Protective Effects of Medicinal Plant-Based Foods against Diabetes: A Review on Pharmacology, Phytochemistry, and Molecular Mechanisms
Abstract
1. Introduction
2. Methods
3. The Pathophysiology of Diabetes
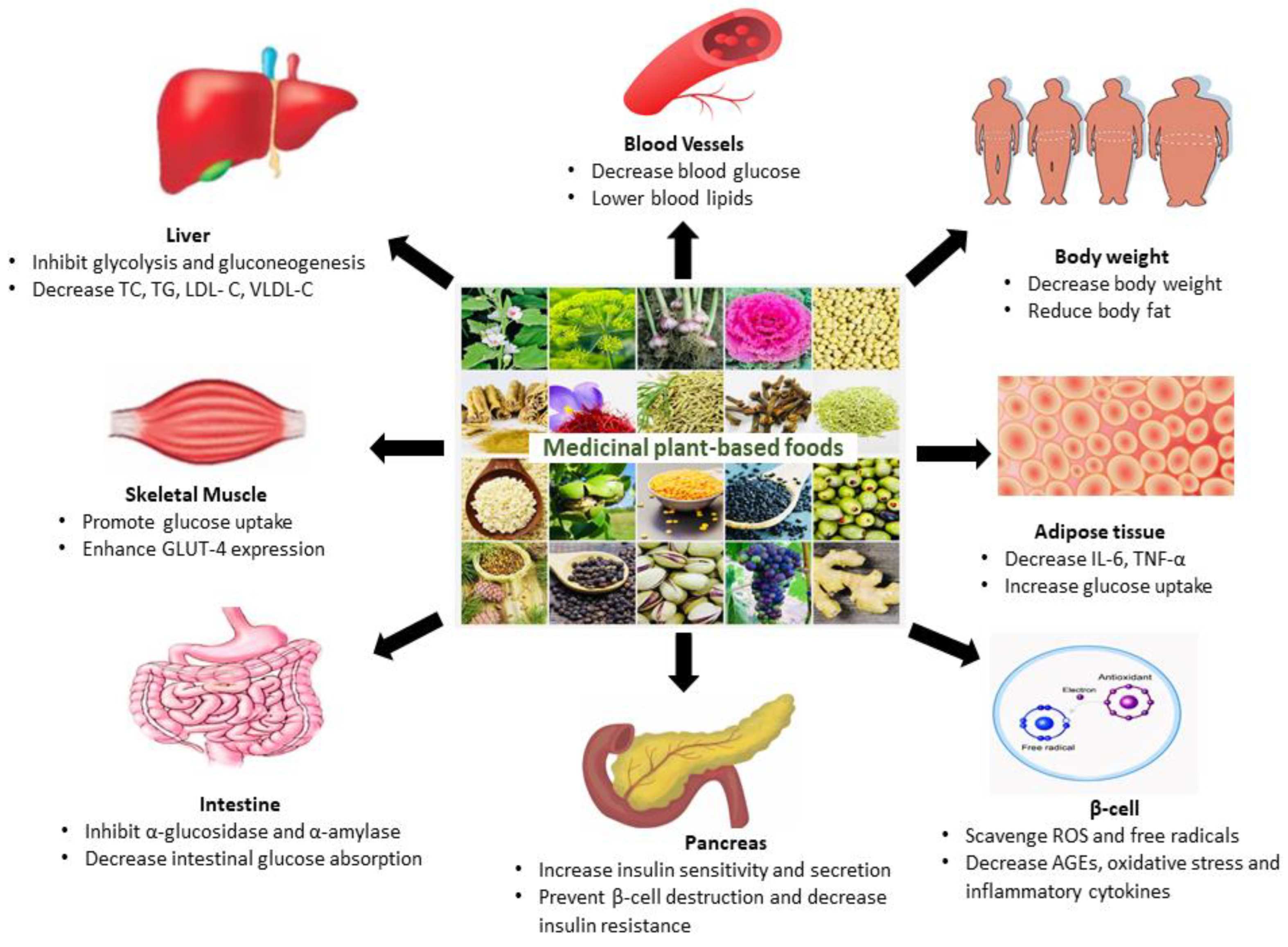
4. Medicinal Plant-Based Foods Recommended for the Treatment of DM
4.1. Althaea officinalis L.
4.2. Anethum graveolens L.
4.3. Allium sativum
4.4. Brassica oleracea L.
4.5. Cicer arietinum L.
4.6. Cinnamomum verum J. Presl.
4.7. Crocus sativus L.
4.8. Cuminum cyminum L.
4.9. Eugenia caryophyllata Thunb.
4.10. Foeniculum vulgare Mill.
4.11. Hordeum vulgare L.
4.12. Juglans regia L.
4.13. Lens culinaris L.
4.14. Nigella sativa L.
4.15. Olea europaea L.
4.16. Pinus gerardiana Wall. ex D. Don
4.17. Piper nigrum L.
4.18. Pistacia vera L.
4.19. Vitis vinifera L.
4.20. Zingiber officinale Roscoe
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AchE | Acetylcholinesterase |
| Akt | Serine/threonine kinase |
| AGEs | Advanced glycated end products |
| BW | Body weight |
| cAMP | Cyclic adenosine monophosphate |
| CVDs | Cardiovascular disorders |
| CREB | cAMP-response element protein |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| FBG | Fasting blood glucose |
| GSH | Glutathione |
| GLUT2 | Glucose transporter 2 |
| HbA1c | Glycated hemoglobin |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PUFA | Polyunsaturated fatty acid |
| PKA | Protein kinase A |
| ROS | Reactive oxygen species |
| STZ | Streptozotocin |
| TC | Total cholesterol |
| TG | Triglyceride |
| VLDL | Very low-density lipoprotein |
| WWI | World war I |
| WWII | World war II |
References
- Rashidi, A.A.; Mirhashemi, S.M.; Taghizadeh, M.; Sarkhail, P. Iranian Medicinal Plants for Diabetes Mellitus: A Systematic Review. Pak. J. Biol. Sci. 2013, 16, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A.; et al. Antidiabetic Phytochemicals from Medicinal Plants: Prospective Candidates for New Drug Discovery and Development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Zarshenas, M.M.; Khademian, S.; Moein, M. Diabetes and Related Remedies in Medieval Persian Medicine. Indian J. Endocrinol. Metab. 2014, 18, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A.; Bancher-Todesca, D.; Pollak, A.; Repa, A.; Lechleitner, M.; Weitgasser, R. Gestational diabetes mellitus. Wien Klin. Wochenschr. 2012, 124 (Suppl. S2), 58–65. [Google Scholar] [CrossRef] [PubMed]
- Cade, W.T. Diabetes-Related Microvascular and Macrovascular Diseases in the Physical Therapy Setting. Phys. Ther. 2008, 88, 1322–1335. [Google Scholar] [CrossRef]
- Dowarah, J.; Singh, V.P. Anti-Diabetic Drugs Recent Approaches and Advancements. Bioorg. Med. Chem. 2020, 28, 115263. [Google Scholar] [CrossRef]
- Tran, L.; Zielinski, A.; Roach, A.H.; Jende, J.A.; Householder, A.M.; Cole, E.E.; Atway, S.A.; Amornyard, M.; Accursi, M.L.; Shieh, S.W.; et al. Pharmacologic Treatment of Type 2 Diabetes: Oral Medications. Ann. Pharmacother. 2015, 49, 540–556. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Nipa, N.; Toma, F.T.; Talukder, A.; Ansari, P. Acute Anti-Hyperglycaemic Activity of Five Traditional Medicinal Plants in High Fat Diet Induced Obese Rats. Front. Biosci. (Schol. Ed.) 2023, 15, 5. [Google Scholar] [CrossRef]
- Heydari, M.; Hashempur, M.H.; Daneshfard, B.; Mosavat, S.H. Chapter 4—Bioactive Foods as Dietary Intervention for Diabetes from the Perspective of Persian Medicine. In Bioactive Food as Dietary Interventions for Diabetes, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 49–68. [Google Scholar] [CrossRef]
- Osadebe, P.; Odoh, U.; Uzor, P. Natural Products as Potential Sources of Antidiabetic Drugs. Br. J. Pharm. Res. 2014, 4, 2075–2095. [Google Scholar] [CrossRef]
- Coulter-Parkhill, A.; McClean, S.; Gault, V.A.; Irwin, N. Therapeutic Potential of Peptides Derived from Animal Venoms: Current Views and Emerging Drugs for Diabetes. Clin. Med. Insights Endocrinol. Diabetes 2021, 14, 11795514211006071. [Google Scholar] [CrossRef]
- Kolhe, S.S.; Rachh, P.R. Review on Potent Anti-diabetic Plants or Herbs from Traditional Medicine. J. Drug Deliv. Ther. 2018, 8, 92–98. [Google Scholar] [CrossRef]
- Rizvi, S.I.; Mishra, N. Traditional Indian medicines used for the management of diabetes mellitus. J. Diabetes Res. 2013, 2013, 712092. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Evaluation of the Antidiabetic and Insulin Releasing Effects of A. squamosa, Including Isolation and Characterization of Active Phytochemicals. Plants 2020, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Brennan, E.; McClelland, A.; Hagiwara, S.; Godson, C.; Kantharidis, P. Chapter 31—MiRNAs in the Pathophysiology of Diabetes and Their Value as Biomarkers. In Epigenetic Biomarkers and Diagnostics; García-Giménez, J.L., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 643–661. [Google Scholar] [CrossRef]
- Lien, C.F.; Chen, S.J.; Tsai, M.C.; Lin, C.S. Potential Role of Protein Kinase C in the Pathophysiology of Diabetes-Associated Atherosclerosis. Front. Pharmacol. 2021, 12, 716332. [Google Scholar] [CrossRef]
- Koya, D.; King, G.L. Protein kinase C activation and the development of diabetic complications. Diabetes 1998, 47, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Zaccardi, F.; Webb, D.R.; Yates, T.; Davies, M.J. Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgrad. Med. J. 2016, 92, 63–69. [Google Scholar] [CrossRef]
- Ohiagu, F.O.; Chikezie, P.C.; Chikezie, C.M. Pathophysiology of diabetes mellitus complications: Metabolic events and control. Biomed. Res. Ther. 2021, 8, 3. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Wolff, S.P.; Dean, R.T. Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem. J. 1987, 245, 243–250. [Google Scholar] [CrossRef]
- Chetyrkin, S.; Mathis, M.; Pedchenko, V.; Sanchez, O.A.; McDonald, W.H.; Hachey, D.L.; Madu, H.; Stec, D.; Hudson, B.; Voziyan, P. Glucose autoxidation induces functional damage to proteins via modification of critical arginine residues. Biochemistry 2011, 50, 6102–6112. [Google Scholar] [CrossRef]
- Verma, S.; Chandra, H.; Banerjee, M. Cyclooxygenase 1 (COX1) expression in Type 2 diabetes mellitus: A preliminary study from north India. Egypt. J. Med. Hum. Genet. 2016, 17, 41–45. [Google Scholar] [CrossRef]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Insulin Secretory and Antidiabetic Actions of Heritiera Fomes Bark Together with Isolation of Active Phytomolecules. PLoS ONE 2022, 17, e0264632. [Google Scholar] [CrossRef] [PubMed]
- Petroni, M.L.; Brodosi, L.; Marchignoli, F.; Sasdelli, A.S.; Caraceni, P.; Marchesini, G.; Ravaioli, F. Nutrition in Patients with Type 2 Diabetes: Present Knowledge and Remaining Challenges. Nutrients 2021, 13, 2748. [Google Scholar] [CrossRef]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Kianitalaei, A.; Feyzabadi, Z.; Hamedi, S.; Qaraaty, M. Althaea Officinalis in Traditional Medicine and modern phytotherapy. J. Adv. Pharm. Educ. Res. 2019, 9, 155. [Google Scholar]
- Bonaterra, G.A.; Schmitt, J.; Schneider, K.; Schwarzbach, H.; Aziz-Kalbhenn, H.; Kelber, O.; Müller, J.; Kinscherf, R. Phytohustil® and Root Extract of Althaea officinalis L. Exert Anti-Inflammatory and Anti-Oxidative Properties and Improve the Migratory Capacity of Endothelial Cells in Vitro. Front. Pharmacol. 2022, 13, 948248. [Google Scholar] [CrossRef]
- Basch, E.; Ulbricht, C.; Hammerness, P.; Vora, M. Marshmallow (Althaea officinalis L.) monograph. J. Herb. Pharmacother. 2003, 3, 71–81. [Google Scholar]
- Kayarohanam, S. Current Trends of Plants Having Antidiabetic Activity: A Review. J. Bioanal. Biomed. 2015, 7, 55–65. [Google Scholar] [CrossRef]
- Khadr, S.; Ahmed, A. Antidiabetic Effect of Marshmallow and Psyllium Leaves in Alloxan Induced Diabetic Rats. J. Home Econ. Menofia Univ. 2016, 26, 225–244. [Google Scholar] [CrossRef]
- Husain, S.; Malik, R.; Javaid, M.; Bibi, S. Ethonobotanical properties and uses of Medicinal plants of Morgah Biodiversity Park, Rawalpindi. Pak. J. Bot. 2008, 40, 1897–1911. [Google Scholar]
- Yousaf, A.; Shahid, S. The study of Anethum graveolens L. (Dill) in the case of Diabetes mellitus (DM). Asian J. Res. Pharm. Sci. 2020, 10, 248–256. [Google Scholar] [CrossRef]
- Mishra, N. Haematological and hypoglycemic potential Anethum graveolens seeds extract in normal and diabetic Swiss albino mice. Vet. World 2013, 6, 502. [Google Scholar] [CrossRef]
- Mansouri, M.; Nayebi, N.; Keshtkar, A.; Hasani-Ranjbar, S.; Taheri, E.; Larijani, B. The effect of 12 weeks Anethum graveolens (dill) on metabolic markers in patients with metabolic syndrome; a randomized double blind controlled trial. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2012, 20, 47. [Google Scholar] [CrossRef]
- Oshaghi, E.A.; Tavilani, H.; Khodadadi, I.; Gobdarzi, M.T. Dill tablet: A potential antioxidant and anti-diabetic medicine. Asian Pac. J. Trop. Biomed. 2015, 5, 696–702. [Google Scholar]
- Goodarzi, M.T.; Khodadadi, I.; Tavilani, H.; Abbasi Oshaghi, E. The Role of Anethum graveolens L. (Dill) in the Management of Diabetes. J. Trop. Med. 2016, 2016, 1098916. [Google Scholar] [CrossRef]
- Oshaghi, E.A.; Khodadadi, I.; Tavilani, H.; Goodarzi, M.T. Aqueous Extract of Anethum graveolens L. has Potential Antioxidant and Antiglycation Effects. Iran. J. Med. Sci. 2016, 41, 328–333. [Google Scholar]
- Kazemi, T.; Panahi Shahri, H.; Hossaini Farash, M.; Darabi, M.; Kashanian, M.; Akbari, H. Effect of Dill Pearl on Serum Lipids. J. Arak Univ. Med. Sci. 2006, 8, 35–41. [Google Scholar]
- Thomson, M.; Amin, Z.; Qattan, K.K.A.; Shaban, L. Anti-diabetic and hypolipidaemic properties of garlic (Allium sativum) in streptozotocin-induced diabetic rats. Semant. Sch. 2007, 15, 108–115. [Google Scholar]
- Hosseini, A.; Hosseinzadeh, H. A review on the effects of Allium sativum (Garlic) in metabolic syndrome. J. Endocrinol. Investig. 2015, 38, 1147–1157. [Google Scholar] [CrossRef]
- Imo, C. Medicinal Properties of Ginger and Garlic: A Review. Curr. Trends Biomed. Eng. Biosci. 2019, 18, 47–52. [Google Scholar] [CrossRef]
- Londhe, V.; Gavasane, A.; Nipate, S.; Bandawane, D.; Chaudhari, P. Role of garlic (Allium sativum) in various diseases: An overview. J. Pharm. Res. Opin. 2011, 1, 129–134. [Google Scholar]
- Liu, C.T.; Sheen, L.Y.; Lii, C.K. Does garlic have a role as an antidiabetic agent? Mol. Nutr. Food Res. 2007, 51, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, L.G.; Elewa, Y.H.A.; Al-Sagan, A.A.; Abd El-Hack, M.E.; Taha, A.E.; Abd-Elhakim, Y.M.; Prasad Devkota, H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef]
- Corzo-Martínez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Eidi, A.; Eidi, M.; Esmaeili, E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomed. Int. J. Phytother. Phytopharm. 2006, 13, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Upadhyay, A.K.; Bahadur, A.; Singh, B.; Singh, K.P.; Rai, M. Antioxidant phytochemicals in cabbage (Brassica oleracea L. var. Capitata). Sci. Hortic. 2006, 108, 233. [Google Scholar] [CrossRef]
- Šamec, D.; Pavlović, I.; Salopek-Sondi, B. White cabbage (Brassica oleracea var. capitata f. alba): Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2017, 16, 117–135. [Google Scholar] [CrossRef]
- Jaiswal, A.K. Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Uuh-Narvaez, J.J.; Segura-Campos, M.R. Cabbage (Brassica oleracea var. capitata): A food with functional properties aimed to type 2 diabetes prevention and management. J. Food Sci. 2021, 86, 4775–4789. [Google Scholar] [CrossRef]
- Al-Saeed, A.A.; Saif, M.; AlAmeer, M.A.; Al-Anazi, B.K. Hypoglycemic and Hypolipidemic Properties of Ethanolic Extract of Brassica oleracea in Streptozotocin-Induced Diabetic Rats. Int. J. Pharm. Res. Allied Sci. 2020, 9, 151–157. [Google Scholar]
- Buko, V.; Zavodnik, I.; Kanuka, O.; Belonovskaya, E.; Naruta, E.; Lukivskaya, O.; Kirko, S.; Budryn, G.; Żyżelewicz, D.; Oracz, J.; et al. Antidiabetic effects and erythrocyte stabilization by red cabbage extract in streptozotocin-treated rats. Food Funct. 2018, 9, 1850–1863. [Google Scholar] [CrossRef]
- Quintero-Soto, M.F.; Chávez-Ontiveros, J.; Garzón-Tiznado, J.A.; Salazar-Salas, N.Y.; Pineda-Hidalgo, K.V.; Delgado-Vargas, F.; López-Valenzuela, J.A. Characterization of peptides with antioxidant activity and antidiabetic potential obtained from chickpea (Cicer arietinum L.) protein hydrolyzates. J. Food Sci. 2021, 86, 2962–2977. [Google Scholar] [CrossRef] [PubMed]
- Acevedo Martinez, K.A.; Yang, M.M.; Gonzalez de Mejia, E. Technological properties of chickpea (Cicer arietinum): Production of snacks and health benefits related to type-2 diabetes. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3762–3787. [Google Scholar] [CrossRef]
- Ramadhani, U.P.; Chandra, B.; Rivai, H. Overview of phytochemistry and pharmacology of chickpeas (Phaseolus vulgaris). World J. Pharm. Pharm. Sci. 2020, 9, 442–461. [Google Scholar]
- Roy, F.; Boye, J.I.; Simpson, B.K. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lenti. Food Res. Int. 2010, 43, 432–442. [Google Scholar] [CrossRef]
- Wood, J.A.; Grusak, M.A. Nutritional value of chickpea. In Chickpea Breeding and Management; Yadav, S.S., Redden, B., Chen, W., Sharma, B., Eds.; CAB International: Wallingford, UK, 2007; pp. 101–142. [Google Scholar] [CrossRef]
- Singh, K.; Gahlot, K. Pharmacognostical and Pharmacological Importance of Cicer arietinum Linn—A Review. Res. J. Pharm. Technol. 2018, 11, 4755–4763. [Google Scholar] [CrossRef]
- Sánchez-Magaña, L.M.; Cuevas-Rodríguez, E.O.; Gutiérrez-Dorado, R.; Ayala-Rodríguez, A.E.; Valdez-Ortiz, A.; Milán-Carrillo, J.; Reyes-Moreno, C. Solid-state bioconversion of chickpea (Cicer arietinum L.) by Rhizopus oligosporus to improve total phenolic content, antioxidant activity and hypoglycemic functionality. Int. J. Food Sci. Nutr. 2014, 65, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Gupta, K.; Sharma, A.; Das, M.; Ansari, I.A.; Dwivedi, P.D. Health Risks and Benefits of Chickpea (Cicer arietinum) Consumption. J. Agric. Food Chem. 2017, 65, 6–22. [Google Scholar] [CrossRef]
- Wei, Y.; Li, P.; Li, B.; Gao, J.; Wang, D.; Qin, L.; Sun, W.; Xu, Y.; Shi, H.; Xu, T.; et al. Study of the Hypoglycemic Activity of Derivatives of Isoflavones from Cicer arietinum L. Evid.-Based Complement. Altern. Med. Ecam 2017, 2017, 8746823. [Google Scholar] [CrossRef]
- Charles, D.J. Antioxidant Properties of Spices, Herbs and Other Sources; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Rafehi, H.; Ververis, K.; Karagiannis, T.C. Controversies surrounding the clinical potential of cinnamon for the management of diabetes. Diabetes Obes. Metab. 2012, 14, 493–499. [Google Scholar] [CrossRef]
- Bandara, T.; Uluwaduge, I.; Jansz, E.R. Bioactivity of cinnamon with special emphasis on diabetes mellitus: A review. Int. J. Food Sci. Nutr. 2012, 63, 380–386. [Google Scholar] [CrossRef]
- Sharma, S.; Mandal, A.; Kant, R.; Jachak, S.; Jagzape, M. Is Cinnamon Efficacious for Glycaemic Control in Type-2 Diabetes Mellitus? JPMA J. Pak. Med. Assoc. 2020, 70, 2065–2069. [Google Scholar]
- Sahib, A.S. Anti-diabetic and antioxidant effect of cinnamon in poorly controlled type-2 diabetic Iraqi patients: A randomized, placebo-controlled clinical trial. J. Intercult. Ethnopharmacol. 2016, 5, 108–113. [Google Scholar] [CrossRef]
- Hayward, N.J.; McDougall, G.J.; Farag, S.; Allwood, J.W.; Austin, C.; Campbell, F.; Horgan, G.; Ranawana, V. Cinnamon Shows Antidiabetic Properties that Are Species-Specific: Effects on Enzyme Activity Inhibition and Starch Digestion. Plant Foods Hum. Nutr. 2019, 74, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, S.; Akilen, R.; Sharma, S.; Tsiami, A. The potential of cinnamon to reduce blood glucose levels in patients with type 2 diabetes and insulin resistance. Diabetes Obes. Metab. 2009, 11, 1100–1113. [Google Scholar] [CrossRef]
- Elgazar, A.F.; Rezq, A.A.; Bukhari, H.M. Anti-Hyperglycemic Effect of Saffron Extract in Alloxan-Induced Diabetic Rats. J. Med. Plants 2013, 5, 14–22. [Google Scholar]
- Pandey, D.K.; Nandy, S.; Mukherjee, A.; Dey, A. Chapter 10—Advances in Bioactive Compounds from Crocus sativus (Saffron): Structure, Bioactivity and Biotechnology. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Bioactive Natural Products; Elsevier: Amsterdam, The Netherlands, 2020; Volume 66, pp. 273–304. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Saffron (Crocus sativus) petal as a new pharmacological target: A review. Iran. J. Basic Med. Sci. 2018, 21, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Samarghandian, S. The effect of saffron (Crocus sativus L.) and its ingredients on the management of diabetes mellitus and dislipidemia. Afr. J. Pharm. Pharmacol. 2014, 8, 541–549. [Google Scholar] [CrossRef][Green Version]
- Razavi, B.M.; Hosseinzadeh, H. Saffron: A promising natural medicine in the treatment of metabolic syndrome. J. Sci. Food Agric. 2017, 97, 1679–1685. [Google Scholar] [CrossRef]
- Srivastava, R.; Ahmed, H.; Dixit, R.K.; Dharamveer; Saraf, S.A. Crocus sativus L.: A comprehensive review. Pharmacogn. Rev. 2010, 4, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.E.; Christodoulou, E.; Kostomitsopoulos, N.; Valsami, G. The cardiovascular-protective properties of saffron and its potential pharmaceutical applications: A critical appraisal of the literature. Phytother. Res. PTR 2021, 35, 6735–6753. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Zare, V.; Butler, A.E.; Barreto, G.E.; Sahebkar, A. Antidiabetic potential of saffron and its active constituents. J. Cell. Physiol. 2019, 234, 8610–8617. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Khan, A.; Aldebasi, Y.; Aldebasi, Y. Saffron (Crocus sativus) and its Active Ingredients: Role in the Prevention and Treatment of Disease. Pharmacogn. J. 2017, 9, 873–879. [Google Scholar] [CrossRef]
- Srinivasan, K. Cumin (Cuminum cyminum) and black cumin (Nigella sativa) seeds: Traditional uses, chemical constituents, and nutraceutical effects. Food Qual. Saf. 2018, 2, 1–16. [Google Scholar] [CrossRef]
- Allaq, A.A.; Sidik, N.J.; Abdul-Aziz, A.; Ahmed, I.A. Cumin (Cuminum cyminum L.): A review of its ethnopharmacology, phytochemistry. Biomed. Res. Ther. 2020, 7, 9. [Google Scholar] [CrossRef]
- Mnif, S.; Aifa, S. Cumin (Cuminum cyminum L.) from traditional uses to potential biomedical applications. Chem. Biodivers. 2015, 12, 733–742. [Google Scholar] [CrossRef]
- Siow, H.-L.; Gan, C.-Y. Functional protein from cumin seed (Cuminum cyminum): Optimization and characterization studies. Food Hydrocoll. 2014, 41, 178–187. [Google Scholar] [CrossRef]
- Singh, N.; Yadav, S.S.; Kumar, S.; Narashiman, B. A review on traditional uses, phytochemistry, pharmacology, and clinical research of dietary spice Cuminum cyminum L. Phytother. Res. PTR 2021, 35, 5007–5030. [Google Scholar] [CrossRef]
- Ebada, M.E. Cuminaldehyde: A Potential Drug Candidate. J. Pharmacol. Clin. Res. 2017, 2, 555585. [Google Scholar] [CrossRef]
- Milind, P.; Deepa, K. Clove: A champion spice. Int. J. Res. Ayurveda Pharm. IJRAP 2011, 2, 47–54. [Google Scholar]
- Hussain, S.; Rahman, R.; Mushtaq, A.; Zerey-Belaskri, A.E. Clove: A review of a precious species with multiple uses. Int. J. Chem. Biochem. Sci. 2017, 11, 129–133. [Google Scholar]
- Cortés-Rojas, D.F.; de Souza, C.R.; Oliveira, W.P. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef]
- Batiha, G.E.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Bouchentouf, S.; Said, G.; Noureddine, M.; Allali, H.; Bouchentouf, A. A Note Study on Antidiabetic Effect of Main Molecules Contained in Clove Using Molecular Modeling Interactions with DPP-4 Enzyme. Int. J. Comput. Theor. Chem. 2017, 5, 9–13. [Google Scholar] [CrossRef][Green Version]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. PTR 2007, 21, 501–506. [Google Scholar] [CrossRef]
- Topal, F. Anticholinergic and antidiabetic effects of isoeugenol from clove (Eugenia caryophylata) oil. Int. J. Food Prop. 2019, 22, 583–592. [Google Scholar] [CrossRef]
- Helal, E.G.E.; AL Jalaud, N.A.A.; El-Aleem, M.A.; Ahmed, S.S. Effect of Phytoestrogen (Fennel) on Some Sex Hormones and Other Physiological Parameters in Male Albino Rats. Egypt. J. Hosp. Med. 2019, 74, 1616–1620. [Google Scholar] [CrossRef]
- Abou, N.; Abou El-Soud, N.; El-Laithy, N.; El-Saeed, G.; Wahby, M.S.; Khalil, M.; Morsy, F.; Shaffie, N.; Abou, C.; Wahby, S. Antidiabetic Activities of Foeniculum vulgare Mill. Essential Oil in Streptozotocin-Induced Diabetic Rats. Maced. J. Med. Sci. 2011, 4, 139–146. [Google Scholar]
- Samadi-Noshahr, Z.; Hadjzadeh, M.A.; Moradi-Marjaneh, R.; Khajavi-Rad, A. The hepatoprotective effects of fennel seeds extract and trans-Anethole in streptozotocin-induced liver injury in rats. Food Sci. Nutr. 2020, 9, 1121–1131. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. BioMed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef]
- Osman, N.N.; Jambi, E.J.; Aseri, N.H. Assessment of antidiabetic and antioxidant activities of Cassia angustifolia and Feoniculum vulgare in diabetic rats. Int. J. Pharm. Res. Allied Sci. 2017, 6, 149–162. [Google Scholar]
- Zeng, Y.; Pu, X.; Yang, J.; Du, J.; Yang, X.; Li, X.; Li, L.; Zhou, Y.; Yang, T. Preventive and Therapeutic Role of Functional Ingredients of Barley Grass for Chronic Diseases in Human Beings. Oxidative Med. Cell. Longev. 2018, 2018, 3232080. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, J.H.; Kim, S.H.; Oh, J.Y.; Seo, W.D.; Kim, K.M.; Jung, J.C.; Jung, Y.S. Barley Sprouts Extract Attenuates Alcoholic Fatty Liver Injury in Mice by Reducing Inflammatory Response. Nutrients 2016, 8, 440. [Google Scholar] [CrossRef] [PubMed]
- Minaiyan, M.; Ghannadi, A.; Movahedian, A.; Hakim-Elahi, I. Effect of Hordeum vulgare L. (Barley) on blood glucose levels of normal and STZ-induced diabetic rats. Res. Pharm. Sci. 2014, 9, 173–178. [Google Scholar]
- Rashid, K.; Kumar, C.S.; Haleel, P.M.M. Healthcare Benefits of Hordeum vulgare L (Barley): A Phyto-Pharmacological Review. Res. J. Pharmacol. Pharmacodyn. 2017, 9, 207–210. [Google Scholar] [CrossRef]
- Boantă, A.; Muntean, L.; Russu, F.; Ona, A.; Porumb, I.; Filip, E. Barley (Hordeum vulgare L.): Medicinal and Therapeutic Uses—Review. Hop Med. Plants 2019, 27, 87–95. [Google Scholar]
- Obadi, M.; Sun, J.; Xu, B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 110065. [Google Scholar] [CrossRef]
- Delaviz, H.; Mohammadi, J.; Ghalamfarsa, G.; Mohammadi, B.; Farhadi, N. A Review Study on Phytochemistry and Pharmacology Applications of Juglans regia Plant. Pharmacogn. Rev. 2017, 11, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Su, C.; Xu, Y.; Shang, K.; Sun, K.; Li, C.; Lu, J. Identifying potential active components of walnut leaf that action diabetes mellitus through integration of UHPLC-Q-Orbitrap HRMS and network pharmacology analysis. J. Ethnopharmacol. 2020, 253, 112659. [Google Scholar] [CrossRef] [PubMed]
- Forino, M.; Stiuso, P.; Lama, S.; Ciminiello, P.; Tenore, G.C.; Novellino, E.; Taglialatela-Scafati, O. Bioassay-guided identification of the antihyperglycaemic constituents of walnut (Juglans regia) leaves. J. Funct. Foods 2016, 26, 731–738. [Google Scholar] [CrossRef]
- Kamyab, H.; Hejrati, S.; Khanavi, M.; Malihi, F.; Mohammadirad, A.; Baeeri, M.; Esmaily, H.; Abdollahi, M. Hepatic mechanisms of the Walnut antidiabetic effect in mice. Open Life Sci. 2010, 5, 304–309. [Google Scholar] [CrossRef]
- Asgary, S.; Parkhideh, S.; Solhpour, A.; Madani, H.; Mahzouni, P.; Rahimi, P. Effect of ethanolic extract of Juglans regia L. on blood sugar in diabetes-induced rats. J. Med. Food 2008, 11, 533–538. [Google Scholar] [CrossRef]
- Magro, A.E.A.; Silva, L.C.; Rasera, G.B.; Ferreira, L.R.; Faria, J.A.F.; Teixeira, J.A. Solid-state fermentation as an efficient strategy for the biotransformation of lentils: Enhancing their antioxidant and antidiabetic potentials. Bioresour. Bioprocess. 2019, 6, 38. [Google Scholar] [CrossRef]
- Singhal, P.; Kaushik, G.; Mathur, P. Antidiabetic potential of commonly consumed legumes: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 655–672. [Google Scholar] [CrossRef]
- Świeca, M.; Baraniak, B.; Gawlik-Dziki, U. In vitro digestibility and starch content, predicted glycemic index and potential in vitro antidiabetic effect of lentil sprouts obtained by different germination techniques. Food Chem. 2013, 138, 1414–1420. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-Rich Lentils and Their Health Promoting Effects. Int. J. Mol. Sci. 2017, 18, 2390. [Google Scholar] [CrossRef]
- Tefera, M.M.; Altaye, B.M.; Yimer, E.M.; Berhe, D.F.; Tadesse Bekele, S. Antidiabetic Effect of Germinated Lens culinaris Medik Seed Extract in Streptozotocin-Induced Diabetic Mice. J. Exp. Pharmacol. 2020, 12, 39–45. [Google Scholar] [CrossRef]
- Casarin, A.L.F.; Rasera, G.B.; de Castro, R.J.S. Combined biotransformation processes affect the antioxidant, antidiabetic and protease inhibitory properties of lentils. Process Biochem. 2021, 102, 250–260. [Google Scholar] [CrossRef]
- Faris, M.A.-I.E.; Takruri, H.R.; Issa, A.Y. Role of lentils (Lens culinaris L.) in human health and nutrition: A review. Mediterr. J. Nutr. Metab. 2013, 6, 3–16. [Google Scholar] [CrossRef]
- Chen, K.; Gao, C.; Han, X.; Li, D.; Wang, H.; Lu, F. Co-fermentation of lentils using lactic acid bacteria and Bacillus subtilis natto increases functional and antioxidant components. J. Food Sci. 2021, 86, 475–483. [Google Scholar] [CrossRef]
- Rchid, H.; Chevassus, H.; Nmila, R.; Guiral, C.; Petit, P.; Chokaïri, M.; Sauvaire, Y. Nigella sativa seed extracts enhance glucose-induced insulin release from rat-isolated Langerhans islets. Fundam. Clin. Pharmacol. 2004, 18, 525–529. [Google Scholar] [CrossRef]
- Benhaddou-Andaloussi, A.; Martineau, L.C.; Spoor, D.; Vuong, T.; Leduc, C.; Joly, E.; Burt, A.; Meddah, B.; Settaf, A.; Arnason, J.T.; et al. Antidiabetic Activity of Nigella sativa Seed Extract in Cultured Pancreatic β-cells, Skeletal Muscle Cells, and Adipocytes. Pharm. Biol. 2008, 46, 96–104. [Google Scholar] [CrossRef]
- Yarnell, E.; Abascal, K. Nigella sativa: Holy Herb of the Middle East. Altern. Complement. Ther. 2011, 17, 99–105. [Google Scholar] [CrossRef]
- Mathur, M.L.; Gaur, J.; Sharma, R.; Haldiya, K.R. Antidiabetic Properties of a Spice Plant Nigella sativa. J. Endocrinol. Metab. 2011, 1, 1–8. [Google Scholar] [CrossRef]
- Ramadan, M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An overview. Int. J. Food Sci. Technol. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- Akhtar, M.T.; Qadir, R.; Bukhari, I.; Ashraf, R.A.; Malik, Z.; Zahoor, S.; Murtaza, M.A.; Siddique, F.; Shah, S.N.H.; Saadia, M. Antidiabetic potential of Nigella sativa L. seed oil in alloxan induced diabetic rabbits. Trop. J. Pharm. Res. 2020, 19, 283–289. [Google Scholar] [CrossRef]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive). Evid. Based Complement. Altern. Med. Ecam 2015, 2015, 541591. [Google Scholar] [CrossRef]
- Caramia, G. Virgin olive oil. From legend to scientific knowledge of the nutraceutical aspects. Pediatr. Med. Chir. 2006, 28, 9–23. [Google Scholar]
- Acar-Tek, N.; Ağagündüz, D. Olive Leaf (Olea europaea L. folium): Potential Effects on Glycemia and Lipidemia. Ann. Nutr. Metab. 2020, 76, 10–15. [Google Scholar] [CrossRef]
- Waterman, E.; Lockwood, B. Active components and clinical applications of olive oil. Altern. Med. Rev. A J. Clin. Ther. 2007, 12, 331–342. [Google Scholar]
- Figueiredo-González, M.; Reboredo-Rodríguez, P.; González-Barreiro, C.; Simal-Gándara, J.; Valentão, P.; Carrasco-Pancorbo, A.; Andrade, P.B.; Cancho-Grande, B. Evaluation of the neuroprotective and antidiabetic potential of phenol-rich extracts from virgin olive oils by in vitro assays. Food Res. Int. 2018, 106, 558–567. [Google Scholar] [CrossRef]
- Collado-González, J.; Grosso, C.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Durand, T.; Guy, A.; Galano, J.M.; Torrecillas, A.; Gil-Izquierdo, Á. Inhibition of α-glucosidase and α-amylase by Spanish extra virgin olive oils: The involvement of bioactive compounds other than oleuropein and hydroxytyrosol. Food Chem. 2017, 235, 298–307. [Google Scholar] [CrossRef]
- Santos-Lozano, J.M.; Rada, M.; Lapetra, J.; Guinda, Á.; Jiménez-Rodríguez, M.C.; Cayuela, J.A.; Ángel-Lugo, A.; Vilches-Arenas, Á.; Gómez-Martín, A.M.; Ortega-Calvo, M.; et al. Prevention of type 2 diabetes in prediabetic patients by using functional olive oil enriched in oleanolic acid: The PREDIABOLE study, a randomized controlled trial. Diabetes Obes. Metab. 2019, 21, 2526–2534. [Google Scholar] [CrossRef]
- Visioli, F.; Davalos, A.; López de Las Hazas, M.C.; Crespo, M.C.; Tomé-Carneiro, J. An overview of the pharmacology of olive oil and its active ingredients. Br. J. Pharmacol. 2020, 177, 1316–1330. [Google Scholar] [CrossRef]
- Alami, K.; Mousavi, S.Y. Afghan Chehelghoza (Pinus gerardiana L.) pine nut diet enhances the learning and memory in male rats. Nutr. Diet. Suppl. 2020, 12, 277–288. [Google Scholar] [CrossRef]
- Singh, G.; Kumar, D.; Dash, A.K. Pinus gerardiana Wallichex. D. Don.—A review. Phytomed. Plus 2021, 1, 100024. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Sharma, R.; Cruz-Martins, N.; Valko, M.; Upadhyay, N.K.; Kuča, K.; Bhardwaj, P. Studies of Phytochemicals, Antioxidant, and Antibacterial Activities of Pinus gerardiana and Pinus roxburghii Seed Extracts. BioMed Res. Int. 2022, 2022, e5938610. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Vali, M.; Haghighi-Zade, M.H.; Siahpoosh, A.; Malihi, R. The Effect of Chilgoza Pine Nut (Pinus gerardiana Wall.) on Blood Glucose and Oxidative Stress in Diabetic Rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2399–2408. [Google Scholar] [CrossRef]
- Zulfqar, F.; Akhtar, M.F.; Saleem, A.; Akhtar, B.; Sharif, A.; Saleem, U. Chemical characterization, antioxidant evaluation, and antidiabetic potential of Pinus gerardiana (Pine nuts) extracts. J. Food Biochem. 2020, 44, e13199. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59(Suppl. S1), S210–S243. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, V.A.; Chempakam, B.; Zachariah, T.J. Chemistry of Spices; CABI Pub.: Wallingford, UK, 2008. [Google Scholar]
- Peter, K.V. Handbook of Herbs and Spices; Woodhead publishing: Sawston, UK, 2006; Volume 3. [Google Scholar]
- Abukawsar, M.; Saleh-E-In, M.; Ahsan, M.; Rahim, M.; Nurul, M.; Huda Bhuiyan, M.N.; Sudhangshu, R.K.; Ghosh, A.; Naher, S. Chemical, pharmacological and nutritional quality assessment of black pepper (Piper nigrum L.) seed cultivars. J. Food Biochem. 2018, 42, e12590. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Pandian, A.; Warkentin, T.D. Phytochemistry and therapeutic potential of black pepper [Piper nigrum (L.)] essential oil and piperine: A review. Clin. Phytosci. 2021, 7, 52. [Google Scholar] [CrossRef]
- Jeena, K.; Liju, V.B.; Umadevi, N.P.; Kuttan, R. Antioxidant, anti-inflammatory and antinociceptive properties of black pepper essential oil (Piper nigrum Linn). J. Essent. Oil Bear. Plants 2014, 17, 1–12. [Google Scholar] [CrossRef]
- Sarfraz, M.; Khaliq, T.; Hafizur, R.M.; Raza, S.A.; Ullah, H. Effect of black pepper, turmeric and ajwa date on the endocrine pancreas of the experimentally induced diabetes in wister albino rats: A histological and immunohistochemical study. Endocr. Metab. Sci. 2021, 4, 100098. [Google Scholar] [CrossRef]
- Shityakov, S.; Bigdelian, E.; Hussein, A.A.; Hussain, M.B.; Tripathi, Y.C.; Khan, M.U.; Shariati, M.A. Phytochemical and pharmacological attributes of piperine: A bioactive ingredient of black pepper. Eur. J. Med. Chem. 2019, 176, 149–161. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Akash, S.; Harun-Or-Rashid, M.; Ray, T.K.; Rahaman, M.S.; Wilairatana, P. Recent advancements of nanoparticles application in cancer and neurodegenerative disorders: At a glance. Biomed. Pharmacother. 2022, 153, 113305. [Google Scholar] [CrossRef]
- Napoli, E.; Gentile, D.; Ruberto, G. GC–MS analysis of terpenes from Sicilian Pistacia vera L. oleoresin. A source of biologically active compounds. Biomed. Chromatogr. 2019, 33, e4381. [Google Scholar] [CrossRef]
- Emlik, H.; Simitcioglu, B.; Cakir, A.; Kilic, I.H.; Karagoz, I.D. Evaluation of Pistacia vera Sap Waste Sections and Its Potential Role on Treatment. Eurasia Proc. Sci. Technol. Eng. Math. 2020, 10, 12–16. [Google Scholar]
- Ozçelik, B.; Aslan, M.; Orhan, I.; Karaoglu, T. Antibacterial, antifungal, and antiviral activities of the lipophylic extracts of Pistacia vera. Microbiol. Res. 2005, 160, 159–164. [Google Scholar] [CrossRef]
- Gok, H.N.; Pekacar, S.; Deliorman Orhan, D. Investigation of Enzyme Inhibitory Activities, Antioxidant Activities, and Chemical Properties of Pistacia vera Leaves Using LC-QTOF-MS and RP-HPLC. Iran. J. Pharm. Res. IJPR 2022, 21, e127033. [Google Scholar] [CrossRef] [PubMed]
- Noguera-Artiaga, L.; Pérez-López, D.; Burgos-Hernández, A.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Phenolic and triterpenoid composition and inhibition of α-amylase of pistachio kernels (Pistacia vera L.) as affected by rootstock and irrigation treatment. Food Chem. 2018, 261, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Cruz, J.F.; Zhu, M.; Kinghorn, A.D.; Wu, C.D. Antimicrobial constituents of Thompson seedless raisins (Vitis vinifera) against selected oral pathogens. Phytochem. Lett. 2008, 1, 151–154. [Google Scholar] [CrossRef]
- Olmo-Cunillera, A.; Escobar-Avello, D.; Pérez, A.J.; Marhuenda-Muñoz, M.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A. Is Eating Raisins Healthy? Nutrients 2020, 12, 54. [Google Scholar] [CrossRef]
- Kandylis, P. Grapes and Their Derivatives in Functional Foods. Foods 2021, 10, 672. [Google Scholar] [CrossRef]
- Kanellos, P.T.; Kaliora, A.C.; Tentolouris, N.K.; Argiana, V.; Perrea, D.; Kalogeropoulos, N.; Kountouri, A.M.; Karathanos, V.T. A pilot, randomized controlled trial to examine the health outcomes of raisin consumption in patients with diabetes. Nutrition 2014, 30, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Liu, R.H.; He, X. Novel triterpenoids isolated from raisins exert potent antiproliferative activities by targeting mitochondrial and Ras/Raf/ERK signaling in human breast cancer cells. Food Funct. 2016, 7, 3244–3251. [Google Scholar] [CrossRef]
- Aderonke Otunola, G.; Jide Afolayan, A. A Review of the Antidiabetic Activities of Ginger; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef][Green Version]
- Anh, N.H.; Kim, S.J.; Long, N.P.; Min, J.E.; Yoon, Y.C.; Lee, E.G.; Kim, M.; Kim, T.J.; Yang, Y.Y.; Son, E.Y.; et al. Ginger on Human Health: A Comprehensive Systematic Review of 109 Randomized Controlled Trials. Nutrients 2020, 12, 157. [Google Scholar] [CrossRef]
- Račková, L.; Cupáková, M.; Tažký, A.; Mičová, J.; Kolek, E.; Košt’álová, D. Redox properties of ginger extracts: Perspectives of use of Zingiber officinale Rosc. as antidiabetic agent. Interdiscip. Toxicol. 2013, 6, 26–33. [Google Scholar] [CrossRef]
- Al-Amin, Z.; Thomson, M.; Al-Qattan, K.; Peltonen-Shalaby, R.; Ali, M. Anti-diabetic and hypolipidemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br. J. Nutr. 2006, 96, 660–666. [Google Scholar] [CrossRef]
- Shanmugam, K.R.; Mallikarjuna, K.; Nishanth, K.; Ku, C.H.; Reddy, K.S. Protective effect of dietary ginger on antioxidant enzymes and oxidative damage in experimental diabetic rat tissues. Food Chem. 2011, 124, 1436–1442. [Google Scholar] [CrossRef]
- Morakinyo, A.O.; Akindele, A.J.; Ahmed, Z. Modulation of antioxidant enzymes and inflammatory cytokines: Possible mechanism of anti-diabetic effect of ginger extracts. Afr. J. Biomed. Res. 2011, 14, 195–202. [Google Scholar]
- Adefegha, A.O.A.S.A.; Oboh, G.; Akinyemi, A.J.; Ademiluyi, A. Inhibitory effects of aqueous extract of two varieties of ginger on some key enzymes linked to type-2 diabetes in vitro. J. Food Nutr. Res. 2010, 49, 14–20. [Google Scholar]
- Balamash, K.S.; Alkreathy, H.M.; Al Gahdali, E.H.; Khoja, S.O.; Ahmad, A. Comparative Biochemical and Histopathological Studies on the Efficacy of Metformin and Virgin Olive Oil against Streptozotocin-Induced Diabetes in Sprague-Dawley Rats. J. Diabetes Res. 2018, 2018, 4692197. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Barreca, D.; Gervasi, T.; Roussell, M.A.; Klein, B.; Feeney, M.J.; Carughi, A. Pistachio Nuts (Pistacia vera L.): Production, Nutrients, Bioactives and Novel Health Effects. Plants 2021, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.Y.; Choo, Q.C.; Muhammad, T.S.T.; Chew, C.H. Lauric acid alleviates insulin resistance by improving mitochondrial biogenesis in THP-1 macrophages. Mol. Biol. Rep. 2020, 47, 9595–9607. [Google Scholar] [CrossRef]
- Muruganathan, U.; Srinivasan, S.; Indumathi, D. Antihyperglycemic Effect of Carvone: Effect on the Levels of Glycoprotein Components in Streptozotocin-Induced Diabetic Rats. J. Acute Dis. 2013, 2, 310–315. [Google Scholar] [CrossRef]
- Abdulghafoor, H.A.; Ramadhan, S.J.; Nawfal, A.J. Therapeutic Effects of Allicin against the Diabetes Mellitus Induced by Streptozotocin in Male Rats. NVEO Nat. Volatiles Essent. Oils J. NVEO 2021, 8, 8934–8945. [Google Scholar]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Ansari, P.; Choudhury, S.T.; Seidel, V.; Rahman, A.B.; Aziz, M.A.; Richi, A.E.; Rahman, A.; Jafrin, U.H.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life 2022, 12, 1146. [Google Scholar] [CrossRef]
- Subash Babu, P.; Prabuseenivasan, S.; Ignacimuthu, S. Cinnamaldehyde—A potential antidiabetic agent. Phytomed. Int. J. Phytother. Phytopharm. 2007, 14, 15–22. [Google Scholar] [CrossRef]
- Behrouz, V.; Dastkhosh, A.; Hedayati, M.; Sedaghat, M.; Sharafkhah, M.; Sohrab, G. The effect of crocin supplementation on glycemic control, insulin resistance and active AMPK levels in patients with type 2 diabetes: A pilot study. Diabetol. Metab. Syndr. 2020, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.B.; Takalikar, S.S.; Joglekar, M.M.; Haldavnekar, V.S.; Arvindekar, A.U. Insulinotropic and β-cell protective action of cuminaldehyde, cuminol and an inhibitor isolated from Cuminum cyminum in streptozotocin-induced diabetic rats. Br. J. Nutr. 2013, 110, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Jayaramaiah, R.H.; Agawane, S.B.; Vannuruswamy, G.; Korwar, A.M.; Anand, A.; Dhaygude, V.S.; Shaikh, M.L.; Joshi, R.S.; Boppana, R.; et al. Potential Dual Role of Eugenol in Inhibiting Advanced Glycation End Products in Diabetes: Proteomic and Mechanistic Insights. Sci. Rep. 2016, 6, 18798. [Google Scholar] [CrossRef]
- Alkhalidy, H.; Moore, W.; Wang, Y.; Luo, J.; McMillan, R.P.; Zhen, W.; Zhou, K.; Liu, D. The Flavonoid Kaempferol Ameliorates Streptozotocin-Induced Diabetes by Suppressing Hepatic Glucose Production. Molecules 2018, 23, 2338. [Google Scholar] [CrossRef] [PubMed]
- Mirjana, M.; Jelena, A.; Aleksandra, U.; Svetlana, D.; Nevena, G.; Jelena, M.; Ibrahim, M.; Ana, Š.D.; Goran, P.; Melita, V. β-Glucan administration to diabetic rats reestablishes redox balance and stimulates cellular pro-survival mechanisms. J. Funct. Foods 2013, 5, 267–278. [Google Scholar] [CrossRef]
- Nimbalkar, V.; Joshi, U.; Shinde, S.; Pawar, G. In-vivo and in-vitro evaluation of therapeutic potential of β- Carotene in diabetes. J. Diabetes Metab. Disord. 2021, 20, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.M.; Abdel Motaal, A.; El Awdan, S.A.W. In vitro and in vivo antidiabetic potential of extracts and a furostanol saponin from Balanites aegyptiaca. Pharm. Biol. 2017, 55, 1931–1936. [Google Scholar] [CrossRef]
- Faisal Lutfi, M.; Abdel-Moneim, A.H.; Alsharidah, A.S.; Mobark, M.A.; Abdellatif, A.A.H.; Saleem, I.Y.; Al Rugaie, O.; Mohany, K.M.; Alsharidah, M. Thymoquinone Lowers Blood Glucose and Reduces Oxidative Stress in a Rat Model of Diabetes. Molecules 2021, 26, 2348. [Google Scholar] [CrossRef]
- Shivarudrappa, A.H.; Ponesakki, G. Lutein reverses hyperglycemia-mediated blockage of Nrf2 translocation by modulating the activation of intracellular protein kinases in retinal pigment epithelial (ARPE-19) cells. J. Cell Commun. Signal. 2020, 14, 207–221. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Ahn, D.; Hwang, J.Y.; Kang, M.J.; Chung, S.J. Linoleic acid exerts antidiabetic effects by inhibiting protein tyrosine phosphatases associated with insulin resistance. J. Funct. Foods 2021, 83, 104532. [Google Scholar] [CrossRef]
- Kumawat, V.S.; Kaur, G. Insulinotropic and antidiabetic effects of β-caryophyllene with l-arginine in type 2 diabetic rats. J. Food Biochem. 2020, 44, e13156. [Google Scholar] [CrossRef]
- Pinent, M.; Blay, M.; Bladé, M.C.; Salvadó, M.J.; Arola, L.; Ardévol, A. Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines. Endocrinology 2004, 145, 4985–4990. [Google Scholar] [CrossRef]
- Panwar, R.; Raghuwanshi, N.; Srivastava, A.K.; Sharma, A.K.; Pruthi, V. In-vivo sustained release of nanoencapsulated ferulic acid and its impact in induced diabetes. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.B.; Mohsin, M.; Bin, N.A.; Razu, B.A.; Hossain, M.T.; Mahzabeen, S.; Unnoor, N.; Muna, I.A.; Akhter, F.; Kabir, A.U. [6]-Gingerol, from Zingiber officinale, potentiates GLP-1 mediated glucose-stimulated insulin secretion pathway in pancreatic β-cells and increases RAB8/RAB10-regulated membrane presentation of GLUT4 transporters in skeletal muscle to improve hyperglycemia in Leprdb/db type 2 diabetic mice. BMC Complement. Altern. Med. 2017, 17, 395. [Google Scholar] [CrossRef]
- Araújo, L.S.; da Silva, M.V.; da Silva, C.A.; Borges, M.F.; Palhares, H.M.D.C.; Rocha, L.P.; Corrêa, R.R.M.; Rodrigues Júnior, V.; Dos Reis, M.A.; Machado, J.R. Analysis of serum inflammatory mediators in type 2 diabetic patients and their influence on renal function. PLoS ONE 2020, 15, e0229765. [Google Scholar] [CrossRef] [PubMed]
- Neufingerl, N.; Eilander, A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients 2021, 14, 29. [Google Scholar] [CrossRef]
- Medawar, E.; Huhn, S.; Villringer, A.; Witte, V.A. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 226. [Google Scholar] [CrossRef]
- Sarian, M.N.; Ahmed, Q.U.; Mat So’ad, S.Z.; Alhassan, A.M.; Murugesu, S.; Perumal, V.; Syed Mohamad, S.N.A.; Khatib, A.; Latip, J. Antioxidant and Antidiabetic Effects of Flavonoids: A Structure-Activity Relationship Based Study. BioMed Res. Int. 2017, 2017, 8386065. [Google Scholar] [CrossRef]
- Shang, Q.; Xiang, J.; Zhang, H.; Li, Q.; Tang, Y. The Effect of Polyhydroxylated Alkaloids on Maltase-Glucoamylase. PLoS ONE 2013, 8, e70841. [Google Scholar] [CrossRef]
| Medicinal Plants | Plants Parts | Traditional Uses | Recipients | Pharmacological Effects | Dose Administered | Duration of Treatment | References |
|---|---|---|---|---|---|---|---|
| Althaea officinalis L. | Leaves, flowers, roots | Diabetes, inflammation, skin infection, digestive and respiratory disorders | Alloxan-induced diabetic rats | ↓ Glutamate pyruvate transaminase (GPT), ↓ cholesterol, ↓ serum glucose levels, ↓ alkaline phosphate level (APL) | Powdered leaves (5% of the diet) | 28 days | [33] |
| Anethum graveolens L. | Leaves, stems, and seeds | Diabetes, digestive disorders, cancer, microbial infections, inflammation, hyperlipidemia, | STZ-induced diabetic rats | ↓ Inflammatory cytokines, ↓ triglycerides, ↓ total cholesterol, ↓ LDL-C, ↓ VLDL-C, ↓ blood glucose, ↓ AGEs, ↓ protein glycation, ↓ fructosamine level, ↓ fasting blood glucose | 300 mg/mL | 56 days | [38,39] |
| Allium sativum L. | Pulp | Diabetes, respiratory tract disorders, bacterial and fungal infections, wounds, cancers, CVDs, abdominal discomfort, diarrhea, cold, asthma, hay fever, inflammation, obesity | STZ-induced diabetic rats | ↓ LDL, ↓ total cholesterol, ↓ oxidative stress, ↑ HDL, ↓ blood glucose, ↓ intestinal glucose absorption, ↓ ROS generation, ↑ intracellular GSH content, ↓ endothelial dysfunction | 200 mg/kg | 21 days | [43,44,45] |
| Brassica oleracea L. | Leaves | Inflammation, digestive disorders, cancer, peptic ulcer, gout, detoxification | STZ-induced diabetic rats | ↑ Glucose homeostatic regulation, ↓ organ damage from T2DM, ↓ oxidative stress, ↓ obesity, ↓ LDL cholesterol, ↓ total cholesterol, ↓ lipid peroxidation, ↑ pancreatic β-cells functions | 250 mg/kg | 40 days | [52,53,55] |
| Cicer arietinum L. | Leaves, fruits, seeds | Diabetes, constipation, diarrhea, dyspepsia, flatulence, weight loss, inflammation, microbial infections | STZ-induced diabetic rats | ↑ Glycemic control, ↓ total cholesterol, ↓ α-amylase and α-glucosidase activity, ↑ insulin secretion and receptor activity, ↓ insulin deficiency, ↓ LDL | 200 mg/kg | 30 days | [58,61,62] |
| Cinnamomum verum J. Presl. | Dried bark (inner part) | Diabetes, arthritis, diarrhea, hemorrhoids, toothache, cough, cold, menstrual irregularities, inflammation, bacterial infections | T2DM patients | ↓ GI enzymes, ↑ insulin response and sensitivity, ↑ glucose uptake, ↑ glycogen synthesis, ↓ gluconeogenesis, ↓ AGE formation, ↑ phosphorylation, ↑ GLUT4 | 1000 mg/day | 84 days | [67,68,69] |
| Crocus sativus L. | Flower stigma | Diabetes, hepatic and cognitive disorders, lumbago, asthma, cough, bronchitis, CVDs, cancer, hyperlipidemia | Alloxan-induced diabetic rats | ↓ ROS, ↓ TG, ↓ TC, ↓ blood glucose, ↓ insulin resistance, ↑ insulin secretion and sensitivity, ↓ LDL, ↑ HDL, ↑ serum insulin, ↓body weight, ↓ lipid level | 25, 50, 100, 200 mg/kg | 60 days | [75,76] |
| Cuminum cyminum L. | Seeds | Diabetes, chronic diarrhea, dyspepsia, asthma, hypertension, inflammation, bronchitis, dizziness, eczema, gastrointestinal disturbances | T2DM patients | ↓ Blood glucose, ↓ glycosylated hemoglobin, ↓ body weight, ↓ phospholipid, ↓ cholesterol, ↓ free fatty acid, ↓ TG, ↑ insulin secretion, ↓ aldose reductase, ↓ α-amylase and ɑ-glucosidase activity, ↓ AGEs | 50,100 mg/kg | 56 days | [83,84,85,86] |
| Eugenia caryophyllata Thunb. | Unopened dried flower buds, stems, leaves, and fruits | Nausea, hepatic, bowel and stomach disorders, vomiting, microbial and protozoal infections, cholera, malaria, tuberculosis | STZ-induced diabetic rats | ↓ PEPCK, ↓ G6Pase gene expression, ↓ AChE, ↓ α-glycosidase, ↓ α-amylase, ↓ elevated blood sugar, ↓ lipid peroxidation | 100 mg/kg | 105 days | [88,90,91,92] |
| Foeniculum vulgare Mill. | Seeds and fruits | Diabetes, lactation, menstruation irregularities, libido, tumor, inflammatory disease, cancer, hepatic disorders | STZ-induced diabetic male rats | ↑ GSH, ↓ α- amylase and α- glucosidase activity, ↓ breakdown of carbohydrates, ↑ glycemic control, ↓ cholesterol, ↓ TG, ↓ LDL, ↑ HDL | 150 mg/kg | 28 days | [95,96,98] |
| Hordeum vulgare L. | Grains, leaves, sprouts | Diabetes, skin infections, arthritis, digestive diseases, weight loss, cancer, detoxification, lipid metabolism | STZ-induced diabetic rats | ↓ Blood glucose, ↓ cholesterol, ↓ hepatic cholesterol synthesis, ↓ α-glucosidase, ↓ α-amylase, ↑ insulin secretion | 100, 250, 500 mg/kg | 11 days | [101,102,103,104] |
| Juglans regia L. | Husks, kernels, shells, seeds, flowers, barks, and leaves | Diabetes, asthma, arthritis, eczema, stomachache, sinusitis, diarrhea, astringent, antiseptic | T2DM patients | ↓ Blood glucose, ↑ insulin, ↓ HbA1c, ↑ GLUT2, ↓ glucose intestinal absorption, ↓ FBG, ↓ TG | 100 mg/kg | 90 days | [105,106,107] |
| Lens culinaris L. | Seeds and sprouts | Diabetes, meat substitutes, obesity, inflammation, hyperlipidemia | STZ-induced diabetic mice | ↓ ROS, ↑ lipoprotein metabolism improvement, ↑ glycemic control, ↓ fasting blood glucose, ↓ serum blood glucose, ↑ gut motility, ↓ body weight | 100, 200, 400 mg/kg | 21 days | [113,114,115,117] |
| Nigella sativa L. | Seeds | Diabetes, digestive disorders, diarrhea, warts, toothaches, swellings, dyspnea, microbial infections, fever, inflammation, hypertension, allergy, infertility, tumors | STZ-induced diabetic rats | ↑ Serum insulin, ↓ serum glucose, ↓ LDL, ↓ TG, ↓ total cholesterol, ↑ proliferation of β-cells, ↓ oxidative stress | 300, 400 mg/kg | 84 days | [118,119,120,121] |
| Olea europaea L. | Fruit, pulp, leaves | Diabetes, hypertension, inflammation, diarrhea, respiratory and urinary tract infections, hemorrhoids, rheumatism, laxative, intestinal diseases, asthma, hyper uremia, hyperlipidemia | STZ-induced diabetic rats | ↓ α-glucosidase and digestive enzymes activity, ↓ postprandial hyperglycemia, ↑ insulin action, ↑ functionality and survival of β-cells | 1mL/100 bw/day (oil) | 42 days | [127,128,129,131,163] |
| Pinus gerardiana Wall. ex D. Don | Seeds, leaves, barks | Diabetes, hypertension, sepsis, fungal and microbial infections | STZ-induced diabetic rats | ↓ Body weight, ↓ oxidative stress, ↓ hyperglycemia, ↑ expression of PPARγ gene, ↑ Akt, ↑ insulin secretion, ↓ malondialdehyde, ↓ fasting blood glucose levels | 3% and 6% w/w, (Powder) | 42 days | [133,135] |
| Piper nigrum L. | Seeds, leaves, flowers, and fruits | Diabetes, menstrual problems, atrophic arthritis, digestive problems, influenza, bacterial infection, inflammation, fever, hypertension, cancer, depressants, diarrhea | Alloxan-induced diabetic rats | ↓ ROS generation, ↓ lipid peroxidation, ↓ lipogenesis, ↑ insulin secretion, ↓ blood glucose, ↓ triglyceride, ↓ total cholesterol, ↑ HDL, ↓ LDL, ↑ total antioxidant capacity | 50 mg/kg | 56 days | [137,141,143] |
| Pistacia vera L. | Seeds, leaves, fruits | Diabetes, coughs, stomach diseases, asthma, sores, chest ailments, rheumatism, trauma, gynecological ailments, hemorrhoids | Pre-diabetic patients | ↓ Fasting blood glucose, ↓ insulinemia, ↓ Serum IL-6, ↓ fructosamine, ↓ insulin resistance, ↓ LDL, ↓ malondialdehyde, ↓ proinflammatory cytokines, ↓ glucose absorption | 57 g/day | 28 days | [145,146,149,164] |
| Vitis vinifera L. | Dried fruits | Diabetes, cancer, obesity, inflammation, hyperlipidemia | T2DM patients | ↓ LDL oxidation and LDL-cholesterol, ↓ blood glucose control, ↓ postprandial glucose levels, ↓ HbA1c, ↓ blood pressure | 36 g/day | 168 days | [152,154,155] |
| Zingiber officinale Roscoe | Roots and rhizomes | Diabetes, digestive disorders, nausea, rheumatism, respiratory tract infection, cough, hypercholesterolemia, neurological diseases, asthma, stroke, constipation, cancer | STZ-induced diabetic rats | ↓ Superoxide anion, ↓ hydroxyl radicals, ↓ α-glucosidase and α-amylase activity, ↓cholesterol, ↓ serum glucose, ↓ triglyceride, ↑ HDL, ↑ insulin sensitivity | 400 mg/kg | 56 days | [158,159,160] |
| Medicinal Plants | Parts Used | Phytoconstituent Studied | Diabetic Model | Dose Administered | Duration of Treatment | Pharmacological Effects of the Phytoconstituents Used | References |
|---|---|---|---|---|---|---|---|
| Althaea officinalis L. | Leaves, roots, seeds | Lauric acid | Insulin resistance induced in macrophage THP-1 cells | 5 μM–50 μM | 1 day | Increases glucose uptake in skeletal muscles and improves mitochondrial dysfunction, insulin sensitivity, and GLUT-1 and GLUT-3 expression | [165] |
| Anethum graveolens L. | Leaves, seeds | Carvone | STZ-induced diabetic rats | 25, 50, 100 mg/kg | 30 days | Alleviates insulin resistance, improves insulin secretion, and reverses glycoprotein abnormalities | [166] |
| Allium sativum L. | Fruits | Allicin | STZ-induced diabetic rats | 15, 30, 45 mg/kg | 84 days | Improves insulin sensitivity and glucose tolerance, ameliorates diabetes-induced morphological alterations in the kidney, and decreases FBG and triglyceride | [167] |
| Brassica oleracea L. | Leaves | Anthocyanin | T2DM patients | 160 mg/kg | 168 days | Improves lipid metabolism and insulin resistance, decreases LDL, total cholesterol, and postprandial glucose, and ameliorates diabetic complications | [168] |
| Cicer arietinum L. | Seeds | Quercetin | Alloxan-induced diabetic rats | 50 mg/kg | 30 days | Decreases blood glucose, total cholesterol, total bilirubin, creatinine, and oxidative stress, regulates glucose homeostasis and improves insulin resistance | [169] |
| Cinnamomum verum J. Presl. | Bark | Cinnamaldehyde | STZ-induced diabetic rats | 5, 10, 20 mg/kg | 45 days | Elevates HDL level, plasma insulin, and hepatic glycogen and decreases serum glucose, total cholesterol, triglyceride, and LDL level | [170] |
| Crocus sativus L. | Flower stigma | Crocin | T2DM patients | 15 mg/kg | 84 days | Enhances GLUT-4 expression, inhibits TNF-α, IL-6, alleviates blood glucose, and improves glucose homeostasis and insulin resistance | [171] |
| Cuminum cyminum L. | Seeds | Cuminaldehyde and cuminol | STZ-induced diabetic rats | 5, 10 mg/kg | 45 days | Increases insulin secretion and insulin sensitivity, lowers blood glucose, provides β-cell protection, and improves lipid profile | [172] |
| Eugenia caryophyllata Thunb. | Flower buds, leaves, stem, fruits | Eugenol | STZ-induced diabetic mice | 100 mg/kg bw (I.P. route) | 45 days | Lowers blood glucose, blood lipids, and AGEs formation and inhibits α-amylase and α-glucosidase enzymes | [173] |
| Foeniculum vulgare Mill. | Seeds | Kaempferol | STZ-induced diabetic mice | 50 mg/kg | 84 days | Suppresses gluconeogenesis, enhances glucose uptake in skeletal muscles, and restores hexokinase activity | [174] |
| Hordeum vulgare L. | Grains, leaves, sprouts | β-glucan | STZ-induced diabetic rats | 80 mg/kg | 28 days | Alleviates diabetic complications, reduces oxidative stress, and lowers blood glucose, total cholesterol, total triglyceride, and LDL level | [175] |
| Juglans regia L. | Husks, kernels, seeds, flowers, bark, leaves | β-carotene | STZ-induced diabetic rats | 10, 20 mg/kg | 14 days | Improves glucose metabolism and lipid accumulation, lowers inflammatory cytokines, nitric oxide production, and oxidative stress, and enhances glucose uptake in skeletal muscle | [176] |
| Lens culinaris L. | Seeds, sprouts | Saponins | STZ-induced diabetic rats | 100, 200 mg/kg | 14 days | Ameliorates postprandial hyperglycemia and diabetic complications and inhibits α-glucosidase and aldose reductase enzymes | [177] |
| Nigella sativa L. | seeds | Thymoquinone | STZ-induced diabetic rats | 50 mg/kg | 28 days | Attenuates blood glucose, lipid peroxidase, nitric oxide production, and oxidative stress and alleviates diabetic nephropathy | [178] |
| Olea europaea L. | Fruits, leaves | Lutein | ARPE-19 cells | 0.5–1 μM | 24 h | Ameliorates diabetic retinopathy and hyperglycemia, Improves SOD2, HO-1, Nrf2, GSH and catalase regulation | [179] |
| Pinus gerardiana Wall. ex D. Don | Nuts | Linoleic acid | PTPN1, PTPN9, PTPN11 cell lines | 0.5–300 μM | 7 days | Inhibits the catalytic activity of PTPN1, PTPN9, and PTPN11 and improves glucose uptake by activating AMPK and Akt pathway | [180] |
| Piper nigrum L. | Seeds, flowers fruits, leaves | β-caryophyllene | STZ-induced diabetic rats | 200 mg/kg | 42 days | Decreases glucose absorption and increases glucose uptake in skeletal muscles, ameliorates glucose tolerance, pancreatic cell damage, oxidative stress, and lipid and blood glucose levels | [181] |
| Pistacia vera L. | Fruits, nuts, leaves | Procyanidins | STZ-induced db/db type 2 diabetic mice | 250 mg/kg | 45 days | Enhances GLUT-4 translocation and glucose uptake on skeletal muscles, possesses insulinotropic ad anti-hyperglycaemic effects | [182] |
| Vitis vinifera L. | Fruits | Ferulic acid | STZ-induced diabetic rats | 10 mg/kg | 14 days | Alleviates body weight, and blood glucose, attenuates diabetes-associated symptoms, and lowers total triglyceride, total cholesterol, LDL and VLDL levels | [183] |
| Zingiber Officinale Roscoe | Roots | Gingerol | Type 2 diabetic mice (Leprdb/db) | 200 mg/kg | 28 days | Induces insulin secretion, elevates plasma GLP-1, activates cAMP, PKA, and CREB in the pancreatic islets, and enhances GLUT-4 translocation | [184] |
| Medicinal Plant | Antidiabetic Phytoconstituent | Chemical Structure |
|---|---|---|
| Althaea officinalis L. | Lauric acid | 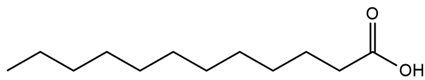 |
| Anethum graveolens L. | Carvone | 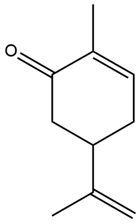 |
| Allium sativum L. | Allicin | 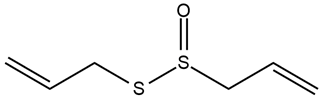 |
| Brassica oleracea L. | Anthocyanins | 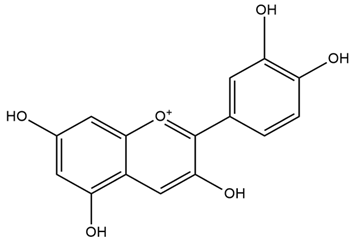 |
| Cicer arietinum L. | Quercetin | 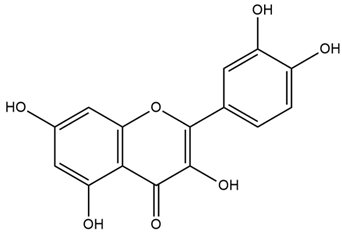 |
| Cinnamomum verum J. Presl. | Cinnamaldehyde |  . . |
| Crocus sativus L. | Crocin |  |
| Cuminum cyminum L. | Cuminaldehyde |  |
| Eugenia caryophyllata Thunb. | Eugenol | 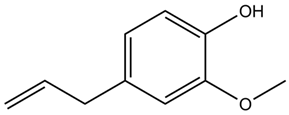 |
| Foeniculum vulgare Mill. | Kaempferol |  |
| Hordeum vulgare L. | β-glucan | 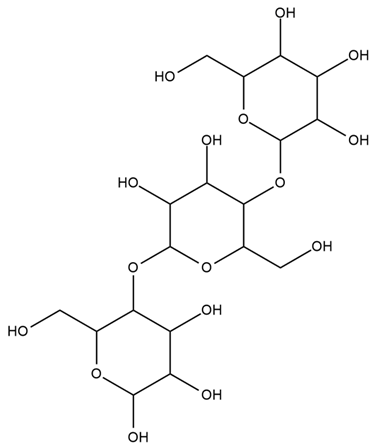 . . |
| Juglans regia L. | β-carotene | 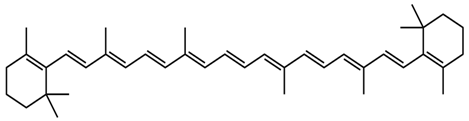 |
| Lens culinaris L. | Saponins | 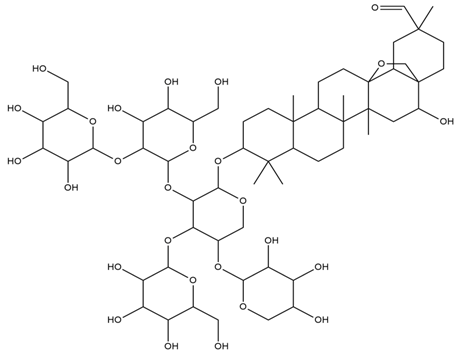 |
| Nigella sativa L. | Thymoquinone | 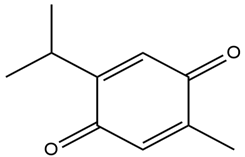 |
| Olea europaea L. | Lutein |  |
| Pinus gerardiana Wall. ex D. Don | Linoleic acid |  |
| Piper nigrum L. | β-caryophyllene |  . . |
| Pistacia vera L. | Procyanidins | 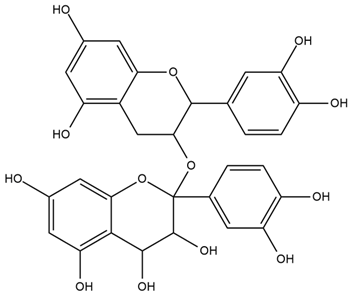 |
| Vitis vinifera L. | Ferulic acid |  |
| Zingiber Officinale Roscoe | Gingerol |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, P.; Samia, J.F.; Khan, J.T.; Rafi, M.R.; Rahman, M.S.; Rahman, A.B.; Abdel-Wahab, Y.H.A.; Seidel, V. Protective Effects of Medicinal Plant-Based Foods against Diabetes: A Review on Pharmacology, Phytochemistry, and Molecular Mechanisms. Nutrients 2023, 15, 3266. https://doi.org/10.3390/nu15143266
Ansari P, Samia JF, Khan JT, Rafi MR, Rahman MS, Rahman AB, Abdel-Wahab YHA, Seidel V. Protective Effects of Medicinal Plant-Based Foods against Diabetes: A Review on Pharmacology, Phytochemistry, and Molecular Mechanisms. Nutrients. 2023; 15(14):3266. https://doi.org/10.3390/nu15143266
Chicago/Turabian StyleAnsari, Prawej, Jannatul F. Samia, Joyeeta T. Khan, Musfiqur R. Rafi, Md. Sifat Rahman, Akib B. Rahman, Yasser H. A. Abdel-Wahab, and Veronique Seidel. 2023. "Protective Effects of Medicinal Plant-Based Foods against Diabetes: A Review on Pharmacology, Phytochemistry, and Molecular Mechanisms" Nutrients 15, no. 14: 3266. https://doi.org/10.3390/nu15143266
APA StyleAnsari, P., Samia, J. F., Khan, J. T., Rafi, M. R., Rahman, M. S., Rahman, A. B., Abdel-Wahab, Y. H. A., & Seidel, V. (2023). Protective Effects of Medicinal Plant-Based Foods against Diabetes: A Review on Pharmacology, Phytochemistry, and Molecular Mechanisms. Nutrients, 15(14), 3266. https://doi.org/10.3390/nu15143266









