Vitamin D—An Effective Antioxidant in an Animal Model of Progressive Multiple Sclerosis
Abstract
:1. Introduction
2. Materials & Methods
2.1. Animals
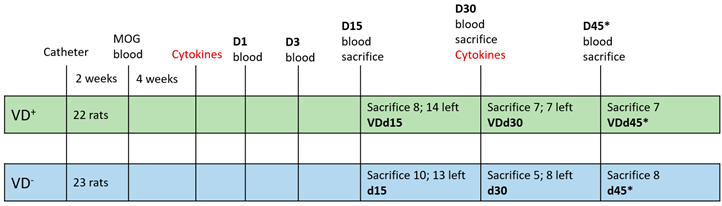
2.2. Experimental Setup
2.3. Blood Sampling, Euthanasia, and Tissue Extraction
2.4. Neuropathology and Immunohistochemistry
2.5. Quantitative Histopathological Evaluation
2.6. Colorimetric Methods for Antioxidative Capacity and Polyphenols
2.7. NfL Measurement Via Single-Molecule Array (SIMOA)
2.8. Statistical Analysis
3. Results
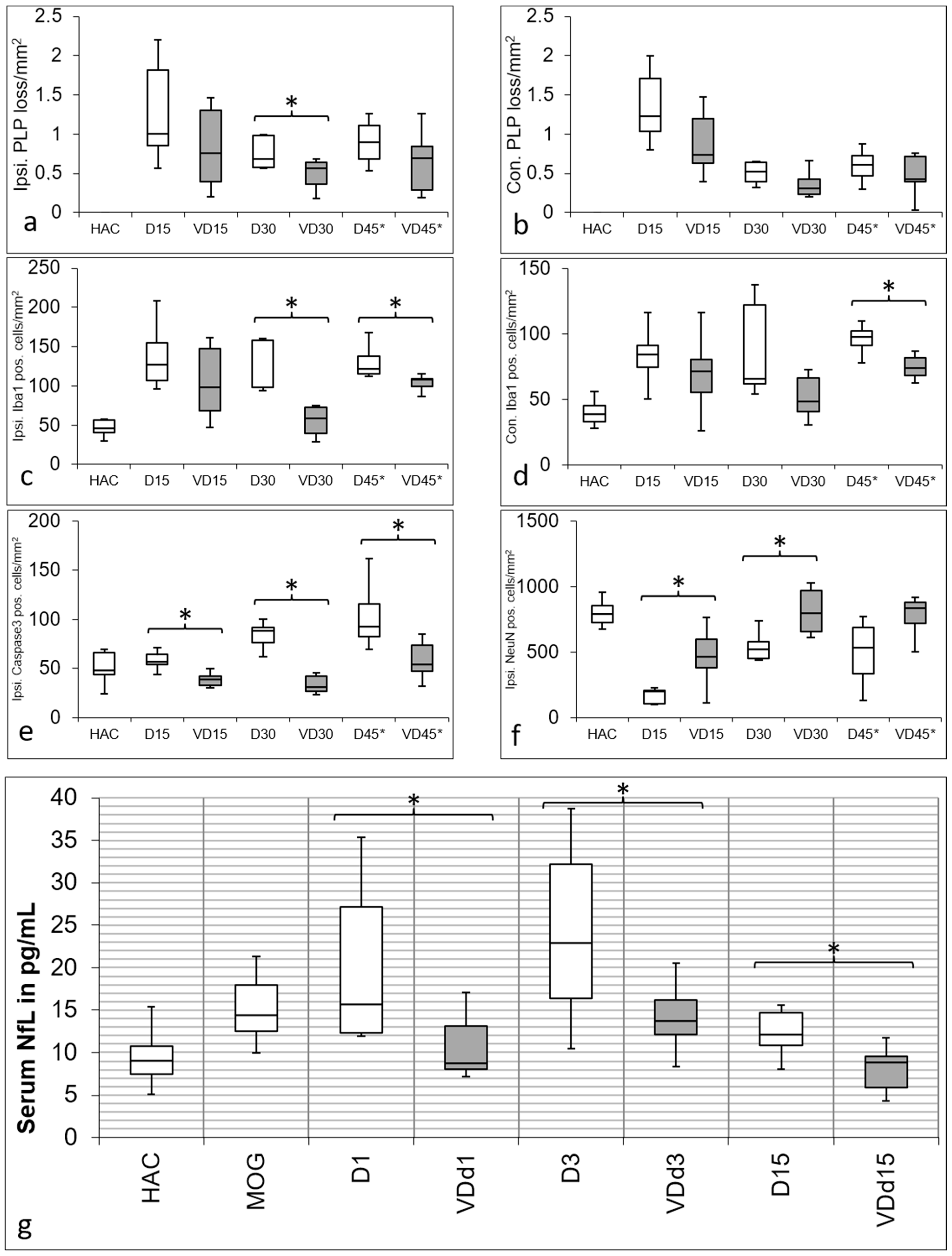
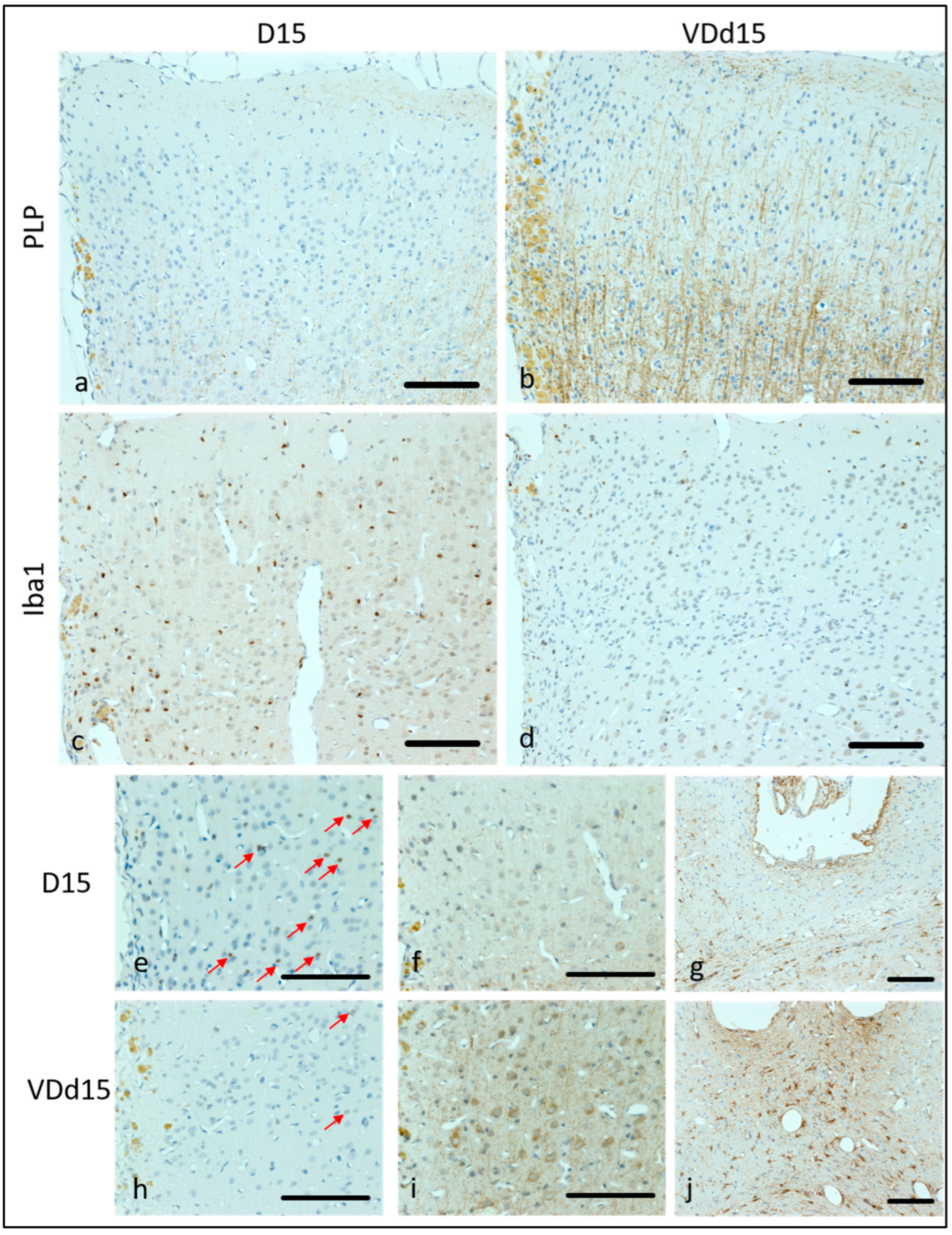
3.1. VD+ Animals Show a Better Preservation of Cortical Cellular Structures and Less Microglia Activation
3.2. NfL Serum Level Is Lower in VD+ Animals, Representing Less Axonal Loss
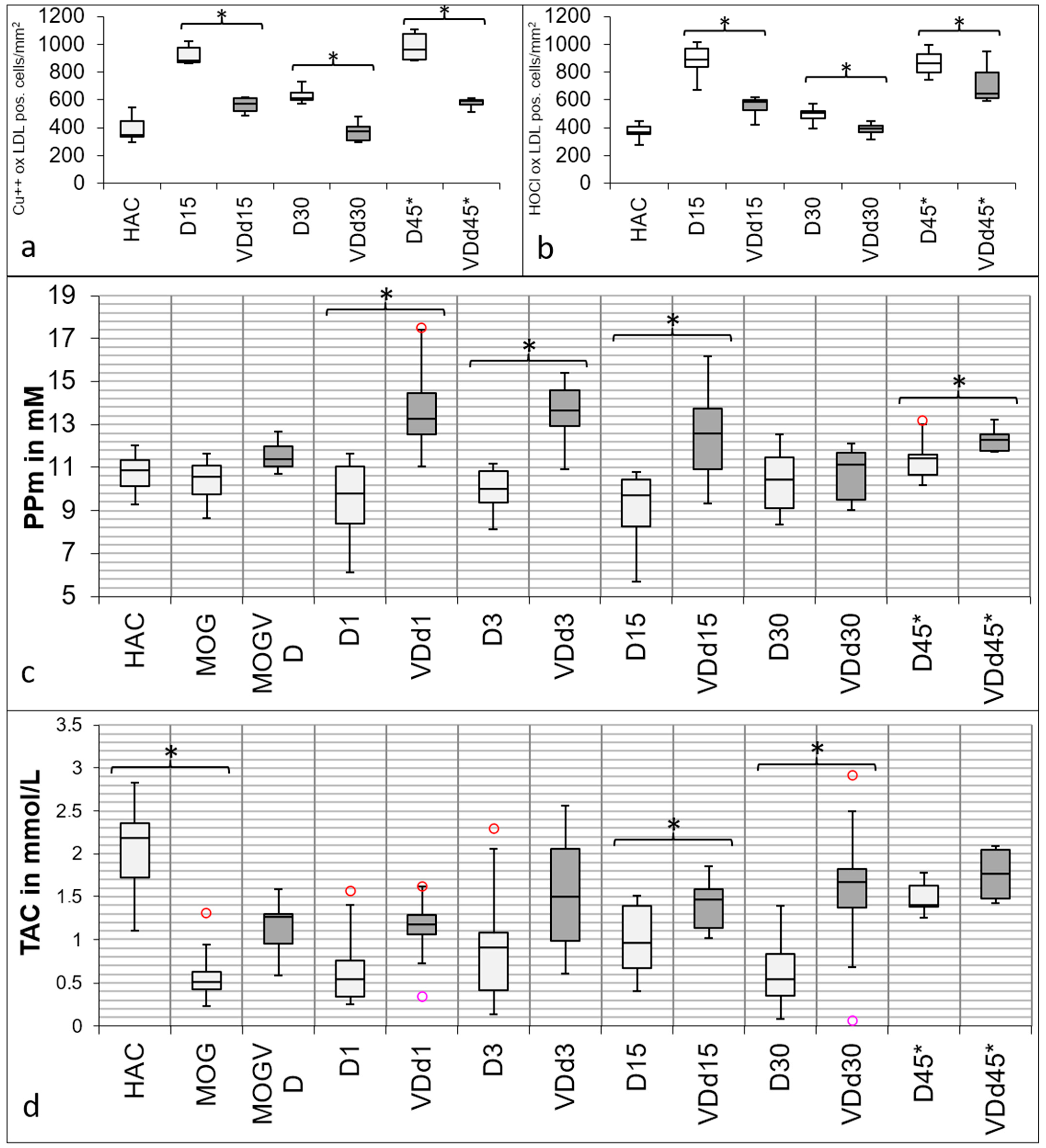
3.3. Histological Oxidative Stress Markers Are Lower in VD+ Animals
3.4. Total Antioxidant Capacity and Polyphenols Are Increased in the Sera of VD+ Animals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adzemovic, M.Z.; Zeitelhofer, M.; Hochmeister, S.; Gustafsson, S.A.; Jagodic, M. Efficacy of Vitamin D in Treating Multiple Sclerosis-like Neuroinflammation Depends on Developmental Stage. Exp. Neurol. 2013, 249, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeitelhofer, M.; Adzemovic, M.Z.; Gomez-Cabrero, D.; Bergman, P.; Hochmeister, S.; N’diaye, M.; Paulson, A.; Ruhrmann, S.; Almgren, M.; Tegnér, J.N.; et al. Functional Genomics Analysis of Vitamin D Effects on CD4+ T Cells in Vivo in Experimental Autoimmune Encephalomyelitis. Proc. Natl. Acad. Sci. USA 2017, 114, E1678–E1687. [Google Scholar] [CrossRef] [PubMed]
- Scazzone, C.; Agnello, L.; Bivona, G.; Lo Sasso, B.; Ciaccio, M. Vitamin D and Genetic Susceptibility to Multiple Sclerosis. Biochem. Genet. 2021, 59, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Ohl, K.; Tenbrock, K.; Kipp, M. Oxidative Stress in Multiple Sclerosis: Central and Peripheral Mode of Action. Exp. Neurol. 2016, 277, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Vitamin D Deficiency Accelerates Ageing and Age-Related Diseases: A Novel Hypothesis. J. Physiol. 2017, 595, 6825–6836. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, T.J.; Mowry, E.M. A Review of Vitamin D Supplementation as Disease-Modifying Therapy. Mult. Scler. 2018, 24, 6–11. [Google Scholar] [CrossRef]
- Pierrot-deseilligny, C.; Souberbielle, J.-C. Contribution of Vitamin D Insufficiency to the Pathogenesis of Multiple Sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 81–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, S.; Taylor, B.; Blizzard, L.; Ponsonby, A.; Pittas, F.; Tremlett, H.; Dwyer, T.; Gies, P.; van der Mei, I. Higher 25-Hydroxyvitamin D Is Associated with Lower Relapse Risk in Multiple Sclerosis. Ann. Neurol. 2010, 68, 193–203. [Google Scholar] [CrossRef]
- Feige, J.; Moser, T.; Bieler, L.; Schwenker, K.; Hauer, L.; Sellner, J. Vitamin D Supplementation in Multiple Sclerosis: A Critical Analysis of Potentials and Threats. Nutrients 2020, 12, 783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasnik, S.; Sharma, I.; Baylink, D.J.; Tang, X. Vitamin D as a Potential Therapy for Multiple Sclerosis: Where Are We? Int. J. Mol. Sci. 2020, 21, 3102. [Google Scholar] [CrossRef]
- Muris, A.H.; Rolf, L.; Broen, K.; Hupperts, R.; Damoiseaux, J.; Smolders, J. A Low Vitamin D Status at Diagnosis Is Associated with an Early Conversion to Secondary Progressive Multiple Sclerosis. J. Steroid Biochem. Mol. Biol. 2016, 164, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Abbatemarco, J.R.; Fox, R.J.; Li, H.; Bermel, R.A.; Ontaneda, D. Vitamin D Levels and Visual System Measurements in Progressive Multiple Sclerosis. Int. J. MS Care 2021, 23, 53–58. [Google Scholar] [CrossRef]
- Abbatemarco, J.R.; Fox, R.J.; Li, H.; Ontaneda, D. Vitamin D and MRI Measures in Progressive Multiple Sclerosis. Mult. Scler. Relat. Disord. 2019, 35, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Ücal, M.; Haindl, M.T.; Adzemovic, M.Z.; Strasser, J.; Theisl, L.; Zeitelhofer, M.; Kraitsy, K.; Ropele, S.; Schäfer, U.; Fazekas, F.; et al. Widespread Cortical Demyelination of Both Hemispheres Can Be Induced by Injection of Pro-Inflammatory Cytokines via an Implanted Catheter in the Cortex of MOG-Immunized Rats. Exp. Neurol. 2017, 294, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Hadžović-Džuvo, A.; Lepara, O.; Valjevac, A.; Avdagić, N.; Hasić, S.; Kiseljaković, E.; Ibragić, S.; Alajbegović, A. Serum Total Antioxidant Capacity in Patients with Multiple Sclerosis. Bosn. J. Basic Med. Sci. 2011, 11, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Marrie, R.A.; Cohen, J.; Stuve, O.; Trojano, M.; Sørensen, P.S.; Reingold, S.; Cutter, G.; Reider, N. A Systematic Review of the Incidence and Prevalence of Comorbidity in Multiple Sclerosis: Overview. Mult. Scler. J. 2015, 21, 263–281. [Google Scholar] [CrossRef]
- Varì, R.; Scazzocchio, B.; Santangelo, C.; Filesi, C.; Galvano, F.; D’Archivio, M.; Masella, R.; Giovannini, C. Protocatechuic Acid Prevents OxLDL-Induced Apoptosis by Activating JNK/Nrf2 Survival Signals in Macrophages. Oxid. Med. Cell. Longev. 2015, 2015, 351827. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as Biomarkers in Neurological Disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Petzold, A.; Bennett, J.L.; Berven, F.S.; Brundin, L.; Comabella, M.; Franciotta, D.; Frederiksen, J.L.; Fleming, J.O.; Furlan, R.; et al. A Consensus Protocol for the Standardization of Cerebrospinal Fluid Collection and Biobanking. Neurology 2009, 73, 1914–1922. [Google Scholar] [CrossRef] [Green Version]
- Zelzer, S.; Tatzber, F.; Herrmann, M.; Wonisch, W.; Rinnerhofer, S.; Kundi, M.; Obermayer-Pietsch, B.; Niedrist, T.; Cvirn, G.; Wultsch, G.; et al. Work Intensity, Low-Grade Inflammation, and Oxidative Status: A Comparison between Office and Slaughterhouse Workers. Oxid. Med. Cell. Longev. 2018, 2018, 2737563. [Google Scholar] [CrossRef] [Green Version]
- Tatzber, F.; Griebenow, S.; Wonisch, W.; Winkler, R. Dual Method for the Determination of Peroxidase Activity and Total Peroxides-Iodide Leads to a Significant Increase of Peroxidase Activity in Human Sera. Anal. Biochem. 2003, 316, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Matías-Guíu, J.; Oreja-Guevara, C.; Matias-Guiu, J.A.; Gomez-Pinedo, U. Vitamin D and Remyelination in Multiple Sclerosis. Neurologia 2018, 33, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.; Dring, A.; Zetterberg, H.; Blennow, K.; Norgren, N.; Gilthorpe, J.; Bergenheim, T.; Svenningsson, A. Neurofilament Light in CSF and Serum Is a Sensitive Marker for Axonal White Matter Injury in MS. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e271. [Google Scholar] [CrossRef] [Green Version]
- Lassmann, H.; van Horssen, J.; Mahad, D. Progressive Multiple Sclerosis: Pathology and Pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Galoppin, M.; Kari, S.; Soldati, S.; Pal, A.; Rival, M.; Engelhardt, B.; Astier, A.; Thouvenot, E. Full spectrum of vitamin D immunomodulation in multiple sclerosis: Mechanisms and therapeutic implications. Brain Commun. 2022, 4, fcac171. [Google Scholar] [CrossRef]
- Sadeghian, N.; Shadman, J.; Moradi, A.; Ghasem Golmohammadi, M.; Panahpour, H. Calcitriol protects the Blood-Brain Barrier integrity against ischemic stroke and reduces vasogenic brain edema via antioxidant and antiapoptotic actions in rats. Brain Res. Bull. 2019, 150, 281–289. [Google Scholar] [CrossRef]
- Uberti, F.; Lattuada, D.; Morsanuto, V.; Nava, U.; Bolis, G.; Vacca, G.; Squarzanti, D.F.; Cisari, C.; Molinari, C. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J Clin Endocrinol Metab. 2014, 99, 1367–1374. [Google Scholar] [CrossRef] [Green Version]
- Engelhardt, B.; Vajkoczy, P.; Weller, R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017, 18, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Anderson, P.; Durbeej, M.; van Rooijen, N.; Ivars, F.; Opdenakker, G.; Sorokin, L.M. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. 2006, 203, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.K. Immune response by microglia in the spinal cord. Ann. N. Y. Acad. Sci. 2010, 1198, 271–278. [Google Scholar] [CrossRef] [PubMed]
| . | Tissue | Serum | ||
|---|---|---|---|---|
| Used for IHC | Used for TAC | Used for PP | Used for NfL SIMOA | |
| HAC | 4 | 8 | 16 | 16 |
| MOG | No sacrifice | 11 | 20 | 10 |
| VDMOG | No sacrifice | 7 | 20 | n.m. |
| d1 | No sacrifice | 8 | 19 | 8 |
| VDd1 | No sacrifice | 5 | 15 | 8 |
| d3 | No sacrifice | 7 | 12 | 11 |
| VDd3 | No sacrifice | 11 | 16 | 7 |
| d15 | 7 | 10 | 14 | 9 |
| VDd15 | 8 | 9 | 20 | 9 |
| d30 | 5 | 10 | 14 | n.m. |
| VDd30 | 7 | 6 | 12 | n.m. |
| d45* | 7 | 5 | 7 | n.m. |
| VDd45* | 7 | 4 | 7 | n.m. |
| TOTAL | 45 | 101 | 192 | 78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haindl, M.T.; Üçal, M.; Wonisch, W.; Lang, M.; Nowakowska, M.; Adzemovic, M.Z.; Khalil, M.; Enzinger, C.; Hochmeister, S. Vitamin D—An Effective Antioxidant in an Animal Model of Progressive Multiple Sclerosis. Nutrients 2023, 15, 3309. https://doi.org/10.3390/nu15153309
Haindl MT, Üçal M, Wonisch W, Lang M, Nowakowska M, Adzemovic MZ, Khalil M, Enzinger C, Hochmeister S. Vitamin D—An Effective Antioxidant in an Animal Model of Progressive Multiple Sclerosis. Nutrients. 2023; 15(15):3309. https://doi.org/10.3390/nu15153309
Chicago/Turabian StyleHaindl, Michaela Tanja, Muammer Üçal, Willibald Wonisch, Michaela Lang, Marta Nowakowska, Milena Z. Adzemovic, Michael Khalil, Christian Enzinger, and Sonja Hochmeister. 2023. "Vitamin D—An Effective Antioxidant in an Animal Model of Progressive Multiple Sclerosis" Nutrients 15, no. 15: 3309. https://doi.org/10.3390/nu15153309







