55) | 3469.59 (2691.21, 4463.24) | 3000.35 (2460.01, 3616.19) | <0.001 |
| Zinc (mg/day) | 9.00 (7.36, 11.99) | 9.47 (7.14, 11.96) | 10.64 (8.40, 12.32) | 0.072 |
| Copper (mg/day) | 1.52 (1.23, 2.53) | 1.57 (1.07, 2.05) | 1.41 (1.09, 1.94) | 0.389 |
| Manganese (mg/day) | 3.48 (2.65, 4.92) | 3.38 (2.62, 3.82) | 2.91 (2.15, 3.60) | 0.001 |
| Selenium (µmol/day) | 1.09 (0.38, 1.14) | 0.65 (0.36, 1.13) | 0.81 (0.50, 1.14) | 0.478 |
| Vitamin B1 (mg/day) | 1.56 (1.24, 2.03) | 1.67 (1.32, 2.04) | 1.63 (1.31, 2.04) | 0.590 |
| Vitamin B2 (mg/day) | 1.59 (1.03, 1.94) | 1.35 (1.04, 1.92) | 1.55 (1.18, 1.96) | 0.401 |
| Vitamin B3 (mg/day) | 16.78 (12.39, 20.90) | 18.08 (14.64, 23.43) | 21.51 (18.06, 26.24) | <0.001 |
| Vitamin B6 (mg/day) | 1.51 (1.15, 1.91) | 1.28 (0.95, 1.78) | 1.19 (0.99, 1.43) | 0.001 |
| Vitamin B12 (mcg/day) | 3.90 (2.53, 6.23) | 4.29 (3.46, 8.01) | 6.20 (4.71, 9.36) | <0.001 |
| Vitamin B5 (mg/day) | 5.58 (3.95, 6.86) | 4.88 (4.14, 6.48) | 5.13 (4.11, 5.98) | 0.299 |
| Vitamin B8 (mcg/day) | 22.20 (16.11, 32.56) | 20.69 (16.56, 27.49) | 20.04 (16.53, 25.52) | 0.518 |
| Vitamin K (mcg/day) | 186.04 (96.52, 292.34) | 128.38 (70.81, 209.20) | 62.76 (44.90, 103.02) | <0.001 |
| Caffeine (mg/day) | 116.13 (97.46, 253.81) | 147.84 (99.31, 199.95) | 106.41 (63.70, 170.85) | 0.067 |
| Indexes of Dietary Acid Load | Odds Ratio of Dietary Indexes of Acid Load (95% Confidence Interval) | |||
|---|---|---|---|---|
| 1st | 2nd | 3rd | p for Trend | |
| Total plant-based protein (gr/day) | ||||
| No. cases/no. controls | 44/43 | 36/44 | 29/43 | |
| Base model b | 1.00 (Ref.) | 0.85 (0.45, 1.58) | 0.73 (0.38, 1.40) | 0.339 |
| Model 1 c | 1.00 (Ref.) | 0.29 (0.13, 0.63) | 0.08 (0.03, 0.23) | <0.001 |
| Model 2 d | 1.00 (Ref.) | 0.29 (0.10, 0.90) | 0.07 (0.01, 0.38) | 0.002 |
| Total animal-based protein (gr/day) | ||||
| No. cases/no. controls | 24/43 | 34/44 | 51/43 | |
| Base model b | 1.00 (Ref.) | 1.47 (0.74, 2.93) | 2.28 (1.18, 4.41) | 0.013 |
| Model 1 c | 1.00 (Ref.) | 1.11 (0.53, 2.30) | 0.87 (0.38, 2.01) | 0.745 |
| Model 2 d | 1.00 (Ref.) | 1.41 (0.47, 4.22) | 0.88 (0.24, 3.26) | 0.833 |
| PRAL (mEq/day) | ||||
| No. cases/no. controls | 14/43 | 24/44 | 71/43 | |
| Base model b | 1.00 (Ref.) | 1.68 (0.76, 3.69) | 4.88 (2.38, 10.02) | <0.001 |
| Model 1 c | 1.00 (Ref.) | 1.83 (0.79, 4.21) | 4.16 (1.94, 8.91) | <0.001 |
| Model 2 d | 1.00 (Ref.) | 0.50 (0.13, 1.74) | 0.86 (0.23, 3.18) | 0.884 |
| NEAP (mEq/day) | ||||
| No. cases/no. controls | 15/43 | 20/44 | 74/43 | |
| Base model b | 1.00 (Ref.) | 1.31 (0.59, 2.91) | 5.03 (2.47, 10.26) | <0.001 |
| Model 1 c | 1.00 (Ref.) | 1.01 (0.43, 2.36) | 3.57 (1.69, 7.53) | <0.001 |
| Model 2 d | 1.00 (Ref.) | 0.41 (0.12, 1.46) | 0.91 (0.25, 3.31) | 0.800 |
| Protein/potassium ratio | ||||
| No. cases/no. controls | 15/43 | 20/44 | 74/43 | |
| Base model b | 1.00 (Ref.) | 1.31 (0.59, 2.91) | 5.03 (2.47, 10.26) | <0.001 |
| Model 1 c | 1.00 (Ref.) | 1.01 (0.43, 2.36) | 3.57 (1.69, 7.53) | <0.001 |
| Model 2 d | 1.00 (Ref.) | 0.41 (0.12, 1.46) | 0.91 (0.25, 3.31) | 0.800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeedirad, Z.; Ariyanfar, S.; Noormohammadi, M.; Ghorbani, Z.; Naser Moghadasi, A.; Shahemi, S.; Ghanaatgar, M.; Rezaeimanesh, N.; Hekmatdoost, A.; Ghaemi, A.; et al. Higher Dietary Acid Load Might Be a Potent Derivative Factor for Multiple Sclerosis: The Results from a Case–Control Study. Nutrients 2023, 15, 3311. https://doi.org/10.3390/nu15153311
Saeedirad Z, Ariyanfar S, Noormohammadi M, Ghorbani Z, Naser Moghadasi A, Shahemi S, Ghanaatgar M, Rezaeimanesh N, Hekmatdoost A, Ghaemi A, et al. Higher Dietary Acid Load Might Be a Potent Derivative Factor for Multiple Sclerosis: The Results from a Case–Control Study. Nutrients. 2023; 15(15):3311. https://doi.org/10.3390/nu15153311
Chicago/Turabian StyleSaeedirad, Zahra, Shadi Ariyanfar, Morvarid Noormohammadi, Zeinab Ghorbani, Abdorreza Naser Moghadasi, Sahar Shahemi, Milad Ghanaatgar, Nasim Rezaeimanesh, Azita Hekmatdoost, Amir Ghaemi, and et al. 2023. "Higher Dietary Acid Load Might Be a Potent Derivative Factor for Multiple Sclerosis: The Results from a Case–Control Study" Nutrients 15, no. 15: 3311. https://doi.org/10.3390/nu15153311
APA StyleSaeedirad, Z., Ariyanfar, S., Noormohammadi, M., Ghorbani, Z., Naser Moghadasi, A., Shahemi, S., Ghanaatgar, M., Rezaeimanesh, N., Hekmatdoost, A., Ghaemi, A., & Razeghi Jahromi, S. (2023). Higher Dietary Acid Load Might Be a Potent Derivative Factor for Multiple Sclerosis: The Results from a Case–Control Study. Nutrients, 15(15), 3311. https://doi.org/10.3390/nu15153311