Concept of an Intervention for Sustainable Weight Loss in Postmenopausal Women with Overweight—Secondary Analysis of a Randomized Dietary Intervention Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design and Participants
2.2. Intervention
2.3. Follow-Up
2.4. Anthropometry and REE
2.5. Statistics
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mensink, G.B.M.; Schienkiewitz, A.; Haftenberger, M.; Lampert, T.; Ziese, T.; Scheidt-Nave, C. Übergewicht und Adipositas in Deutschland: Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013, 56, 786–794. [Google Scholar] [CrossRef]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Schienkiewitz, A.; Mensink, G.B.M.; Scheidt-Nave, C. Comorbidity of overweight and obesity in a nationally representative sample of German adults aged 18–79 years. BMC Public Health 2012, 12, 658. [Google Scholar] [CrossRef]
- Villareal, D.T.; Chode, S.; Parimi, N.; Sinacore, D.R.; Hilton, T.; Armamento-Villareal, R.; Napoli, N.; Qualls, C.; Shah, K. Weight loss, exercise, or both and physical function in obese older adults. N. Engl. J. Med. 2011, 364, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.M.; Wells, J.C.K.; Smith, S.R.; Stephan, B.C.M.; Siervo, M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin. Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef]
- Poehlman, E.T. Menopause, energy expenditure, and body composition. Acta Obstet. Gynecol. Scand. 2002, 81, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Yadigar, S.; Yavuzer, H.; Yavuzer, S.; Cengiz, M.; Yürüyen, M.; Döventaş, A.; Erdinçler, D.S. Primary Sarcopenia in Older People with Normal Nutrition. J. Nutr. Health Aging 2016, 20, 234–238. [Google Scholar] [CrossRef]
- Porter Starr, K.N.; Pieper, C.F.; Orenduff, M.C.; McDonald, S.R.; McClure, L.B.; Zhou, R.; Payne, M.E.; Bales, C.W. Improved Function With Enhanced Protein Intake per Meal: A Pilot Study of Weight Reduction in Frail, Obese Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1369–1375. [Google Scholar] [CrossRef]
- Bales, C.W.; Porter Starr, K.N.; Orenduff, M.C.; McDonald, S.R.; Molnar, K.; Jarman, A.K.; Onyenwoke, A.; Mulder, H.; Payne, M.E.; Pieper, C.F. Influence of Protein Intake, Race, and Age on Responses to a Weight-Reduction Intervention in Obese Women. Curr. Dev. Nutr. 2017, 1, e000703. [Google Scholar] [CrossRef]
- Leidy, H.J.; Carnell, N.S.; Mattes, R.D.; Campbell, W.W. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity 2007, 15, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Armstrong, C.L.H.; Leidy, H.J.; Campbell, W.W. Normal vs. high-protein weight loss diets in men: Effects on body composition and indices of metabolic syndrome. Obesity 2013, 21, E204–E210. [Google Scholar] [CrossRef]
- Mathers, J.C. Paving the way to better population health through personalised nutrition. EFSA J. 2019, 17, e170713. [Google Scholar] [CrossRef]
- Rein, M.; Ben-Yacov, O.; Godneva, A.; Shilo, S.; Zmora, N.; Kolobkov, D.; Cohen-Dolev, N.; Wolf, B.-C.; Kosower, N.; Lotan-Pompan, M.; et al. Effects of personalized diets by prediction of glycemic responses on glycemic control and metabolic health in newly diagnosed T2DM: A randomized dietary intervention pilot trial. BMC Med. 2022, 20, 56. [Google Scholar] [CrossRef]
- Verma, M.; Hontecillas, R.; Tubau-Juni, N.; Abedi, V.; Bassaganya-Riera, J. Challenges in Personalized Nutrition and Health. Front. Nutr. 2018, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Yoon, S.R.; Lee, J.H.; Kim, H.; Kim, O.Y. Importance of Adherence to Personalized Diet Intervention in Obesity Related Metabolic Improvement in Overweight and Obese Korean Adults. Clin. Nutr. Res. 2019, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, C.; Dawczynski, C.; Henze, A.; Simon, M.-C. Personalized dietary recommendations for weight loss: A scientific perspective from various angles. Ernährungsumschau 2021, 68, 26–35. [Google Scholar] [CrossRef]
- Wilson-Barnes, S.; Gymnopoulos, L.; Dimitropoulos, K.; Solachidis, V.; Rouskas, K.; Russell, D.; Oikonomidis, Y.; Hadjidimitriou, S.; Maria Botana, J.; Brkic, B.; et al. PeRsOnalised nutriTion for hEalthy livINg: The PROTEIN project. Nutr. Bull. 2021, 77–87. [Google Scholar] [CrossRef]
- Celis-Morales, C.; Livingstone, K.M.; Marsaux, C.F.; Macready, A.L.; Fallaize, R.; O’Donovan, C.B.; Woolhead, C.; Forster, H.; Walsh, M.C.; Navas-Carretero, S.; et al. Effect of personalized nutrition on health-related behaviour change: Evidence from the Food4Me European randomized controlled trial. Int. J. Epidemiol. 2017, 46, 578–588. [Google Scholar] [CrossRef]
- Hoffmann, L.; Peuker, M.; Hager, U.; Wiegand, T.; Amerschläger, K.; Weidenbach, I.; Radziwill, R.; Kohlenberg-Müller, K. What are the challenges and potential advantages of implementing process guided methods in the practice of nutrition counselling and dietetic therapy?: Results for Dietetic Assessment and Dietetic Diagnosis. Ernährungsumschau 2023, 70, 2–11. [Google Scholar] [CrossRef]
- Peuker, M.; Lachmann, K.; Hoffmann, L.; Wiegand, T.; Siebert, H.; Kohlenberg-Müller, K. Implementing process-guided methods in nutrition counselling and dietetic therapy—What does current practice look like?: Results of a descriptive pilot study. Ernährungsumschau 2022, 69, 176–183. [Google Scholar] [CrossRef]
- Kohlenberg-Müller, K.; Ramminger, S.; Kolm, A.; Barkmeijer, A.; Gast, C.; Adam, M.; Le Bruyn, B.; Heine-Bröring, R.; Rachman-Elbaum, S.; Werkman, A.; et al. Nutrition assessment in process-driven, personalized dietetic intervention—The potential importance of assessing behavioural components to improve behavioural change: Results of the EU-funded IMPECD project. Clin. Nutr. ESPEN 2019, 32, 125–134. [Google Scholar] [CrossRef]
- Page, N.; Czuba, C.E. Empowerment: What Is It? J. Extension 1999, 37, 1–5. [Google Scholar]
- Street, S.; Avenell, A. Are individual or group interventions more effective for long-term weight loss in adults with obesity? A systematic review. Clin. Obes. 2022, 12, e12539. [Google Scholar] [CrossRef]
- Englert, I.; Bosy-Westphal, A.; Bischoff, S.C.; Kohlenberg-Müller, K. Impact of Protein Intake during Weight Loss on Preservation of Fat-Free Mass, Resting Energy Expenditure, and Physical Function in Overweight Postmenopausal Women: A Randomized Controlled Trial. Obes. Facts 2021, 14, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Bellg, A.J.; Borrelli, B.; Resnick, B.; Hecht, J.; Minicucci, D.S.; Ory, M.; Ogedegbe, G.; Orwig, D.; Ernst, D.; Czajkowski, S. Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004, 23, 443–451. [Google Scholar] [CrossRef]
- Hall, K.D.; Sacks, G.; Chandramohan, D.; Chow, C.C.; Wang, Y.C.; Gortmaker, S.L.; Swinburn, B.A. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011, 378, 826–837. [Google Scholar] [CrossRef]
- Oberritter, H.; Schäbethal, K.; von Ruesten, A.; Boeing, H. The DGE Nutrition Circle—Presentation and Basis of the Food-Related Recommendations from the German Nutrition Society (DGE). Ernährungs Umschau Int. 2013, 60, 24–29. [Google Scholar] [CrossRef]
- Academy of Nutrition and Dietetics. Measurement of Resting Metabolic Rate in the Non-Critically Ill. Evidence Analysis Library. 2014. Available online: http://www.andeal.org/search.cfm?keywords=resting+metabolic+rate (accessed on 30 July 2016).
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; de Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [CrossRef]
- Weber, M.A.; Schiffrin, E.L.; White, W.B.; Mann, S.; Lindholm, L.H.; Kenerson, J.G.; Flack, J.M.; Carter, B.L.; Materson, B.J.; Ram, C.V.S.; et al. Clinical practice guidelines for the management of hypertension in the community: A statement by the American Society of Hypertension and the International Society of Hypertension. J. Clin. Hypertens. 2014, 16, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Darsini, D.; Hamidah, H.; Notobroto, H.B.; Cahyono, E.A. Health Risks Associated with High Waist Circumference: A Systematic Review. J. Public Health Res. 2020, 9, 1811. [Google Scholar] [CrossRef] [PubMed]
- Paul-Ebhohimhen, V.; Avenell, A. A systematic review of the effectiveness of group versus individual treatments for adult obesity. Obes. Facts 2009, 2, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.A.; Desai, M.; King, A.C. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association With Genotype Pattern or Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA 2018, 319, 667–679. [Google Scholar] [CrossRef]
- Greendale, G.A.; Sternfeld, B.; Huang, M.; Han, W.; Karvonen-Gutierrez, C.; Ruppert, K.; Cauley, J.A.; Finkelstein, J.S.; Jiang, S.-F.; Karlamangla, A.S. Changes in body composition and weight during the menopause transition. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Seimon, R.V.; Wild-Taylor, A.L.; Keating, S.E.; McClintock, S.; Harper, C.; Gibson, A.A.; Johnson, N.A.; Fernando, H.A.; Markovic, T.P.; Center, J.R.; et al. Effect of Weight Loss via Severe vs. Moderate Energy Restriction on Lean Mass and Body Composition Among Postmenopausal Women With Obesity: The TEMPO Diet Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e1913733. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Bagheri, R.; Abbasnezhad, A.; Tinsley, G.M.; Alipour, M.; Wong, A. Effects of gradual weight loss v. rapid weight loss on body composition and RMR: A systematic review and meta-analysis. Br. J. Nutr. 2020, 124, 1121–1132. [Google Scholar] [CrossRef]
- Deutsche Adipositas-Gesellschaft (DAG) e.V., Deutsche Diabetes Gesellschaft. Interdisziplinäre Leitlinie der Qualität S3 zur „Prävention und Therapie der Adipositas“. 2014. Available online: https://register.awmf.org/assets/guidelines/050-001l_S3_Adipositas_Pr%C3%A4vention_Therapie_2014-11-abgelaufen.pdf (accessed on 30 November 2022).
- Celis-Morales, C.; Abraham, S.; Keenan, P.; Ashor, A.W.; Livingstone, K.M.; Lara, J.; Mathers, J.C. Effect of web-based tailored lifestyle interventions on fruit and vegetable consumption in adults: A systematic review and meta-analysis of randomised controlled trials. Proc. Nutr. Soc. 2015, 74, E41. [Google Scholar] [CrossRef]
- Celis-Morales, C.; Lara, J.; Mathers, J.C. Personalising nutritional guidance for more effective behaviour change. Proc. Nutr. Soc. 2015, 74, 130–138. [Google Scholar] [CrossRef]
- Hassapidou, M.; Vlassopoulos, A.; Kalliostra, M.; Govers, E.; Mulrooney, H.; Ells, L.; Salas, X.R.; Muscogiuri, G.; Darleska, T.H.; Busetto, L.; et al. European Association for the Study of Obesity Position Statement on Medical Nutrition Therapy for the Management of Overweight and Obesity in Adults Developed in Collaboration with the European Federation of the Associations of Dietitians. Obes. Facts 2023, 16, 11–28. [Google Scholar] [CrossRef]
- Hartmann-Boyce, J.; Johns, D.J.; Jebb, S.A.; Aveyard, P. Effect of behavioural techniques and delivery mode on effectiveness of weight management: Systematic review, meta-analysis and meta-regression. Obes. Rev. 2014, 15, 598–609. [Google Scholar] [CrossRef]
- Schulz, K.F.; Grimes, D.A. Sample size slippages in randomised trials: Exclusions and the lost and wayward. Lancet 2002, 359, 781–785. [Google Scholar] [CrossRef]
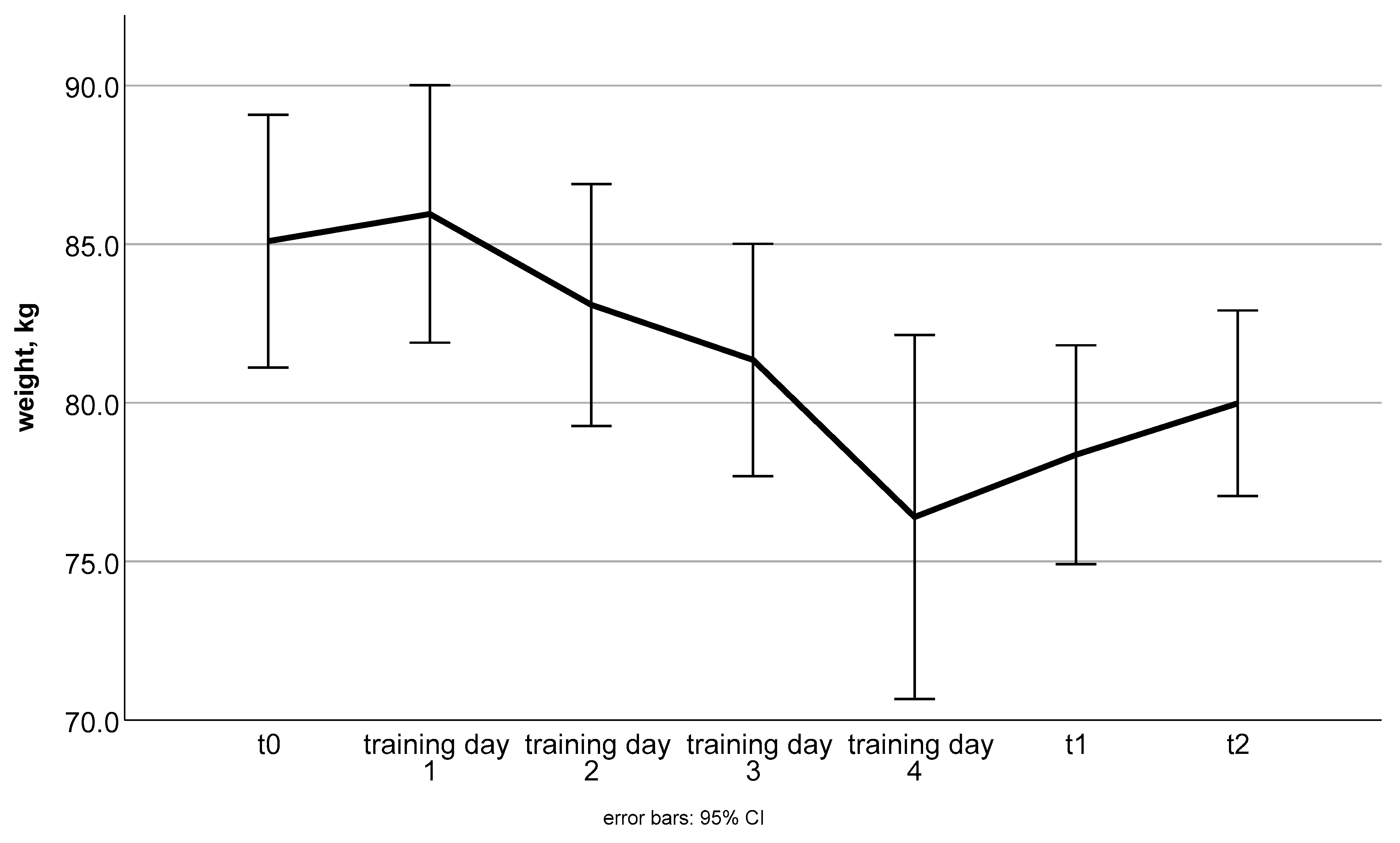

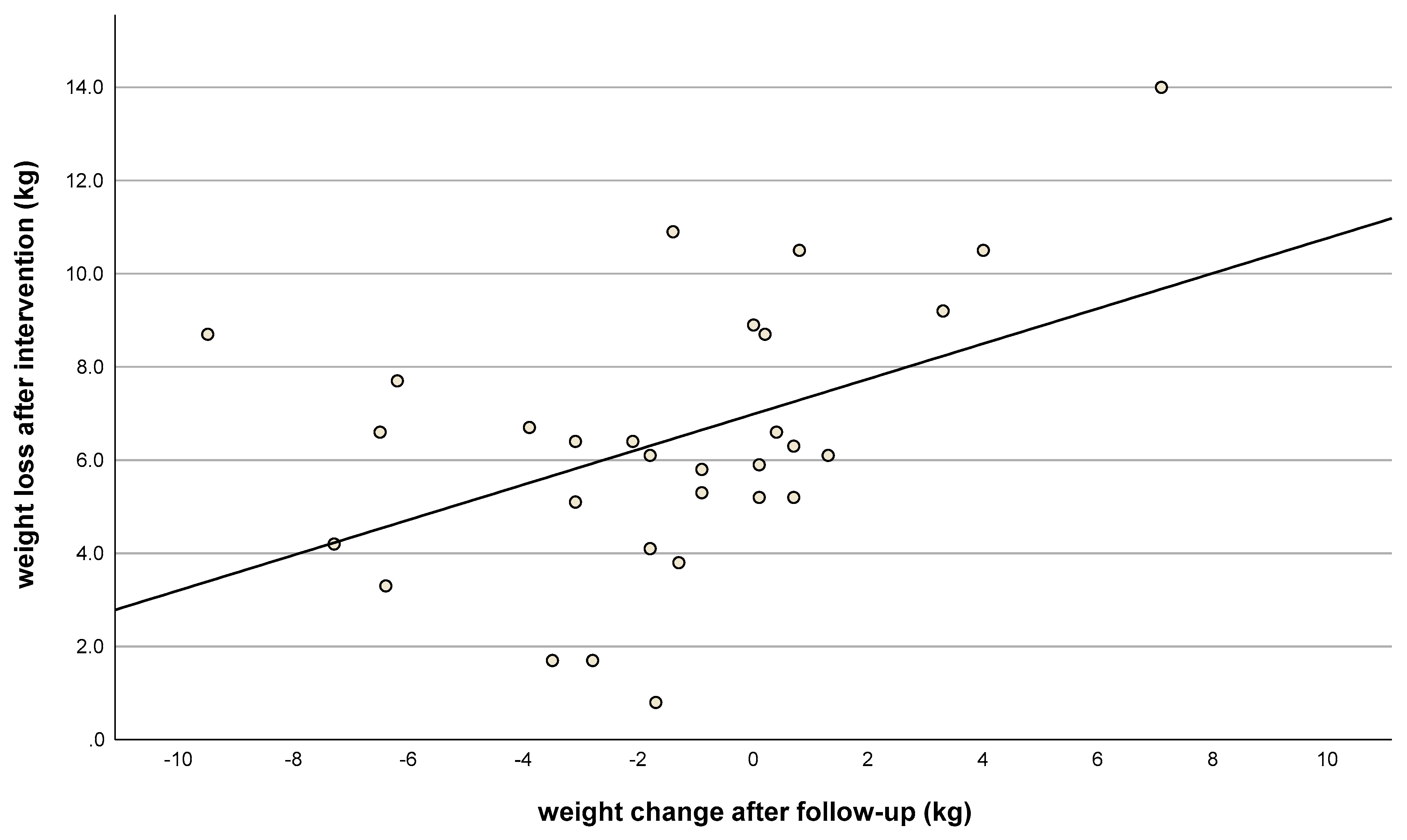
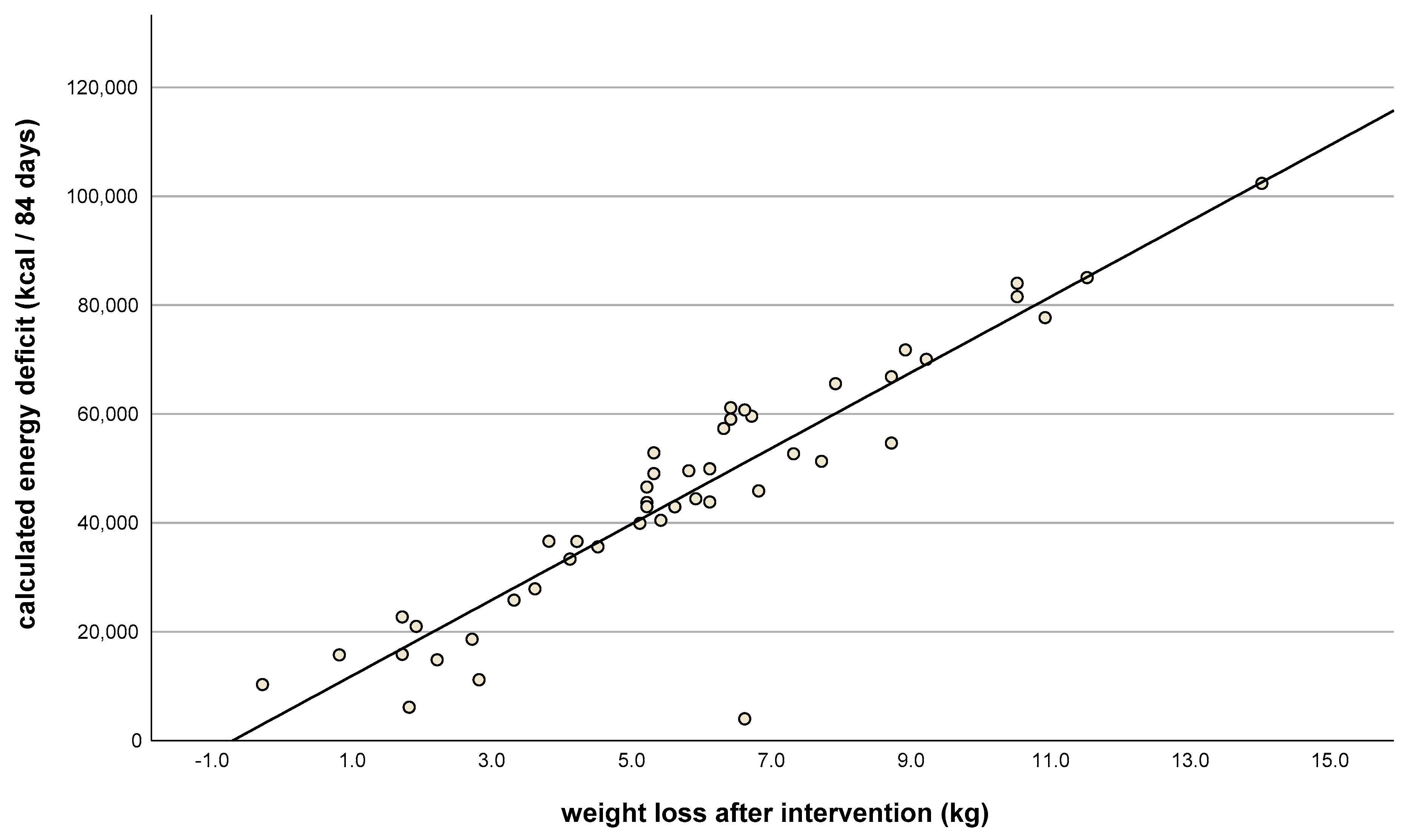
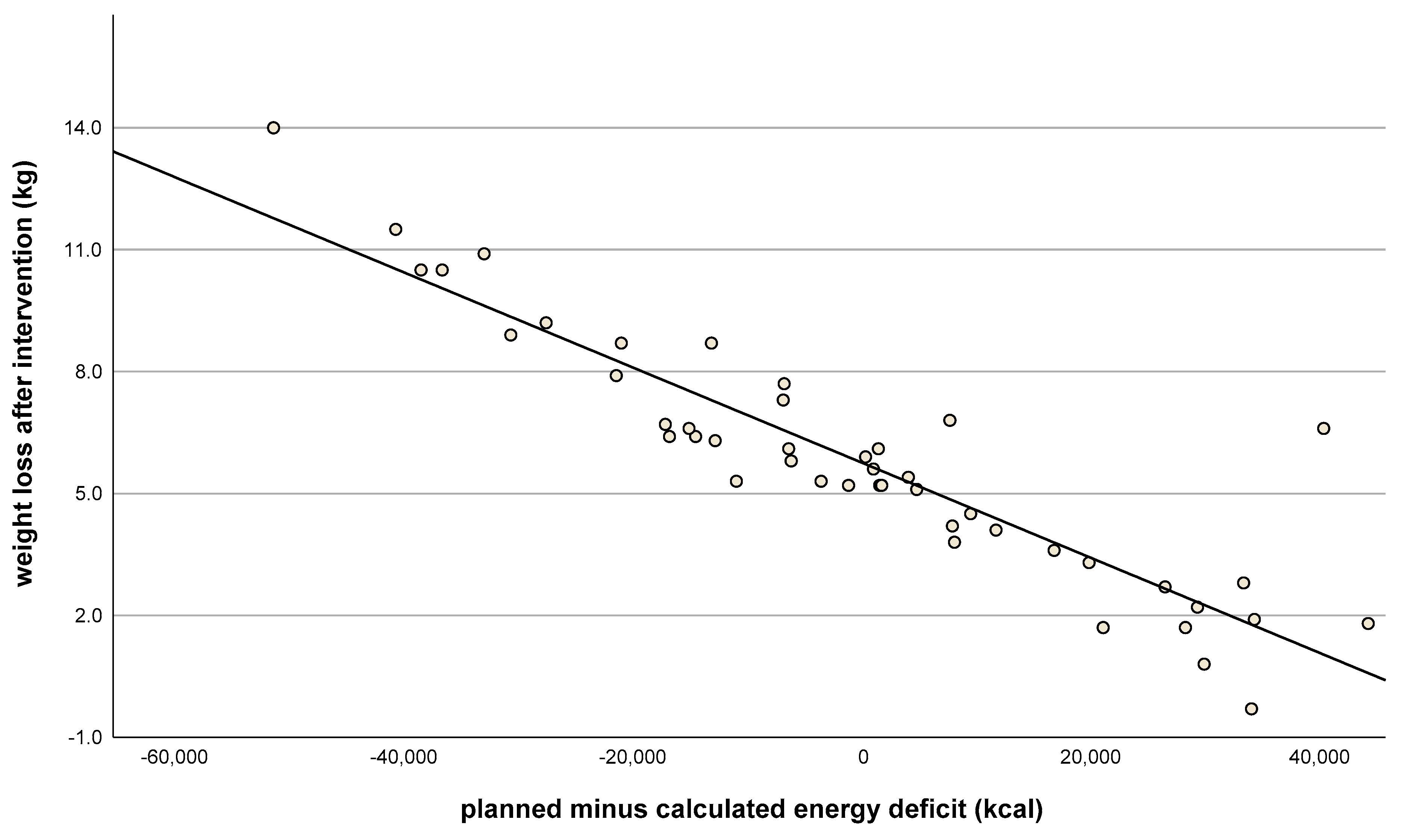
| Consecutive Meal Plan Number | Meal Overview |
|---|---|
| 22 | Potato salad with dill mustard sauce Pear crumble |
| 23 | Sweet potato soup served with baguette Fruit yogurt |
| 24 | Cauliflower gratin with crispy crust Fruit salad Bread with plum jam |
| 25 | Spicy mushroom rice pan Jam roll Fruit |
| 26 | Pasta cream cheese pan Marble cake |
| 27 | Macaroni in ham and leek sauce served with salad Chocolate cookie and fruit |
| 28 | Spinach and potato casserole Wild berry jelly Pretzel roll with jam |
| 29 | Potato and carrot cakes with herb curd cheese Crumble cake Fruit |
| 30 | Risotto with spinach and gorgonzola cheese Jam roll Fruit |
| 31 | Stuffed peppers with rosemary potatoes Cake |
| Body Weight, kg | Baseline (n = 46) | Change at t1 (n = 46) | Change at t2 (n = 30) |
| mean ± SD | 83.9 ± 8.8 | −5.8 ± 3.0 | −1.5 ± 3.5 |
| P 1 | <0.001 | 0.024 | |
| FFM, kg | Baseline (n = 46) | Change at t1 (n = 46) | Change at t2 (n = 30) |
| mean ± SD | 46.1 ± 4.8 | −1.1 ± 1.2 | +0.3 ± 1.5 |
| P 1 | <0.001 | 0.237 | |
| SMM, kg | Baseline (n = 42) | Change at t1 (n = 42) | Change at t2 (n = 30) |
| mean ± SD | 22.4 ± 2.4 | −0.8 ± 0.9 | + 0.4 ± 1.0 |
| P 1 | <0.001 | 0.063 | |
| FM, kg | Baseline (n = 46) | Change at t1 (n = 46) | Change at t2 (n = 30) |
| mean ± SD | 44.9 ± 4.1 | −2.7 ± 1.4 | +0.7 ± 1.8 |
| P 1 | <0.001 | 0.041 | |
| REE, kJ/24 h (kcal/24 h) | Baseline (n = 46) | Change at t1 (n = 46) | Change at t2 (n = 29) |
| mean ± SD | 1687 ± 181 | −1096 ± 439 (−262 ± 105) | +150 ± 385 (+36 ± 92) |
| P 1 | <0.001 | 0.042 | |
| Waist Circumference, cm | Baseline (n = 43) | Change at t1 (n = 43) | Change at t2 (n = 27) |
| mean ± SD | 97.8 ± 9.4 | −8.0 ± 3.2 | −0.2 ± 3.4 |
| P 1 | <0.001 | 0.816 | |
| Blood Pressure (Systole), mmHg | Baseline (n = 46) | Change at t1 (n = 46) | Change at t2 (n = 28) |
| mean ± SD | 137 ± 23 | −12 ± 13 | 9 ± 11 |
| P 1 | <0.001 | <0.001 | |
| Blood Pressure (Diastole), mmHg | Baseline (n = 46) | Change at t1 (n = 46) | Change at t2 (n = 28) |
| mean ± SD | 89 ± 13 | −6 ± 7 | 3 ± 7 |
| P 1 | <0.001 | <0.024 |
| Training day | 1 | 2 | 3 | 4 |
| Number of absent women | 1 | 2 | 7 | 10 |
| Number of protocol violations | 0 | 14 | 21 | 24 |
| First conversation | Statements | ||||||||
| Strengths | Everything is good | Adaptation of recipes required | Recipes are good | ||||||
| n | 5 | 2 | 2 | ||||||
| Challenges | Too much food | Hunger | Bad mood | Fatigue | Compliance | Boredom (with shakes) | Flatulence | Fullness | |
| n | 7 | 3 | 1 | 1 | 5 | 2 | 3 | 2 | |
| Second conversation | Statements | ||||||||
| Strengths | Everything is good | ||||||||
| n | 1 | ||||||||
| Challenges | Too much food | Hunger | Fatigue | Compliance | Flatulence | ||||
| n | 3 | 3 | 2 | 2 | 6 | ||||
| Third conversation | Statements | ||||||||
| Strengths | Everything is good | Belly is tighter | |||||||
| n | 2 | 1 | |||||||
| Challenges | Hunger | Fatigue | Compliance | Flatulence | |||||
| n | 2 | 1 | 4 | 2 | |||||
| Fourth conversation | Statements | ||||||||
| Strengths | Everything is good | Feels good | Not hungry | ||||||
| n | 1 | 1 | 1 | ||||||
| Challenges | Too much food | Hunger | Fatigue | Compliance | Flatulence | Boredom (with shakes) | |||
| n | 1 | 2 | 1 | 6 | 1 | 1 | |||
| Statements during follow-up phase | |||||||||
| Strengths | Would like to lose more weight | Trying to maintain weight loss | Trying to implement recommendations | Proud of what has been achieved | Doing more sports/movement | ||||
| n | 1 | 3 | 6 | 1 | 7 | ||||
| Challenges | Weight gain after stopping shakes | Increased hunger/appetite | Hard to maintain diet without support | Increased sweet cravings after stopping shakes | Returned to old eating behavior | ||||
| n | 1 | 2 | 1 | 1 | 1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Englert, I.; Egert, S.; Hoffmann, L.; Kohlenberg-Müller, K. Concept of an Intervention for Sustainable Weight Loss in Postmenopausal Women with Overweight—Secondary Analysis of a Randomized Dietary Intervention Study. Nutrients 2023, 15, 3250. https://doi.org/10.3390/nu15143250
Englert I, Egert S, Hoffmann L, Kohlenberg-Müller K. Concept of an Intervention for Sustainable Weight Loss in Postmenopausal Women with Overweight—Secondary Analysis of a Randomized Dietary Intervention Study. Nutrients. 2023; 15(14):3250. https://doi.org/10.3390/nu15143250
Chicago/Turabian StyleEnglert, Isabell, Sarah Egert, Laura Hoffmann, and Kathrin Kohlenberg-Müller. 2023. "Concept of an Intervention for Sustainable Weight Loss in Postmenopausal Women with Overweight—Secondary Analysis of a Randomized Dietary Intervention Study" Nutrients 15, no. 14: 3250. https://doi.org/10.3390/nu15143250
APA StyleEnglert, I., Egert, S., Hoffmann, L., & Kohlenberg-Müller, K. (2023). Concept of an Intervention for Sustainable Weight Loss in Postmenopausal Women with Overweight—Secondary Analysis of a Randomized Dietary Intervention Study. Nutrients, 15(14), 3250. https://doi.org/10.3390/nu15143250







