Research Progress on the Anti-Aging Potential of the Active Components of Ginseng
Abstract
:1. Introduction
2. Aging Process
3. Anti-Aging Properties of the Active Components of Ginseng
4. Anti-Aging Mechanism of Ginseng
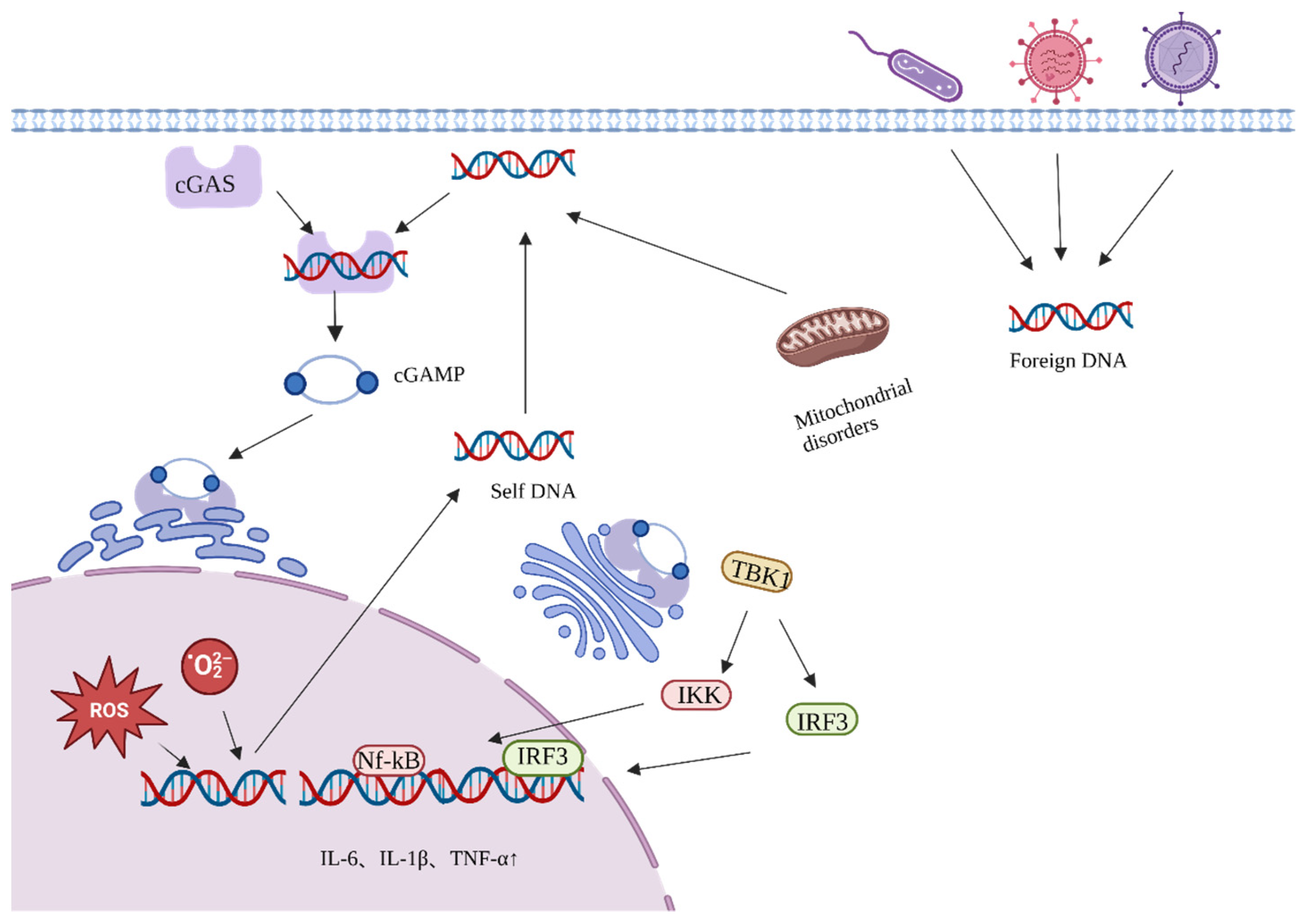
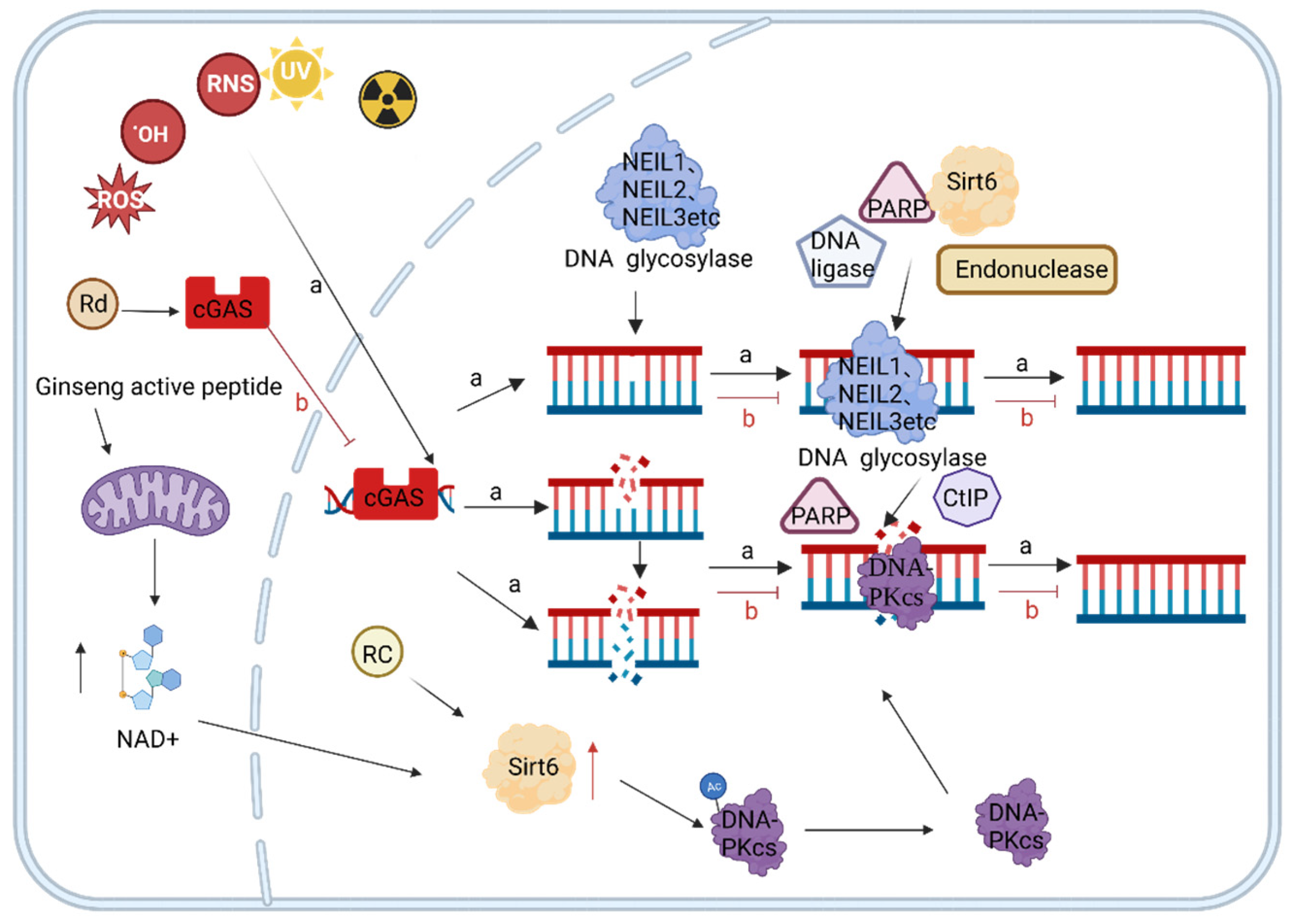
4.1. Active Ingredients of Ginseng Delay Aging by Reducing Endogenous Oxidative DNA Damage
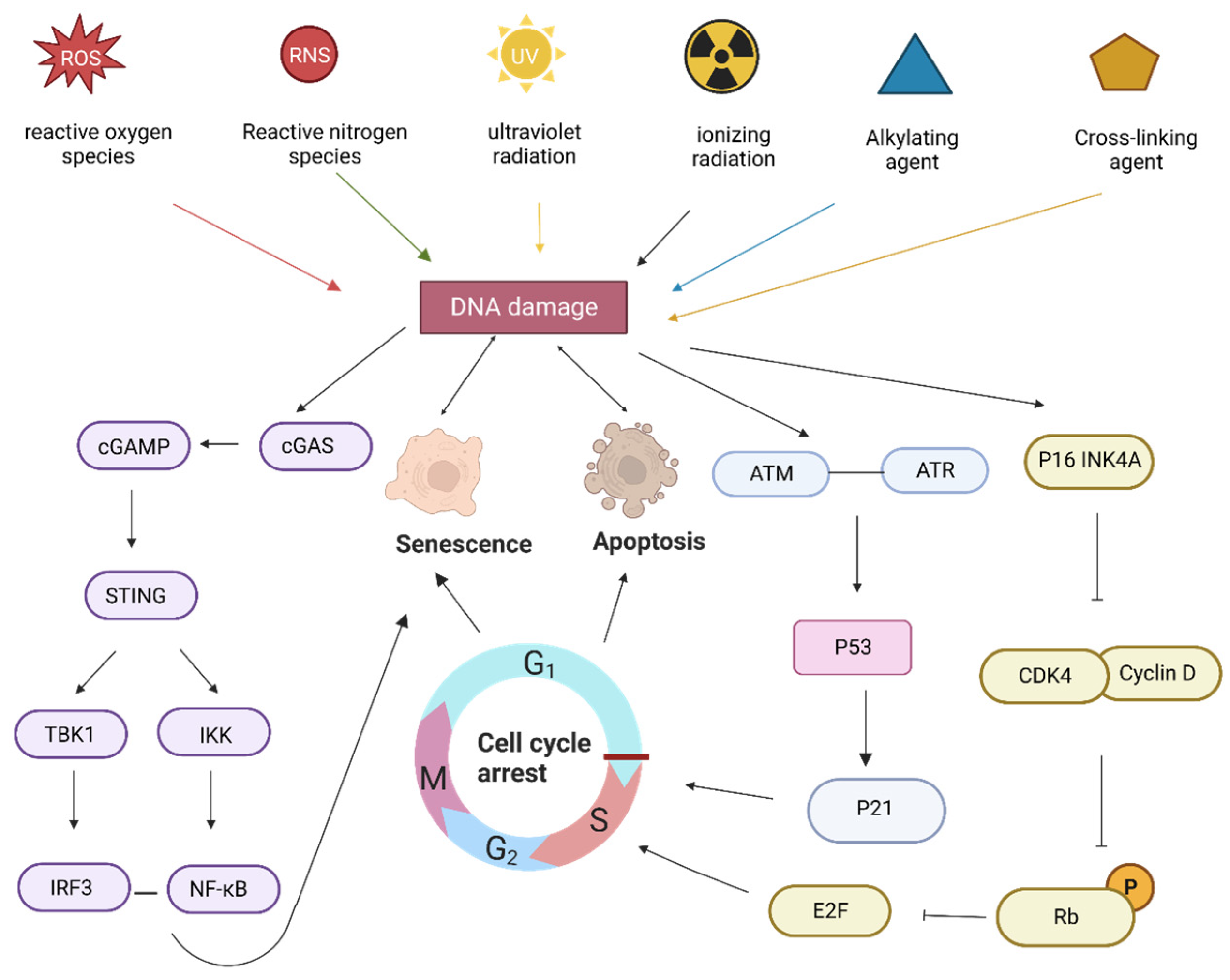
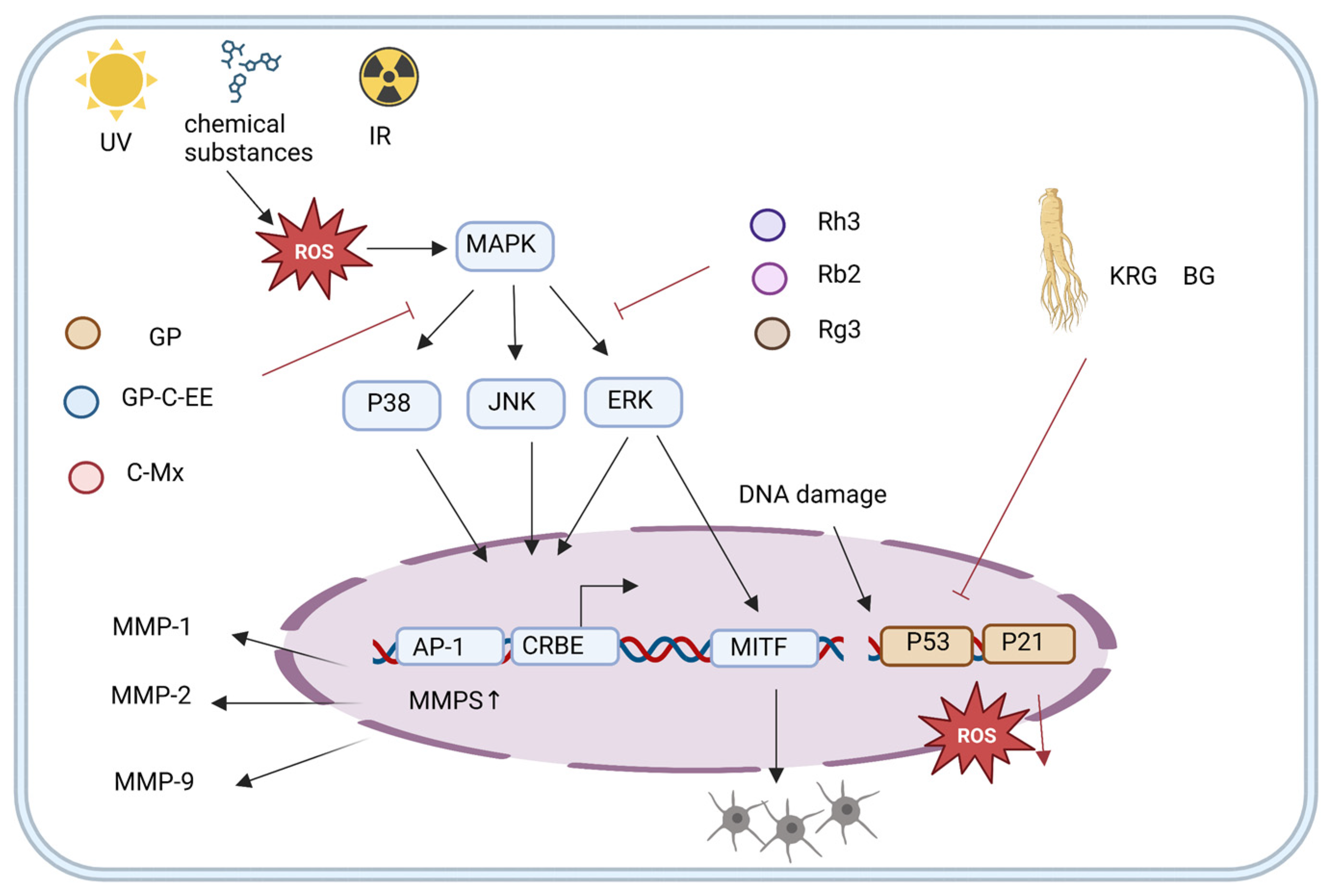
4.2. Ginseng Active Ingredients Delay Aging by Reducing Exogenous Oxidative DNA Damage
4.3. Active Ingredients of Ginseng Slow Down Aging by Regulating DNA Damage Repair
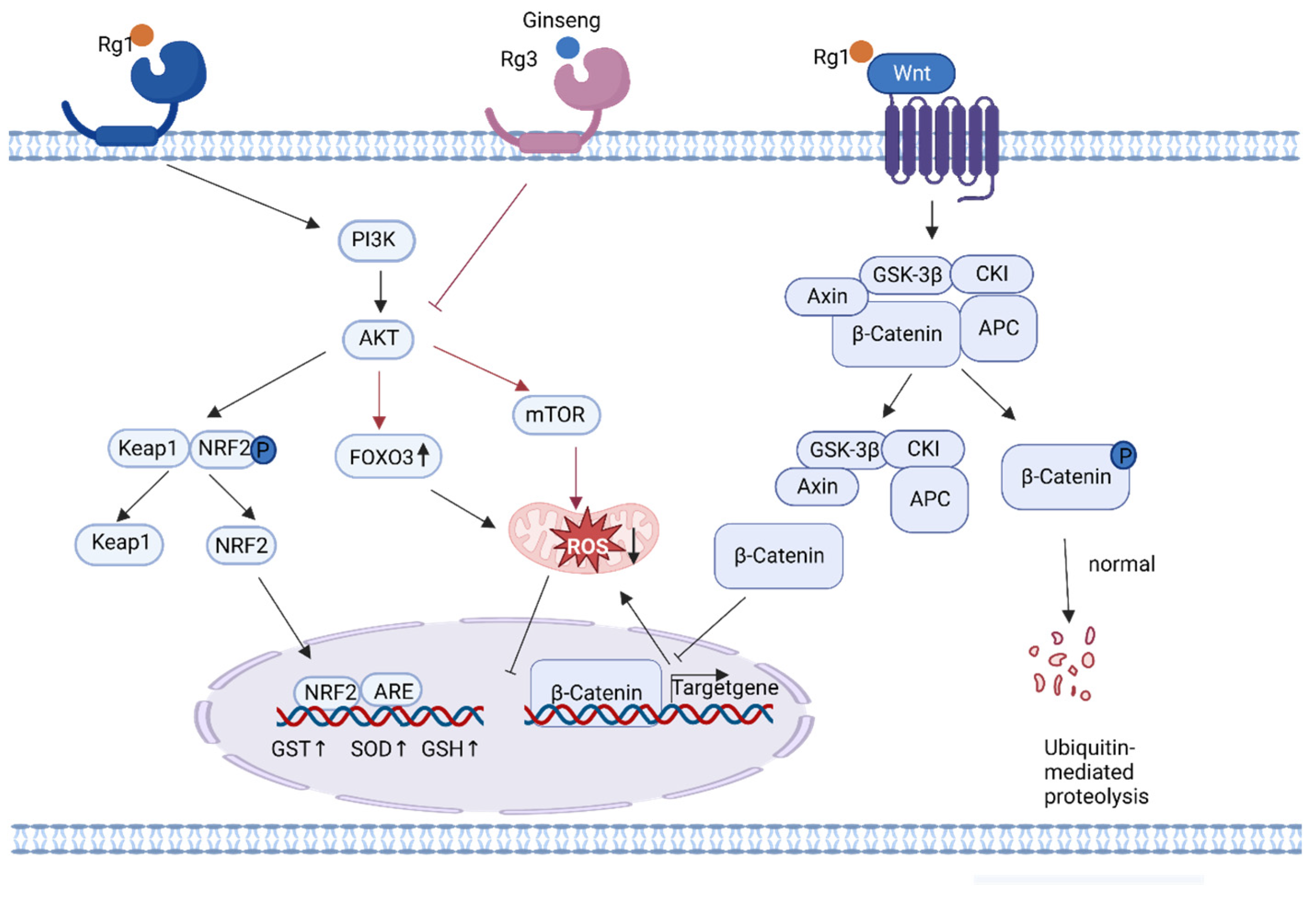
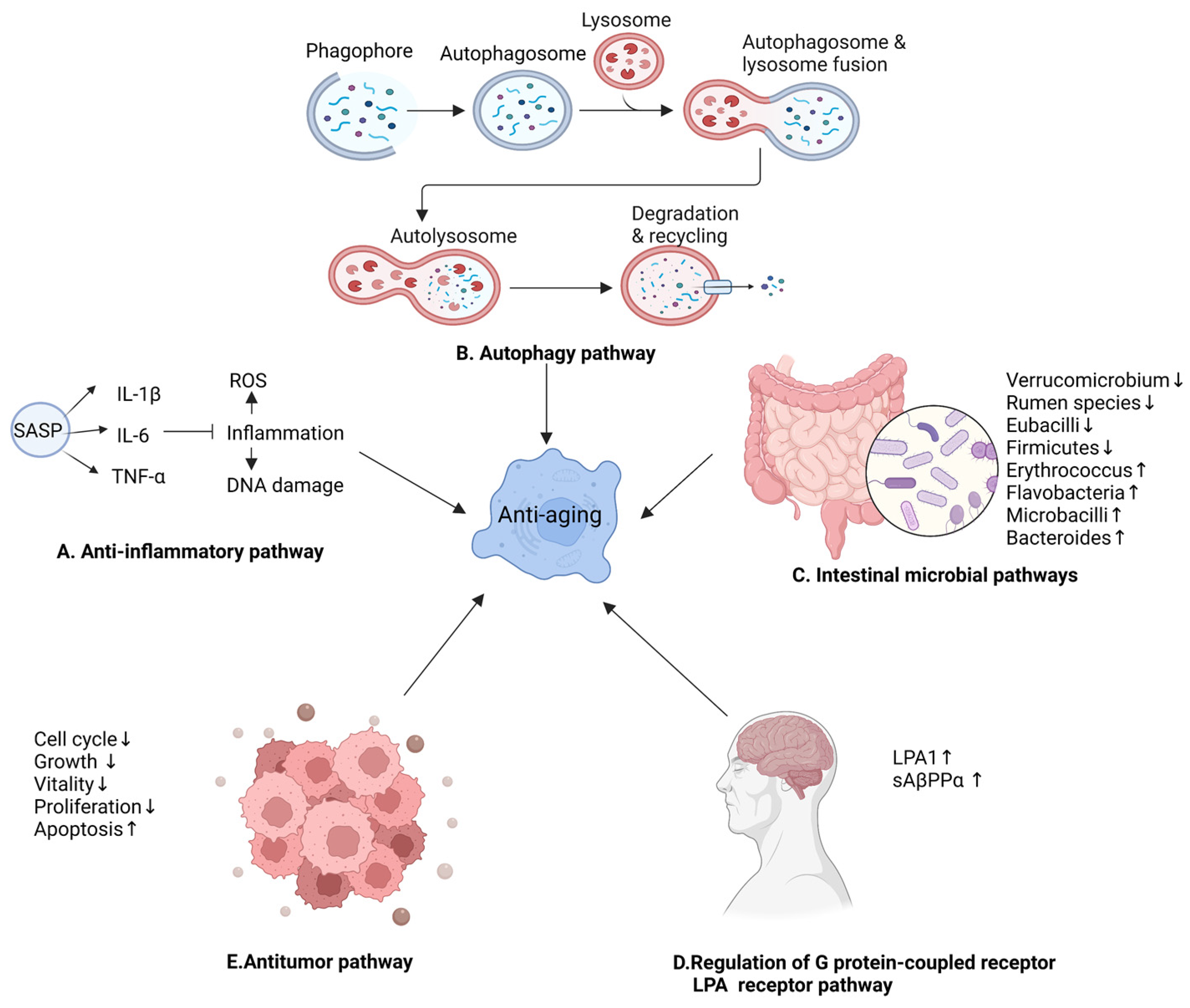
4.4. Other Anti-Aging Mechanisms of Ginseng
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto-Imoto, H.; Minami, S.; Shioda, T.; Yamashita, Y.; Sakai, S.; Maeda, S.; Yamamoto, T.; Oki, S.; Takashima, M.; Yamamuro, T.; et al. Age-associated decline of MondoA drives cellular senescence through impaired autophagy and mitochondrial homeostasis. Cell Rep. 2022, 38, 110444. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular senescence: Defining a path forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef]
- An, M.Y.; Lee, S.R.; Hwang, H.J.; Yoon, J.G.; Lee, H.J.; Cho, J.A. Antioxidant and anti-inflammatory effects of Korean Black ginseng extract through ER stress pathway. Antioxidants 2021, 10, 62. [Google Scholar] [CrossRef]
- Sun, G.; Wang, J.; Xu, X.; Zhai, L.; Li, Z.; Liu, J.; Zhao, D.; Jiang, R.; Sun, L. Panax ginseng Meyer cv. Silvatica phenolic acids protect DNA from oxidative damage by activating Nrf2 to protect HFF-1 cells from UVA-induced photoaging. J. Ethnopharmacol. 2023, 302, 115883. [Google Scholar] [CrossRef]
- Li, S.; Qi, Y.; Chen, L.; Qu, D.; Li, Z.; Gao, K.; Chen, J.; Sun, Y. Effects of Panax ginseng polysaccharides on the gut microbiota in mice with antibiotic-associated diarrhea. Int. J. Biol. Macromol. 2019, 124, 931–937. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y.; Zhang, S.; Zhao, Y.; Wang, C.; Li, K.; Jin, Z.; Qiao, J.; Liu, M. Studies on the regulation and molecular mechanism of Panax ginseng saponins on senescence and related behaviors of Drosophila melanogaster. Front. Aging Neurosci. 2022, 14, 870326. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Zhai, L.; Sun, L.; Zhao, D.; Wang, Z.; Li, X. Ginsenoside extract from ginseng extends lifespan and health span in Caenorhabditis elegans. Food Funct. 2021, 12, 6793–6808. [Google Scholar] [CrossRef]
- Yousefzadeh, M.; Henpita, C.; Vyas, R.; Soto-Palma, C.; Robbins, P.; Niedernhofer, L. DNA damage-how and why we age? eLife 2021, 10, e62852. [Google Scholar] [CrossRef]
- Ciccia, A.; Elledge, S.J. The DNA damage response: Making it safe to play with knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Sharpless, N.E. Senescence in health and disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef] [Green Version]
- Wiggins, K.A.; Parry, A.J.; Cassidy, L.D.; Humphry, M.; Webster, S.J.; Goodall, J.C.; Narita, M.; Clarke, M.C.H. IL-1α cleavage by inflammatory caspases of the noncanonical inflammasome controls the senescence-associated secretory phenotype. Aging Cell 2019, 18, e12946. [Google Scholar] [CrossRef] [Green Version]
- Pignolo, R.J.; Passos, J.F.; Khosla, S.; Tchkonia, T.; Kirkland, J.L. Reducing senescent cell burden in aging and disease. Trends Mol. Med. 2020, 26, 630–638. [Google Scholar] [CrossRef]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef]
- Paez-Ribes, M.; González-Gualda, E.; Doherty, G.J.; Muñoz-Espín, D. Targeting senescent cells in translational medicine. EMBO Mol. Med. 2019, 11, e10234. [Google Scholar] [CrossRef]
- Oost, W.; Talma, N.; Meilof, J.F.; Laman, J.D. Targeting senescence to delay progression of multiple sclerosis. J. Mol. Med. 2018, 96, 1153–1166. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Misra Sen, J.; Gorospe, M.; et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 2019, 22, 719–728. [Google Scholar] [CrossRef]
- Thompson, L.H. Recognition, signaling, and repair of DNA double-strand breaks produced by ionizing radiation in mammalian cells: The molecular choreography. Mutat. Res. 2012, 751, 158–246. [Google Scholar] [CrossRef]
- Nikitaki, Z.; Holá, M.; Donà, M.; Pavlopoulou, A.; Michalopoulos, I.; Angelis, K.J.; Georgakilas, A.G.; Macovei, A.; Balestrazzi, A. Integrating plant and animal biology for the search of novel DNA damage biomarkers. Mutat. Res. Rev. Mutat. Res. 2018, 775, 21–38. [Google Scholar] [CrossRef]
- Shang, D.; Sun, D.; Shi, C.; Xu, J.; Shen, M.; Hu, X.; Liu, H.; Tu, Z. Activation of epidermal growth factor receptor signaling mediates cellular senescence induced by certain pro-inflammatory cytokines. Aging Cell 2020, 19, e13145. [Google Scholar] [CrossRef] [Green Version]
- Sendama, W. The effect of ageing on the resolution of inflammation. Ageing Res. Rev. 2020, 57, 101000. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Wu, D.; Ning, X.; Yang, G.; Lin, Z.; Tian, M.; Zhou, Y. α-Amylase-assisted extraction of polysaccharides from Panax ginseng. Int. J. Biol. Macromol. 2015, 75, 152–157. [Google Scholar] [CrossRef]
- Zhao, B.; Lv, C.; Lu, J. Natural occurring polysaccharides from Panax ginseng C. A. Meyer: A review of isolation, structures, and bioactivities. Int. J. Biol. Macromol. 2019, 133, 324–336. [Google Scholar] [CrossRef]
- Sun, H.; Liu, F.; Sun, L.; Liu, J.; Wang, M.; Chen, X.; Xu, X.; Ma, R.; Feng, K.; Jiang, R. Proteomic analysis of amino acid metabolism differences between wild and cultivated Panax ginseng. J. Ginseng Res. 2016, 40, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Lu, X.; Pang, T.; Ma, C.; Li, X.; Xu, G. Determination of radix ginseng volatile oils at different ages by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. J. Sep. Sci. 2008, 31, 3451–3457. [Google Scholar] [CrossRef]
- Hyun, S.H.; Kim, S.W.; Seo, H.W.; Youn, S.H.; Kyung, J.S.; Lee, Y.Y.; In, G.; Park, C.K.; Han, C.K. Physiological and pharmacological features of the non-saponin components in Korean Red Ginseng. J. Ginseng Res. 2020, 44, 527–537. [Google Scholar] [CrossRef]
- Resetar, M.; Liu, X.; Herdlinger, S.; Kunert, O.; Pferschy-Wenzig, E.M.; Latkolik, S.; Steinacher, T.; Schuster, D.; Bauer, R.; Dirsch, V.M. Polyacetylenes from Oplopanax horridus and Panax ginseng: Relationship between Structure and PPARγ Activation. J. Nat. Prod. 2020, 83, 918–926. [Google Scholar] [CrossRef] [Green Version]
- Jang, G.Y.; Kim, M.Y.; Lee, Y.J.; Li, M.; Shin, Y.S.; Lee, J.; Jeong, H.S. Influence of organic acids and heat treatment on ginsenoside conversion. J. Ginseng Res. 2018, 42, 532–539. [Google Scholar] [CrossRef]
- Yoon, D.; Shin, W.C.; Oh, S.M.; Choi, B.R.; Young Lee, D. Integration of multiplatform metabolomics and multivariate analysis for geographical origin discrimination of Panax ginseng. Food Res. Int. 2022, 159, 111610. [Google Scholar] [CrossRef]
- Liu, X.; Mi, X.; Wang, Z.; Zhang, M.; Hou, J.; Jiang, S.; Wang, Y.; Chen, C.; Li, W. Ginsenoside Rg3 promotes regression from hepatic fibrosis through reducing inflammation-mediated autophagy signaling pathway. Cell Death Dis. 2020, 11, 454. [Google Scholar] [CrossRef]
- Xiong, X.; Huang, G.; Huang, H. The antioxidant activities of phosphorylated polysaccharide from native ginseng. Int. J. Biol. Macromol. 2019, 126, 842–845. [Google Scholar] [CrossRef]
- Chu, S.F.; Zhang, Z.; Zhou, X.; He, W.B.; Chen, C.; Luo, P.; Liu, D.D.; Ai, Q.D.; Gong, H.F.; Wang, Z.Z.; et al. Ginsenoside Rg1 protects against ischemic/reperfusion-induced neuronal injury through miR-144/Nrf2/ARE pathway. Acta Pharmacol. Sin. 2019, 40, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Long, J.; Liu, X.K.; Kang, Z.P.; Wang, M.X.; Zhao, H.M.; Huang, J.Q.; Xiao, Q.P.; Liu, D.Y.; Zhong, Y.B. Ginsenoside Rg1 ameliorated experimental colitis by regulating the balance of M1/M2 macrophage polarization and the homeostasis of intestinal flora. Eur. J. Pharmacol. 2022, 917, 174742. [Google Scholar] [CrossRef]
- Szeto, Y.T.; Sin, Y.S.; Pak, S.C.; Kalle, W. American ginseng tea protects cellular DNA within 2 h from consumption: Results of a pilot study in healthy human volunteers. Int. J. Food Sci. Nutr. 2015, 66, 815–818. [Google Scholar] [CrossRef]
- Wang, L.; Qiao, P.; Ouyang, Z.; Li, D.; Zheng, J.; Wang, G.; Wang, F. Ginseng volatile oil prolongs the lifespan and healthspan of Caenorhabditis elegans. Biogerontology 2022, 23, 485–497. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Jiang, R.; Li, C.; Chen, X.; Xiao, H.; Hou, J.; Hu, L.; Huang, C.; Wang, Y. Ginsenoside Rg1 prevents bone marrow mesenchymal stem cell senescence via NRF2 and PI3K/Akt signaling. Free Radic. Biol. Med. 2021, 174, 182–194. [Google Scholar] [CrossRef]
- Wang, Z.L.; Chen, L.B.; Qiu, Z.; Chen, X.B.; Liu, Y.; Li, J.; Wang, L.; Wang, Y.P. Ginsenoside Rg1 ameliorates testicular senescence changes in D-gal-induced aging mice via anti-inflammatory and antioxidative mechanisms. Mol. Med. Rep. 2018, 17, 6269–6276. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Yao, H.; Chen, X.; Wang, Z.; Xiang, Y.; Xia, J.; Liu, Y.; Wang, Y. Ginsenoside Rg1 decreases oxidative stress and down-regulates Akt/mTOR signalling to attenuate cognitive impairment in mice and senescence of neural stem cells induced by D-galactose. Neurochem. Res. 2018, 43, 430–440. [Google Scholar] [CrossRef]
- Li, J.; Cai, D.; Yao, X.; Zhang, Y.; Chen, L.; Jing, P.; Wang, L.; Wang, Y. Protective effect of ginsenoside Rg1 on hematopoietic stem/progenitor cells through attenuating oxidative stress and the Wnt/β-catenin signaling pathway in a mouse model of d-galactose-induced aging. Int. J. Mol. Sci. 2016, 17, 849. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhang, L.; Zhang, J.; Ran, R.; Shao, Y.; Li, J.; Jia, D.; Zhang, Y.; Zhang, M.; Wang, L.; et al. Protective effects of ginsenoside Rg1 on splenocytes and thymocytes in an aging rat model induced by d-galactose. Int. Immunopharmacol. 2018, 58, 94–102. [Google Scholar] [CrossRef]
- Yang, K.E.; Jang, H.J.; Hwang, I.H.; Hong, E.M.; Lee, M.G.; Lee, S.; Jang, I.S.; Choi, J.S. Stereoisomer-specific ginsenoside 20(S)-Rg3 reverses replicative senescence of human diploid fibroblasts via Akt-mTOR-sirtuin signaling. J. Ginseng Res. 2020, 44, 341–349. [Google Scholar] [CrossRef]
- Yu, S.; Xia, H.; Guo, Y.; Qian, X.; Zou, X.; Yang, H.; Yin, M.; Liu, H. Ginsenoside Rb1 retards aging process by regulating cell cycle, apoptotic pathway and metabolism of aging mice. J. Ethnopharmacol. 2020, 255, 112746. [Google Scholar] [CrossRef]
- Yang, K.E.; Nam, S.B.; Jang, M.; Park, J.; Lee, G.E.; Cho, Y.Y.; Jang, B.C.; Lee, C.J.; Choi, J.S. Ginsenoside Rb2 suppresses cellular senescence of human dermal fibroblasts by inducing autophagy. J. Ginseng Res. 2023, 47, 337–346. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Shin, E.J.; Jeong, J.H.; Sharma, N.; Nah, S.Y.; Ko, S.K.; Byun, J.K.; Lee, Y.; Lei, X.G.; Kim, D.J.; et al. Ginsenoside Re attenuates memory impairments in aged klotho deficient mice via interactive modulations of angiotensin II AT1 receptor, Nrf2 and GPx-1 gene. Free Radic. Biol. Med. 2022, 189, 2–19. [Google Scholar] [CrossRef]
- Kang, H.; Lim, J.W.; Kim, H. Inhibitory effect of Korean Red ginseng extract on DNA damage response and apoptosis in Helicobacter pylori-infected gastric epithelial cells. J. Ginseng Res. 2020, 44, 79–85. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, J.Y.; Bae, J.; Kim, Y.M.; Won, M.H.; Ha, K.S.; Kwon, Y.G.; Kim, Y.M. Korean Red ginseng prevents endothelial senescence by downregulating the HO-1/NF-κB/miRNA-155-5p/eNOS pathway. J. Ginseng Res. 2021, 45, 344–353. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Yu, Y.; Wang, Y.; Xue, D.; Zhou, Y.; Li, X. A ginseng-derived rhamnogalacturonan I (RG-I) pectin promotes longevity via TOR signalling in Caenorhabditis elegans. Carbohydr. Polym. 2023, 312, 120818. [Google Scholar] [CrossRef]
- Lee, Y.; Oh, S. Administration of red ginseng ameliorates memory decline in aged mice. J. Ginseng Res. 2015, 39, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Xu, M.H.; Li, Y. Bioactive oligopeptides from ginseng (Panax ginseng Meyer) Suppress Oxidative Stress-Induced Senescence in Fibroblasts via NAD+/SIRT1/PGC-1α Signaling Pathway. Nutrients 2022, 14, 5289. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, D.Y.; O’Connell, J.F.; Egan, J.M.; Kim, Y. Black ginseng ameliorates cellular senescence via p53-p21/p16 pathway in aged mice. Biology 2022, 11, 1108. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Pei, J.; Wang, Y.; Zhang, J.; Zheng, H.; Cui, R. Anti-ageing effects of red ginseng on female Drosophila melanogaster. J. Cell Mol. Med. 2020, 24, 3751–3755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, Y.H.; Jeong, S.Y.; Kim, Y.H.; Rodriguez, I.; Nuankaew, W.; Bhawal, U.K.; Hong, B.N.; Kang, T.H. Anti-aging effects of Korean Red Ginseng (KRG) in differentiated embryo chondrocyte (DEC) knockout mice. J. Ginseng Res. 2021, 45, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, A.; Nussenzweig, A. Endogenous DNA damage as a source of genomic instability in cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Chen, D.; Zhang, X.; Yang, R.; Yang, Y.; Chen, P.; Shen, Z. Oxidative stress and ginsenosides: An update on the molecular mechanisms. Oxid. Med. Cell Longev. 2022, 2022, 9299574. [Google Scholar] [CrossRef]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Oxidative DNA damage induced by ROS-modulating agents with the ability to target DNA: A comparison of the biological characteristics of citrus pectin and apple pectin. Sci. Rep. 2018, 8, 13902. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Carusillo, A.; Mussolino, C.D.N.A. DNA Damage: From threat to treatment. Cells 2020, 9, 1665. [Google Scholar] [CrossRef]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer’s disease. Mol. Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef]
- Dai, X.; Yan, X.; Wintergerst, K.A.; Cai, L.; Keller, B.B.; Tan, Y. Nrf2: Redox and metabolic regulator of stem cell state and function. Trends Mol. Med. 2020, 26, 185–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabouny, R.; Fraunberger, E.; Geoffrion, M.; Ng, A.C.; Baird, S.D.; Screaton, R.A.; Milne, R.; McBride, H.M.; Shutt, T.E. The Keap1-Nrf2 stress response pathway promotes mitochondrial hyperfusion through degradation of the mitochondrial fission protein Drp1. Antioxid. Redox Signal. 2017, 27, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q.; Jiang, B.; Feng, J.; Li, J.; Gu, Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia. Mol. Med. Rep. 2019, 19, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Morris, B.J.; Willcox, D.C.; Donlon, T.A.; Willcox, B.J. FOXO3: A major gene for human longevity—A mini-review. Gerontology 2015, 61, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sun, Z. Current understanding of klotho. Ageing Res. Rev. 2009, 8, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.W.; Shin, Y.J.; Luo, K.; Quan, Y.; Cui, S.; Ko, E.J.; Chung, B.H.; Yang, C.W. Ginseng increases klotho expression by FoxO3-mediated manganese superoxide dismutase in a mouse model of tacrolimus-induced renal injury. Aging. 2019, 11, 5548–5569. [Google Scholar] [CrossRef]
- Katoh, M.; Katoh, M. Wnt signaling pathway and stem cell signaling network. Clin. Cancer Res. 2007, 13, 4042–4045. [Google Scholar] [CrossRef] [Green Version]
- Hao, M.; Ding, C.; Peng, X.; Chen, H.; Dong, L.; Zhang, Y.; Chen, X.; Liu, W.; Luo, Y. Ginseng under forest exerts stronger anti-aging effects compared to garden ginseng probably via regulating PI3K/AKT/mTOR pathway, SIRT1/NF-κB pathway and intestinal flora. Phytomedicine 2022, 105, 154365. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, W.; Han, G.; Zhou, S.; Li, J.; Chen, M.; Li, H. Panax notoginseng saponins prevent senescence and inhibit apoptosis by regulating the PI3K-AKT-mTOR pathway in osteoarthritic chondrocytes. Int. J. Mol. Med. 2020, 45, 1225–1236. [Google Scholar] [CrossRef]
- Keijzers, G.; Bakula, D.; Scheibye-Knudsen, M. Monogenic diseases of DNA repair. N. Engl. J. Med. 2017, 377, 1868–1876. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, H.; Zhang, Y.; Zheng, Z.; Xiao, W.; Hong, X.; Chen, F.; Peng, X.; Pei, Y.; Rong, J.; et al. Ginsenoside-Rg1 attenuates sepsis-induced cardiac dysfunction by modulating mitochondrial damage via the P2X7 receptor-mediated Akt/GSK-3β signaling pathway. J. Biochem. Mol. Toxicol. 2022, 36, e22885. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, L.; Zhang, C.; Guo, Y.; Li, J.; Wu, C.; Jiao, J.; Zheng, H. Ginsenoside Rg1 improves Alzheimer’s disease by regulating oxidative stress, apoptosis, and neuroinflammation through Wnt/GSK-3β/β-catenin signaling pathway. Chem. Biol. Drug Des. 2022, 99, 884–896. [Google Scholar] [CrossRef]
- Cadet, J.; Wagner, J.R. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignard, J.; Mirey, G.; Salles, B. Ionizing-radiation induced DNA double-strand breaks: A direct and indirect lighting up. Radiother. Oncol. 2013, 108, 362–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida, L.G.N.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 712–768. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suto, M.; Masutomi, H.; Ishihara, K.; Masaki, H. A Potato Peel Extract Stimulates Type I Collagen Synthesis via Akt and ERK Signaling in Normal Human Dermal Fibroblasts. Biol. Pharm. Bull. 2019, 42, 1510–1516. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Y.; Xiao, Y.K.; Hwang, E.; Haeng, J.J.; Yi, T.H. Antiphotoaging and Antimelanogenesis Properties of Ginsenoside C-Y, a Ginsenoside Rb2 Metabolite from American Ginseng PDD-ginsenoside. Photochem. Photobiol. 2019, 95, 1412–1423. [Google Scholar] [CrossRef]
- Nam, J.J.; Min, J.E.; Son, M.H.; Oh, J.H.; Kang, S. Ultraviolet- and infrared-induced 11 beta-hydroxysteroid dehydrogenase type 1 activating skin photoaging is inhibited by red ginseng extract containing high concentration of ginsenoside Rg3(S). Photodermatol. Photoimmunol. Photomed. 2017, 33, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Park, S.Y.; Yin, C.S.; Kim, H.T.; Kim, Y.M.; Yi, T.H. Antiaging effects of the mixture of Panax ginseng and Crataegus pinnatifida in human dermal fibroblasts and healthy human skin. J. Ginseng Res. 2017, 41, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.; Xu, X.; Sun, Z.; Wang, F.; Ma, R.; Feng, K.; Li, T.; Sun, L. Protective Effects of Ginseng Proteins on Photoaging of Mouse Fibroblasts Induced by UVA. Photochem. 2020, 96, 113–123. [Google Scholar] [CrossRef]
- Lee, J.O.; Kim, E.; Kim, J.H.; Hong, Y.H.; Kim, H.G.; Jeong, D.; Kim, J.; Kim, S.H.; Park, C.; Seo, D.B.; et al. Antimelanogenesis and skin-protective activities of Panax ginseng calyx ethanol extract. J. Ginseng Res. 2018, 42, 389–399. [Google Scholar] [CrossRef]
- Liu, X.Y.; Hwang, E.; Park, B.; Ngo, H.T.T.; Xiao, Y.K.; Yi, T.H. Ginsenoside C-Mx Isolated from Notoginseng Stem-leaf Ginsenosides Attenuates Ultraviolet B-mediated Photoaging in Human Dermal Fibroblasts. Photochem. Photobiol. 2018, 94, 1040–1048. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef]
- Dilshat, R.; Fock, V.; Kenny, C.; Gerritsen, I.; Lasseur, R.M.J.; Travnickova, J.; Eichhoff, O.M.; Cerny, P.; Möller, K.; Sigurbjörnsdóttir, S.; et al. MITF reprograms the extracellular matrix and focal adhesion in melanoma. eLife 2021, 10, e63093. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, W.J.; Chang, S.E.; Lee, G.Y. Antimelanogenic effect of ginsenoside Rg3 through extracellular signal-regulated kinase-mediated inhibition of microphthalmia-associated transcription factor. J. Ginseng Res. 2015, 39, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.Y.; Jeong, Y.T.; Jeong, S.C.; Lee, M.K.; Min, J.W.; Lee, J.W.; Kim, G.S.; Lee, S.E.; Ahn, Y.S.; Kang, H.C.; et al. Melanin Biosynthesis Inhibition Effects of Ginsenoside Rb2 Isolated from Panax ginseng Berry. J. Microbiol. Biotechnol. 2015, 25, 2011–2015. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, J.I.; Myung, C.H.; Hwang, J.S. Inhibitory effects of ginsenosides on basic fibroblast growth factor-induced melanocyte proliferation. J. Ginseng Res. 2017, 41, 268–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aitken, G.R.; Henderson, J.R.; Chang, S.C.; McNeil, C.J.; Birch-Machin, M.A. Direct monitoring of UV-induced free radical generation in HaCaT keratinocytes. Clin. Exp. Dermatol. 2007, 32, 722–727. [Google Scholar] [CrossRef]
- Oh, S.J.; Oh, Y.; Ryu, I.W.; Kim, K.; Lim, C.J. Protective properties of ginsenoside Rb3 against UV-B radiation-induced oxidative stress in HaCaT keratinocytes. Biosci. Biotechnol. Biochem. 2016, 80, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Hernández Borrero, L.J.; El-Deiry, W.S. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim. Biophys. Acta-Rev. Cancer. 2021, 1876, 188556. [Google Scholar] [CrossRef]
- You, L.; Cho, J.Y. The regulatory role of Korean ginseng in skin cells. J. Ginseng Res. 2021, 45, 363–370. [Google Scholar] [CrossRef]
- McKinnon, P.J. Genome integrity and disease prevention in the nervous system. Genes. Dev. 2017, 31, 1180–1194. [Google Scholar] [CrossRef] [Green Version]
- Chow, H.M.; Herrup, K. Genomic integrity and the ageing brain. Nat. Rev. Neurosci. 2015, 16, 672–684. [Google Scholar] [CrossRef]
- Ticli, G.; Cazzalini, O.; Stivala, L.A.; Prosperi, E. Revisiting the function of p21CDKN1A in DNA repair: The influence of protein interactions and stability. Int. J. Mol. Sci. 2022, 23, 7058. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.X.; Zhang, X.; Zhao, G. Ginsenoside Rd attenuates DNA damage by increasing expression of DNA glycosylase endonuclease VIII-like proteins after focal cerebral ischemia. Chin. Med. J. 2016, 129, 1955–1962. [Google Scholar] [CrossRef]
- Pao, P.C.; Patnaik, D.; Watson, L.A.; Gao, F.; Pan, L.; Wang, J.; Adaikkan, C.; Penney, J.; Cam, H.P.; Huang, W.C.; et al. HDAC1 modulates OGG1-initiated oxidative DNA damage repair in the aging brain and Alzheimer’s disease. Nat. Commun. 2020, 11, 2484. [Google Scholar] [CrossRef]
- Jiang, H.; Xue, X.; Panda, S.; Kawale, A.; Hooy, R.M.; Liang, F.; Sohn, J.; Sung, P.; Gekara, N.O. Chromatin-bound cGAS is an inhibitor of DNA repair and hence accelerates genome destabilization and cell death. EMBO J. 2019, 38, e102718. [Google Scholar] [CrossRef] [PubMed]
- Sykora, P.; Misiak, M.; Wang, Y.; Ghosh, S.; Leandro, G.S.; Liu, D.; Tian, J.; Baptiste, B.A.; Cong, W.N.; Brenerman, B.M.; et al. DNA polymerase β deficiency leads to neurodegeneration and exacerbates Alzheimer disease phenotypes. Nucleic Acids Res. 2015, 43, 943–959. [Google Scholar] [CrossRef] [Green Version]
- Morigi, M.; Perico, L.; Benigni, A. Sirtuins in renal health and disease. J. Am. Soc. Nephrol. 2018, 29, 1799–1809. [Google Scholar] [CrossRef] [Green Version]
- Krokan, H.E.; Bjørås, M. Base excision repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012583. [Google Scholar] [CrossRef]
- Chakraborty, A.; Wakamiya, M.; Venkova-Canova, T.; Pandita, R.K.; Aguilera-Aguirre, L.; Sarker, A.H.; Singh, D.K.; Hosoki, K.; Wood, T.G.; Sharma, G.; et al. Neil2-null mice accumulate oxidized DNA bases in the transcriptionally active sequences of the genome and are susceptible to innate inflammation. J. Biol. Chem. 2015, 290, 24636–24648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Z.; Hine, C.; Tian, X.; Van Meter, M.; Au, M.; Vaidya, A.; Seluanov, A.; Gorbunova, V. SIRT6 promotes DNA repair under stress by activating PARP1. Science 2011, 332, 1443–1446. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.; Guo, J.; Tang, K.; Chen, Y.; Gong, X.; Chen, Y.; Zhong, Y.; Xiao, X.; Duan, S.; Cui, T.; et al. Ginsenoside Rc modulates SIRT6-NRF2 interaction to alleviate alcoholic liver disease. J. Agric. Food Chem. 2022, 70, 14220–14234. [Google Scholar] [CrossRef]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef]
- Reginato, G.; Cannavo, E.; Cejka, P. Physiological protein blocks direct the Mre11-Rad50-Xrs2 and Sae2 nuclease complex to initiate DNA end resection. Genes. Dev. 2017, 31, 2325–2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cagnetta, A.; Soncini, D.; Orecchioni, S.; Talarico, G.; Minetto, P.; Guolo, F.; Retali, V.; Colombo, N.; Carminati, E.; Clavio, M.; et al. Depletion of SIRT6 enzymatic activity increases acute myeloid leukemia cells’ vulnerability to DNA-damaging agents. Haematologica. 2018, 103, 80–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Liu, N.; Zhang, H.; Zhang, H.; Qiao, J.; Jia, W.; Zhu, S.; Mao, Z.; Kang, J. Sirt6 promotes DNA end joining in iPSCs derived from old mice. Cell Rep. 2017, 18, 2880–2892. [Google Scholar] [CrossRef] [Green Version]
- Ronowska, A.; Szutowicz, A.; Bielarczyk, H.; Gul-Hinc, S.; Klimaszewska-Łata, J.; Dyś, A.; Zyśk, M.; Jankowska-Kulawy, A. The regulatory effects of acetyl-CoA distribution in the healthy and diseased brain. Front. Cell Neurosci. 2018, 12, 169. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Stokasimov, E.; Cui, Y.; Pellman, D. Breakage of cytoplasmic chromosomes by pathological DNA base excision repair. Nature 2022, 606, 930–936. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Wu, X.; Ma, D.; Wu, J.; Wang, L.; Jiang, Y.; Fei, Y.; Zhu, C.; Tan, R.; et al. Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature 2018, 563, 131–136. [Google Scholar] [CrossRef]
- Aliper, A.M.; Bozdaganyan, M.E.; Orekhov, P.S.; Zhavoronkov, A.; Osipov, A.N. Replicative and radiation-induced aging: A comparison of gene expression profiles. Aging 2019, 11, 2378–2387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Tang, C.; Zhang, M.Y.; Huang, W.L.; Xu, Y.; Sun, H.Y.; Yang, F.; Song, L.L.; Wang, H.; Mu, L.L.; et al. Blocking ATM-dependent NF-κB pathway overcomes niche protection and improves chemotherapy response in acute lymphoblastic leukemia. Leukemia 2019, 33, 2365–2378. [Google Scholar] [CrossRef]
- Dunphy, G.; Flannery, S.M.; Almine, J.F.; Connolly, D.J.; Paulus, C.; Jønsson, K.L.; Jakobsen, M.R.; Nevels, M.M.; Bowie, A.G.; Unterholzner, L. Non-canonical activation of the DNA sensing adaptor STING by ATM and IFI16 mediates NF-κB signaling after nuclear DNA damage. Mol. Cell 2018, 71, 745–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Li, H.; Qiu, T.; Dai, J.; Zhang, Y.; Chen, J.; Cai, H. Loss of PTEN induces lung fibrosis via alveolar epithelial cell senescence depending on NF-κB activation. Aging Cell 2019, 18, e12858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uyar, B.; Palmer, D.; Kowald, A.; Murua Escobar, H.; Barrantes, I.; Möller, S.; Akalin, A.; Fuellen, G. Single-cell analyses of aging, inflammation and senescence. Ageing Res. Rev. 2020, 64, 101156. [Google Scholar] [CrossRef]
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Aging, inflammation and the environment. Exp. Gerontol. 2018, 105, 10–18. [Google Scholar] [CrossRef]
- Pezone, A.; Olivieri, F.; Napoli, M.V.; Procopio, A.; Avvedimento, E.V.; Gabrielli, A. Inflammation and DNA damage: Cause, effect or both. Nat. Rev. Rheumatol. 2023, 19, 200–211. [Google Scholar] [CrossRef]
- Yu, T.; Yang, Y.; Kwak, Y.S.; Song, G.G.; Kim, M.Y.; Rhee, M.H.; Cho, J.Y. Ginsenoside Rc from Panax ginseng exerts anti-inflammatory activity by targeting TANK-binding kinase 1/interferon regulatory factor-3 and p38/ATF-2. J. Ginseng Res. 2017, 41, 127–133. [Google Scholar] [CrossRef]
- Yu, T.; Rhee, M.H.; Lee, J.; Kim, S.H.; Yang, Y.; Kim, H.G.; Kim, Y.; Kim, C.; Kwak, Y.S.; Kim, J.H.; et al. Ginsenoside Rc from Korean Red ginseng (Panax ginseng C.A. Meyer) Attenuates inflammatory Symptoms of Gastritis, Hepatitis and Arthritis. Am. J. Chin. Med. 2016, 44, 595–615. [Google Scholar] [CrossRef]
- Kaushik, S.; Tasset, I.; Arias, E.; Pampliega, O.; Wong, E.; Martinez-Vicente, M.; Cuervo, A.M. Autophagy and the hallmarks of aging. Ageing Res. Rev. 2021, 72, 101468. [Google Scholar] [CrossRef]
- Kirkin, V. History of the selective autophagy research: How did it begin and where does it stand today? J. Mol. Biol. 2020, 432, 3–27. [Google Scholar] [CrossRef]
- Gatica, D.; Lahiri, V.; Klionsky, D.J. Cargo recognition and degradation by selective autophagy. Nat. Cell Biol. 2018, 20, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Eckhart, L.; Tschachler, E.; Gruber, F. Autophagic control of skin aging. Front. Cell Dev. Biol. 2019, 7, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Green, D.R. Autophagy-independent functions of the autophagy machinery. Cell 2019, 177, 1682–1699. [Google Scholar] [CrossRef]
- Li, W.; He, P.; Huang, Y.; Li, Y.F.; Lu, J.; Li, M.; Kurihara, H.; Luo, Z.; Meng, T.; Onishi, M.; et al. Selective autophagy of intracellular organelles: Recent research advances. Theranostics 2021, 11, 222–256. [Google Scholar] [CrossRef] [PubMed]
- Leidal, A.M.; Levine, B.; Debnath, J. Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 2018, 20, 1338–1348. [Google Scholar] [CrossRef]
- Kim, J.K.; Shin, K.K.; Kim, H.; Hong, Y.H.; Choi, W.; Kwak, Y.S.; Han, C.K.; Hyun, S.H.; Cho, J.Y. Korean Red Ginseng exerts anti-inflammatory and autophagy-promoting activities in aged mice. J. Ginseng Res. 2021, 45, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Chen, K.C.; Zhou, Y.; Wei, H.; Qi, M.H.; Wang, Z.; Zheng, Y.N.; Chen, R.X.; Liu, S.; Li, W. Evaluating the effects of mitochondrial autophagy flux on ginsenoside Rg2 for delaying D-galactose induced brain aging in mice. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 104, 154341. [Google Scholar] [CrossRef]
- Kim, J.; Cho, S.Y.; Kim, S.H.; Cho, D.; Kim, S.; Park, C.W.; Shimizu, T.; Cho, J.Y.; Seo, D.B.; Shin, S.S. Effects of Korean ginseng berry on skin antipigmentation and antiaging via FoxO3a activation. J. Ginseng Res. 2017, 41, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Kim, H.S.; Park, S.H.; Kim, D.; Hong, Y.D.; Kim, J.H.; Cho, J.Y. Syringaresinol derived from Panax ginseng berry attenuates oxidative stress-induced skin aging via autophagy. J. Ginseng Res. 2022, 46, 536–542. [Google Scholar] [CrossRef]
- Cho, Y.J.; Choi, S.H.; Lee, R.; Hwang, H.; Rhim, H.; Cho, I.H.; Kim, H.C.; Lee, J.I.; Hwang, S.H.; Nah, S.Y. Ginseng Gintonin Contains Ligands for GPR40 and GPR55. Molecules 2020, 25, 1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.H.; Kim, H.J.; Cho, H.J.; Park, S.D.; Lee, N.E.; Hwang, S.H.; Cho, I.H.; Hwang, H.; Rhim, H.; Kim, H.C.; et al. Gintonin, a Ginseng-Derived Exogenous Lysophosphatidic Acid Receptor Ligand, Protects Astrocytes from Hypoxic and Re-oxygenation Stresses Through Stimulation of Astrocytic Glycogenolysis. Mol. Neurobiol. 2019, 56, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.M.; Hwang, H.; Seo, M.; Chang, B.J.; Kim, H.J.; Choi, S.H.; Rhim, H.; Kim, H.C.; Cho, I.H.; Nah, S.Y. Gintonin Attenuates D-Galactose-Induced Hippocampal Senescence by Improving Long-Term Hippocampal Potentiation, Neurogenesis, and Cognitive Functions. Gerontology 2018, 64, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Shin, E.J.; Shin, T.J.; Lee, B.H.; Choi, S.H.; Kang, J.; Kim, H.J.; Kwon, S.H.; Jang, C.G.; Lee, J.H.; et al. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates Alzheimer’s disease-related neuropathies: Involvement of non-amyloidogenic processing. JAD 2012, 31, 207–223. [Google Scholar] [CrossRef]
- Gao, Q.; Zheng, J. Ginsenoside Rh2 inhibits prostate cancer cell growth through suppression of microRNA-4295 that activates CDKN1A. Cell Prolif. 2018, 51, e12438. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Hong, B.; Wu, S.; Niu, T. Inhibition of prostatic cancer growth by ginsenoside Rh2. Tumor. Biol. 2015, 36, 2377–2381. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, J.; Li, J.; Zheng, Y.; Zhong, X.; Huang, S.; Chen, B.; Peng, B.; Zou, X.; Chen, X. Pristimerin synergistically sensitizes conditionally reprogrammed patient derived-primary hepatocellular carcinoma cells to sorafenib through endoplasmic reticulum stress and ROS generation by modulating Akt/FoxO1/p27(kip1) signaling pathway. Phytomedicine 2021, 86, 153563. [Google Scholar] [CrossRef]
- Xiaodan, S.; Ying, C. Role of ginsenoside Rh2 in tumor therapy and tumor microenvironment immunomodulation. Biomed. Pharmacother. 2022, 156, 113912. [Google Scholar] [CrossRef]
- Lev-Ari, S.; Starr, A.N.; Vexler, A.; Kalich-Philosoph, L.; Yoo, H.S.; Kwon, K.R.; Yadgar, M.; Bondar, E.; Bar-Shai, A.; Volovitz, I.; et al. Rh2-enriched Korean ginseng (Ginseng Rh2+) inhibits tumor growth and development of metastasis of non-small cell lung cancer. Food Funct. 2021, 12, 8068–8077. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.S.; Zhang, E.T.; Li, G.A.; Liu, W.Y.; Li, Y.; Jin, Y.H. (20S) Ginsenoside Rh2 Exerts Its Anti-Tumor Effect by Disrupting the HSP90A-Cdc37 System in Human Liver Cancer Cells. Int. J. Mol. Sci. 2021, 22, 13170. [Google Scholar] [CrossRef]
- Huang, J.; Liu, D.; Wang, Y.; Liu, L.; Li, J.; Yuan, J.; Jiang, Z.; Jiang, Z.; Hsiao, W.W.; Liu, H.; et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 2022, 71, 734–745. [Google Scholar] [CrossRef]
- Zhai, F.G.; Liang, Q.C.; Wu, Y.Y.; Liu, J.Q.; Liu, J.W. Red ginseng polysaccharide exhibits anticancer activity through GPX4 downregulation-induced ferroptosis. Pharm. Biol. 2022, 60, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.S.; Hwang, S.H.; Yoon, T.J.; Kim, S.H.; Shin, K.S. Polysaccharides from ginseng leaves inhibit tumor metastasis via macrophage and NK cell activation. Int. J. Biol. Macromol. 2017, 103, 1327–1333. [Google Scholar] [CrossRef]
- Lee, D.Y.; Park, C.W.; Lee, S.J.; Park, H.R.; Seo, D.B.; Park, J.Y.; Park, J.; Shin, K.S. Immunostimulating and Antimetastatic Effects of Polysaccharides Purified from Ginseng Berry. Am. J. Chin. Med. 2019, 47, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, Q.; Zhao, W.; Gong, Y.; Su, A.; Liu, F.; Liu, Y.; Li, Z.; Zhu, J. Ginsenoside Rg3 Inhibition of Thyroid Cancer Metastasis Is Associated with Alternation of Actin Skeleton. J. Med. Food. 2018, 21, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Ji, R.; Dong, X.; Xu, X.; Xin, Y.; Jiang, X. Antitumor activity of ginsenoside Rg3 in melanoma through downregulation of the ERK and Akt pathways. Int. J. Oncol. 2019, 54, 2069–2079. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Jin, Y.; Feng, T.; Wang, H.; Liu, D.; Zhou, Z.; Yan, Q.; Yang, H.; Yang, J.; Yang, J.; et al. Ginsenoside Rg3 Inhibits the Growth of Osteosarcoma and Attenuates Metastasis through the Wnt/β-Catenin and EMT Signaling Pathway. eCAM 2020, 2020, 6065124. [Google Scholar] [CrossRef] [PubMed]
- Carmody, R.N.; Gerber, G.K.; Luevano, J.M., Jr.; Gatti, D.M.; Somes, L.; Svenson, K.L.; Turnbaugh, P.J. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 2015, 17, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Conway, J.; Duggal, A.N. Ageing of the gut microbiome: Potential influences on immune senescence and inflammageing. Ageing Res. Rev. 2021, 68, 101323. [Google Scholar] [CrossRef]
- Jeon, H.; Bae, C.H.; Lee, Y.; Kim, H.Y.; Kim, S. Korean red ginseng suppresses 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced inflammation in the substantia nigra and colon. Brain Behav. Immun. 2021, 94, 410–423. [Google Scholar] [CrossRef]
- Xu, H.Y.; Li, Q.C.; Zhou, W.J.; Zhang, H.B.; Chen, Z.X.; Peng, N.; Gong, S.Y.; Liu, B.; Zeng, F. Anti-oxidative and anti-aging effects of probiotic fermented ginseng by modulating gut microbiota and metabolites in Caenorhabditis elegans. Plant Foods Hum. Nutr. 2023, 78, 320–328. [Google Scholar] [CrossRef]
- Bai, X.; Fu, R.; Duan, Z.; Liu, Y.; Zhu, C.; Fan, D. Ginsenoside Rh4 alleviates antibiotic-induced intestinal inflammation by regulating the TLR4-MyD88-MAPK pathway and gut microbiota composition. Food Funct. 2021, 12, 2874–2885. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, Y.; Zheng, H.; Li, S.; Li, Z.; Yuan, L.; Cheng, X.; He, C.; Sun, J. Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: Pharmacology and mechanisms. Biomed. Pharmacother. 2020, 132, 110915. [Google Scholar] [CrossRef]
- Zheng, Q.; Bao, X.Y.; Zhu, P.C.; Tong, Q.; Zheng, G.Q.; Wang, Y. Ginsenoside Rb1 for myocardial ischemia/reperfusion injury: Preclinical evidence and possible mechanisms. Oxid. Med. Cell Longev. 2017, 2017, 6313625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Zeng, G.; Hong, L.; Ye, Q.; Chen, X.; Zhang, J. Ginsenoside Rg1 and astaxanthin act on the hypothalamus to protect female mice against reproductive aging. Chin. Med. J. 2022, 135, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, C.; Li, S.; Jiang, C. Ginsenoside Rf relieves mechanical hypersensitivity, depression-like behavior, and inflammatory reactions in chronic constriction injury rats. Phytother. Res. 2019, 33, 1095–1103. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Chu, S.; Zhang, Z.; Chen, N.; Li, L.; Zhang, L. Ginsenoside Rg1 protects mice against streptozotocin-induced type 1 diabetic by modulating the NLRP3 and Keap1/Nrf2/HO-1 pathways. Eur. J. Pharmacol. 2020, 866, 172801. [Google Scholar] [CrossRef]
- Zhou, T.; Zu, G.; Zhang, X.; Wang, X.; Li, S.; Gong, X.; Liang, Z.; Zhao, J. Neuroprotective effects of ginsenoside Rg1 through the Wnt/β-catenin signaling pathway in both in vivo and in vitro models of Parkinson’s disease. Neuropharmacology 2016, 101, 480–489. [Google Scholar] [CrossRef]
- Xie, L.; Zhai, R.; Chen, T.; Gao, C.; Xue, R.; Wang, N.; Wang, J.; Xu, Y.; Gui, D. Panax Notoginseng Ameliorates podocyte EMT by targeting the Wnt/β-catenin signaling pathway in STZ-induced diabetic rats. Drug Des. Dev. Ther. 2020, 14, 527–538. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.; Jeon, H.; Kim, H.Y.; Koo, S.; Kim, S. Korean red ginseng promotes hippocampal neurogenesis in mice. Neural Regen. Res. 2020, 15, 887–893. [Google Scholar] [CrossRef]
- Cho, D.E.; Choi, G.M.; Lee, Y.S.; Hong, J.P.; Yeom, M.; Lee, B.; Hahm, D.H. Long-term administration of red ginseng non-saponin fraction rescues the loss of skeletal muscle mass and strength associated with aging in mice. J. Ginseng Res. 2022, 46, 657–665. [Google Scholar] [CrossRef] [PubMed]
| Active Ingredient | Biological Effects | In Vivo Model | In Vitro Model | Testing Index | Source |
|---|---|---|---|---|---|
| Rg1 | Mitigation of DNA damage and antioxidant and anti-aging effects | NRF2−/−, C57BL/6 mice intraperitoneally injected with D-galactose (D-gal) for 42 days | D-gal induced primary bone marrow mesenchymal stem cells treated for 24 h | β-Galactosidase, γ-H2AX, p16, p53, p21, IL-6, IL-1β | [40] |
| Antioxidant, anti-apoptotic, free radical-scavenging, and anti-inflammatory effects | C57BL/6 intraperitoneally injected with D-gal for 42 days | β-Galactosidase, MDA, SOD, IL-1β, IL-6, TNF-α, p53, p21 | [41] | ||
| Antioxidant effect and mitigation of oxidative stress | C57BL/6 intraperitoneally injected with D-gal for 42 days | D-gal stimulation of primary neural stem cells | MDA, SOD, GSH-px, p53, p21, Rb | [42] | |
| Inhibition of excessive activation of the Wnt/β-linked protein signaling pathway | C57BL/6 mice injected with D-gal for 42 days | ROS, SOD, GSH-px, MDA, c-Myc, GSK-3β, p53, p16, p21 | [43] | ||
| Antioxidant and downregulation of aging-related proteins | Sprague Dawley rats injected with D-gal for 42 days | IL-2, IL-6, TNF-α, GSH, SOD, MDA | [44] | ||
| Rg3 | Downregulation of AKT and regulation of NAD/NADH | Human dermal fibroblasts undergo continuous passaging up to 34–36 generations, allowing them to become senescent cells | SA-β-gal, ROS, sirt1/3/6, NAD/NADH, p21, p53 | [45] | |
| Rb1 | Regulation of the p53-p21-Cdk2 pathway, cell cycle regulation, and anti-apoptotic effect | C57BL/6 mice fed for 10 months | p53, p21, Cdk2, bax, NF-κB | [46] | |
| Rb2 | Induction of autophagy | Human dermal fibroblasts undergo passaging until they become senescent cells in 34 to 36 generations | SA-β-gal, p53, p21, p16, CDK4, p62 | [47] | |
| Re | Upregulation of Nrf2/GPx-1/ERK/CERB signaling | Klotho mutant mice | NOX, ROS, GPx, Nrf2, ERK, CERB | [48] |
| Active Ingredient | Biological Effects | In Vivo Model | In Vitro Model | Testing Index | Source |
|---|---|---|---|---|---|
| Ginsenosides | Anti-apoptotic and antioxidant effect and inhibition of oxidative DNA damage | Helicobacter pylori stimulated AGS human gastric epithelial cells (bacteria:cells = 3:1) for 1 h | ROS, Bax/Bcl-2, caspase-3, ATM, Mdm2, ARF | [49] | |
| Total Ginsenoside Aqueous Extract | Inhibition of oxidative stress | Caenorhabditis elegans and worms | ROS, NAD+, SIRT1, NRF2 | [11] | |
| Ginsenoside aqueous extract | Anti-inflammatory and antioxidant effects | Mir-155-5p inhibitor, human umbilical vein endothelial cells | SA-β-gal, ROS NO, NF-κB, p53, p21 | [50] | |
| Ginseng rhamnogalacturonic acid I | Upregulation of DAF-16 and skn-1 activities | C. elegans | ROS, Nrf2, DAF-16 | [51] | |
| Red ginseng extracts | Anti-inflammatory effect and regulation of antioxidant enzyme activity | C57BL/6 20–21 months | NOS, COX, TNF-α, IL-1β | [52] | |
| Ginseng oligopeptide | Adjustment of the NAD/SIRT1/PGC-1 α pathways to improve mitochondrial function | Embryonic NIH/3T3 fibroblasts treated with H2O2 for 4 h | γ-H2A.X, ROS, GSH-Px, SOD, MDA | [53] | |
| Ginseng volatile oil | Elimination of free radicals and suppression of oxidation | C. elegans | SOD, MDA | [39] | |
| Black ginseng | Inhibition of p53-p21/p16 activation and anti-inflammatory effect | 18-month-old C57BL/6 mice | 20 Gy γ radiation-induced senescence of primary mouse embryonic fibroblasts and 30 passages of HEK293 cells | SA-β-percentage of gal-positive cells, p53 | [54] |
| Red ginseng | Inhibition of the Akt pathway | 36-day-old female Drosophila melanogaster | Raf1, ERK, p-ERK, AKT, p-AKT | [55] | |
| Korean ginseng | Regulation of PPAR signaling and antioxidant effect | Dec2−/− mice | HEI-OC1 cells treated with neomycin for 24 h | Dec1, Dec2, Dec25, Il1β, Fabp2 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Su, Q.; Hu, S.; Ruan, X.; Ouyang, S. Research Progress on the Anti-Aging Potential of the Active Components of Ginseng. Nutrients 2023, 15, 3286. https://doi.org/10.3390/nu15153286
Su J, Su Q, Hu S, Ruan X, Ouyang S. Research Progress on the Anti-Aging Potential of the Active Components of Ginseng. Nutrients. 2023; 15(15):3286. https://doi.org/10.3390/nu15153286
Chicago/Turabian StyleSu, Jingqian, Qiaofen Su, Shan Hu, Xinglin Ruan, and Songying Ouyang. 2023. "Research Progress on the Anti-Aging Potential of the Active Components of Ginseng" Nutrients 15, no. 15: 3286. https://doi.org/10.3390/nu15153286
APA StyleSu, J., Su, Q., Hu, S., Ruan, X., & Ouyang, S. (2023). Research Progress on the Anti-Aging Potential of the Active Components of Ginseng. Nutrients, 15(15), 3286. https://doi.org/10.3390/nu15153286







