Interaction of Iron Homeostasis and Fatty Acid Metabolism in the Development of Glucose Intolerance in Women with Previous Gestational Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
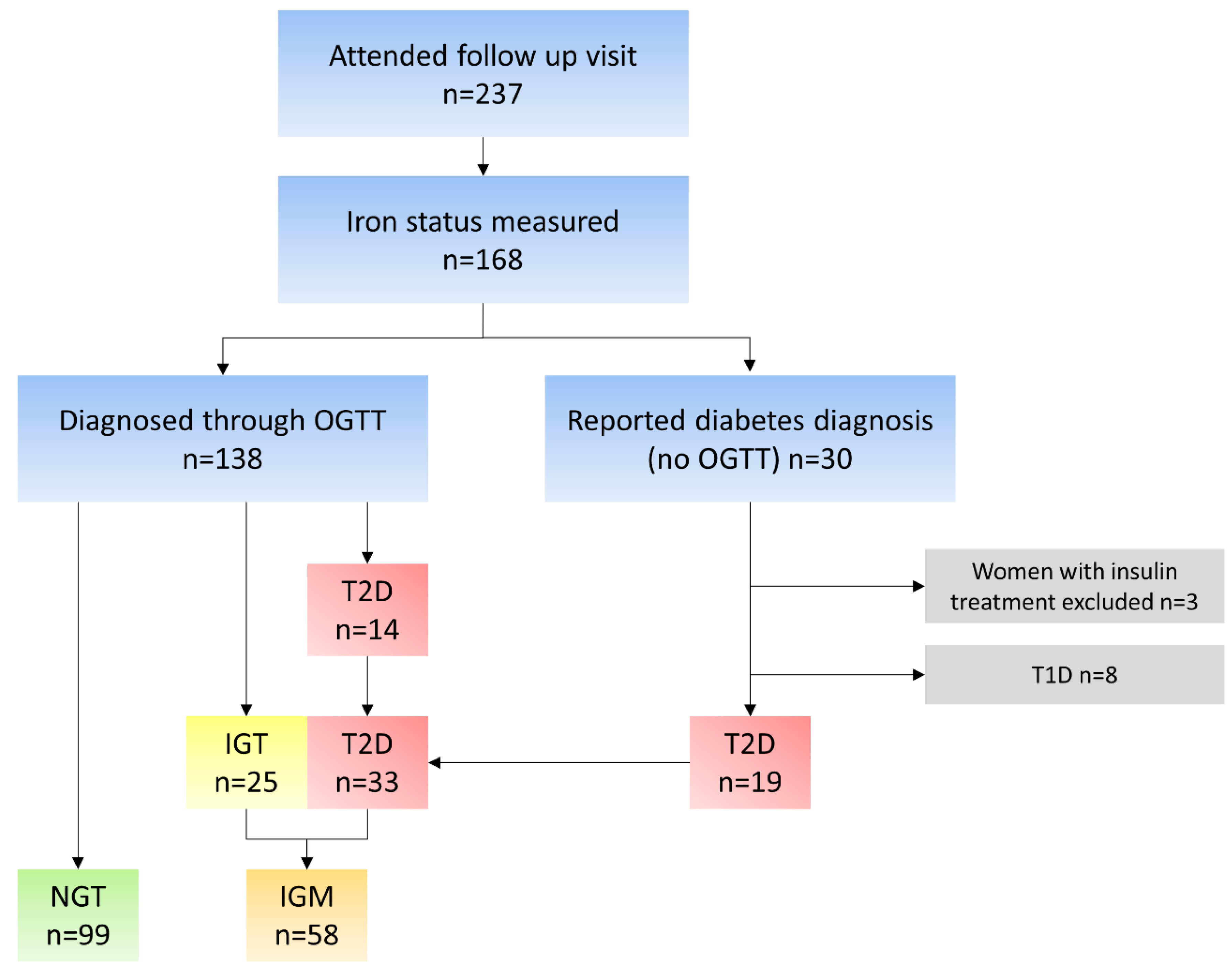
2.2. Collection of Data and Data Processing
2.3. Glucose Tolerance Groups
2.4. Biochemical Measurements
2.5. Statistical Analyses
3. Results
3.1. Background Characteristics
3.2. Iron Status
3.3. Fatty Acid Profiles
3.4. Associations between Fas, Desaturase Activity and Iron Parameters
4. Discussion
4.1. Differences between Glucose Tolerance Groups
4.2. Associations between Iron Parameters and Fatty Acid Metabolism
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; McIntyre, H.D.; et al. Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Kenny, L.C.; Myres, J.E. Obstetrics: By Ten Teachers, 20th ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Andersson-Hall, U.; Gustavsson, C.; Pedersen, A.; Malmodin, D.; Joelsson, L.; Holmäng, A. Higher Concentrations of BCAAs and 3-HIB Are Associated with Insulin Resistance in the Transition from Gestational Diabetes to Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 4207067. [Google Scholar] [CrossRef]
- Ben-Haroush, A.; Yogev, Y.; Hod, M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet. Med. 2004, 21, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef]
- Eitel, K.; Staiger, H.; Brendel, M.D.; Brandhorst, D.; Bretzel, R.G.; Häring, H.-U.; Kellerer, M. Different role of saturated and unsaturated fatty acids in β-cell apoptosis. Biochem. Biophys. Res. Commun. 2002, 299, 853–856. [Google Scholar] [CrossRef]
- Gerst, F.; Wagner, R.; Oquendo, M.B.; Siegel-Axel, D.; Fritsche, A.; Heni, M.; Staiger, H.; Häring, H.-U.; Ullrich, S. What role do fat cells play in pancreatic tissue? Mol. Metab. 2019, 25, 1–10. [Google Scholar] [CrossRef]
- Sampath, H.; Ntambi, J.M. The role of stearoyl-CoA desaturase in obesity, insulin resistance, and inflammation. Ann. N. Y. Acad. Sci. 2011, 1243, 47–53. [Google Scholar] [CrossRef]
- Liao, T.; Xu, X.; Zhang, Y.; Yan, J. Interactive effects of gestational diabetes mellitus and maximum level of total bile acid in maternal serum on adverse pregnancy outcomes in women with intrahepatic cholestasis of pregnancy. BMC Pregnancy Childbirth 2023, 23, 326. [Google Scholar] [CrossRef]
- Andersson-Hall, U.; Carlsson, N.-G.; Sandberg, A.-S.; Holmäng, A. Circulating Linoleic Acid is Associated with Improved Glucose Tolerance in Women after Gestational Diabetes. Nutrients 2018, 10, 1629. [Google Scholar] [CrossRef]
- Backe, M.B.; Moen, I.W.; Ellervik, C.; Hansen, J.B.; Mandrup-Poulsen, T. Iron Regulation of Pancreatic Beta-Cell Functions and Oxidative Stress. Annu. Rev. Nutr. 2016, 36, 241–273. [Google Scholar] [CrossRef]
- Feng, Y.; Qi, R.; Xu, M.; Shen, Z.; Li, M. Dietary iron supplements may affect stress adaptation and aggravate stress hyperglycemia in a rat model of psychological stress. Nutrition 2012, 28, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Feng, Q.; Lv, Y.; Song, X.; Qu, H.; Chen, Y. The relationship between iron metabolism, stress hormones, and insulin resistance in gestational diabetes mellitus. Nutr. Diabetes 2020, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Simcox, J.A.; McClain, D.A. Iron and Diabetes Risk. Cell Metab. 2013, 17, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, X.; Zheng, W.; Zhou, J.; Song, Z.; Xu, M.; Min, J.; Wang, F. Genetic Support of A Causal Relationship Between Iron Status and Type 2 Diabetes: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2021, 106, e4641–e4651. [Google Scholar] [CrossRef]
- Alkhatib, B.; Al Domi, H.; Shomaf, M.; Abu Irmaileh, B.E. Iron Overload Effect on Serum Levels of Glucose, Insulin and HOMA-IR was Ifluenced by the Type of Fat in Female Sprague-Dawley Rats Fed High-Fat-Diets. J. Clin. Nutr. Diet. 2017, 3, 100058. [Google Scholar] [CrossRef]
- Valenzuela, R.; Rincón-Cervera, M.Á.; Echeverría, F.; Barrera, C.; Espinosa, A.; Hernández-Rodas, M.C.; Ortiz, M.; Valenzuela, A.; Videla, L.A. Iron-induced pro-oxidant and pro-lipogenic responses in relation to impaired synthesis and accretion of long-chain polyunsaturated fatty acids in rat hepatic and extrahepatic tissues. Nutrition 2017, 45, 49–58. [Google Scholar] [CrossRef]
- Wu, Y.; Baylin, A.; Colacino, J.A. Iron, oxidative stress, and Δ9 stearoyl-coenzymeA desaturase index (C16:1/C16:0): An analysis applying the National Health and Nutrition Examination Survey 2003–04. Curr. Dev. Nutr. 2018, 2, nzx001. [Google Scholar] [CrossRef]
- Sha, W.; Hu, F.; Xi, Y.; Chu, Y.; Bu, S. Mechanism of Ferroptosis and Its Role in Type 2 Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 9999612. [Google Scholar] [CrossRef]
- Krümmel, B.; von Hanstein, A.-S.; Plötz, T.; Lenzen, S.; Mehmeti, I. Differential effects of saturated and unsaturated free fatty acids on ferroptosis in rat β-cells. J. Nutr. Biochem. 2022, 106, 109013. [Google Scholar] [CrossRef]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Hoppe, M.; Hulthén, L.; Samuelson, G. Is cord blood hepcidin influenced by the low-grade acute-phase response occurring during delivery? A small-scale longitudinal study. J. Matern. Neonatal Med. 2019, 32, 2166–2172. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.N.; Wylie-Rosett, J.; Gunter, M.J.; Negassa, A.; Kabat, G.C.; Rohan, T.E.; Crandall, J. Diabetes Prevention Program (DPP) Research Group Biomarkers of body iron stores and risk of developing type 2 diabetes. Diabetes Obes. Metab. 2009, 11, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cao, J.C.; Arija, V.; Aranda, N.; Basora, J.; Diez-Espino, J.; Estruch, R.; Fitó, M.; Corella, D.; Salas-Salvadó, J. Soluble transferrin receptor and risk of type 2 diabetes in the obese and nonobese. Eur. J. Clin. Investig. 2017, 47, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Montonen, J.; Boeing, H.; Steffen, A.; Lehmann, R.; Fritsche, A.; Joost, H.-G.; Schulze, M.B.; Pischon, T. Body iron stores and risk of type 2 diabetes: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Diabetologia 2012, 55, 2613–2621. [Google Scholar] [CrossRef]
- Fernández-Real, J.M.; Izquierdo, M.; Moreno-Navarrete, J.M.; Gorostiaga, E.; Ortega, F.; Martínez, C.; Idoate, F.; Ricart, W.; Ibañez, J. Circulating soluble transferrin receptor concentration decreases after exercise-induced improvement of insulin sensitivity in obese individuals. Int. J. Obes. 2009, 33, 768–774. [Google Scholar] [CrossRef]
- Slomka, A.; Giemza-Kucharska, P.; Żekanowska, E.; Korbal, P. Gestational diabetes mellitus has no affect on prohepcidin and other iron status parameters in infants. Przegląd Med. Uniw. Rzesz. I Nar. Inst. Leków W Warszawie 2012, 1, 70–73. [Google Scholar]
- Vessby, B.; Gustafsson, I.-B.; Tengblad, S.; Boberg, M.; Andersson, A. Desaturation and Elongation of Fatty Acids and Insulin Action. Ann. N. Y. Acad. Sci. 2002, 967, 183–195. [Google Scholar] [CrossRef]
- Hodge, A.M.; English, D.R.; O’Dea, K.; Sinclair, A.J.; Makrides, M.; Gibson, R.A.; Giles, G.G. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: Interpreting the role of linoleic acid. Am. J. Clin. Nutr. 2007, 86, 189–197. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Lanti, C.; Gatti, S.; Rametta, R.; Recalcati, S.; Maggioni, M.; Fracanzani, A.L.; Riso, P.; Cairo, G.; Fargion, S. High Fat Diet Subverts Hepatocellular Iron Uptake Determining Dysmetabolic Iron Overload. PLoS ONE 2015, 10, e0116855. [Google Scholar] [CrossRef]
- Cui, R.; Choi, S.-E.; Kim, T.H.; Lee, H.J.; Lee, S.J.; Kang, Y.; Jeon, J.Y.; Kim, H.J.; Lee, K.-W. Iron overload by transferrin receptor protein 1 regulation plays an important role in palmitate-induced insulin resistance in human skeletal muscle cells. FASEB J. 2018, 33, 1771–1786. [Google Scholar] [CrossRef]
- Zhang, Z.; Funcke, J.-B.; Zi, Z.; Zhao, S.; Straub, L.G.; Zhu, Y.; Zhu, Q.; Crewe, C.; An, Y.A.; Chen, S. Adipocyte iron levels impinge on a fat-gut crosstalk to regulate intestinal lipid absorption and mediate protection from obesity. Cell Metab. 2021, 33, 1624–1639.e9. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.G.; Dhayal, S.; Diakogiannaki, E.; Welters, H.J. The cytoprotective actions of long-chain mono-unsaturated fatty acids in pancreatic β-cells. Biochem. Soc. Trans. 2008, 36, 905–908. [Google Scholar] [CrossRef] [PubMed]
- R’Zik, S.; Beguin, Y. Serum soluble transferrin receptor concentration is an accurate estimate of the mass of tissue receptors. Exp. Hematol. 2001, 29, 677–685. [Google Scholar] [CrossRef] [PubMed]
| NGT | IGM | NGT vs. IGM | |||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | p-Value | |
| Age (years) | 99 | 39.4 | 4.9 | 58 | 38.5 | 5.6 | 0.289 |
| Body mass index (kg/m2) | 99 | 26.7 | 4.9 | 58 | 29.6 | 5.3 | 0.001 * |
| Waist circumference (cm) | 98 | 87.7 | 11.0 | 57 | 94.7 | 12.7 | 0.001 * |
| Hip circumference (cm) | 98 | 103.4 | 10.4 | 57 | 107.4 | 10.8 | 0.021 * |
| s-Insulin fasting (mU/L) | 96 | 8.13 | 4.46 | 57 | 12.91 | 6.52 | <0.001 * |
| s-Insulin 120 min (mU/L) | 84 | 38.38 | 26.41 | 30 * | 79.07 | 56.54 | <0.001 * |
| p-Glucose fasting (mM) | 99 | 5.37 | 0.37 | 58 | 7.19 | 2.47 | <0.001 * |
| p-Glucose 120 min (mM) | 91 | 5.49 | 1.09 | 31 a | 8.58 | 2.32 | <0.001 * |
| HOMA-IR | 95 | 1.97 | 1.10 | 57 | 4.20 | 2.64 | <0.001 * |
| hs-CRP (mg/L) | 99 | 2.41 | 4.03 | 58 | 4.87 | 7.51 | 0.006 * |
| NGT | IGM | NGT vs. IGM | |||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | p-Value | |
| s-Ferritin (µg/L) | 98 | 59.61 | 45.43 | 58 | 61.50 | 80.37 | 0.064 |
| s-Transferrin (g/L) | 97 | 2.80 | 0.42 | 57 | 2.87 | 0.44 | 0.330 |
| s-Iron (µmol/L) | 95 | 16.81 | 5.41 | 57 | 15.04 | 5.77 | 0.037 * |
| s-Transferrin receptor (mg/L) | 98 | 3.29 | 1.15 | 58 | 3.87 | 1.68 | 0.023 * |
| Transferrin saturation | 94 | 0.25 | 0.09 | 57 | 0.22 | 0.09 | 0.038 * |
| s-TIBC (µmol/L) | 98 | 67.88 | 14.30 | 58 | 70.27 | 14.02 | 0.229 |
| s-Hepcidin (ng/mL) | 87 | 40.20 | 41.06 | 55 | 33.60 | 33.24 | 0.394 |
| NGT | IGM | NGT vs. IGM | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | p-Value | ||
| Saturated FAs. % | ||||||||
| Myristic acid | (14:0) | 96 | 0.60 | 0.28 | 57 | 0.71 | 0.28 | 0.007 * |
| Palmitic acid | (16:0) | 96 | 23.46 | 2.24 | 57 | 24.35 | 2.08 | 0.001 * |
| Stearic acid | (18:0) | 96 | 8.35 | 0.98 | 57 | 8.55 | 1.03 | 0.189 |
| MUFAs. % | ||||||||
| Palmitoleic acid (POA) | POA (16:1n7) | 96 | 1.44 | 0.53 | 57 | 1.68 | 0.75 | 0.068 |
| Vaccenic acid | (18:1n7) | 96 | 2.03 | 0.30 | 57 | 2.04 | 0.32 | 0.961 |
| Oleic acid | (18:1n9) | 96 | 22.46 | 2.55 | 57 | 23.16 | 3.17 | 0.116 |
| ω-3 PUFAs. % | ||||||||
| α-Linolenic acid | ALA (18:3n3) | 96 | 0.71 | 0.23 | 57 | 0.78 | 0.28 | 0.179 |
| Eicosapentaenoic acid | EPA (20:5n3) | 96 | 0.92 | 0.59 | 57 | 0.95 | 0.42 | 0.310 |
| Docosapentaenoic acid | DPA (22:5n3) | 96 | 0.40 | 0.09 | 57 | 0.44 | 0.11 | 0.039 * |
| Docosahexaenoic acid | DHA (22:6n3) | 96 | 1.74 | 0.63 | 57 | 1.64 | 0.53 | 0.592 |
| ω-6 PUFAs. % | ||||||||
| Linoleic acid | LA (18:2n6) | 96 | 30.31 | 4.10 | 57 | 27.68 | 3.97 | <0.001 * |
| γ-Linolenic acid | GLA (18:3n6) | 96 | 0.30 | 0.16 | 57 | 0.38 | 0.14 | <0.001 * |
| Dihomo-γ-linolenic acid | DGLA (20:3n6) | 96 | 1.25 | 0.32 | 57 | 1.40 | 0.37 | 0.021 * |
| Arachidonic acid | AA (20:4n6) | 96 | 6.03 | 1.27 | 57 | 6.23 | 1.24 | 0.323 |
| Desaturase activity | ||||||||
| Delta-5 desaturase | D5D (20:4n6/20:3n6) | 96 | 5.25 | 1.77 | 57 | 4.87 | 1.70 | 0.155 |
| Delta-6 desaturase | D6D (20:3n6/18:2n6) | 96 | 0.05 | 0.02 | 57 | 0.06 | 0.02 | <0.001 * |
| Stearoyl-CoA desaturase | SCD (16:1n7/16:0) | 96 | 0.06 | 0.01 | 57 | 0.07 | 0.03 | 0.153 |
| Delta-9 desaturase | D9D (18:1n9/18:0) | 96 | 2.73 | 0.48 | 57 | 2.76 | 0.57 | 0.621 |
| Dependent | Independent | Unadjusted | Adjusted | |
|---|---|---|---|---|
| Docosapentaenoic acid (22:5n3) | Ferritin | β-coefficient | 0.210 * | 0.213 * |
| p-Value | 0.041 | 0.032 | ||
| Docosahexaenoic acid (22:6n3) | Iron | β-coefficient | 0.232 * | 0.147 |
| p-Value | 0.026 | 0.166 | ||
| Docosahexaenoic acid (22:6n3) | Transferrin saturation | β-coefficient | 0.221 * | 0.131 |
| p-Value | 0.035 | 0.218 | ||
| Oleic acid (18:1n9) | Hepcidin | β-coefficient | −0.291 * | −0.275 * |
| p-Value | 0.007 | 0.010 | ||
| Delta-6 desaturase (20:3n6/18:2n6) | Hepcidin | β-coefficient | −0.250 * | −0.228 * |
| p-Value | 0.022 | 0.032 |
| Dependent | Independent | Unadjusted | Adjusted | |
|---|---|---|---|---|
| Palmitoleic acid (16:1n7) | Ferritin | β-coefficient | 0.298 * | 0.228 |
| p-Value | 0.024 | 0.070 | ||
| Stearoyl-CoA desaturase (16:1n7/16:0) | Ferritin | β-coefficient | 0.271 * | 0.206 |
| p-Value | 0.042 | 0.104 | ||
| Palmitoleic acid (16:1n7) | Iron | β-coefficient | 0.232 | 0.250 * |
| p-Value | 0.085 | 0.048 | ||
| Vaccenic acid (18:1n7) | Iron | β-coefficient | 0.319 * | 0.333 * |
| p-Value | 0.017 | 0.014 | ||
| Oleic acid (18:1n9) | Iron | β-coefficient | 0.321 * | 0.302 * |
| p-Value | 0.016 | 0.018 | ||
| Stearoyl-CoA desaturase (16:1n7/16:0) | Iron | β-coefficient | 0.299 * | 0.313 * |
| p-Value | 0.025 | 0.013 | ||
| Delta-9 desaturase (18:1n9/18:0) | Iron | β-coefficient | 0.308 * | 0.317 * |
| p-Value | 0.021 | 0.018 | ||
| Myristic acid (14:0) | Transferrin receptor | β-coefficient | 0.284 * | 0.274 * |
| p-Value | 0.033 | 0.039 | ||
| Palmitic acid (16:0) | Transferrin receptor | β-coefficient | 0.305 * | 0.273 * |
| p-Value | 0.021 | 0.034 | ||
| Vaccenic acid (18:1n7) | Transferrin receptor | β-coefficient | −0.248 | −0.265 * |
| p-Value | 0.063 | 0.048 | ||
| Oleic acid (18:1n9) | Transferrin receptor | β-coefficient | −0.236 | −0.251 * |
| p-Value | 0.077 | 0.046 | ||
| Vaccenic acid (18:1n7) | Transferrin saturation | β-coefficient | 0.303 * | 0.322 * |
| p-Value | 0.023 | 0.018 | ||
| Oleic acid (18:1n9) | Transferrin saturation | β-coefficient | 0.283 * | 0.281 * |
| p-Value | 0.035 | 0.027 | ||
| Stearoyl-CoA desaturase (16:1n7/16:0) | Transferrin saturation | β-coefficient | 0.252 | 0.285 * |
| p-Value | 0.061 | 0.024 | ||
| Delta-9 desaturase (18:1n9/18:0) | Transferrin saturation | β-coefficient | 0.299 * | 0.322 * |
| p-Value | 0.025 | 0.016 | ||
| Stearic acid (18:0) | TIBC | β-coefficient | 0.257 | 0.276 * |
| p-Value | 0.054 | 0.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Källner, K.; Krook, R.; Sandberg, A.-S.; Hulthén, L.; Andersson-Hall, U.; Holmäng, A. Interaction of Iron Homeostasis and Fatty Acid Metabolism in the Development of Glucose Intolerance in Women with Previous Gestational Diabetes Mellitus. Nutrients 2023, 15, 3214. https://doi.org/10.3390/nu15143214
Källner K, Krook R, Sandberg A-S, Hulthén L, Andersson-Hall U, Holmäng A. Interaction of Iron Homeostasis and Fatty Acid Metabolism in the Development of Glucose Intolerance in Women with Previous Gestational Diabetes Mellitus. Nutrients. 2023; 15(14):3214. https://doi.org/10.3390/nu15143214
Chicago/Turabian StyleKällner, Kristin, Rasmus Krook, Ann-Sofie Sandberg, Lena Hulthén, Ulrika Andersson-Hall, and Agneta Holmäng. 2023. "Interaction of Iron Homeostasis and Fatty Acid Metabolism in the Development of Glucose Intolerance in Women with Previous Gestational Diabetes Mellitus" Nutrients 15, no. 14: 3214. https://doi.org/10.3390/nu15143214
APA StyleKällner, K., Krook, R., Sandberg, A.-S., Hulthén, L., Andersson-Hall, U., & Holmäng, A. (2023). Interaction of Iron Homeostasis and Fatty Acid Metabolism in the Development of Glucose Intolerance in Women with Previous Gestational Diabetes Mellitus. Nutrients, 15(14), 3214. https://doi.org/10.3390/nu15143214





