Abstract
Background: Nutrition plays a key role in modulating the likelihood of healthy ageing. In the present study, we aimed to conduct a systematic review to assess the impact of nutrition on healthy ageing in Asia. Methods: The systematic review was registered in the International Prospective Register of Systematic Reviews database (CRD42023408936) and conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The PubMed, Web of Science, and Embase databases were searched up to February 2023 without language restrictions. We included prospective cohort studies that evaluated the associations of intake of a single food or consumption of a single nutrient at midlife; adherence to various dietary patterns at midlife; and improved adherence to dietary patterns from mid- to late life with the likelihood of healthy ageing and its components. Results: Out of 16,373 records, we included 71 papers comprising 24 cohorts from Singapore, China, Japan, and Thailand. The healthy ageing components included cognitive function, physical function, and depression. The majority of studies supported the observation that the likelihood of healthy ageing and its components in late life was positively increased by a higher consumption of healthy foods, such as vegetables, fruits, fish, nuts, legumes, tea, milk, and dairy, at midlife, and also by greater adherence to dietary patterns with high diversity scores or high total antioxidant capacities. Furthermore, improved adherence to healthy dietary patterns from mid- to late life also increased the likelihood of healthy ageing in late life. Conclusion: Consuming healthy foods and adhering to healthy dietary patterns at midlife can promote the likelihood of healthy ageing. Moreover, improving diet quality from mid- to late life can still be beneficial.
1. Introduction
An increase in life expectancy and a decline in fertility rates have resulted in accelerated ageing of the population in many countries, including those in Asia. By 2050, a quarter of Asia’s population is predicted to be ≥60 years old, which will inevitably lead to an increased number of older adults with chronic diseases and disability, and with profound consequences for health, health systems, the workforce, and budgeting for many Asian countries [1]. To provide a public health framework for action, World Health Organization has released the “World report on ageing and health”, which calls for comprehensive public health action to promote healthy ageing, the latter being defined as developing and maintaining the functional ability that enables well-being in older age [2].
Nutrition and diet have been established as possessing some of the most important influences on health and ageing, with the overwhelming majority of evidence coming from Western populations [3,4]. However, there is still limited evidence on the associations between diet and nutrition at midlife and the likelihood of a multidimensional concept of healthy ageing and its components in late life in Asian populations.
In the present review, we aimed to conduct a comprehensive overview of Asian studies on the prospective associations of consumption of a single food or nutrient at midlife; adherence to various dietary patterns at midlife; and improved adherence to dietary patterns from mid- to late life with the likelihood of healthy ageing and its components. The results of this review could provide important evidence to develop better region-specific strategies aimed at promoting healthy ageing in Asia.
2. Methods
This systematic review was registered in the International Prospective Register of Systematic Reviews database (CRD42023408936). We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. The first two authors (Y.F.Z. and X.Y.S.) independently performed the study selection, data extraction, and assessment of study quality, and divergences were solved by discussion or consulting a third author (K.W.P.).
2.1. Data Sources and Searches
PubMed, EMBASE, and Web of Science were searched for studies investigating the relationship between nutrition and healthy ageing from the database’s inception to February 2023. Table S1 shows the strategies used for each database. In brief, the search terms included the Medical Subject Heading terms and related exploded versions, as well as keywords in titles or abstracts related to the following themes: ‘diet’, ‘nutrition’, ‘food’, ‘dietary pattern’, ‘healthy aging’, ‘dementia’, ‘cognitive’, ‘depression’, ‘activities of daily living’, ‘physical function’, ‘self-perceived health’, ‘ function-limiting’, ‘major chronic diseases’, ‘cohort’, ‘prospective’, ‘follow up’, and ‘longitudinal’. No language restriction was applied. In addition, reference lists of the included studies and relevant reviews were searched to identify further publications. We included cohort studies conducted in countries of Asia (defined as Eastern Asia, Southern Asia, and Southeastern Asia) and outcome measures assessed in older adults (aged ≥ 60 years). Although the global cut-off for older persons is ≥65 years, we included those aged 60–65 years as well, in order to account for a different definition of ‘older adults’ in some Asian countries [5].
2.2. Study Selection
The following types of studies were excluded: (1) duplicate publications or those reporting from the same cohort (the one with smaller sample size or shorter follow-up duration would be excluded); (2) unrelated to nutrition or healthy ageing; (3) not a prospective design; (4) not from a peer-reviewed publication; (5) ageing outcomes measured in those below aged <60 years; (6) and not conducted in Asia.
2.3. Data Extraction and Quality Assessment
Predesigned tables were used to extract information, including cohort name, country, sample size, age, median/mean follow-up duration, definition and acquisition of exposure, and assessment of outcome. The Newcastle–Ottawa Scale was used to assess the quality of the studies. A study was considered high quality if it received ≥6 points out of 9 points [6].
3. Results
3.1. Study Selection and Characteristics
We identified 16,373 studies in the literature search. Among these, 3875 duplicates were excluded. After screening the titles and abstracts, 12,129 citations were excluded, and the remaining 369 studies were included for full-text assessment. We further excluded 298 articles after full-text reading (reasons are shown in Figure 1) and included 71 studies comprising 24 cohorts in this review (Figure 1).
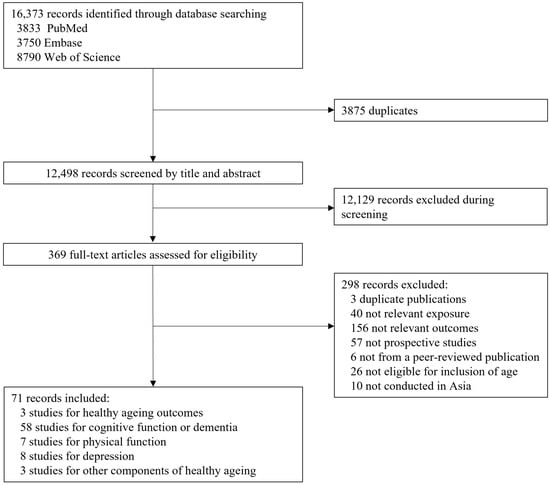
Figure 1.
Flow chart of the study selection.
The quality of the included studies, as assessed using the Newcastle–Ottawa Scale, was considered to be high for all 71 studies (Table S2). The characteristics of the eligible studies are shown in Table 1. Sixteen cohorts were from China, five from Japan, two from Singapore, and one from Thailand. Most of the studies were conducted among middle-aged or older participants, ranging in age from 40 to 89.2 years. The sample size ranged between 427 and 41,447, and the follow-up period ranged between 1.4 and 25.0 years. Food frequency questionnaires (FFQs) were used for data collection in most cohorts, except for the China Health and Nutrition Survey (CHNS), the Singapore Longitudinal Aging Studies, the National Institute for Longevity Sciences—Longitudinal Study of Aging (NILS-LSA), and the Zhejiang Ageing and Health Cohort Study. In these studies, 24 h dietary recalls for 3 consecutive days [7], 3-day dietary records [8,9,10], or simple food consumption questions [11,12,13] were used.

Table 1.
Characteristics of studies included in the systematic review.
3.2. Association between Nutrition and Healthy Ageing
Three studies [7,14,15], which included 17,244 participants in two cohorts, investigated the multidimensional concept of healthy ageing. In the SCHS, healthy ageing was defined as the absence of specific chronic diseases; good mental and overall self-perceived health; good physical functioning; and a lack of adverse outcomes of cognitive impairment, limitations in instrumental activities of daily living (IADL), or function-limiting pain [14,15]. Data from the SCHS reported that a greater adherence to various healthy dietary patterns at midlife, defined by the alternate Mediterranean diet (aMED), the Dietary Approaches to Stop Hypertension (DASH) diet, the Alternative Healthy Eating Index (AHEI)-2010, the overall plant-based diet index (PDI), and the healthful plant-based diet index (hPDI), was associated with a higher likelihood of healthy ageing in late life, with the odds ratio (OR) comparing the highest with the lowest quartile of diet quality scores ranging from 34% to 53% for healthy ageing [14]. Furthermore, consistent or improved adherence to the DASH diet from mid- to late life was associated with a 19% to 108% higher likelihood of healthy ageing [15]. In the CHNS, a healthy ageing score was calculated by adding up the standardized scores for physical functional limitation, comorbidity, cognitive function, and psychological stress, with a lower score indicating a healthier ageing process [7]. Data from the CHNS revealed that a higher level of dietary diversity was associated with a lower score, representing healthier ageing (T3 vs. T1: β, −0.16; 95% confidence interval [CI], −0.20 to −0.11) [7]. A summary of the associations between diet/nutrition and the outcomes of ageing is presented in Figure 2.
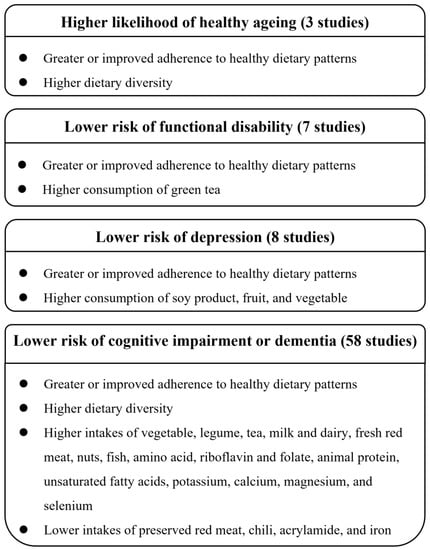
Figure 2.
Summary of major findings regarding the associations between diet/nutrition and outcomes of ageing.
3.3. Association between Nutrition and Physical Function
Seven studies [14,15,16,17,18,19,20], which included 48,674 participants, studied physical function components and how they are affected by ageing. Among these, physical function was assessed using the eight-item IADL scale [14,15,16], the Long-Term Care Insurance (LTCI) certification [17,19,20], or by the self-reported ability to conduct five self-care tasks (standing up after sitting for a long time, dressing, toileting, bathing, and feeding) [18]. Inconsistent findings were found regarding the association between the dietary diversity score and IADL limitation or incident disability, with one study showing a higher average dietary diversity score to be associated with a decreased risk of ADL disability (T3 vs. T1: hazard ratio, 0.50; 95% CI, 0.39–0.66) [18], while other studies reported null associations [16,17]. Regarding dietary patterns, greater adherence to various healthy dietary patterns [14,16,19], such as aMED, DASH, AHEI-2010, PDI, hPDI diet, fruit–egg–milk pattern, vegetable–meat–fish pattern, condiment and tea pattern, and the improved Japanese Diet Index, as well as increased adherence to the DASH diet [15], was significantly associated with a lower risk of IADL limitation or functional disability. For individual nutrients, data from the Ohsaki Cohort 2006 study showed that a higher consumption of green tea was significantly associated with a lower risk of incidents of functional disability, with a hazard ratio (95% CI) of 0.90 (0.77–1.06) among respondents who consumed 1–2 cups green tea/d; 0.75 (0.64–0.88) for those who consumed 3–4 cups/d; and 0.67 (0.57–0.79) for those who consumed ≥5 cups/d in comparison with those who consumed <1 cup/d (p-trend < 0.001) [20].
3.4. Association between Nutrition and Depression
Eight studies [13,14,15,21,22,23,24,25], which included 33,935 participants, investigated the components of depression in ageing. Among these, depression was assessed using the Center for Epidemiological Scale—Depression (CES-D) score [23,24,25], the Geriatric Depression Scale (GDS) [14,15,21], the Patient Health Questionnaire-9 (PHQ-9) [13], or the PhenX Toolkit [22]. As for dietary patterns, greater adherence to established healthy dietary patterns, such as the aMED, DASH, AHEI-2010, PDI, and hPDI diets [14], as well as an improvement in diet quality measured by these patterns [15], was associated with a lower risk of depression. However, for dietary patterns identified through a posteriori analytic methodology, while there were no significant associations of ‘vegetables-fruits’, ‘snacks-drinks-milk products’ and ‘meat-fish’ dietary patterns with a subsequent report of depressive symptoms among Chinese in Hong Kong [21], the vegetable–egg–beans–milk dietary pattern was associated with a lower risk of depression (OR, 0.65; 95% CI, 0.49–0.87), and the salt-preserved vegetable–garlic dietary pattern was associated with a higher risk of depression (OR, 1.33; 95% CI, 1.00–1.77) according to a study from the CLHLS [22]. For individual foods, higher intakes of soy products, fruits, and vegetables were associated with a lower risk of depression [13,24,25], whereas other food categories, including eggs, meat/poultry, seafood, dairy, legumes, grains, and tea, showed no significant associations [24]. Inconsistent results were shown for fish intake, with some studies reporting an inverse association [23] and others reporting null association [24].
3.5. Association between Nutrition and Cognitive Function or Dementia
Fifty-eight studies, which included 488,056 participants, investigated cognitive function components of ageing. Among these, cognitive function was assessed using the Telephone Interview for Cognitive Status—modified (TICS-m) [7,26,27,28,29,30,31,32,33,34,35,36,37], the Mini-Mental State Examination (MMSE) [8,9,11,12,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,59,61,65,73,77], the Montreal Cognitive Assessment (MoCA) [64,68,74,75], the Short Portable Mental Status Questionnaire (SPMSQ) [69,70], or the World Health Organization/University of California-Los Angeles Auditory Verbal Learning Test (AVLT) [71], or was evaluated by asking questions about walking capability, hearing/vision, memory, and decision-making ability [72]. Diagnoses of dementia were made in accordance with the Diagnostic and Statistical Manual of Mental Disorders [53,54,55,56,60,76]; the criteria of the LTCI certification [10,57,58,62,63]; or the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) [67]; or were obtained from the National Health Insurance Database [66].
For dietary diversity, a higher score was associated with a lower risk of cognitive impairment [7,38], bad memory [26], and disabling dementia [57]. Regarding dietary patterns, greater adherence to healthy dietary patterns, such as the aMED, DASH, AHEI-2010, modified AHEI, PDI, hPDI diet [39,40,43,64,71], Chinese Food Pagoda [72], “vegetable” [68] or “vegetable-pork” dietary pattern [30], “protein-rich” dietary pattern [29], beans and mushroom dietary pattern [28], Japanese dietary pattern [10,53,63], or wheat-based diverse diet [27], as well as improvements in diet quality [44], were associated with a lower risk of cognitive impairment, cognitive/memory decline, and incident dementia. However, the Western dietary pattern [69], animal-based dietary pattern [41], unhealthful PDI [39], and starch-rich dietary pattern [29] increased the risk of cognitive decline.
For individual foods, higher intakes of vegetables and their constituent nutrients [50,54,64,65,67,70], legumes [42], tea [8,34,66], milk and dairy [48,55], fresh red meat [47,74], nuts [31,51], and fish [32,60,62,66] were associated with a lower risk of cognitive impairment, cognitive decline, and dementia. For individual nutrients, a higher dietary total antioxidant capacity [45] and higher intakes of amino acids [61], riboflavin and folate [49,75], animal protein [33], unsaturated fatty acids, polyunsaturated fatty acids (PUFAs), and n-3 PUFA supplements [11,52,60,75] were associated with a lower risk of cognitive impairment, cognitive decline, and dementia. In contrast, higher consumption of preserved red meat [47], chili [35], acrylamide [73], and protein intake from grains [33] were associated with a higher risk of cognitive impairment. No significant associations were found for thiamine, niacin, vitamin B-6 [49], sugar-sweetened beverages [46], or coffee [8]. Inconsistent findings were found for intakes of fruit, soy, and isoflavones, as well as vitamin B-12, with different studies showing either inverse associations [9,50,67,70], positive associations [59], or a U-shaped relation [77], and others reporting null associations [48,49,54,58,65].
For dietary minerals, higher intakes of potassium, calcium, magnesium, and selenium were associated with a lower risk of dementia [54,56,76] or a reduced likelihood of reporting memory decline [37], whereas a higher iron intake was associated with poorer cognitive function [36].
3.6. Association between Nutrition and Other Components of Healthy Ageing
For other components of healthy ageing, greater adherence to various healthy dietary patterns at midlife, as well as consistent or improved adherence to the DASH diet from mid- to late life, was associated with a higher likelihood of having good self-perceived health and physical functioning and a lower likelihood of having chronic diseases and function-limiting pain [14,15]. In addition, a higher dietary diversity score was associated with less psychological stress (T3 vs. T1: OR, 0.59; 95% CI, 0.49–0.72); however, the association between the dietary diversity score and the number of comorbidities was insignificant [7].
4. Discussion
In this systematic review, we used data from population-based longitudinal cohort studies to investigate the prospective associations between nutrition at midlife and the likelihood of healthy ageing and its components in late life in Asia. Most of the current evidence has supported the positive associations of higher intakes of healthy foods at midlife, such as vegetables, fruits, fish, nuts, legumes, tea, milk, and dairy. Furthermore, a higher dietary diversity or total dietary antioxidant capacity at midlife, as well as greater or improved adherence to healthy dietary patterns from mid- to late life, was also associated with the likelihood of healthy ageing and its components in late life.
The currently available literature supports that adherence to various healthy dietary patterns is associated with a higher likelihood of healthy ageing. These healthy dietary patterns, either determined a priori or identified through a posteriori analytic methodology, are similar in that they recommend high consumption of fruits, vegetables, and whole grains; moderate consumption of dairy products, fish, and poultry; and low consumption of sugary beverages, saturated fat, added sodium, red meat, and processed food [14,16,19,39,40,43,54,64,71]. However, these results should be interpreted with caution, given that differences exist in the major ingredients and culinary methods used between Asian and Western cuisines. For example, the Mediterranean diet emphasizes fruits, vegetables, whole grains, and olive oil as staples, while Asian diets commonly rely on white rice, noodles, and other grains as primary sources of energy [72]. This variation in staple foods may significantly impact nutrient composition and overall dietary patterns.
In addition, the findings confirmed that maintaining consistently high DASH scores was related to a greater likelihood of healthy ageing than keeping consistently low DASH scores [15]. Moreover, those who managed to improve their DASH scores by >10% from mid- to late life were able to increase their likelihood of healthy ageing [15]. Hence, our findings provide evidence for the recommendation of the 2020–2025 Dietary Guidelines Advisory Committee that “it is never too late to eat healthfully” [78]. More studies are warranted to explore strategies in order to achieve a sustained change in dietary behaviours in the real world and to create an environment in which to make healthy eating affordable and accessible.
Dietary diversity is an important index reflecting nutrient adequacy. Increasing dietary diversity can ensure sufficient nutrient intake and improve dietary quality to promote healthy ageing [7,38]. However, mixed findings were observed regarding the association between dietary diversity score and IADL limitation or incident disability. Data from the CLHLS, including 2285 subjects aged >60 years with a maximum follow-up of 7 years, reported that dietary diversity had no effect on the occurrence of IADL limitation [16]. The Ota Genki Senior Project, including 10,318 Japanese adults aged >65 years with a median follow-up of 5.1 years, found that dietary variety was not independently associated with incident disability [17]. However, data from 5004 participants in a study of the CHNS reported that higher dietary diversity scores were associated with fewer physical functional limitations [18]. There are several potential reasons for these mixed findings. First, there is substantial variability in the measures of physical function and functional disability due to the use of different scales and instruments in different studies. Second, the intake frequency and scoring criteria of dietary diversity scores varied substantially across studies. For example, the dietary diversity score was calculated according to the intake frequency of 13 food groups, and the low group was defined as <7 in the CLHLS [16], whereas it was calculated according to the intake frequency of 10 food groups and a low group was defined as <3 in the Ota Genki Senior Project [17]. Nevertheless, our review concurs with the World Health Organization [79] and Chinese dietary guidelines [80] in terms of recommending adherence to a diverse diet to achieve healthy ageing in later life.
The associations between the intakes of fruits and fish and the likelihood of healthy aging components were inconsistent, and this could be explained by differences in the ranges of consumption among different populations. For example, the Hisayama study, which included 1071 Japanese participants, observed small differences among quintiles of fruit intake, with the range of the highest quartile of fruit intake being ≥115 g/d for men (≥100 g/d for women) and the lowest quartile being ≤32 g/d for men (≤21 g/d for women) [54]. However, there were substantial differences among the quartiles of fruit intake in the SCHS, with the median fruit intake in the highest and lowest quartile being 383.44 g/d and 76.30 g/d, respectively [50]. Notably, the SCHS applied a 165-item FFQ which included 14 fruits [50], whereas the Hisayama study applied a 70-item FFQ, and might have underestimated the fruit intake in this population [54]. Differences in methods of categorizing the intake of fish across studies could also explain these inconsistent results. For example, fish intake was divided into <3 times/week and ≥3 times/week in the Survey of Health and Living Status of the Elderly, and a null effect was reported for fish intake and risk of depression [24]. In contrast, fish intake was divided according to quartile consumption in the JPHC study, and a reduced risk of major depressive disorder was found in the third quartile (111.1 g/d) [23].
To the best of our knowledge, this is the first study which has systematically reviewed the association between nutrition in midlife and the likelihood of healthy ageing in late life according to Asian cohort studies. In addition, the quality of the included studies was considered to be high. Several limitations should be considered. First, except for the analyses of the association between nutrition and cognitive function, analyses related to healthy ageing, physical function, depression, and other components of healthy ageing only included limited studies. In addition, although we included 71 studies from 24 cohorts, these cohorts were situated in China, Japan, Singapore and Thailand, and represented a small proportion of the diverse Asian population. Second, substantial variations existed across the studies in terms of the measures of exposure, definitions of outcomes, sample sizes, and follow-up durations. Nonetheless, the overall results are consistent in that they recommend the consumption of healthy foods and adherence to healthy dietary patterns at midlife for healthy ageing. Moreover, improving the quality of one’s diet from mid- to late life can still be beneficial.
5. Conclusions
The present study identified associations between nutrition at midlife and the likelihood of healthy ageing in late life using robust data from cohort studies in Asia. Our study’s results provide important evidence for policymaking and dietary guidelines aimed at promoting healthy ageing in Asia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15143153/s1, Table S1: Search strategy; Table S2: Risk of bias of the included studies: the Newcastle–Ottawa Scale.
Author Contributions
Y.-F.Z. and W.-P.K. conceived and designed the study. Y.-F.Z. wrote the first draft of the manuscript. Y.-F.Z. and X.-Y.S. selected the studies and extracted the data. A.P. provided critical revision for the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This publication is supported by the Healthy Aging Science Program of the International Life Sciences Institute Southeast Asia Region (ILSI SEA Region). A. Pan is supported by the National Natural Science Foundation of China [82192902, 81930124 and 82021005] and the National Key Research and Development Program of China (2022YFC3600600). W.-P. Koh is supported by the National Medical Research Council, Singapore [CSA-SI (MOH-000434)]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The literature search was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses, and the review was registered in the International Prospective Register of Systematic Reviews database (CRD42023408936).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- ESCAP. Ageing in Asia and the Pacific: Overview; ESCAP: Bangkok, Thailand, 2017. [Google Scholar]
- WHO. World Report on Ageing and Health; WHO/FWC/ALC/15.01; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Knight, A.; Bryan, J.; Murphy, K. Is the Mediterranean diet a feasible approach to preserving cognitive function and reducing risk of dementia for older adults in Western countries? New insights and future directions. Ageing Res. Rev. 2016, 25, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.; Molero, P.; Ortuno Sanchez-Pedreno, F.; Van der Does, W.; Angel Martinez-Gonzalez, M. Diet quality and depression risk: A systematic review and dose-response meta-analysis of prospective studies. J. Affect. Disord. 2018, 226, 346–354. [Google Scholar] [CrossRef]
- Chen, L.K.; Arai, H.; Assantachai, P.; Akishita, M.; Chew, S.T.H.; Dumlao, L.C.; Duque, G.; Woo, J. Roles of nutrition in muscle health of community-dwelling older adults: Evidence-based expert consensus from Asian Working Group for Sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 1653–1672. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S.; Longnecker, M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992, 135, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, A. Dietary Diversity and Healthy Aging: A Prospective Study. Nutrients 2021, 13, 1787. [Google Scholar] [CrossRef]
- Shirai, Y.; Kuriki, K.; Otsuka, R.; Kato, Y.; Nishita, Y.; Tange, C.; Tomida, M.; Imai, T.; Ando, F.; Shimokata, H. Green tea and coffee intake and risk of cognitive decline in older adults: The National Institute for Longevity Sciences, Longitudinal Study of Aging. Public Health Nutr. 2020, 23, 1049–1057. [Google Scholar] [CrossRef]
- Nakamoto, M.; Otsuka, R.; Nishita, Y.; Tange, C.; Tomida, M.; Kato, Y.; Imai, T.; Sakai, T.; Ando, F.; Shimokata, H. Soy food and isoflavone intake reduces the risk of cognitive impairment in elderly Japanese women. Eur. J. Clin. Nutr. 2018, 72, 1458–1462. [Google Scholar] [CrossRef]
- Zhang, S.; Otsuka, R.; Nishita, Y.; Tange, C.; Tomida, M.; Ando, F.; Shimokata, H.; Arai, H. Twenty-year prospective cohort study of the association between a Japanese dietary pattern and incident dementia: The NILS-LSA project. Eur. J. Nutr. 2023, 62, 1719–1729. [Google Scholar] [CrossRef]
- Gao, Q.; Niti, M.; Feng, L.; Yap, K.B.; Ng, T.P. Omega-3 Polyunsaturated Fatty Acid Supplements and Cognitive Decline: Singapore Longitudinal Aging Studies. J. Nutr. Health Aging 2011, 15, 32–35. [Google Scholar] [CrossRef]
- Li, F.D.; Tong, Z.D.; Chang, Y.; Li, K.F.; Gu, X.; Zhang, T.; Lin, J.F. Eggs Consumption in Relation to Lower Risk of Cognitive Impairment in Elderly: Findings from a 6-Year Cohort Study. J. Nutr. Health Aging 2022, 26, 771–777. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, G.J.; Li, F.D.; Gu, X.; Zhai, Y.J.; Xu, L.; Wu, M.N.; Shen, H.W.; Lin, J.F. Soy product consumption and the risk of major depressive disorder in older adults: Evidence from a cohort study. Front. Psychiatry 2022, 13, 888667. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F.; Song, X.Y.; Wu, J.; Chen, G.C.; Neelakantan, N.; van Dam, R.M.; Feng, L.; Yuan, J.M.; Pan, A.; Koh, W.P. Association Between Dietary Patterns in Midlife and Healthy Ageing in Chinese Adults: The Singapore Chinese Health Study. J. Am. Med. Dir. Assoc. 2021, 22, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F.; Lai, J.S.; Chong, M.F.; Tong, E.H.; Neelakantan, N.; Pan, A.; Koh, W.P. Association between changes in diet quality from mid-life to late-life and healthy ageing: The Singapore Chinese Health Study. Age Ageing 2022, 51, afac232. [Google Scholar] [CrossRef]
- Aihemaitijiang, S.; Zhang, L.; Ye, C.; Halimulati, M.; Huang, X.; Wang, R.; Zhang, Z. Long-Term High Dietary Diversity Maintains Good Physical Function in Chinese Elderly: A Cohort Study Based on CLHLS from 2011 to 2018. Nutrients 2022, 14, 1730. [Google Scholar] [CrossRef]
- Hata, T.; Seino, S.; Yokoyama, Y.; Narita, M.; Nishi, M.; Hida, A.; Shinkai, S.; Kitamura, A.; Fujiwara, Y. Interaction of Eating Status and Dietary Variety on Incident Functional Disability among Older Japanese Adults. J. Nutr. Health Aging 2022, 26, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, A.; Wu, W.; Ren, Z.X.; Yang, C.L.; Wang, P.Y.; Zhang, Y.M. Beneficial Effect of Dietary Diversity on the Risk of Disability in Activities of Daily Living in Adults: A Prospective Cohort Study. Nutrients 2020, 12, 3263. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Zhang, S.; Tomata, Y.; Abe, S.; Tanji, F.; Sugawara, Y.; Tsuji, I. Association between improved adherence to the Japanese diet and incident functional disability in older people: The Ohsaki Cohort 2006 Study. Clin. Nutr. 2020, 39, 2238–2245. [Google Scholar] [CrossRef] [PubMed]
- Tomata, Y.; Kakizaki, M.; Nakaya, N.; Tsuboya, T.; Sone, T.; Kuriyama, S.; Hozawa, A.; Tsuji, I. Green tea consumption and the risk of incident functional disability in elderly Japanese: The Ohsaki Cohort 2006 Study. Am. J. Clin. Nutr. 2012, 95, 732–739. [Google Scholar] [CrossRef]
- Chan, R.; Chan, D.; Woo, J. A prospective cohort study to examine the association between dietary patterns and depressive symptoms in older Chinese people in Hong Kong. PLoS ONE 2014, 9, e105760. [Google Scholar] [CrossRef]
- Pei, Z.F.; Zhang, J.J.; Qin, W.Z.; Hu, F.F.; Zhao, Y.; Zhang, X.H.; Cong, X.X.; Liu, C.L.; Xu, L.Z. Association between Dietary Patterns and Depression in Chinese Older Adults: A Longitudinal Study Based on CLHLS. Nutrients 2022, 14, 5230. [Google Scholar] [CrossRef]
- Matsuoka, Y.J.; Sawada, N.; Mimura, M.; Shikimoto, R.; Nozaki, S.; Hamazaki, K.; Uchitomi, Y.; Tsugane, S. Dietary fish, n-3 polyunsaturated fatty acid consumption, and depression risk in Japan: A population-based prospective cohort study. Transl. Psychiatry 2017, 7, e1242. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.C.; Chang, T.L.; Chi, S.H. Frequent consumption of vegetables predicts lower risk of depression in older Taiwanese—Results of a prospective population-based study. Public Health Nutr. 2012, 15, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Fann, L.Y.; Huang, S.H.; Huang, Y.C.; Chen, C.F.; Sun, C.A.; Wang, B.L.; Chien, W.C.; Lu, C.H. The Synergetic Impact of Physical Activity and Fruit and Vegetable Consumption on the Risk of Depression in Taiwanese Adults. Int. J. Environ. Res. Public Health 2022, 19, 7300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, A.; Wu, W.; Yang, C.L.; Ren, Z.X.; Wang, M.C.; Wang, P.Y.; Zhang, Y.M. Dietary Diversity Is Associated with Memory Status in Chinese Adults: A Prospective Study. Front. Aging Neurosci. 2020, 12, 580760. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Adair, L.S.; Plassman, B.L.; Batis, C.; Edwards, L.J.; Popkin, B.M.; Mendez, M.A. Dietary Patterns and Cognitive Decline Among Chinese Older Adults. Epidemiology 2015, 26, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.W.; Hodge, A.M.; Hill, E.; Zhu, Z.T.; He, M.G. Associations of Dietary Pattern and Sleep Duration with Cognitive Decline in Community-Dwelling Older Adults: A Seven-Year Follow-Up Cohort Study. J. Alzheimer’s Dis. 2021, 82, 1559–1571. [Google Scholar] [CrossRef]
- Xu, X.Y.; Parker, D.; Shi, Z.M.; Byles, J.; Hall, J.; Hickman, L. Dietary Pattern, Hypertension and Cognitive Function in an Older Population: 10-Year Longitudinal Survey. Front. Public Health 2018, 6, 201. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, F.; Zhang, J.; Wei, Y.; Bai, J.; Wang, H.; Jia, X. Association between Micronutrient-Related Dietary Pattern and Cognitive Function among Persons 55 Years and Older in China: A Longitudinal Study. Nutrients 2023, 15, 481. [Google Scholar] [CrossRef]
- Li, M.; Shi, Z. A Prospective Association of Nut Consumption with Cognitive Function in Chinese Adults aged 55+ _ China Health and Nutrition Survey. J. Nutr. Health Aging 2019, 23, 211–216. [Google Scholar] [CrossRef]
- Qin, B.; Plassman, B.L.; Edwards, L.J.; Popkin, B.M.; Adair, L.S.; Mendez, M.A. Fish intake is associated with slower cognitive decline in Chinese older adults. J. Nutr. 2014, 144, 1579–1585. [Google Scholar] [CrossRef]
- Gao, R.; Yang, Z.; Yan, W.; Du, W.; Zhou, Y.; Zhu, F. Protein intake from different sources and cognitive decline over 9 years in community-dwelling older adults. Front. Public Health 2022, 10, 1016016. [Google Scholar] [CrossRef] [PubMed]
- Sukik, L.; Liu, J.H.; Shi, Z.M. Tea Consumption Is Associated with Reduced Cognitive Decline and Interacts with Iron Intake: A Population-Based Longitudinal Study on 4,820 Old Adults. J. Alzheimer’s Dis. 2022, 90, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.M.; El-Obeid, T.; Riley, M.; Li, M.; Page, A.; Liu, J.H. High Chili Intake and Cognitive Function among 4582 Adults: An Open Cohort Study over 15 Years. Nutrients 2019, 11, 1183. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.M.; Li, M.; Wang, Y.F.; Liu, J.H.; El-Obeid, T. High iron intake is associated with poor cognition among Chinese old adults and varied by weight status-a 15-y longitudinal study in 4852 adults. Am. J. Clin. Nutr. 2019, 109, 109–116. [Google Scholar] [CrossRef]
- Jiang, K.; Xie, C.X.; Li, Z.R.; Zeng, H.; Zhao, Y.; Shi, Z.M. Selenium Intake and its Interaction with Iron Intake Are Associated with Cognitive Functions in Chinese Adults: A Longitudinal Study. Nutrients 2022, 14, 3005. [Google Scholar] [CrossRef]
- Zheng, J.Z.; Zhou, R.; Li, F.R.; Chen, L.R.; Wu, K.Y.; Huang, J.H.; Liu, H.M.; Huang, Z.W.; Xu, L.; Yuan, Z.L.; et al. Association between dietary diversity and cognitive impairment among the oldest-old: Findings from a nationwide cohort study. Clin. Nutr. 2021, 40, 1452–1462. [Google Scholar] [CrossRef]
- Zhu, A.N.; Yuan, C.Z.; Pretty, J.; Ji, J.S. Plant-based dietary patterns and cognitive function: A prospective cohort analysis of elderly individuals in China (2008–2018). Brain Behav. 2022, 12, e2670. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pang, Y.; Liu, J.; Wang, J.; Xie, Z.; Huang, T. Association of healthy lifestyle with cognitive function among Chinese older adults. Eur. J. Clin. Nutr. 2021, 75, 325–334. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, H.; Ni, R.; Cao, Y.; Fang, W.; Chen, Y.; Pan, G. Interaction between the animal-based dietary pattern and green space on cognitive function among Chinese older adults: A prospective cohort study. Int. J. Hyg. Environ. Health 2023, 250, 114147. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Cheng, H.G. Lower intake of vegetables and legumes associated with cognitive decline among illiterate elderly Chinese: A 3-year cohort study. J. Nutr. Health Aging 2012, 16, 549–552. [Google Scholar] [CrossRef]
- Wu, J.; Song, X.Y.; Chen, G.C.; Neelakantan, N.; van Dam, R.M.; Feng, L.; Yuan, J.M.; Pan, A.; Koh, W.P. Dietary pattern in midlife and cognitive impairment in late life: A prospective study in Chinese adults. Am. J. Clin. Nutr. 2019, 110, 912–920. [Google Scholar] [CrossRef]
- Tong, E.H.; Lai, J.S.; Whitton, C.; Neelakantan, N.; Zhou, Y.F.; Chen, C.; van Dam, R.M.; Feng, L.; Pan, A.; Chong, M.F.F.; et al. Changes in Diet Quality from Mid- to Late Life Are Associated with Cognitive Impairment in the Singapore Chinese Health Study. J. Nutr. 2021, 151, 2800–2807. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.T.; Jiang, Y.W.; Feng, L.; Pan, A.; Koh, W.P. Dietary Total Antioxidant Capacity and Late-Life Cognitive Impairment: The Singapore Chinese Health Study. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Wu, J.; Feng, L.; Yuan, J.M.; Koh, E.P.; Pan, A. Sugar-sweetened beverages consumption in midlife and risk of late-life cognitive impairment in Chinese adults. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 55–61. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Sheng, L.T.; Pan, X.F.; Feng, L.; Yuan, J.M.; Pan, A.; Koh, W.P. Meat consumption in midlife and risk of cognitive impairment in old age: The Singapore Chinese Health Study. Eur. J. Nutr. 2020, 59, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Talaei, M.; Feng, L.; Yuan, J.M.; Pan, A.; Koh, W.P. Dairy, soy, and calcium consumption and risk of cognitive impairment: The Singapore Chinese Health Study. Eur. J. Nutr. 2020, 59, 1541–1552. [Google Scholar] [CrossRef]
- Sheng, L.T.; Jiang, Y.W.; Pan, X.F.; Feng, L.; Yuan, J.M.; Pan, A.; Koh, W.P. Association Between Dietary Intakes of B Vitamins in Midlife and Cognitive Impairment in Late-Life: The Singapore Chinese Health Study. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1222–1227. [Google Scholar] [CrossRef]
- Sheng, L.T.; Jiang, Y.W.; Alperet, D.J.; Feng, L.; Pan, A.; Koh, W.P. Quantity and variety of fruit and vegetable intake in midlife and cognitive impairment in late life: A prospective cohort study. Br. J. Nutr. 2022, 129, 2084–2093. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Sheng, L.T.; Feng, L.; Pan, A.; Koh, W.P. Consumption of dietary nuts in midlife and risk of cognitive impairment in late-life: The Singapore Chinese Health Study. Age Ageing 2020, 50, 1215–1221. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Sheng, L.T.; Pan, X.F.; Feng, L.; Yuan, J.M.; Pan, A.; Koh, W.P. Midlife Dietary Intakes of Monounsaturated Acids, n-6 Polyunsaturated Acids, and Plant-Based Fat Are Inversely Associated with Risk of Cognitive Impairment in Older Singapore Chinese Adults. J. Nutr. 2020, 150, 901–909. [Google Scholar] [CrossRef]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Doi, Y.; Uchida, K.; Shirota, T.; Yonemoto, K.; Kitazono, T.; Kiyohara, Y. Dietary patterns and risk of dementia in an elderly Japanese population: The Hisayama Study. Am. J. Clin. Nutr. 2013, 97, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Yoshida, D.; Ohara, T.; Hata, J.; Honda, T.; Hirakawa, Y.; Shibata, M.; Oishi, E.; Sakata, S.; Furuta, Y.; et al. Long-term association of vegetable and fruit intake with risk of dementia in Japanese older adults: The Hisayama study. BMC Geriatr. 2022, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ohara, T.; Ninomiya, T.; Hata, J.; Yoshida, D.; Mukai, N.; Nagata, M.; Uchida, K.; Shirota, T.; Kitazono, T.; et al. Milk and dairy consumption and risk of dementia in an elderly Japanese population: The Hisayama Study. J. Am. Geriatr. Soc. 2014, 62, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Hirakawa, Y.; Doi, Y.; Hata, J.; Uchida, K.; Shirota, T.; Kitazono, T.; Kiyohara, Y. Self-reported dietary intake of potassium, calcium, and magnesium and risk of dementia in the Japanese: The Hisayama Study. J Am. Geriatr. Soc. 2012, 60, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, R.; Zhang, S.; Ihira, H.; Sawada, N.; Inoue, M.; Yamagishi, K.; Yasuda, N.; Tsugane, S. Dietary diversity and risk of late-life disabling dementia in middle-aged and older adults. Clin. Nutr. 2023, 42, 541–549. [Google Scholar] [CrossRef]
- Murai, U.; Sawada, N.; Charvat, H.; Inoue, M.; Yasuda, N.; Yamagishi, K.; Tsugane, S. Soy product intake and risk of incident disabling dementia: The JPHC Disabling Dementia Study. Eur. J. Nutr. 2022, 61, 4045–4057. [Google Scholar] [CrossRef]
- Svensson, T.; Sawada, N.; Mimura, M.; Nozaki, S.; Shikimoto, R.; Tsugane, S. Midlife intake of the isoflavone genistein and soy, and the risk of late-life cognitive impairment: The JPHC Saku Mental Health Study. J. Epidemiol. 2021, 33, 342–349. [Google Scholar] [CrossRef]
- Nozaki, S.; Sawada, N.; Matsuoka, Y.J.; Shikimoto, R.; Mimura, M.; Tsugane, S. Association between Dietary Fish and PUFA Intake in Midlife and Dementia in Later Life: The JPHC Saku Mental Health Study. J. Alzheimer’s Dis. 2021, 79, 1091–1104. [Google Scholar] [CrossRef]
- Kinoshita, K.; Otsuka, R.; Takada, M.; Tsukamoto-Yasui, M.; Nishita, Y.; Tange, C.; Tomida, M.; Shimokata, H.; Kuzuya, M.; Imaizumi, A.; et al. The Association between Dietary Amino Acid Intake and Cognitive Decline 8 Years Later in Japanese Community-Dwelling Older Adults. J. Nutr. Health Aging 2021, 25, 165–171. [Google Scholar] [CrossRef]
- Tsurumaki, N.; Zhang, S.; Tomata, Y.; Abe, S.; Sugawara, Y.; Matsuyama, S.; Tsuji, I. Fish consumption and risk of incident dementia in elderly Japanese: The Ohsaki cohort 2006 study. Br. J. Nutr. 2019, 122, 1182–1191. [Google Scholar] [CrossRef]
- Tomata, Y.; Sugiyama, K.; Kaiho, Y.; Honkura, K.; Watanabe, T.; Zhang, S.; Sugawara, Y.; Tsuji, I. Dietary Patterns and Incident Dementia in Elderly Japanese: The Ohsaki Cohort 2006 Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Lee, M.S.; Chiou, J.M.; Chen, T.F.; Chen, Y.C.; Chen, J.H. Association of Diet Quality and Vegetable Variety with the Risk of Cognitive Decline in Chinese Older Adults. Nutrients 2019, 11, 1666. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Kwok, T.; Woo, J. Higher fruit and vegetable variety associated with lower risk of cognitive impairment in Chinese community-dwelling older men: A 4-year cohort study. Eur. J. Nutr. 2022, 61, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.Y.; Lo, Y.L.; Wu, S.Y.; Wang, P.N.; Pan, W.H. Dietary Patterns and Foods Associated with Cognitive Function in Taiwanese Older Adults: The Cross-sectional and Longitudinal Studies. J. Am. Med. Dir. Assoc. 2019, 20, 544–550.e4. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.T.C.; Richards, M.; Chan, W.C.; Chiu, H.F.K.; Lee, R.S.Y.; Lam, L.C.W. Lower risk of incident dementia among Chinese older adults having three servings of vegetables and two servings of fruits a day. Age Ageing 2017, 46, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Jung, C.C.; Chen, J.H.; Chiou, J.M.; Chen, T.F.; Chen, Y.F.; Tang, S.C.; Yeh, S.J.; Lee, M.S. Association of Dietary Patterns with Global and Domain-Specific Cognitive Decline in Chinese Elderly. J. Am. Geriatr. Soc. 2017, 65, 1159–1167. [Google Scholar] [CrossRef]
- Tsai, H.J. Dietary patterns and cognitive decline in Taiwanese aged 65 years and older. Int. J. Geriatr. Psychiatry 2015, 30, 523–530. [Google Scholar] [CrossRef]
- Wang, R.S.; Wang, B.L.; Huang, Y.N.; Wan, T.T.H. The combined effect of physical activity and fruit and vegetable intake on decreasing cognitive decline in older Taiwanese adults. Sci. Rep. 2022, 12, 9825. [Google Scholar] [CrossRef]
- Jia, J.; Zhao, T.; Liang, Y.; Liu, W.; Li, F.; Shi, S.; Zhou, C.; Yang, H.; Liao, Z.; Li, Y.; et al. Association between healthy lifestyle and memory decline in older adults: 10 year, population based, prospective cohort study. BMJ 2023, 380, e072691. [Google Scholar] [CrossRef]
- Zhu, J.; Xiang, Y.B.; Cai, H.; Li, H.; Gao, Y.T.; Zheng, W.; Shu, X.O. A Prospective Investigation of Dietary Intake and Functional Impairments among the Elderly. Am. J. Epidemiol. 2018, 187, 2372–2386. [Google Scholar] [CrossRef]
- Liu, Z.M.; Tse, L.A.; Chen, B.; Wu, S.; Chan, D.; Kowk, T.; Woo, J.; Xiang, Y.T.; Wong, S.Y.S. Dietary acrylamide exposure was associated with mild cognition decline among non-smoking Chinese elderly men. Sci. Rep. 2017, 7, 6395. [Google Scholar] [CrossRef] [PubMed]
- Manacharoen, A.; Jayanama, K.; Ruangritchankul, S.; Vathesatogkit, P.; Sritara, P.; Warodomwichit, D. Association of body mass index and dietary intake with mild cognitive impairment and dementia: A retrospective cohort study. BMC Geriatr. 2023, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.W.; Liu, K.; Chen, S.; Yu, H.Y.; An, Y.; Wang, Y.; Zhang, X.N.; Wang, Y.S.; Qin, Z.S.; Xiao, R. Dietary Intake of Riboflavin and Unsaturated Fatty Acid Can Improve the Multi-Domain Cognitive Function in Middle-Aged and Elderly Populations: A 2-Year Prospective Cohort Study. Front. Aging Neurosci. 2019, 11, 226. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, C.; Zhao, Q.; Wu, W.; Liang, X.; Xiao, Z.; Mortimer, J.A.; Borenstein, A.R.; Dai, Q.; Ding, D. Dietary calcium and magnesium intake and risk for incident dementia: The Shanghai Aging Study. Alzheimer’s Dement. 2022, 8, e12362. [Google Scholar] [CrossRef]
- Wang, L.J.; Liu, K.; Zhang, X.N.; Wang, Y.S.; Liu, W.; Wang, T.; Hao, L.; Ju, M.W.; Xiao, R. The Effect and Mechanism of Cholesterol and Vitamin B-12 on Multi-Domain Cognitive Function: A Prospective Study on Chinese Middle-Aged and Older Adults. Front. Aging Neurosci. 2021, 13, 707958. [Google Scholar] [CrossRef] [PubMed]
- U.S. Departments of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Departments of Health and Human Services: Washington, DC, USA, 2020.
- WHO. Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 25 May 2023).
- Chinese Nutrition Society. Dietary Guidelines for Chinese (2016); People’s Medical Publishing House Co., Ltd.: Beijing, China, 2016; ISBN 978-7-117-22214-3. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).