Carvacrol Improves Vascular Function in Hypertensive Animals by Modulating Endothelial Progenitor Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Experimental Design
2.2. Indirect Measurement of Systolic Blood Pressure (SBP) and Heart Rate (HR)
2.3. Isolation and In Vitro Culture of EPC Derived from Bone Marrow and Peripheral Blood
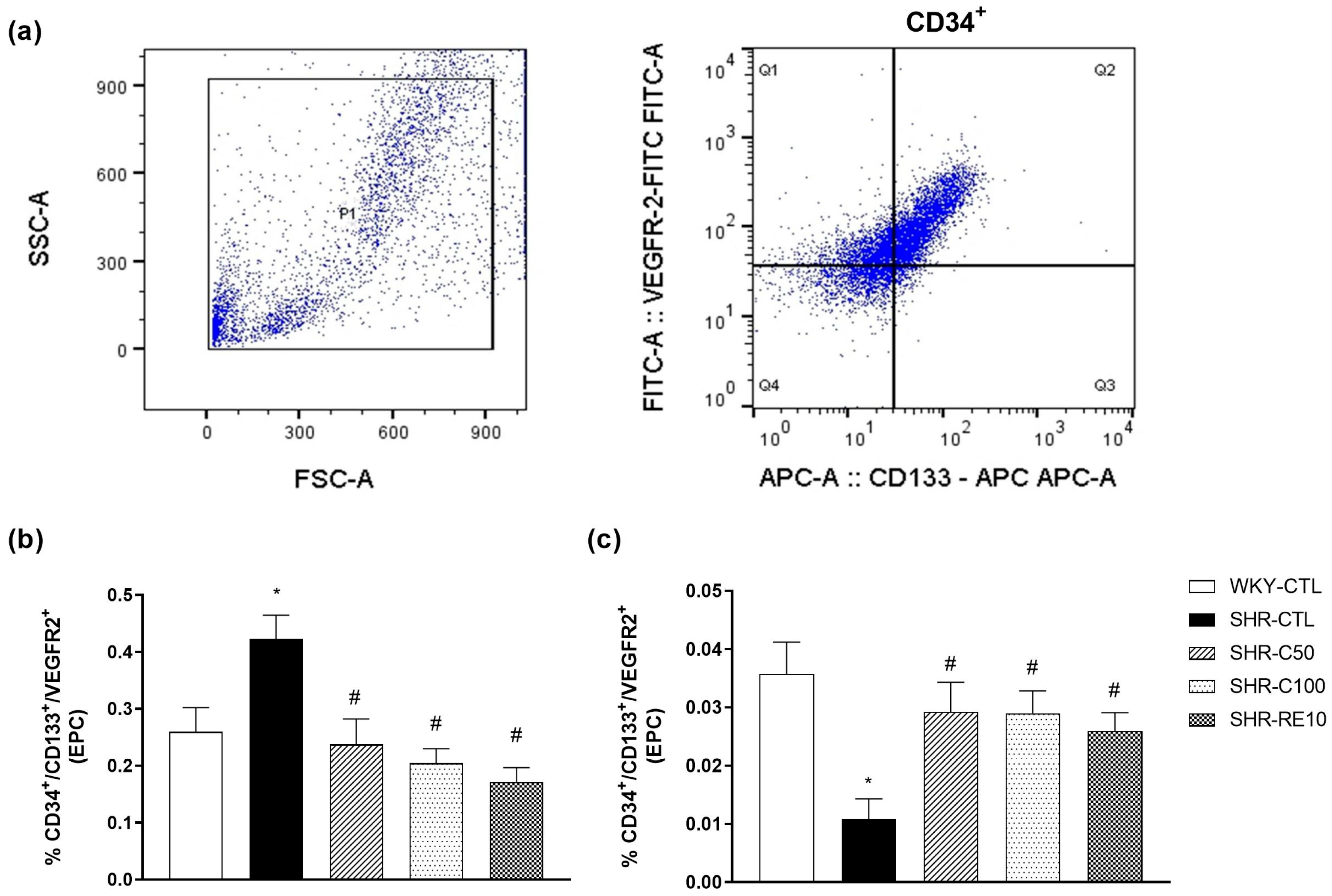
2.4. EPC Quantification and Characterization after Treatment with Carvacrol
2.5. Functional Evaluation of Endothelial Progenitor Cells (EPCs) by Colony-Forming Units
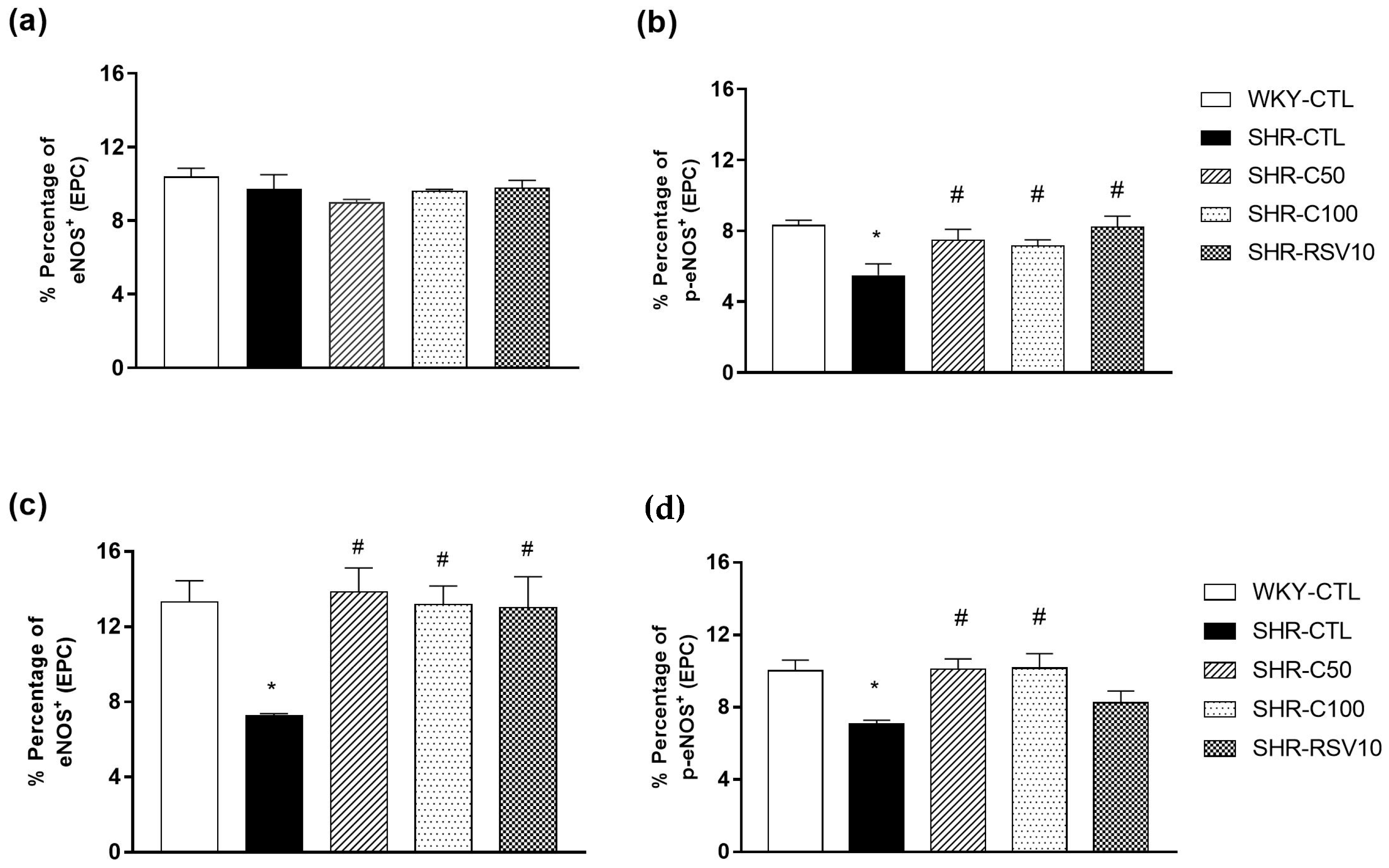
2.6. Evaluation of EPC-eNOS Expression after Carvacrol Treatment
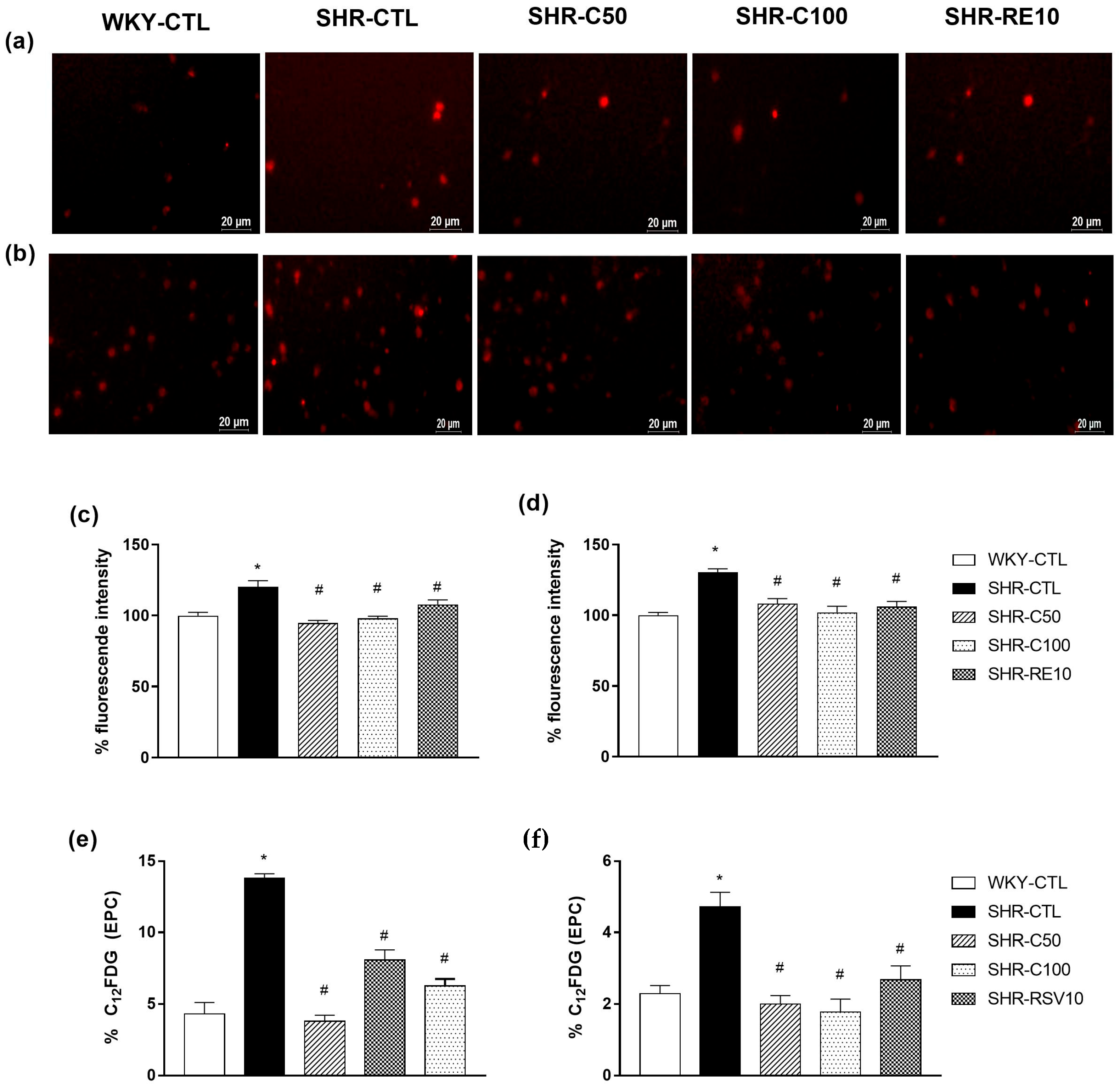
2.7. Evaluation of the Effect of Carvacrol Treatment on ROS Production
2.8. Evaluation of Cellular Senescence by Flow Cytometry
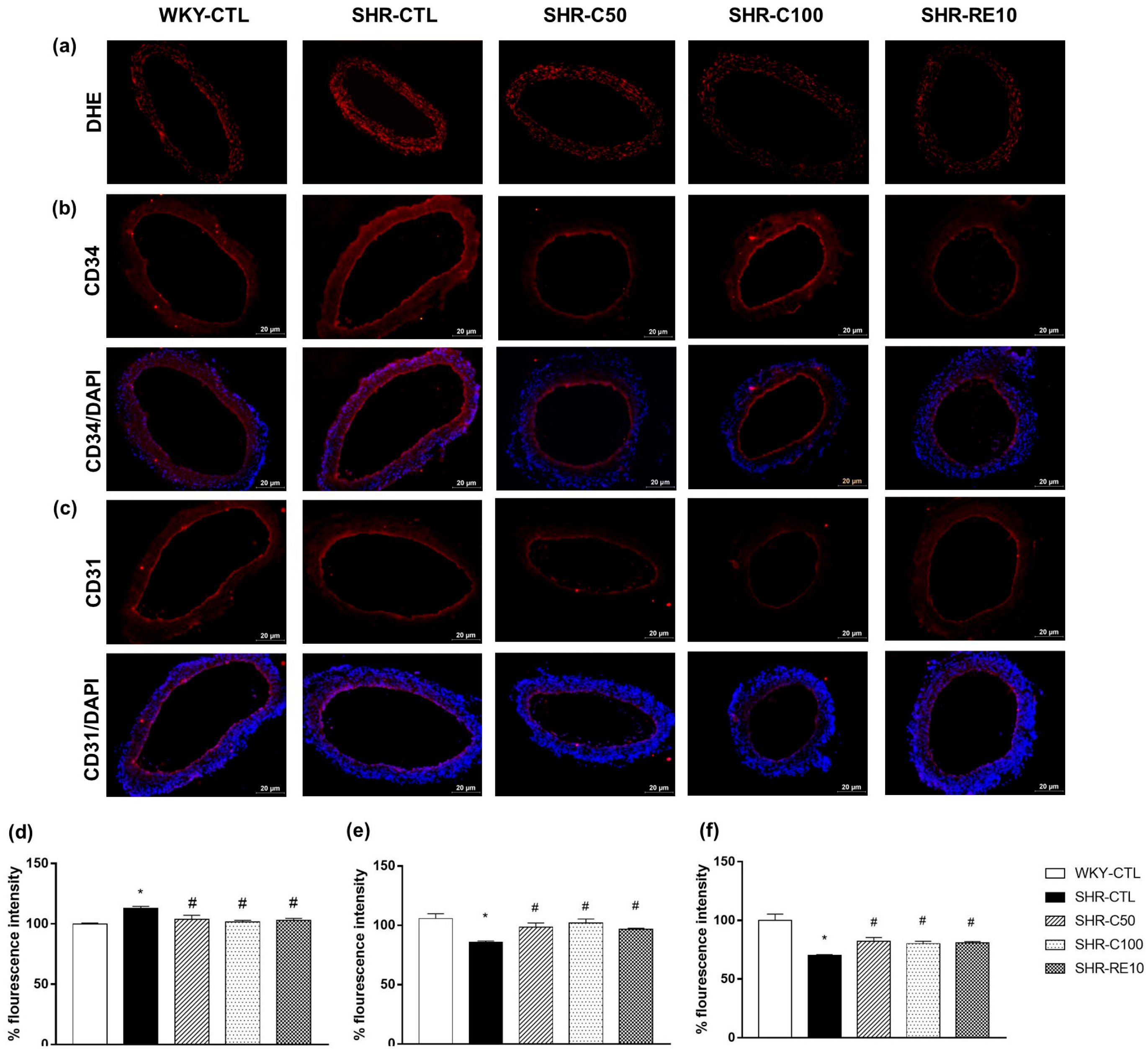
2.9. Immunofluorescence
2.10. Statistical Analysis
3. Results
3.1. Carvacrol Reduces SBP in SHR Animals
3.2. Carvacrol Enhances EPC Mobilization to the Peripheral Circulation
3.3. Characterization of EPC after Cell Culture
3.4. Carvacrol Treatment Increases eNOS Activity and Expression
3.5. Carvacrol Reduced Oxidative Stress and Cellular Senescence in EPCs
3.6. Carvacrol Reduces Vascular Oxidative Stress and Induces EPC-Mediated Reendothelialization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, F.; Liu, X.; Ding, H.; Zhang, W. Paracrine mechanisms of endothelial progenitor cells in vascular repair. Acta Histochem. 2022, 124, 151833. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, Y.; Miao, X.-H.; Guo, J.-D.; Li, D.-W. Neovascularization and tissue regeneration by endothelial progenitor cells in ischemic stroke. Neurol. Sci. 2021, 42, 3585–3593. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.B. Endothelial Progenitor Cells for the Vascularization of Engineered Tissues. Tissue Eng. Part B Rev. 2018, 24, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Peyter, A.C.; Armengaud, J.B.; Guillot, E.; Yzydorczyk, C. Endothelial Progenitor Cells Dysfunctions and Cardiometabolic Disorders: From Mechanisms to Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 6667. [Google Scholar] [CrossRef]
- Suzuki, R.; Fukuda, N.; Katakawa, M.; Tsunemi, A.; Tahira, Y.; Matsumoto, T.; Ueno, T.; Soma, M. Effects of an angiotensin II receptor blocker on the impaired function of endothelial progenitor cells in patients with essential hypertension. Am. J. Hypertens. 2014, 27, 695–701. [Google Scholar] [CrossRef]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef]
- De Cavanagh, E.M.; González, S.A.; Inserra, F.; Forcada, P.; Castellaro, C.; Chiabaut-Svane, J.; Obregón, S.; Casarini, M.J.; Kempny, P.; Kotliar, C. Sympathetic predominance is associated with impaired endothelial progenitor cells and tunneling nanotubes in controlled-hypertensive patients. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H207–H215. [Google Scholar] [CrossRef]
- Budzyn, M.; Gryszczynska, B.; Boruczkowski, M.; Kaczmarek, M.; Begier-Krasinska, B.; Osinska, A.; Bukowska, A.; Iskra, M.; Kasprzak, M.P. The endothelial status reflected by circulating endothelial cells, circulating endothelial progenitor cells and soluble thrombomodulin in patients with mild and resistant hypertension. Vasc. Pharmacol. 2019, 113, 77–85. [Google Scholar] [CrossRef]
- Luo, S.; Xia, W.; Chen, C.; Robinson, E.A.; Tao, J. Endothelial progenitor cells and hypertension: Current concepts and future implications. Clin. Sci. 2016, 130, 2029–2042. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F.; Li, Z.; Huang, L.; Cao, Q.; Chen, S. MeCP2 mediated dysfunction in senescent EPCs. Oncotarget 2017, 8, 78289–78299. [Google Scholar] [CrossRef]
- Reskiawan, A.K.R.; Alwjwaj, M.; Ahmad Othman, O.; Rakkar, K.; Sprigg, N.; Bath, P.M.; Bayraktutan, U. Inhibition of oxidative stress delays senescence and augments functional capacity of endothelial progenitor cells. Brain Res. 2022, 1787, 147925. [Google Scholar] [CrossRef]
- Veenstra, J.P.; Johnson, J.J. Oregano (Origanum vulgare) extract for food preservation and improvement in gastrointestinal health. Int. J. Nutr. 2019, 3, 43–52. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Berkay Yilmaz, Y.; Antika, G.; Salehi, B.; Tumer, T.B.; Kulandaisamy Venil, C.; Das, G.; Patra, J.K.; Karazhan, N.; Akram, M.; et al. Phytochemical constituents, biological activities, and health-promoting effects of the genus Origanum. Phytother. Res. PTR 2021, 35, 95–121. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef]
- Naziroglu, M. A novel antagonist of TRPM2 and TRPV4 channels: Carvacrol. Metab. Brain Dis. 2022, 37, 711–728. [Google Scholar] [CrossRef]
- Peixoto-Neves, D.; Silva-Alves, K.S.; Gomes, M.D.; Lima, F.C.; Lahlou, S.; Magalhaes, P.J.; Ceccatto, V.M.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fundam. Clin. Pharmacol. 2010, 24, 341–350. [Google Scholar] [CrossRef]
- Dantas, B.P.; Alves, Q.L.; de Assis, K.S.; Ribeiro, T.P.; de Almeida, M.M.; de Vasconcelos, A.P.; de Araujo, D.A.; de Andrade Braga, V.; de Medeiros, I.A.; Alencar, J.L.; et al. Participation of the TRP channel in the cardiovascular effects induced by carvacrol in normotensive rat. Vasc. Pharmacol. 2015, 67–69, 48–58. [Google Scholar] [CrossRef]
- Costa, H.A.; Dias, C.J.M.; Martins, V.A.; de Araujo, S.A.; da Silva, D.P.; Mendes, V.S.; de Oliveira, M.N.S., Jr.; Mostarda, C.T.; Borges, A.C.R.; Ribeiro, R.M.; et al. Effect of treatment with carvacrol and aerobic training on cardiovascular function in spontaneously hypertensive rats. Exp. Physiol. 2021, 106, 891–901. [Google Scholar] [CrossRef]
- Olmezturk Karakurt, T.C.; Emir, I.; Bedir, Z.; Ozkaloglu Erdem, K.T.; Suleyman, H.; Sarigul, C.; Mendil, A.S. Effects of carvacrol on ketamine-induced cardiac injury in rats: An experimental study. Drug. Chem. Toxicol. 2022, 13, 1–6. [Google Scholar] [CrossRef]
- El-Sayed, E.M.; Abd-Allah, A.R.; Mansour, A.M.; El-Arabey, A.A. Thymol and carvacrol prevent cisplatin-induced nephrotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. JBMT 2015, 29, 165–172. [Google Scholar] [CrossRef]
- Lee, K.P.; Sudjarwo, G.W.; Jung, S.H.; Lee, D.; Lee, D.Y.; Lee, G.B.; Baek, S.; Kim, D.Y.; Lee, H.M.; Kim, B.; et al. Carvacrol inhibits atherosclerotic neointima formation by downregulating reactive oxygen species production in vascular smooth muscle cells. Atherosclerosis 2015, 240, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ba, L.; Huang, W.; Liu, Y.; Pan, H.; Mingyao, E.; Shi, P.; Wang, Y.; Li, S.; Qi, H.; et al. Role of carvacrol in cardioprotection against myocardial ischemia/reperfusion injury in rats through activation of MAPK/ERK and Akt/eNOS signaling pathways. Eur. J. Pharmacol. 2017, 796, 90–100. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.H.; Mohamed, H.H.; Elsabagh, D.A.; Mohamed, S.A.; Noreldin, A.E.; Al Jaouni, S.K.; Alsenosy, A.A. Eugenol and carvacrol attenuate brain D-galactose-induced aging-related oxidative alterations in rats. Env. Sci. Pollut. Res. Int. 2022, 29, 47436–47447. [Google Scholar] [CrossRef] [PubMed]
- Matluobi, D.; Araghi, A.; Maragheh, B.F.A.; Rezabakhsh, A.; Soltani, S.; Khaksar, M.; Siavashi, V.; Feyzi, A.; Bagheri, H.S.; Rahbarghazi, R.; et al. Carvacrol promotes angiogenic paracrine potential and endothelial differentiation of human mesenchymal stem cells at low concentrations. Microvasc. Res. 2018, 115, 20–27. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, X.; Shao, Y.; Su, C.; Tao, J.; Liu, X. Berberine reduces endothelial injury and arterial stiffness in spontaneously hypertensive rats. Clin. Exp. Hypertens. 2020, 42, 257–265. [Google Scholar] [CrossRef]
- Wang, Y.; Thatcher, S.E.; Cassis, L.A. Measuring Blood Pressure Using a Noninvasive Tail Cuff Method in Mice. Methods Mol. Biol. 2017, 1614, 69–73. [Google Scholar] [CrossRef]
- Kundu, N.; Domingues, C.C.; Chou, C.; Ahmadi, N.; Houston, S.; Jerry, D.J.; Sen, S. Use of p53-Silenced Endothelial Progenitor Cells to Treat Ischemia in Diabetic Peripheral Vascular Disease. J. Am. Heart Assoc. 2017, 6, e005146. [Google Scholar] [CrossRef]
- Souza, L.V.; De Meneck, F.; Oliveira, V.; Higa, E.M.; Akamine, E.H.; Franco, M.D.C. Detrimental Impact of Low Birth Weight on Circulating Number and Functional Capacity of Endothelial Progenitor Cells in Healthy Children: Role of Angiogenic Factors. J. Pediatr. 2019, 206, 72–77.e71. [Google Scholar] [CrossRef]
- Chen, C.; Song, C.; Zhang, D.; Yin, D.; Zhang, R.; Chen, J.; Dou, K. Effect of resveratrol combined with atorvastatin on re-endothelialization after drug-eluting stents implantation and the underlying mechanism. Life Sci. 2020, 245, 117349. [Google Scholar] [CrossRef]
- Radziwon-Balicka, A.; Lesyk, G.; Back, V.; Fong, T.; Loredo-Calderon, E.L.; Dong, B.; El-Sikhry, H.; El-Sherbeni, A.A.; El-Kadi, A.; Ogg, S.; et al. Differential eNOS-signalling by platelet subpopulations regulates adhesion and aggregation. Cardiovasc. Res. 2017, 113, 1719–1731. [Google Scholar] [CrossRef]
- Khemais-Benkhiat, S.; Belcastro, E.; Idris-Khodja, N.; Park, S.H.; Amoura, L.; Abbas, M.; Auger, C.; Kessler, L.; Mayoux, E.; Toti, F.; et al. Angiotensin II-induced redox-sensitive SGLT1 and 2 expression promotes high glucose-induced endothelial cell senescence. J. Cell. Mol. Med. 2020, 24, 2109–2122. [Google Scholar] [CrossRef]
- Soh, B.S.; Ng, S.Y.; Wu, H.; Buac, K.; Park, J.H.; Lian, X.; Xu, J.; Foo, K.S.; Felldin, U.; He, X.; et al. Endothelin-1 supports clonal derivation and expansion of cardiovascular progenitors derived from human embryonic stem cells. Nat. Commun. 2016, 7, 10774. [Google Scholar] [CrossRef]
- Castela, A.; Gomes, P.; Silvestre, R.; Guardao, L.; Leite, L.; Chilro, R.; Rodrigues, I.; Vendeira, P.; Virag, R.; Costa, C. Vasculogenesis and Diabetic Erectile Dysfunction: How Relevant Is Glycemic Control? J. Cell. Biochem. 2017, 118, 82–91. [Google Scholar] [CrossRef]
- Aimad, A.; Youness, E.A.; Sanae, R.; El Moussaoui, A.; Bourhia, M.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Nafidi, H.A.; et al. Chemical Composition and Antifungal, Insecticidal and Repellent Activity of Essential Oils From Origanum compactum Benth. Used in the Mediterranean Diet. Front. Plant. Sci. 2022, 13, 798259. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A.; Sabzalian, M.R. Thymol, carvacrol, and antioxidant accumulation in Thymus species in response to different light spectra emitted by light-emitting diodes. Food Chem. 2020, 307, 125521. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.Z.; Gu, C.X.; Liu, G.L.; Tian, K. Carvacrol suppresses inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes. J. Cell. Biochem. 2018, 120, 8169–8176. [Google Scholar] [CrossRef]
- Sadeghzadeh, S.; Hejazian, S.H.; Jamhiri, M.; Hafizibarjin, Z.; Sadeghzadeh, S.; Safari, F.; Pharmacology. The effect of carvacrol on transcription levels of Bcl-2 family proteins in hypertrophied heart of rats. J. Physiol. 2018, 22, 54–62. [Google Scholar]
- Dias, C.J.; Costa, H.A.; Alves Dias-Filho, C.A.; Ferreira, A.C.; Rodrigues, B.; Irigoyen, M.C.; Romao Borges, A.C.; de Andadre Martins, V.; Branco Vidal, F.C.; Ribeiro, R.M.; et al. Carvacrol reduces blood pressure, arterial responsiveness and increases expression of MAS receptors in spontaneously hypertensive rats. Eur. J. Pharmacol. 2022, 917, 174717. [Google Scholar] [CrossRef]
- Heinisch, P.P.; Bello, C.; Emmert, M.Y.; Carrel, T.; Dreßen, M.; Hörer, J.; Winkler, B.; Luedi, M.M.J.C. Endothelial progenitor cells as biomarkers of cardiovascular pathologies: A narrative review. Cells 2022, 11, 1678. [Google Scholar] [CrossRef]
- Naito, H.; Iba, T.; Takakura, N. Mechanisms of new blood-vessel formation and proliferative heterogeneity of endothelial cells. Int. Immunol. 2020, 32, 295–305. [Google Scholar] [CrossRef]
- Huang, P.H.; Chen, Y.H.; Tsai, H.Y.; Chen, J.S.; Wu, T.C.; Lin, F.Y.; Sata, M.; Chen, J.W.; Lin, S.J. Intake of red wine increases the number and functional capacity of circulating endothelial progenitor cells by enhancing nitric oxide bioavailability. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, G.; Doerries, C.; Mocharla, P.S.; Mueller, M.F.; Bahlmann, F.H.; Horvath, T.; Jiang, H.; Sorrentino, S.A.; Steenken, N.; Manes, C.; et al. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: Relation to endothelial dysfunction. Hypertension 2010, 55, 1389–1397. [Google Scholar] [CrossRef]
- Huang, P.H.; Huang, S.S.; Chen, Y.H.; Lin, C.P.; Chiang, K.H.; Chen, J.S.; Tsai, H.Y.; Lin, F.Y.; Chen, J.W.; Lin, S.J. Increased circulating CD31+/annexin V+ apoptotic microparticles and decreased circulating endothelial progenitor cell levels in hypertensive patients with microalbuminuria. J. Hypertens. 2010, 28, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Magen, E.; Feldman, A.; Cohen, Z.; Alon, D.B.; Minz, E.; Chernyavsky, A.; Linov, L.; Mishal, J.; Schlezinger, M.; Sthoeger, Z. Circulating endothelial progenitor cells, Th1/Th2/Th17-related cytokines, and endothelial dysfunction in resistant hypertension. Am. J. Med. Sci. 2010, 339, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Oak, M.H.; Jung, S.H.; Park, D.H.; Auger, C.; Kim, K.R.; Lee, S.W.; Schini-Kerth, V.B. An ethanolic extract of Lindera obtusiloba stems causes NO-mediated endothelium-dependent relaxations in rat aortic rings and prevents angiotensin II-induced hypertension and endothelial dysfunction in rats. Naunyn Schmiedebergs Arch. Pharm. 2011, 383, 635–645. [Google Scholar] [CrossRef]
- Fernandes, T.; Nakamuta, J.S.; Magalhaes, F.C.; Roque, F.R.; Lavini-Ramos, C.; Schettert, I.T.; Coelho, V.; Krieger, J.E.; Oliveira, E.M. Exercise training restores the endothelial progenitor cells number and function in hypertension: Implications for angiogenesis. J. Hypertens. 2012, 30, 2133–2143. [Google Scholar] [CrossRef]
- Yu, Y.; Fukuda, N.; Yao, E.H.; Matsumoto, T.; Kobayashi, N.; Suzuki, R.; Tahira, Y.; Ueno, T.; Matsumoto, K. Effects of an ARB on endothelial progenitor cell function and cardiovascular oxidation in hypertension. Am. J. Hypertens. 2008, 21, 72–77. [Google Scholar] [CrossRef]
- MacEneaney, O.J.; DeSouza, C.A.; Weil, B.R.; Kushner, E.J.; Van Guilder, G.P.; Mestek, M.L.; Greiner, J.J.; Stauffer, B.L. Prehypertension and endothelial progenitor cell function. J. Hum. Hypertens. 2011, 25, 57–62. [Google Scholar] [CrossRef]
- Bogdanski, P.; Miller-Kasprzak, E.; Pupek-Musialik, D.; Jablecka, A.; Lacinski, M.; Jagodzinski, P.P.; Jakubowski, H. Plasma total homocysteine is a determinant of carotid intima-media thickness and circulating endothelial progenitor cells in patients with newly diagnosed hypertension. Clin. Chem. Lab. Med. 2012, 50, 1107–1113. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; He, X.; Li, Y.; Zou, Y. Effects of metoprolol, methyldopa, and nifedipine on endothelial progenitor cells in patients with gestational hypertension and preeclampsia. Clin. Exp. Pharmacol. Physiol. 2019, 46, 302–312. [Google Scholar] [CrossRef]
- Aicher, A.; Heeschen, C.; Mildner-Rihm, C.; Urbich, C.; Ihling, C.; Technau-Ihling, K.; Zeiher, A.M.; Dimmeler, S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat. Med. 2003, 9, 1370–1376. [Google Scholar] [CrossRef]
- Georgescu, A. Vascular dysfunction in diabetes: The endothelial progenitor cells as new therapeutic strategy. World J. Diabetes 2011, 2, 92–97. [Google Scholar] [CrossRef]
- Cao, W.; Cui, J.; Li, S.; Zhang, D.; Guo, Y.; Li, Q.; Luan, Y.; Liu, X. Crocetin restores diabetic endothelial progenitor cell dysfunction by enhancing NO bioavailability via regulation of PI3K/AKT-eNOS and ROS pathways. Life Sci. 2017, 181, 9–16. [Google Scholar] [CrossRef]
- He, J.; Liu, X.; Su, C.; Wu, F.; Sun, J.; Zhang, J.; Yang, X.; Zhang, C.; Zhou, Z.; Zhang, X.; et al. Inhibition of Mitochondrial Oxidative Damage Improves Reendothelialization Capacity of Endothelial Progenitor Cells via SIRT3 (Sirtuin 3)-Enhanced SOD2 (Superoxide Dismutase 2) Deacetylation in Hypertension. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1682–1698. [Google Scholar] [CrossRef]
- Li, C.; Lin, L.; Zhang, L.; Xu, R.; Chen, X.; Ji, J.; Li, Y. Long noncoding RNA p21 enhances autophagy to alleviate endothelial progenitor cells damage and promote endothelial repair in hypertension through SESN2/AMPK/TSC2 pathway. Pharmacol. Res. 2021, 173, 105920. [Google Scholar] [CrossRef]
- Peng, J.; Liu, B.; Ma, Q.-L.; Luo, X.-J. Dysfunctional endothelial progenitor cells in cardiovascular diseases: Role of NADPH oxidase. J. Cardiovasc. Pharmacol. 2015, 65, 80–87. [Google Scholar] [CrossRef]
- Marketou, M.E.; Kalyva, A.; Parthenakis, F.I.; Pontikoglou, C.; Maragkoudakis, S.; Kontaraki, J.E.; Chlouverakis, G.; Zacharis, E.A.; Patrianakos, A.; Papadaki, H.A.; et al. Circulating endothelial progenitor cells in hypertensive patients with increased arterial stiffness. J. Clin. Hypertens. 2014, 16, 295–300. [Google Scholar] [CrossRef]
- Gu, J.; Wang, C.; Fan, H.; Ding, H.; Xie, X.; Xu, Y.; Wang, B.; Huang, D. Effects of resveratrol on endothelial progenitor cells and their contributions to reendothelialization in intima-injured rats. J. Cardiovasl Pharm. 2006, 47, 711–721. [Google Scholar] [CrossRef]

| Groups | Initial SBP | Final SBP |
|---|---|---|
| WKY-CTL (n = 6) | 138 ± 2.8 | 137 ± 3.4 |
| SHR-CTL (n = 7) | 186 ± 5.1 * | 202 ± 5.1 |
| SHR-C50 (n = 6) | 193 ± 3.7 * | 171 ± 3.8 # † ‡ |
| SHR-C100 (n = 7) | 188 ± 3.2 * | 163 ± 2.7 # † ‡ |
| SHR-RE10 (n = 6) | 194 ± 3.2 * | 168 ± 5.2 # † ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, T.A.F.; Lima, V.S.; de Almeida, A.J.P.O.; de Arruda, A.V.; Veras, A.C.M.F.; Lima, T.T.; Soares, E.M.C.; Santos, A.C.d.; Vasconcelos, M.E.C.d.; de Almeida Feitosa, M.S.; et al. Carvacrol Improves Vascular Function in Hypertensive Animals by Modulating Endothelial Progenitor Cells. Nutrients 2023, 15, 3032. https://doi.org/10.3390/nu15133032
Gonçalves TAF, Lima VS, de Almeida AJPO, de Arruda AV, Veras ACMF, Lima TT, Soares EMC, Santos ACd, Vasconcelos MECd, de Almeida Feitosa MS, et al. Carvacrol Improves Vascular Function in Hypertensive Animals by Modulating Endothelial Progenitor Cells. Nutrients. 2023; 15(13):3032. https://doi.org/10.3390/nu15133032
Chicago/Turabian StyleGonçalves, Tays Amanda Felisberto, Viviane Silva Lima, Arthur José Pontes Oliveira de Almeida, Alinne Villar de Arruda, Ana Caroline Meneses Ferreira Veras, Thaís Trajano Lima, Evyllen Myllena Cardoso Soares, Adhonias Correia dos Santos, Maria Eduarda Costa de Vasconcelos, Mathania Silva de Almeida Feitosa, and et al. 2023. "Carvacrol Improves Vascular Function in Hypertensive Animals by Modulating Endothelial Progenitor Cells" Nutrients 15, no. 13: 3032. https://doi.org/10.3390/nu15133032
APA StyleGonçalves, T. A. F., Lima, V. S., de Almeida, A. J. P. O., de Arruda, A. V., Veras, A. C. M. F., Lima, T. T., Soares, E. M. C., Santos, A. C. d., Vasconcelos, M. E. C. d., de Almeida Feitosa, M. S., Veras, R. C., & de Medeiros, I. A. (2023). Carvacrol Improves Vascular Function in Hypertensive Animals by Modulating Endothelial Progenitor Cells. Nutrients, 15(13), 3032. https://doi.org/10.3390/nu15133032






