Chlorogenic Acid Inhibits Ceramide Accumulation to Restrain Hepatic Glucagon Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Treatment
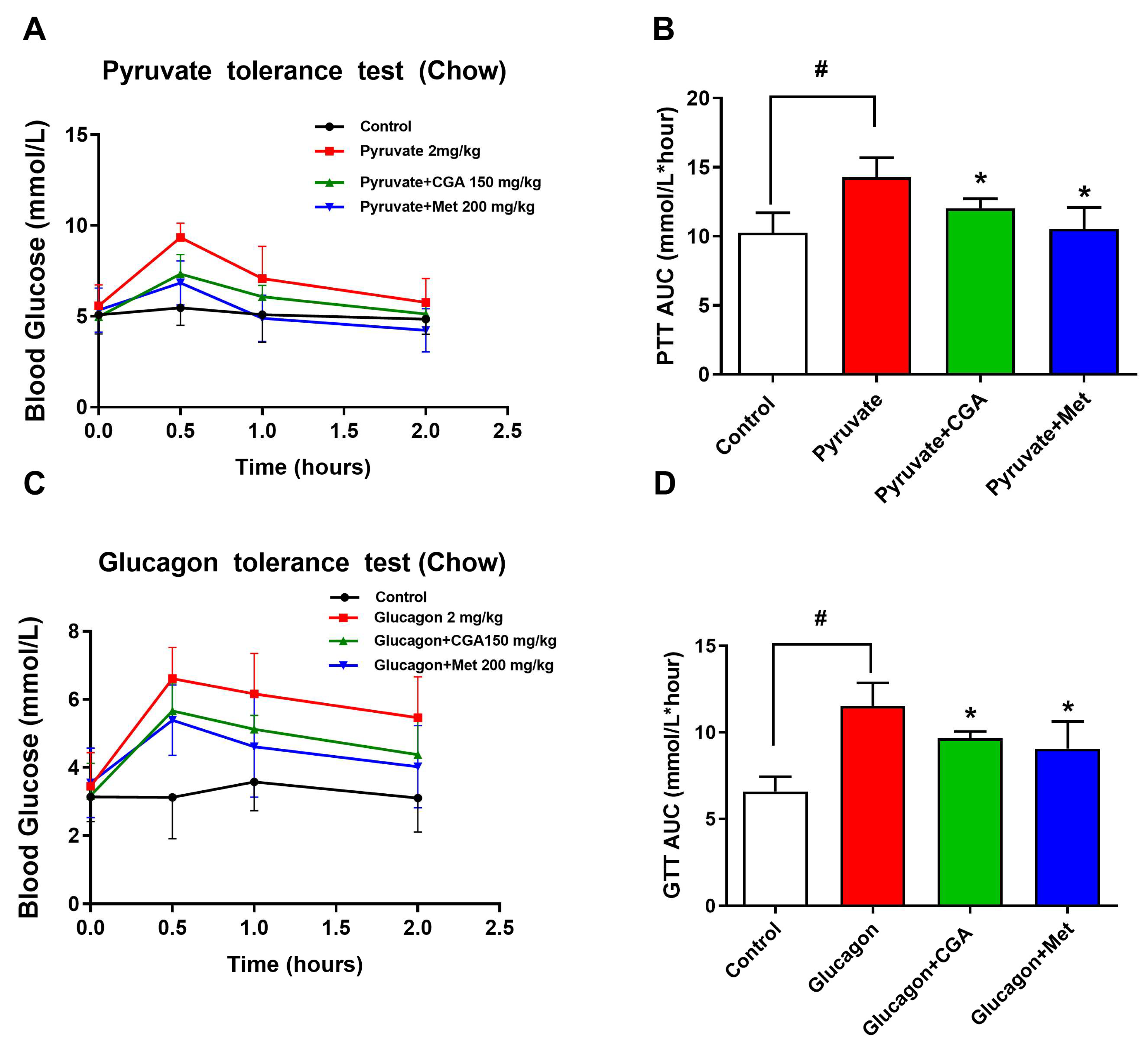
2.3. Glucagon, Pyruvate, and Oral Glucose Tolerance Tests
2.4. Preparation of Primary Hepatocytes and Treatment
2.5. Glucose Output
2.6. Western Blot Analysis
2.7. Measurement of Ceramide, IL-6 and TNF-α
2.8. Quantitative Real-Time PCR
2.9. Immunofluorescence
2.10. Statistical Analysis
3. Results
3.1. CGA Inhibits Endogenous Glucose Production in Mice
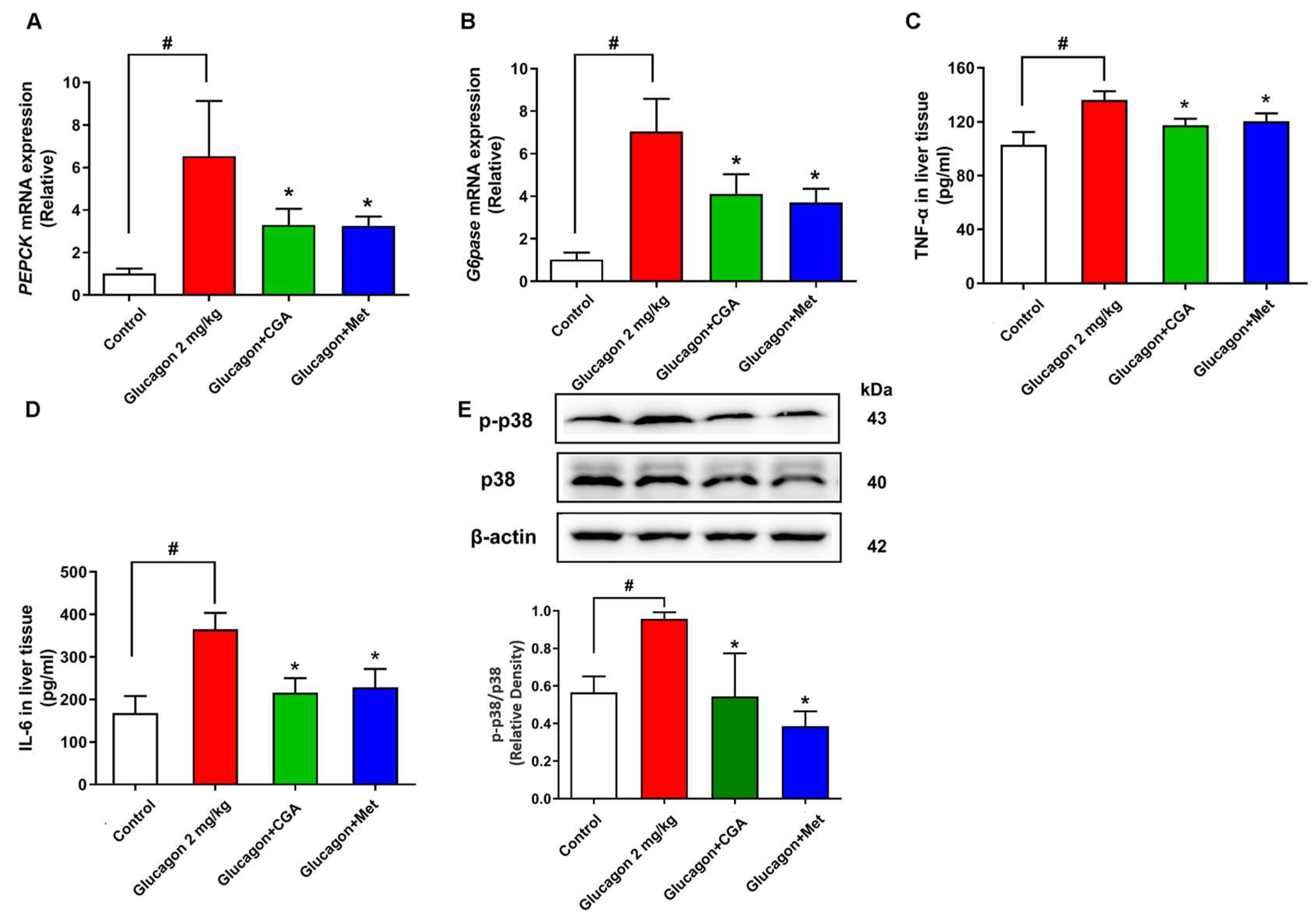
3.2. CGA Inhibits Hepatic Glucagon Response and Inflammation Response
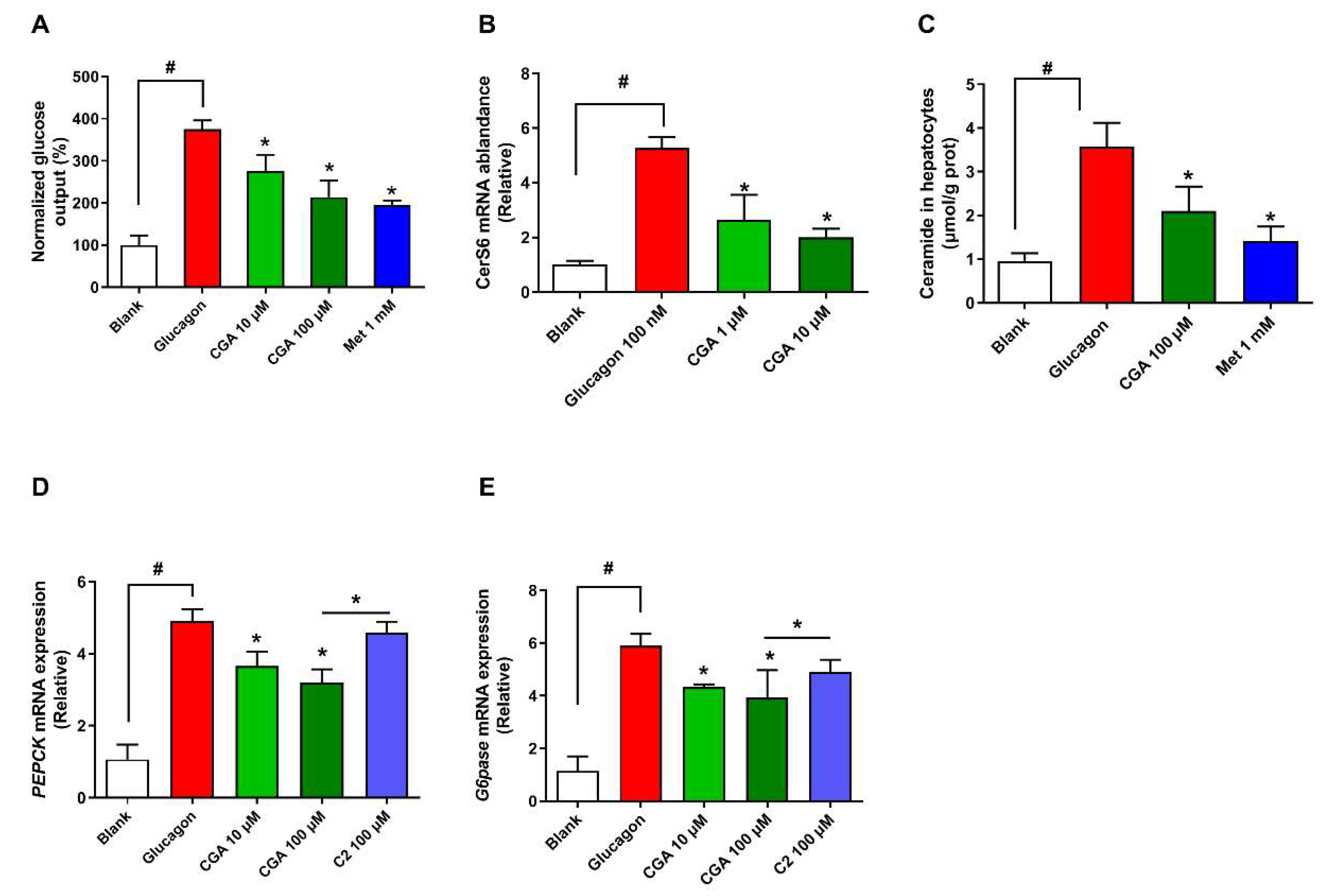
3.3. CGA Inhibits Expression of Ceramide Synthase 6 and Ceramide Accumulation
3.4. CGA Inhibits Glucose Output and Ceramide Accumulation in Primary Hepatocytes
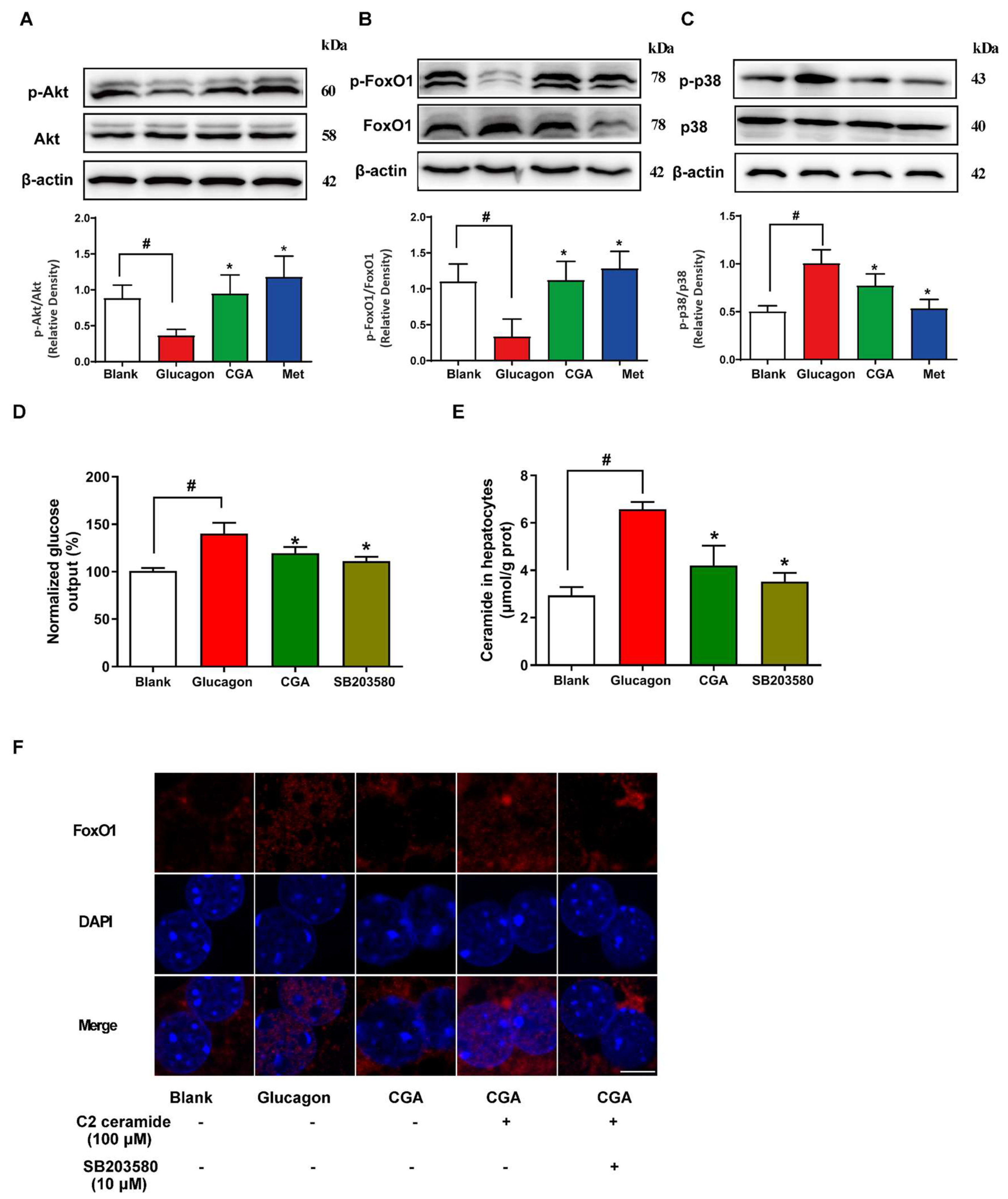
3.5. CGA Protects Akt Phosphorylation and Inhibits FoxO1 Activation in Hepatocytes
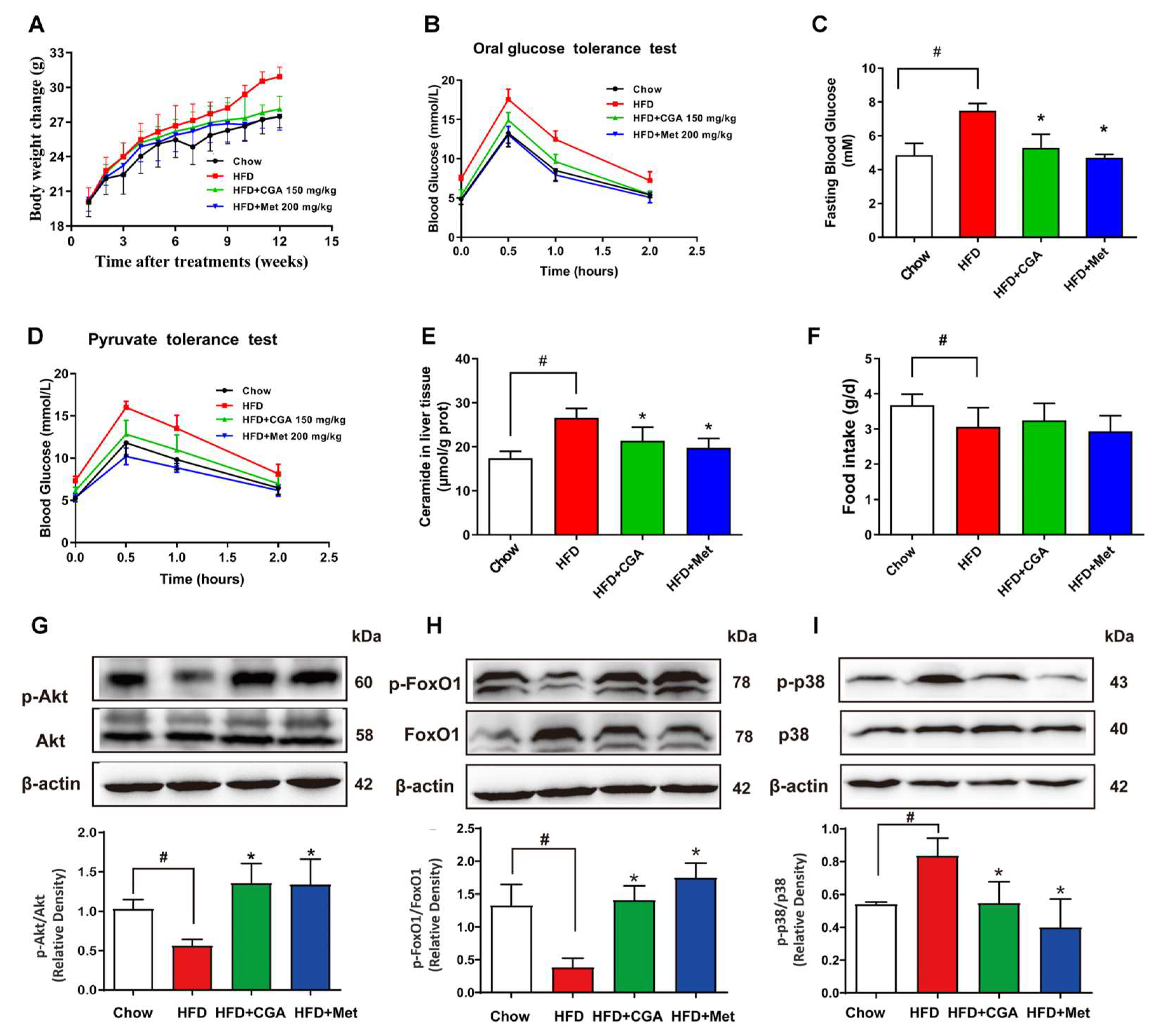
3.6. CGA Inhibited Endogenous Glucose Production and Ceramide Accumulation in HFD-Fed Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CerS6 | ceramide synthetase 6 |
| CGA | Chlorogenic acid |
| Degs1 | Dihydroceramide desulfurase 1 |
| G6pase | Glucose-6-phosphatase |
| GTT | Glucagon tolerance test |
| HFD | High-fat diet |
| OGTT | Oral glucose tolerance test |
| PEPCK | Phosphoenol pyruvate carboxykinase |
| PTT | Pyruvate tolerance test |
| T2DM | Type 2 diabetes mellitus |
References
- Laakso, M. Biomarkers for type 2 diabetes. Mol. Metab. 2019, 27, S139–S146. [Google Scholar] [CrossRef] [PubMed]
- Norton, L.; Shannon, C.; Gastaldelli, A.; DeFronzo, R.A. Insulin: The master regulator of glucose metabolism. Metabolism 2022, 129, 155142. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I.; Yazdi, Z.S.; Beitelshees, A.L. Pharmacological treatment of hyperglycemia in type 2 diabetes. J. Clin. Investig. 2021, 131, e142243. [Google Scholar] [CrossRef]
- Xiao, N.; Lou, M.-D.; Lu, Y.-T.; Yang, L.-L.; Liu, Q.; Liu, B.; Qi, L.-W.; Li, P. Ginsenoside Rg5 attenuates hepatic glucagon response via suppression of succinate-associated HIF-1α induction in HFD-fed mice. Diabetologia 2017, 60, 1084–1093. [Google Scholar] [CrossRef]
- Pan, A.; Sun, X.-M.; Huang, F.-Q.; Liu, J.-F.; Cai, Y.-Y.; Wu, X.; Alolga, R.N.; Li, P.; Liu, B.-L.; Liu, Q.; et al. The mitochondrial β-oxidation enzyme HADHA restrains hepatic glucagon response by promoting β-hydroxybutyrate production. Nat. Commun. 2022, 13, 386. [Google Scholar] [CrossRef]
- Zhang, W.-S.; Pan, A.; Zhang, X.; Ying, A.; Ma, G.; Liu, B.-L.; Qi, L.-W.; Liu, Q.; Li, P. Inactivation of NF-κB2 (p52) restrains hepatic glucagon response via preserving PDE4B induction. Nat. Commun. 2019, 10, 4303–4313. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, F.-G.; Zhang, W.-S.; Pan, A.; Yang, Y.-L.; Liu, J.-F.; Li, P.; Liu, B.-L.; Qi, L.-W. Ginsenoside Rg1 inhibits glucagon-induced hepatic gluconeogenesis through Akt-FoxO1 interaction. Theranostics 2017, 7, 4001–4012. [Google Scholar] [CrossRef]
- Unger, R.H.; Cherrington, A.D. Glucagonocentric restructuring of diabetes: A pathophysiologic and therapeutic makeover. J. Clin. Investig. 2012, 122, 4–12. [Google Scholar] [CrossRef]
- Perry, R.J. Regulation of Hepatic Lipid and Glucose Metabolism by INSP3R1. Diabetes 2022, 71, 1834–1841. [Google Scholar] [CrossRef]
- Chaurasia, B.; Tippetts, T.S.; Mayoral Monibas, R.; Liu, J.; Li, Y.; Wang, L.; Wilkerson, J.L.; Sweeney, C.R.; Pereira, R.F.; Sumida, D.H.; et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 2019, 365, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.L.; Brozinick, J.T.; Wang, L.-P.; Hawkins, E.D.; Sargent, K.M.; Liu, Y.; Narra, K.; Hoehn, K.L.; Knotts, T.A.; Siesky, A.; et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007, 5, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulou, M.; Gordillo, R.; Koliaki, C.; Gancheva, S.; Jelenik, T.; De Filippo, E.; Herder, C.; Markgraf, D.; Jankowiak, F.; Esposito, I.; et al. Specific Hepatic Sphingolipids Relate to Insulin Resistance, Oxidative Stress, and Inflammation in Nonalcoholic Steatohepatitis. Diabetes Care 2018, 41, 1235–1243. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Zhou, Y.; Chen, Z.; Ren, D.; Cho, K.W.; Jiang, L.; Shen, H.; Sasaki, Y.; Rui, L. NF-κB–inducing kinase (NIK) promotes hyperglycemia and glucose intolerance in obesity by augmenting glucagon action. Nat. Med. 2012, 18, 943–949. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, B.; Zhao, X.; Lin, Y.; Wang, J.; Wang, X.; Hu, N.; Wang, S. Chlorogenic acid supplementation ameliorates hyperuricemia, relieves renal inflammation, and modulates intestinal homeostasis. Food Funct. 2021, 12, 5637–5649. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wei, J.; Sheng, F.; Li, P. Attenuation of Palmitic Acid–Induced Lipotoxicity by Chlorogenic Acid through Activation of SIRT1 in Hepatocytes. Mol. Nutr. Food Res. 2019, 63, e1801432. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef]
- Bhandarkar, N.S.; Brown, L.; Panchal, S.K. Chlorogenic acid attenuates high-carbohydrate, high-fat diet–induced cardiovascular, liver, and metabolic changes in rats. Nutr. Res. 2019, 62, 78–88. [Google Scholar] [CrossRef]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Raichur, S. Ceramide Synthases Are Attractive Drug Targets for Treating Metabolic Diseases. Front. Endocrinol. 2020, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Iqbal, M.; Karam, J.; Salifu, M.O.; McFarlane, S.I. Oxidative stress, glucose metabolism, and the prevention of type 2 diabetes: Pathophysiological insights. Antioxid. Redox Signal. 2007, 9, 911–929. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]
- Carlson, C.J.; Koterski, S.; Sciotti, R.J.; Poccard, G.B.; Rondinone, C.M. Enhanced basal activation of mitogen-activated protein kinases in adipocytes from type 2 diabetes—Potential role of p38 in the downregulation of GLUT4 expression. Diabetes 2003, 52, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Collins, Q.F.; Becker, T.C.; Robidoux, J.; Lupo, E.G.; Xiong, Y.; Daniel, K.W.; Floering, L.; Collins, S. p38 mitogen-activated protein kinase plays a stimulatory role in hepatic gluconeogenesis. J. Biol. Chem. 2005, 280, 42731–42737. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, S.; Xiao, B.; Zuo, S.; Zhang, Q.; Chen, G.; Yu, Y.; Chen, D.; Liu, Q.; Liu, Y.; et al. Prostaglandin F2α Facilitates Hepatic Glucose Production Through CaMKIIγ/p38/FOXO1 Signaling Pathway in Fasting and Obesity. Diabetes 2018, 67, 1748–1760. [Google Scholar] [CrossRef]
- Donath, M.Y. Targeting inflammation in the treatment of type 2 diabetes: Time to start. Nat. Rev. Drug Discov. 2014, 13, 465–476. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Chlorogenic Acid (CGA) Isomers Alleviate Interleukin 8 (IL-8) Production in Caco-2 Cells by Decreasing Phosphorylation of p38 and Increasing Cell Integrity. Int. J. Mol. Sci. 2018, 19, 3873. [Google Scholar] [CrossRef]
- Shi, H.; Shi, A.; Dong, L.; Lu, X.; Wang, Y.; Zhao, J.; Dai, F.; Guo, X. Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 2016, 35, 1366–1373. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, L.; Zhang, H.; Wu, G.; Zhang, Z.; Lv, J. Chlorogenic acid against palmitic acid in endoplasmic reticulum stress-mediated apoptosis resulting in protective effect of primary rat hepatocytes. Lipids Health Dis. 2018, 17, 270. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-D.; Wang, J.-W. Ceramide de novo synthesis in non-alcoholic fatty liver disease: Pathogenic mechanisms and therapeutic perspectives. Biochem. Pharmacol. 2022, 202, 115157. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Yang, L.-L.; Yang, Y.-L.; Liu, L.-W.; Li, J.; Liu, B.; Liu, K.; Qi, L.-W.; Li, P. Ginsenoside Rg5 Inhibits Succinate-Associated Lipolysis in Adipose Tissue and Prevents Muscle Insulin Resistance. Front. Pharmacol. 2017, 8, 43. [Google Scholar] [CrossRef]
- Turpin-Nolan, S.M.; Brüning, J.C. The role of ceramides in metabolic disorders: When size and localization matters. Nat. Rev. Endocrinol. 2020, 16, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Raichur, S.; Wang, S.T.; Chan, P.W.; Li, Y.; Ching, J.; Chaurasia, B.; Dogra, S.; Öhman, M.K.; Takeda, K.; Sugii, S.; et al. CerS2 Haploinsufficiency Inhibits β-Oxidation and Confers Susceptibility to Diet-Induced Steatohepatitis and Insulin Resistance. Cell Metab. 2014, 20, 919. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Kuba, M.; Koyasu, S.; Yamamoto, Y.; Motomura, K.; Arulmozhiraja, S.; Ohno, H.; Sharma, R.; Shimura, T.; Okajima, Y.; et al. Hepatocyte ELOVL Fatty Acid Elongase 6 Determines Ceramide Acyl-Chain Length and Hepatic Insulin Sensitivity in Mice. Hepatology 2020, 71, 1609–1625. [Google Scholar] [CrossRef]

| Gene | Sequence 5′–3′ | |
|---|---|---|
| CerS5 (mice) | Forward | TCAGACTTCTTGCTGGAGGT |
| Reverse | AGAGTCCACAGAAGGTCAGC | |
| CerS6 (mice) | Forward | AGAACTTGAGGATTGGAGGGTT |
| Reverse | CTGACAACACGTTCTCCAGC | |
| Degs1 (mice) | Forward | GAATGGGTCTACACGGACCAG |
| Reverse | CGAGAAGCATCATGGCTACAA | |
| G6pase (mice) | Forward | CGACTCGCTATCTCCAAGTGA |
| Reverse | GTTGAACCAGTCTCCGACCA | |
| PEPCK (mice) | Forward | AAGCATTCAACGCCAGGTTC |
| Reverse | GGGCGAGTCTGTCAGTTCAAT | |
| β-actin (mice) | Forward | AGTGTGACGTTGACATCCGTA |
| Reverse | GCCAGAGCAGTAATCTCCTTCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, N.; Zhang, T.; Han, M.; Tian, D.; Liu, J.; Li, S.; Yang, L.; Pan, G. Chlorogenic Acid Inhibits Ceramide Accumulation to Restrain Hepatic Glucagon Response. Nutrients 2023, 15, 3173. https://doi.org/10.3390/nu15143173
Xiao N, Zhang T, Han M, Tian D, Liu J, Li S, Yang L, Pan G. Chlorogenic Acid Inhibits Ceramide Accumulation to Restrain Hepatic Glucagon Response. Nutrients. 2023; 15(14):3173. https://doi.org/10.3390/nu15143173
Chicago/Turabian StyleXiao, Na, Tengfei Zhang, Mingli Han, Dan Tian, Jiawei Liu, Shan Li, Lele Yang, and Guojun Pan. 2023. "Chlorogenic Acid Inhibits Ceramide Accumulation to Restrain Hepatic Glucagon Response" Nutrients 15, no. 14: 3173. https://doi.org/10.3390/nu15143173
APA StyleXiao, N., Zhang, T., Han, M., Tian, D., Liu, J., Li, S., Yang, L., & Pan, G. (2023). Chlorogenic Acid Inhibits Ceramide Accumulation to Restrain Hepatic Glucagon Response. Nutrients, 15(14), 3173. https://doi.org/10.3390/nu15143173





