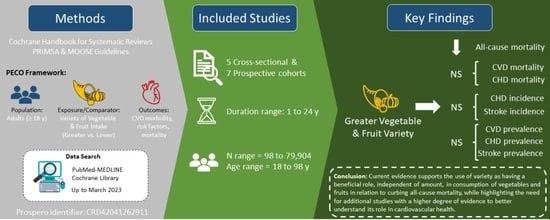
Vegetable and Fruit Intake Variety and Cardiovascular Health and Mortality: A Systematic Review and Meta-Analysis of Observational Studies
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Eligibility
2.3. Data Sources, Search, and Screening
2.4. Data Extraction
2.5. Outcomes
2.6. Quality Assessment
2.7. Data Synthesis and Analysis
2.8. Grading the Evidence
3. Results
3.1. Search Results
3.2. Study Characteristics
3.3. Study Quality Assessment
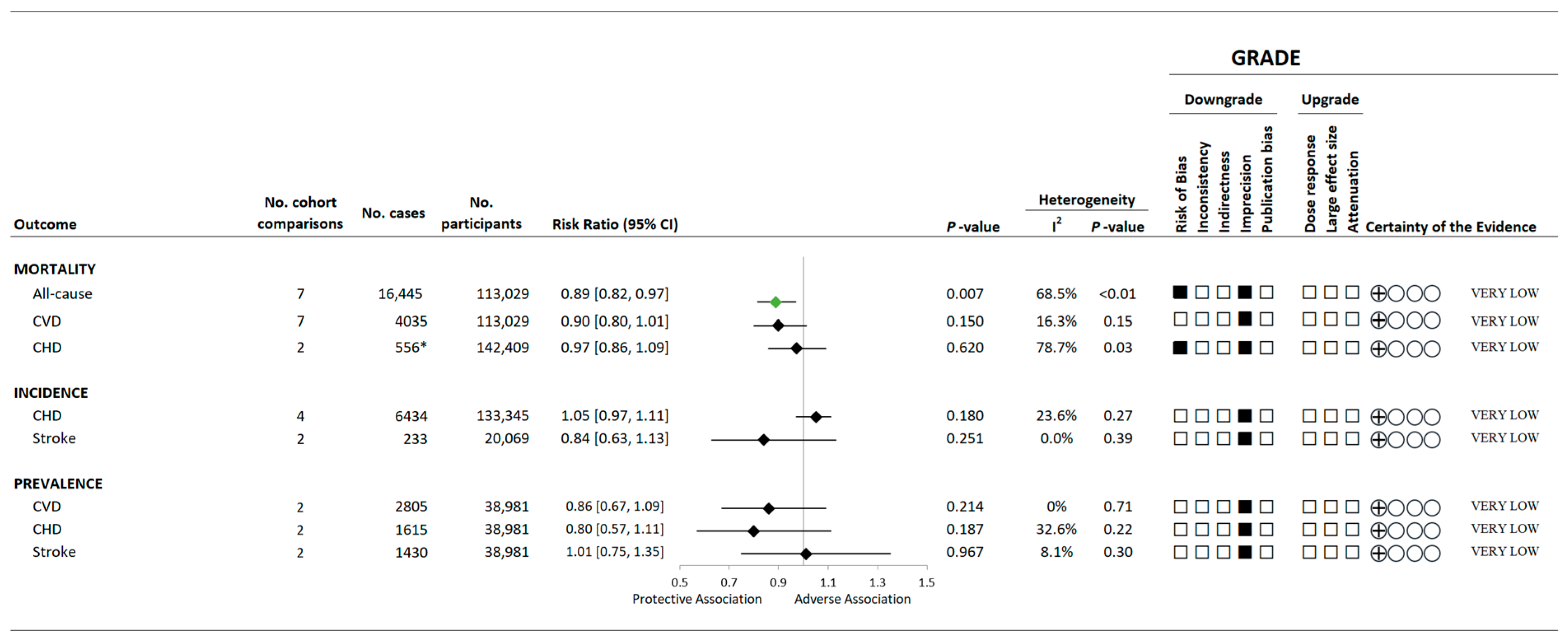
3.4. Variety in Vegetable and Fruit Consumption and Mortality
3.5. Variety in Vegetable and Fruit Consumption and CVD Morbidity
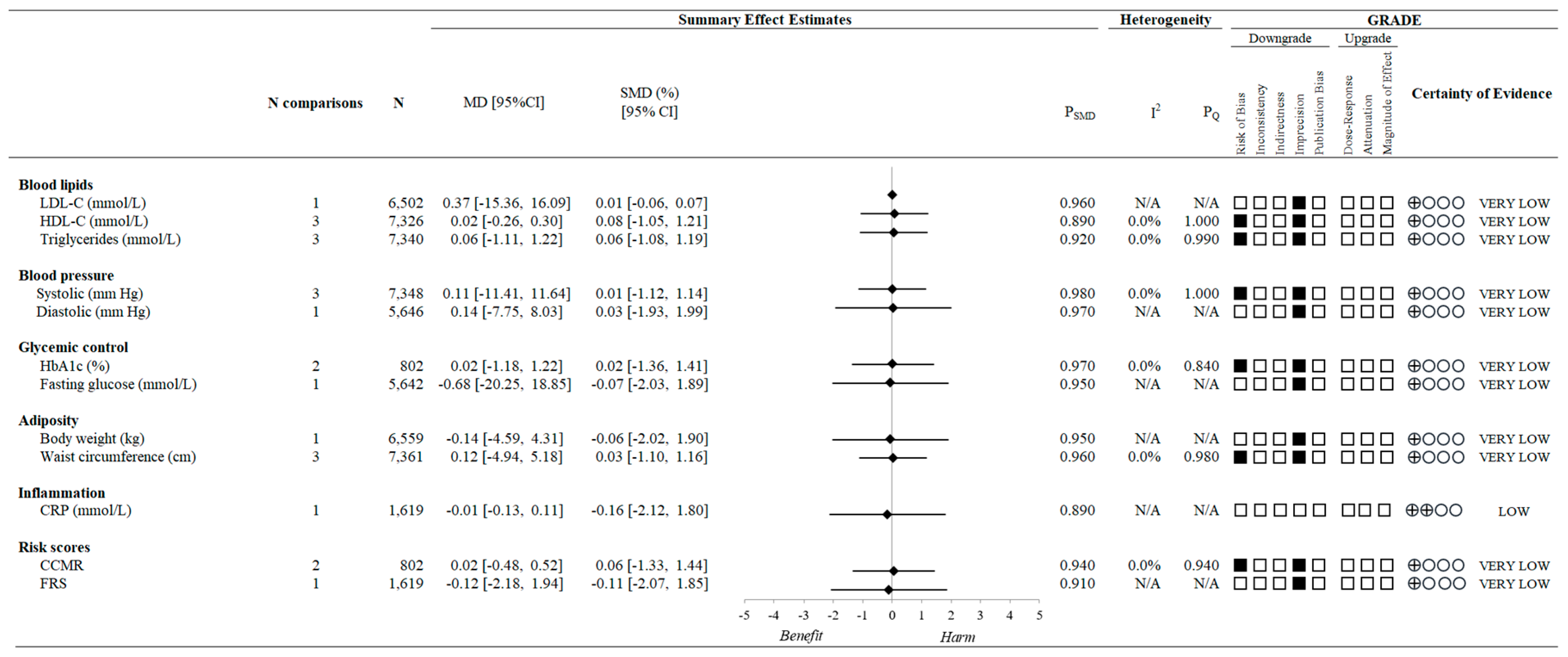
3.6. Variety in Vegetable and Fruit Consumption and CVD Risk Factors
3.7. Sensitivity and Subgroup Analyses
3.8. Publication Bias
3.9. Certainty of the Evidence
4. Discussion
4.1. Findings in Relation to the Existing Literature
4.2. Limitations and Strengths
4.3. Practical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Higgins, J.; Thomas, J.; Chandler, J.; Cumptston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, version 6.4 (updated August 2023); Cochrane: London, UK, 2023; Available online: www.training.cochrane.org/handbook (accessed on 21 September 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Porbital PlotDigitizer. Available online: https://plotdigitizer.com/app (accessed on 21 September 2023).
- NIH. National Heart Lung and Blood Institute Quality Assessment Tool for Obsercational Cohort and Cross-Sectional Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 21 September 2023).
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 21 September 2023).
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials Revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; Wiley: New York, NY, USA, 2009; ISBN 9780470057247. [Google Scholar]
- Lee, J.E.; Männistö, S.; Spiegelman, D.; Hunter, D.J.; Bernstein, L.; van den Brandt, P.A.; Buring, J.E.; Cho, E.; English, D.R.; Flood, A.; et al. Intakes of Fruit, Vegetables, and Carotenoids and Renal Cell Cancer Risk: A Pooled Analysis of 13 Prospective Studies. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Analysing Data and Undertaking Meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: New York, NY, USA, 2019; pp. 241–284. [Google Scholar]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 21 September 2023).
- Schünemann, H.J.; Wiercioch, W.; Brozek, J.; Etxeandia-Ikobaltzeta, I.; Mustafa, R.A.; Manja, V.; Brignardello-Petersen, R.; Neumann, I.; Falavigna, M.; Alhazzani, W.; et al. GRADE Evidence to Decision (EtD) Frameworks for Adoption, Adaptation, and de Novo Development of Trustworthy Recommendations: GRADE-ADOLOPMENT. J. Clin. Epidemiol. 2017, 81, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef]
- European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP) 3 Guideline on Clinical Investigation of Medicinal Products. 2018. Available online: www.ema.europa.eu/contact (accessed on 27 May 2023).
- Borg, R.; Kuenen, J.C.; Carstensen, B.; Zheng, H.; Nathan, D.M.; Heine, R.J.; Nerup, J.; Borch-Johnsen, K.; Witte, D.R. Associations between features of glucose exposure and A1C: The A1C-Derived Average Glucose (ADAG) study. Diabetes 2010, 59, 1585–1590. [Google Scholar] [CrossRef]
- Ge, L.; Sadeghirad, B.; Ball, G.D.C.; da Costa, B.R.; Hitchcock, C.L.; Svendrovski, A.; Kiflen, R.; Quadri, K.; Kwon, H.Y.; Karamouzian, M.; et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: Systematic review and network meta-analysis of randomised trials. BMJ 2020, 369, m696. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.C.; Kanters, S.; Bandayrel, K.; Wu, P.; Naji, F.; Siemieniuk, R.A.; Ball, G.D.C.; Busse, J.W.; Thorlund, K.; Guyatt, G.; et al. Comparison of weight loss among named diet programs in overweight and obese adults: A meta-analysis. JAMA 2014, 312, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Reynolds Risk Score: Calculating Heart and Stroke Risk for Women and Men. Available online: http://www.reynoldsriskscore.org/ (accessed on 27 May 2023).
- Ridker, P.M.; Paynter, N.P.; Rifai, N.; Gaziano, J.M.; Cook, N.R. C-reactive protein and parental history improve global cardiovascular risk prediction: The Reynolds risk score for men. Circulation 2008, 118, 2243–2251. [Google Scholar] [CrossRef]
- Ridker, P.M.; Buring, J.E.; Rifai, N.; Cook, N.R. Development and Validation of Improved Algorithms for the Assessment of Global Cardiovascular Risk in Women the Reynolds Risk Score. Available online: https://jamanetwork.com/ (accessed on 27 May 2023).
- Canadian Cardiovascular Society, Framingham Risk Score (FRS). 2017. Available online: https://ccs.ca/app/uploads/2020/12/FRS_eng_2017_fnl1.pdf (accessed on 27 May 2023).
- Santesso, N.; Glenton, C.; Dahm, P.; Garner, P.; Akl, E.A.; Alper, B.; Brignardello-Petersen, R.; Carrasco-Labra, A.; De Beer, H.; Hultcrantz, M.; et al. GRADE Guidelines 26: Informative Statements to Communicate the Findings of Systematic Reviews of Interventions. J. Clin. Epidemiol. 2020, 119, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- McCrory, M.A.; Fuss, P.J.; McCallum, J.E.; Yao, M.; Vinken, A.G.; Hays, N.P.; Roberts, S.B. Dietary Variety within Food Groups: Association with Energy Intake and Body Fatness in Men and Women. Am. J. Clin. Nutr. 1999, 69, 440–447. [Google Scholar] [CrossRef]
- Bernstein, M.A.; Tucker, K.L.; Ryan, N.D.; O’Neill, E.F.; Clements, K.M.; Nelson, M.E.; Evans, W.J.; Singh, M.A.F. Higher Dietary Variety Is Associated with Better Nutritional Status in Frail Elderly People. J. Am. Diet Assoc. 2002, 102, 1096–1104. [Google Scholar] [CrossRef]
- Azadbakht, L.; Mirmiran, P.; Esmaillzadeh, A.; Azizi, F. Dietary Diversity Score and Cardiovascular Risk Factors in Tehranian Adults. Public Health Nutr. 2006, 9, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Bertoia, M.L.; Mukamal, K.J.; Cahill, L.E.; Hou, T.; Ludwig, D.S.; Mozaffarian, D.; Willett, W.C.; Hu, F.B.; Rimm, E.B. Changes in Intake of Fruits and Vegetables and Weight Change in United States Men and Women Followed for Up to 24 Years: Analysis from Three Prospective Cohort Studies. PLoS Med. 2015, 12, e1001878. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Wedick, N.M.; Pan, A.; Manson, J.E.; Rexrode, K.M.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Quantity and Variety in Fruit and Vegetable Intake and Risk of Coronary Heart Disease1-3. Am. J. Clin. Nutr. 2013, 98, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Conrad, Z.; Thomson, J.; Jahns, L. Prospective Analysis of Vegetable Amount and Variety on the Risk of All-Cause and Cause-Specific Mortality among US Adults, 1999–2011. Nutrients 2018, 10, 1377. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sasazuki, S.; Shimazu, T.; Sawada, N.; Yamaji, T.; Iwasaki, M.; Mizoue, T.; Tsugane, S. Association of Dietary Diversity with Total Mortality and Major Causes of Mortality in the Japanese Population: JPHC Study. Eur. J. Clin. Nutr. 2020, 74, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Oude Griep, L.M.; Verschuren, W.M.M.; Kromhout, D.; Ocké, M.C.; Geleijnse, J.M. Variety in Fruit and Vegetable Consumption and 10-Year Incidence of CHD and Stroke. Public Health Nutr. 2012, 15, 2280–2286. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.; Zhu, Z.; Chan, R.; Kwok, T.; Woo, J. Prospective Analysis of Fruit and Vegetable Variety on Health Outcomes in Community-Dwelling Chinese Older Adults. J. Nutr. Health Aging 2021, 25, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Conrad, Z.; Raatz, S.; Jahns, L. Greater Vegetable Variety and Amount Are Associated with Lower Prevalence of Coronary Heart Disease: National Health and Nutrition Examination Survey, 1999–2014. Nutr. J. 2018, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Tucker, K.L. Greater Variety in Fruit and Vegetable Intake Is Associated with Lower Inflammation in Puerto Rican Adults. Am. J. Clin. Nutr. 2011, 93, 37–46. [Google Scholar] [CrossRef]
- Lamb, M.J.E.; Griffin, S.J.; Sharp, S.J.; Cooper, A.J.M. Fruit and Vegetable Intake and Cardiovascular Risk Factors in People with Newly Diagnosed Type 2 Diabetes. Eur. J. Clin. Nutr. 2017, 71, 115–121. [Google Scholar] [CrossRef]
- López-González, L.; Becerra-Tomás, N.; Babio, N.; Martínez-González, M.Á.; Nishi, S.K.; Corella, D.; Goday, A.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; et al. One-Year Changes in Fruit and Vegetable Variety Intake and Cardiometabolic Risk Factors Changes in a Middle-Aged Mediterranean Population at High Cardiovascular Risk. Eur. J. Clin. Nutr. 2022, 76, 1393–1402. [Google Scholar] [CrossRef]
- Kegler, M.C.; Hermstad, A.; Haardörfer, R. Home Food Environment and Associations with Weight and Diet among U.S. Adults: A Cross-Sectional Study. BMC Public Health 2021, 21, 1032. [Google Scholar] [CrossRef]
- Zurbau, A.; Au-Yeung, F.; Mejia, S.B.; Khan, T.A.; Vuksan, V.; Jovanovski, E.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Sievenpiper, J.L. Relation of Different Fruit and Vegetable Sources with Incident Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2020, 9, e017728. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Li, Y.; Bhupathiraju, S.N.; Rosner, B.A.; Sun, Q.; Giovannucci, E.L.; Rimm, E.B.; Manson, J.A.E.; Willett, W.C.; Stampfer, M.J.; et al. Fruit and Vegetable Intake and Mortality Results from 2 Prospective Cohort Studies of US Men and Women and a Meta-Analysis of 26 Cohort Studies. Circulation 2021, 143, 1642–1654. [Google Scholar] [CrossRef]
- He, F.J.; Nowson, C.A.; Lucas, M.; MacGregor, G.A. Increased Consumption of Fruit and Vegetables Is Related to a Reduced Risk of Coronary Heart Disease: Meta-Analysis of Cohort Studies. J. Hum. Hypertens. 2007, 21, 717–728. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Nowson, C.A.; MacGregor, G.A. Fruit and Vegetable Consumption and Stroke: Meta-Analysis of Cohort Studies. Lancet 2006, 367, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Aljadani, H.M.; Patterson, A.; Sibbritt, D.; Taylor, R.M.; Collins, C.E. Frequency and Variety of Usual Intakes of Healthy Foods, Fruit, and Vegetables Predicts Lower 6-Year Weight Gain in Young Women. Eur. J. Clin. Nutr. 2020, 74, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Shashirekha, M.N.; Mallikarjuna, S.E.; Rajarathnam, S. Status of Bioactive Compounds in Foods, with Focus on Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2015, 55, 1324–1339. [Google Scholar] [CrossRef]
- Miller, V.; Webb, P.; Micha, R.; Mozaffarian, D. Global Dietary Database Defining Diet Quality: A Synthesis of Dietary Quality Metrics and Their Validity for the Double Burden of Malnutrition. Lancet Planet. Health 2020, 4, e352–e370. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Blekkenhorst, L.C.; Liu, A.H.; Bondonno, N.P.; Ward, N.C.; Croft, K.D.; Hodgson, J.M. Vegetable-Derived Bioactive Nitrate and Cardiovascular Health. Mol. Aspects Med. 2018, 61, 83–91. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, H.; Nilcham, P.; Bucur, O.; Vogt, F.; Slabu, I.; Liehn, E.A.; Rusu, M. Vitamin C Regulates the Profibrotic Activity of Fibroblasts in In Vitro Replica Settings of Myocardial Infarction. Int. J. Mol. Sci. 2023, 24, 8379. [Google Scholar] [CrossRef]
- Morelli, M.B.; Gambardella, J.; Castellanos, V.; Trimarco, V.; Santulli, G. Vitamin C and Cardiovascular Disease: An Update. Antioxidants 2020, 9, 1227. [Google Scholar] [CrossRef]
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.F.; Vesa, C.M.; Behl, T.; Bungau, A.F.; et al. Oxidative Stress—Complex Pathological Issues Concerning the Hallmark of Cardiovascular and Metabolic Disorders. Biomed. Pharmacother. 2022, 152, 113238. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and Lycopene Supplementation and Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef]
- Greene, C.M.; Waters, D.; Clark, R.M.; Contois, J.H.; Fernandez, M.L. Plasma LDL and HDL Characteristics and Carotenoid Content Are Positively Influenced by Egg Consumption in an Elderly Population1. Nutr. Metab. 2006, 3, 6. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary Intake and Blood Concentrations of Antioxidants and the Risk of Cardiovascular Disease, Total Cancer, and All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef] [PubMed]
- Muiesan, M.L.; Buso, G.; Agabiti Rosei, C. Less Sodium and More Potassium to Reduce Cardiovascular Risk. Eur. Heart J. Suppl. 2023, 25, B108–B110. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.; Kumar, S.; Diep Pham, H.T.; Coffey, S.; Mann, J. Dietary Fibre in Hypertension and Cardiovascular Disease Management: Systematic Review and Meta-Analyses. BMC Med. 2022, 20, 139. [Google Scholar] [CrossRef]
- Hall, J.N.; Moore, S.; Harper, S.B.; Lynch, J.W. Global Variability in Fruit and Vegetable Consumption. Am. J. Prev. Med. 2009, 36, 402–409.e5. [Google Scholar] [CrossRef]
- Baldwin, J.N.; Ashton, L.M.; Forder, P.M.; Haslam, R.L.; Hure, A.J.; Loxton, D.J.; Patterson, A.J.; Collins, C.E. Increasing Fruit and Vegetable Variety over Time Is Associated with Lower 15-Year Healthcare Costs: Results from the Australian Longitudinal Study on Women’s Health. Nutrients 2021, 13, 2829. [Google Scholar] [CrossRef]
- Ekwaru, J.P.; Ohinmaa, A.; Loehr, S.; Setayeshgar, S.; Thanh, N.X.; Veugelers, P.J. The Economic Burden of Inadequate Consumption of Vegetables and Fruit in Canada. Public Health Nutr. 2017, 20, 515–523. [Google Scholar] [CrossRef]
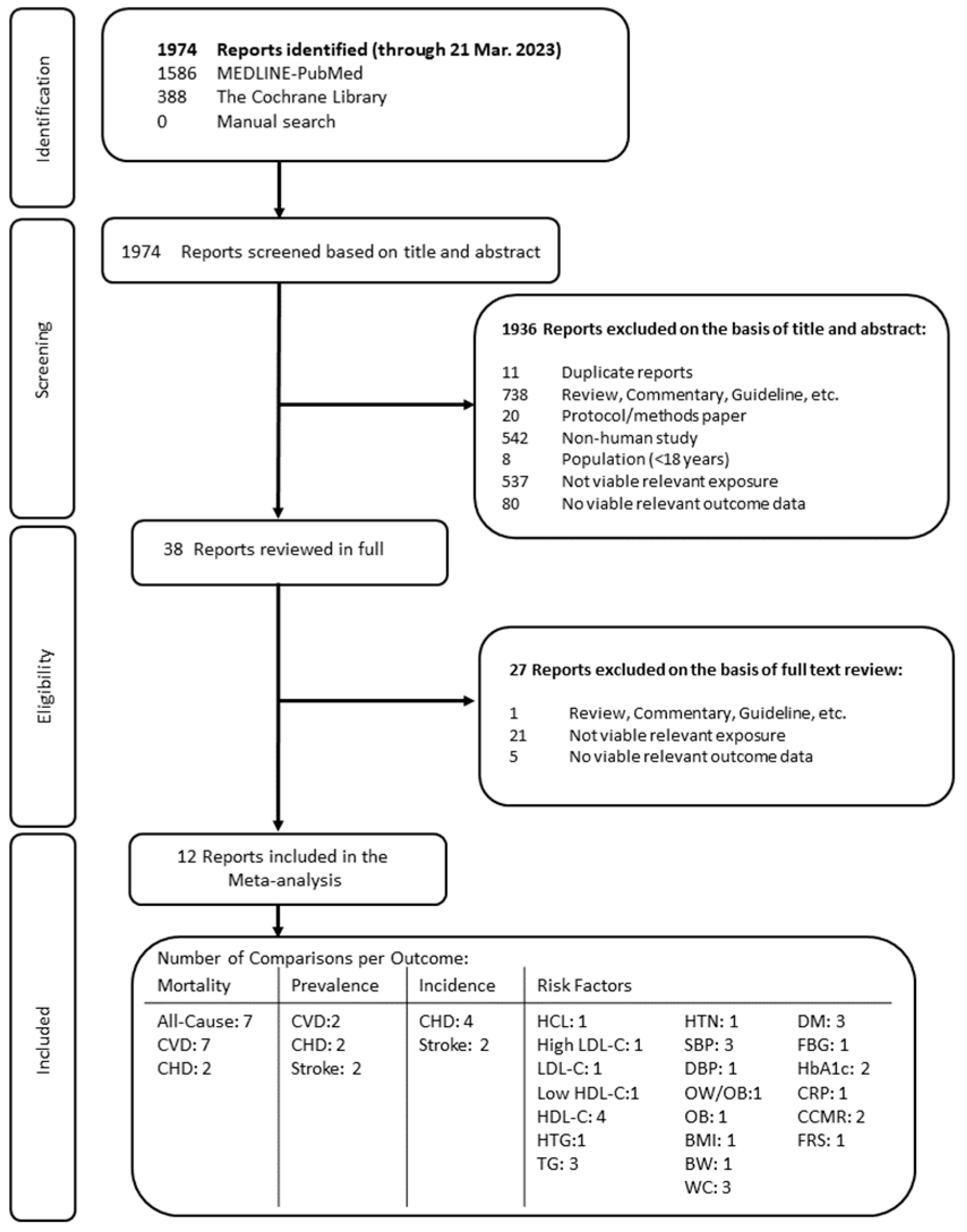

| Characteristic | Cross-Sectional Studies | Prospective Cohort Studies |
|---|---|---|
| Number of reports | 5 | 7 |
| Study location | Iran (1), United States (4) | China (1), Japan (1), the Netherlands (1), Spain (1), the United Kingdom (1), The United States (2) |
| Sample size (range) | 1159 (98 to 38,981) | 24,601 (401 to 79,904) |
| Baseline age (range) | 46.8 y (18 to 98 y) | 54.7 y (20 to 76 y) |
| Duration (range) | N/A | 12 y (1 to 24 y) |
| Dietary assessment method | FFQ (2), 24-h recall (1), Food Record (1), Survey (1) | FFQ (6), 24-h recall (1) |
| Outcome assessment method | Conducted by investigators (3), self-reported (2) | Confirmed or conducted by investigators (7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishi, S.K.; Khoury, N.; Valle Hita, C.; Zurbau, A.; Salas-Salvadó, J.; Babio, N. Vegetable and Fruit Intake Variety and Cardiovascular Health and Mortality: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2023, 15, 4913. https://doi.org/10.3390/nu15234913
Nishi SK, Khoury N, Valle Hita C, Zurbau A, Salas-Salvadó J, Babio N. Vegetable and Fruit Intake Variety and Cardiovascular Health and Mortality: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients. 2023; 15(23):4913. https://doi.org/10.3390/nu15234913
Chicago/Turabian StyleNishi, Stephanie K., Nadine Khoury, Cristina Valle Hita, Andreea Zurbau, Jordi Salas-Salvadó, and Nancy Babio. 2023. "Vegetable and Fruit Intake Variety and Cardiovascular Health and Mortality: A Systematic Review and Meta-Analysis of Observational Studies" Nutrients 15, no. 23: 4913. https://doi.org/10.3390/nu15234913
APA StyleNishi, S. K., Khoury, N., Valle Hita, C., Zurbau, A., Salas-Salvadó, J., & Babio, N. (2023). Vegetable and Fruit Intake Variety and Cardiovascular Health and Mortality: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients, 15(23), 4913. https://doi.org/10.3390/nu15234913