In this study, we performed a bibliometric analysis of the effects of intestinal microbes on obesity-related studies from 2013 to 2022 using the core collection of WoSCC to comprehensively understand global research trends and hotspots and provide references for researchers in this field or those who want to become involved in the field.
4.1. Global Trends in Effects of Intestinal Microbes on Obesity
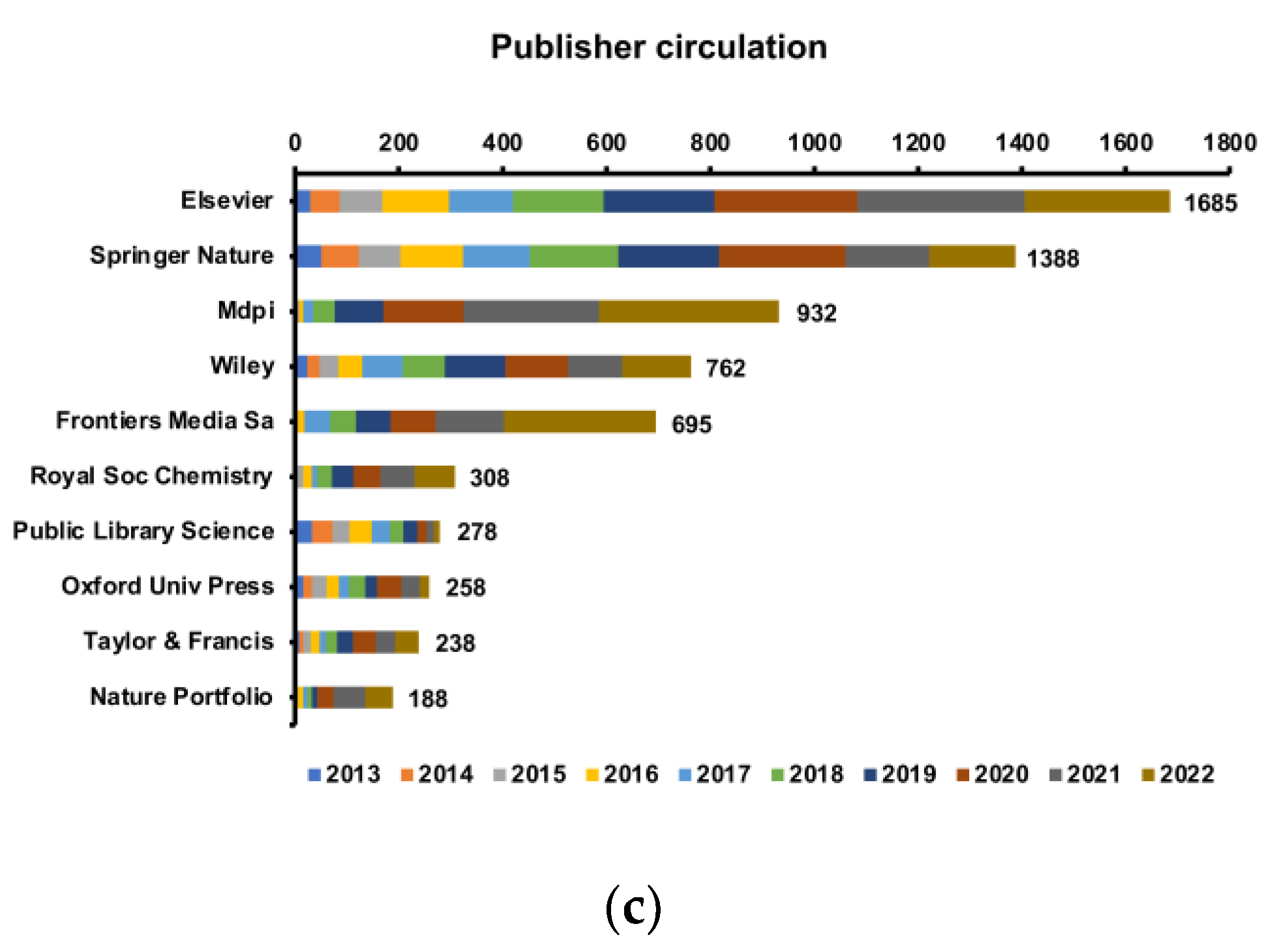
The amount of annual scientific production is an important indicator of development in an academic field. In our study, a total of 8888 original articles from 1392 journals met the inclusion criteria. The article annual growth rate reached 22.93%, and the annual growth rate from 2019 to 2020 was the highest, shedding light on the increasing attention and expanding research exploration dedicated to this field.
The closeness of collaboration between countries/regions, institutions, authors, and journals was assessed, which can help to find the laws of scientific research cooperation, guiding more effective scientific research activities, and promoting potential collaborative opportunities for other groups.
According to
Table S1, the total number of publications in the USA (87,065) was greater than that in China (65,250) and far greater than that in France (13,809), Canada (13,496), and the other top 10 most cited countries. The total citations of the United States and China are far more than the other top 10 most cited country citations combined. Without doubt, the United States and China demonstrate their position as leaders in the field. Notably, Israel, with 49 publications, was the most cited country per article (average article citations, 124.18), suggesting that the quality of research on the effects of intestinal microbes on obesity in Israel is very high. A similar story is unfolding in many European countries such as Belgium (average article citations, 107.85) and Sweden (average article citations, 103.95), etc., which sets a model for other countries/regions in this research field (
Tables S1 and S4). To improve the quality of articles, the reliability of the data source and the rigor of the experimental design may be the first factors affecting the quality of publications, and more attention should be given to these aspects in future studies.
According to
Figure 2b, the bottom right is most dominated by universities from China, while the upper left is most dominated by schools in North America or Europe. Nevertheless, more cooperation between the two clusters could be conducted to catalyze breakthrough progress in research on the effects of intestinal microbes on obesity. Four of the top 10 institutions are based in China, followed by the United States with three, which are thereby maximizing regional advantages and demonstrating the dominance of the United States and China in the field. This may partly explain why China and the United States consistently maintain a high quantity of publications. The University of Copenhagen in Denmark was the most productive institution worldwide, followed by the Chinese Academy of Sciences in China, indicating that these two institutions participated in the most collaborations with other institutions worldwide. Although Spain and Canada ranked third and fourth in terms of total publications, only one of the Spanish research institutions ranked in the top 10, indicating a lack of institutions with professional and research stature in terms of the effects of intestinal microbes on obesity research (
Table 1 and
Table 2). The most effective organizations and groups are leading the trends on the effects of intestinal microbes on obesity research; thus, further study at these institutions will ensure continuous future development in this field.
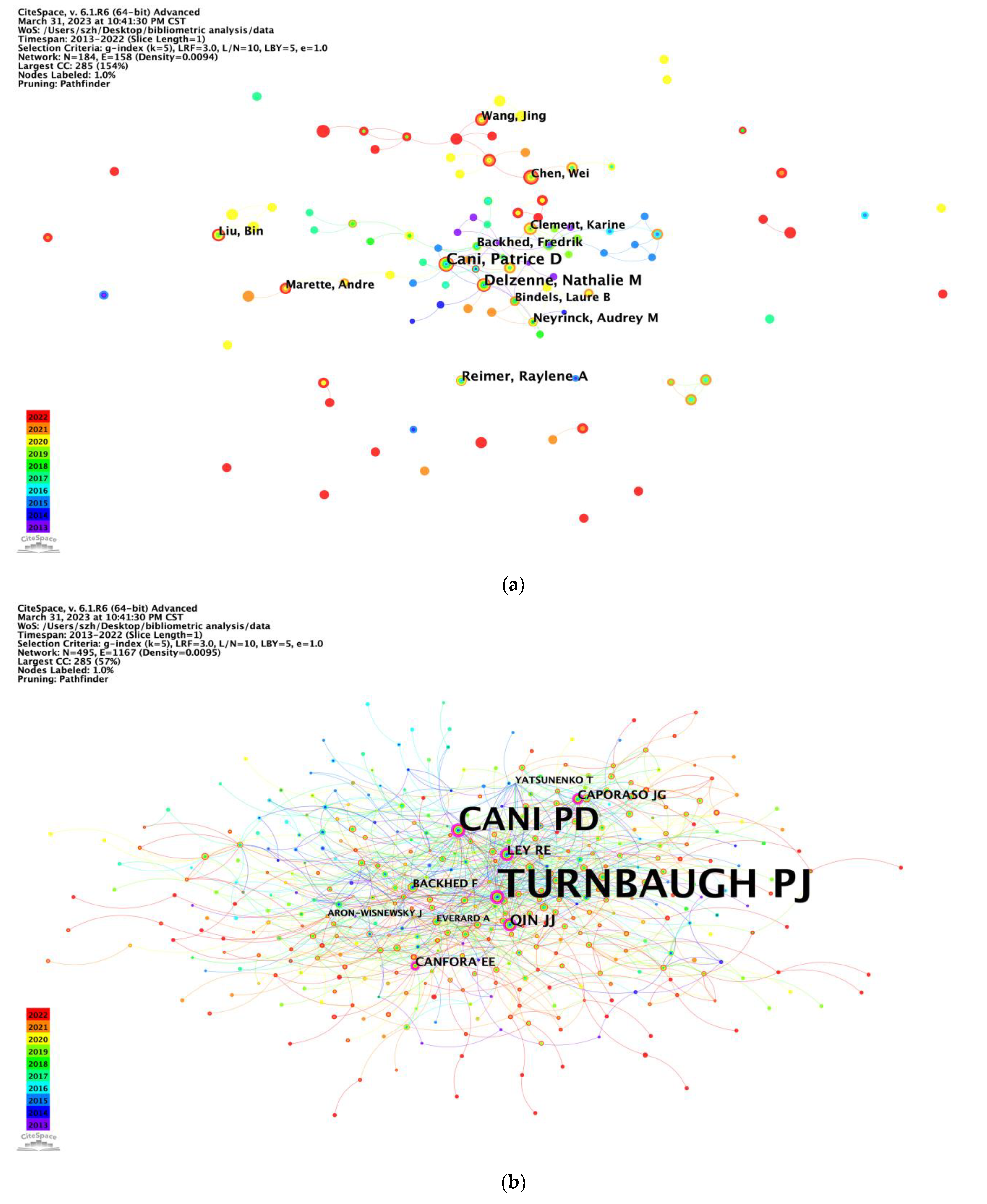
Notably, Prof. Patrice D. Cani from Université Catholique de Louvain, Belgium, has published the most articles with the highest centrality, mainly focusing on physiology, molecular metabolism, and nutrition. Researchers have emphasized the significant roles played by the intestinal microbiota in the development of diseases associated with overweight and obesity, including type 2 diabetes, cardiovascular diseases, and certain cancers. Prof. Patrice D. Cani discovered a very particular bacterium called
Akkermansia muciniphila, which has beneficial effects on health by strengthening the intestinal barrier, decreasing body weight and fat mass gain while decreasing insulin resistance and diabetes [
16]. Recently, his team discovered a new bacterium called
Dysosmobacter welbionis, a completely new genus isolated from the human intestine. Interestingly, they found that this bacterium was present in the intestinal microbiota of the general population but was less abundant in the intestines of individuals with obesity and type 2 diabetes. Experimental studies have demonstrated that the administration of this bacterium improves the health of obese and diabetic mice [
17]. The most cited co-author, Peter J. Turnbaugh, is from the University of California San Francisco, United States. He employed interdisciplinary approaches utilizing preclinical models and human cohorts to investigate the mechanisms by which the gut microbiome influences nutrition and pharmacology. His team observed that the consumption of diets exclusively composed of animal or plant products led to rapid changes in the structure of the gut microbial community. These changes were significant enough to override any pre-existing inter-individual differences in microbial community gene expression [
18]. This research finding was published in the prestigious journal Nature and has become the most highly cited article in the field.
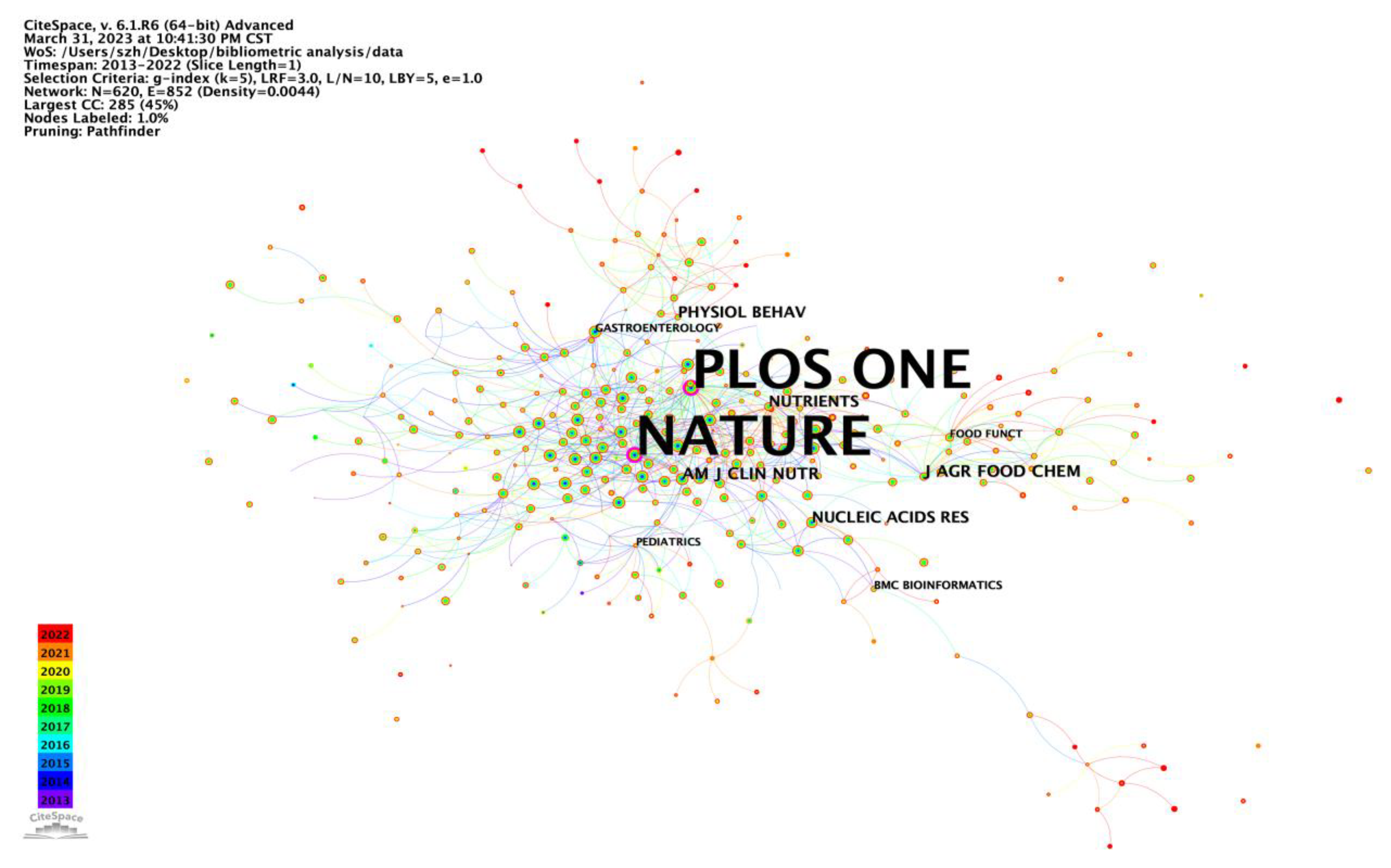
Conducting an analysis of the characteristics of international peer-reviewed journals enables us to gain insights into current research directions and keep pace with the cutting-edge research in the field. Among the top 10 influential journals in the field of intestinal microbe effects on obesity research, three of them (Nutrients, Scientific reports, PLOS ONE) were also among the top 10 co-cited journals. Undoubtedly, Nutrients, Scientific Reports, Food & Function, and PLOS ONE have emerged as the leading publications, publishing the most relevant articles and maintaining their popularity among researchers who wish to stay updated on the latest research trends. Notably, three of the top 10 co-cited references were published in Nature, one in PNAS, and one in Gut (
Table 8). Remarkably, Nature had an impact factor (IF) of 69.504 (2021), followed by Gut (IF, 31.795) and PNAS (IF, 12.779), underscoring their significant contributions in the field of intestinal microbe effects on obesity research.
4.3. Current Hotspots and Field Development Predictions of the Effects of Intestinal Microbes on Obesity Research
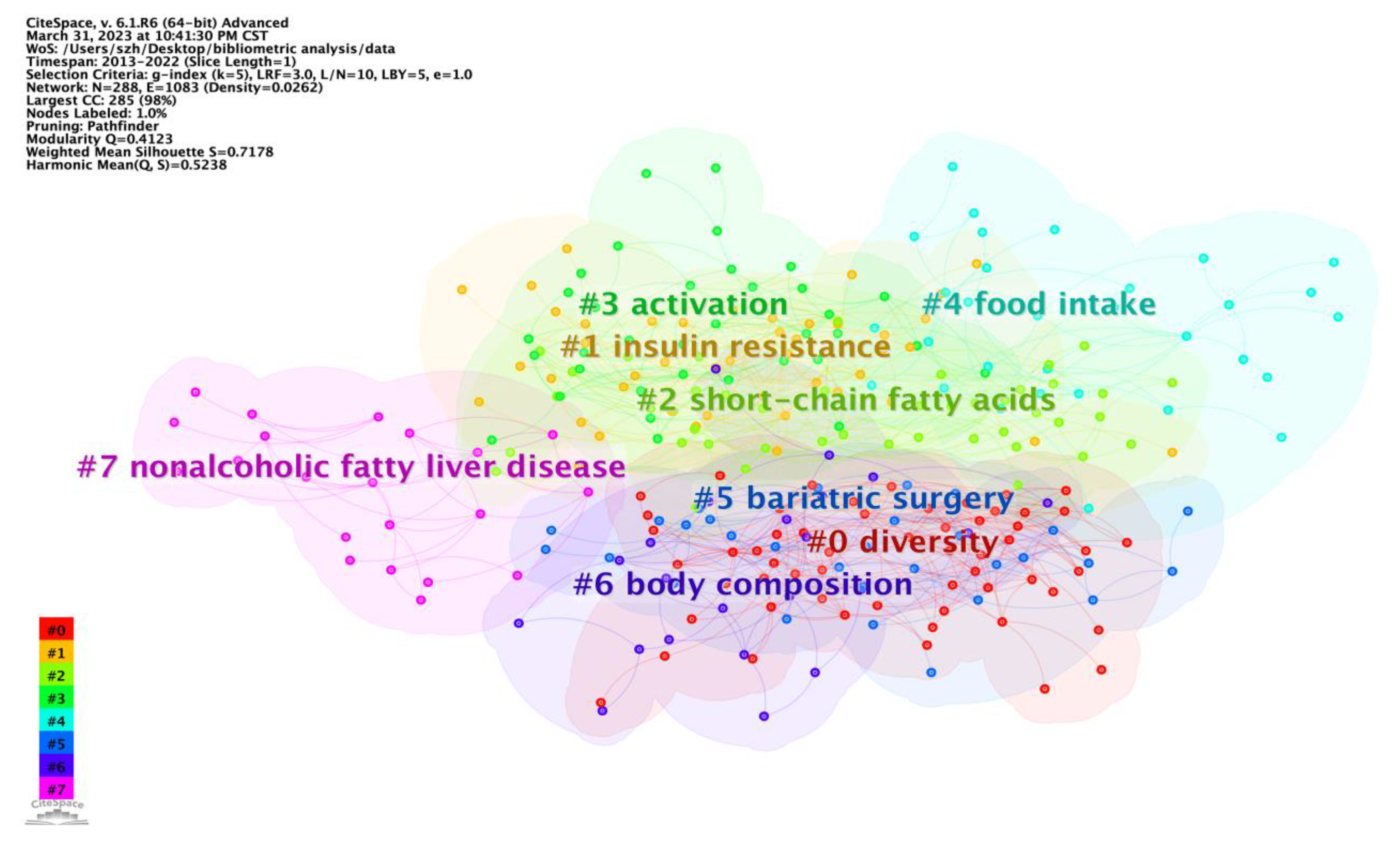
Keywords with high frequency are usually used to accurately reveal the main research interests and hotspots in the field within a certain period. Combined with burst detection, which helps researchers to precisely catch up to the research trends from numerous studies, could effectively capture the dramatic increases in references or keywords in one research field within a specified period. Therefore, it served as an important indicator of research hotspots or research frontiers over time (
Table S2). The “obese patient” and “serum” burst separately in 2019 and 2020 and continue to burst until now, which indicated that the two topics have received continuous attention in recent years and might be the main trends of research on the effect of intestinal microbes on obesity. During the co-citation reference burst value analysis, five references stood out prominently, focusing primarily on key bacterial metabolites such as short-chain fatty acids, succinate, and secondary bile acids. These references also discuss the benefits of
A. muciniphila supplementation in improving metabolic parameters in obese patients. Additionally, these references present the latest tools for microbiome data analysis, which warrants thorough exploration and investigation (
Table S3). In conjunction with the keywords that continue to exhibit high burst values, we conducted an analysis of the current research hotspots and identified future research trends.
4.3.1. Intestinal Microbes May Regulate Obesity through Bacterial Metabolites
There are complex interactions between the host and intestinal microbes in carbohydrate, amino acid, lipid, and nucleic acid metabolism. Intestinal microbes can use their respective metabolites to maintain intestinal viability while affecting the development, homeostasis and function of the host immune system through nutrition- and metabolite-dependent mechanisms [
27]. Small molecules, such as vitamins, fatty acids, amino acids, and bile acids, regulate host-intestinal metabolic homeostasis by binding to specific host membranes or nuclear receptors [
28,
29]. Short-chain fatty acids (SCFAs), as one of the major products from microbial fermentative activity in the gut [
30], can directly activate G protein-coupled receptors, inhibit histone deacetylases, and serve as energy substrates [
31,
32]. G protein-coupled receptors are also called free fatty acid receptors (FFARs) since they sense free fatty acids. SCFAs facilitate gut–brain axis signaling by activating FFAR2 and FFAR3. This leads to increased satiety and reduced food intake, ultimately impacting the host’s body weight [
33,
34] and other physiological responses, such as immunity, intestinal transit time, and inflammation [
35,
36,
37,
38].
Bile acids, such as cholic acid and chenodeoxycholic acid, can facilitate dietary fat and fat-soluble vitamin absorption. Modified bile acids are referred to as secondary bile acids and include deoxycholic acid, lithocholic acid, and ursodeoxycholic acid [
39]. Bile acids can directly and rapidly affect the metabolism of bacteria, including membrane damage and disruption of amino acid, nucleotide and carbohydrate metabolism, while short-term exposure to bile acids significantly affects host metabolism by altering the bacterial community structure [
40]. Research has found that supplementation with
Parabacteroides distasonis in mice can modulate the composition of bile acids in the gut, resulting in decreased weight gain, reduced hyperglycemia, and alleviated hepatic steatosis in ob/ob and high-fat diet (HFD)-fed mice [
41].
According to the above analysis, although notable advances in the effects of intestinal microbes on obesity have been made, our understanding of the interrelationships between them remains descriptive, and we still have numerous gaps to fill.
4.3.2. Microbiomes May Act as Metabolic Markers of Obesity
Human obesity is a heterogeneous condition in the context of pathogenesis, pathophysiology, and therapeutic responsiveness. Studies of alterations in the genome—the microbial gut metagenome—may define subsets of adult individuals with different metabolic risk profiles, which could contribute to resolving some of the heterogeneity associated with adiposity-related phenotypes [
21]. With the help of sequencing, recent research found that the richness of the human gut microbiome correlated with human metabolic markers. Individuals with a low bacterial richness were characterized by more marked overall adiposity, insulin resistance, and dyslipidemia as well as a more pronounced inflammatory phenotype when compared with high bacterial richness individuals [
21]. Correspondingly, higher gut microbiome gene richness and
A. muciniphila abundance could exhibit the healthiest metabolic status, particularly in fasting plasma glucose, plasma triglycerides, and body fat distribution [
19]. Research has also proven that transmissible and modifiable interactions between diet and microbiota influence host biology. The transformation correlated with invasion of members of
Bacteroidales from Ln into Ob microbiota that prevented development of increased adiposity and body mass in Ob cage mates and transformed their microbiota metabolic profile to a lean-like state. Therefore, it may be possible to intervene in obesity by targeting the gut microbiota [
22]. The gut microbiome can also rapidly respond to an altered diet, and a dynamic balance can be achieved between intestinal microbes and diet immediately, indicating that not only a long-term diet but also a short-term diet can influence the gut microbiome [
18].
All these findings suggest that gut microbiome richness is a key factor in maintaining the homeostasis of human health and may act as a metabolic marker of obesity
4.3.3. Gut Microbes Could Be a Target for Obesity Treatment
Bariatric surgery is considered the only effective and sustainable weight loss method for obese patients. There is a 50–70% decrease in body weight and fat mass after surgical procedures such as Roux-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) [
42]. Nevertheless, obese patients undergoing bariatric surgery may experience overgrowth of small intestinal bacteria [
43], a condition that snags with weight loss and increases the risk of micronutrient deficiency that appears to be harmful for the configuration and composition of intestinal microbiota [
44,
45]. To maintain the effect of surgery and avoid weight rebound, it is important for obese patients to correct the microbial balance and improve microbiota-host interactions with specific interventions [
46]. Research has also shown that the elevated pH resulting from RYGB could ensure the survival of probiotic bacteria, making it possible for surgical patients to receive probiotic therapy (80). Probiotics are a kind of active microorganism beneficial to the host that colonizes the human intestinal tract and reproductive system, and they can improve the host microecological balance and play a beneficial role. Bariatric surgery is often followed by an increase in
Streptococcaceae and a decline in
Bifidobacteriaceae [
42]. Proper supplementation with probiotics can compensate for the intestinal microbial imbalance caused by surgery. Moreover, recent research showed that probiotic intervention could increase the levels of peptide YY and GLP-1 in mice [
47], reduce the level of intestinal inflammation [
48], and induce the production of anti-inflammatory cytokines [
49]. Meanwhile, intestinal peptide signals activate the gut–brain axis, which, in turn, exerts endocrine effects on other organ systems, especially the brain, regulating appetite, metabolism, and other dietary behaviors [
33,
34,
50,
51]. Consequently, weight loss and reduced insulin resistance occur.
Weight-loss intervention by bariatric surgery partially reversed obesity-associated microbial and metabolic alterations in obese individuals [
26]. This means that external interventions can affect intestinal microbes. It may be possible to intervene in obesity by targeting the gut microbiota [
22], and there is already evidence proving this speculation.
A. muciniphila is a mucin-degrading bacterium that resides in the mucus layer. A research study found that
A. muciniphila decreased in obese and type 2 diabetic mice, and
A. muciniphila treatment reversed high-fat diet-induced metabolic disorders, including fat-mass gain, metabolic endotoxemia, adipose tissue inflammation, and insulin resistance [
16]. Pasteurized
A. muciniphila could enhance its capacity to reduce fat mass development, insulin resistance, and dyslipidemia. Moreover, Amuc_1100, a purified membrane protein from
A. muciniphila, could improve the gut barrier and partly recapitulate the beneficial effects of the bacterium [
23]. All these findings are of great driving significance, indicating that it is feasible to use microbes to treat obesity, but more research is needed to support this hypothesis. However, due to the heterogeneity of experimental techniques, methods, and objects, the effectiveness and safety of microbial product intervention in improving obesity against intestinal microbiota need to be further verified, and the mechanism remains unclear. Few studies have focused on the development of new functional microbiological products, and most of them have been carried out in mice. Before microbial products can be reasonably and effectively used as treatment for obesity, a large number of studies are needed, especially randomized controlled clinical trials.
4.3.4. New Technology Promotes In-Depth Research on the Role of Intestinal Microbes in Obesity
Among the top 10 co-cited references, we found that second-generation sequencing was a vital technology that relies on its high-throughput characteristics, making it easy to sequence the transcriptome or genome of a species [
52]. With the development of sequencing technology, a large amount of data is generated. To make more accurate and full use of sequencing data, software and data analysis platforms have also been developed. An open-source software package named DADA2 was used for modeling and correcting Ill umina-sequenced amplicon errors. With the help of this software, researchers could accurately reconstruct amplicon-sequenced communities at the highest resolution, which ensured the accuracy of the research to the greatest extent [
24]. Another utility tool named QIIME 2 could serve not only as a marker-gene analysis tool but also as a multidimensional and powerful data science platform that can be rapidly adapted to analyze diverse microbiome features [
25]. These tools help to drive rapid development in microbiome research (
Table 8). The serum metabolome has emerged as a technique that focuses on defining the functional status of host–microbial relationships in biological specimens, which can reflect the dynamic changes in metabolites and explore disease-related metabolites or dysregulated metabolic pathways (metabolome analysis for investigating host–gut microbiota interactions). A top 10 co-cited reference showed the gut microbiome and serum metabolome alterations in obesity that patients after bariatric surgery not only appeared partially to reverse obesity-associated microbial alterations but also were accompanied by metabolic alterations, including the decreased abundance of
Bacteroides thetaiotaomicron and the elevated serum glutamate concentration, which also suggests that it may be possible to intervene in obesity by targeting the gut microbiota [
26].
4.4. Strengths and Limitations
This bibliometric study conducted a systematic analysis of the basic situation, research hotspots, and trends in effects of intestinal microbes on obesity from a visualization perspective. The results of the bibliometric study were objective and accurate, which could provide a comprehensive guide for academics who are already working or wish to work in this field. Nevertheless, there are still some limitations in our study. First, owing to the nature of the CiteSpace software, our data are filtered only from the WoSCC database, which is not sufficiently comprehensive and may lead to data omission. Second, our results were processed by CiteSpace software with certain algorithms, which could lead to bias in some of the results. Finally, only English articles were included from the database and analysis, potentially leading to a source bias.