Dietary Plant Polysaccharides for Cancer Prevention: Role of Immune Cells and Gut Microbiota, Challenges and Perspectives
Abstract
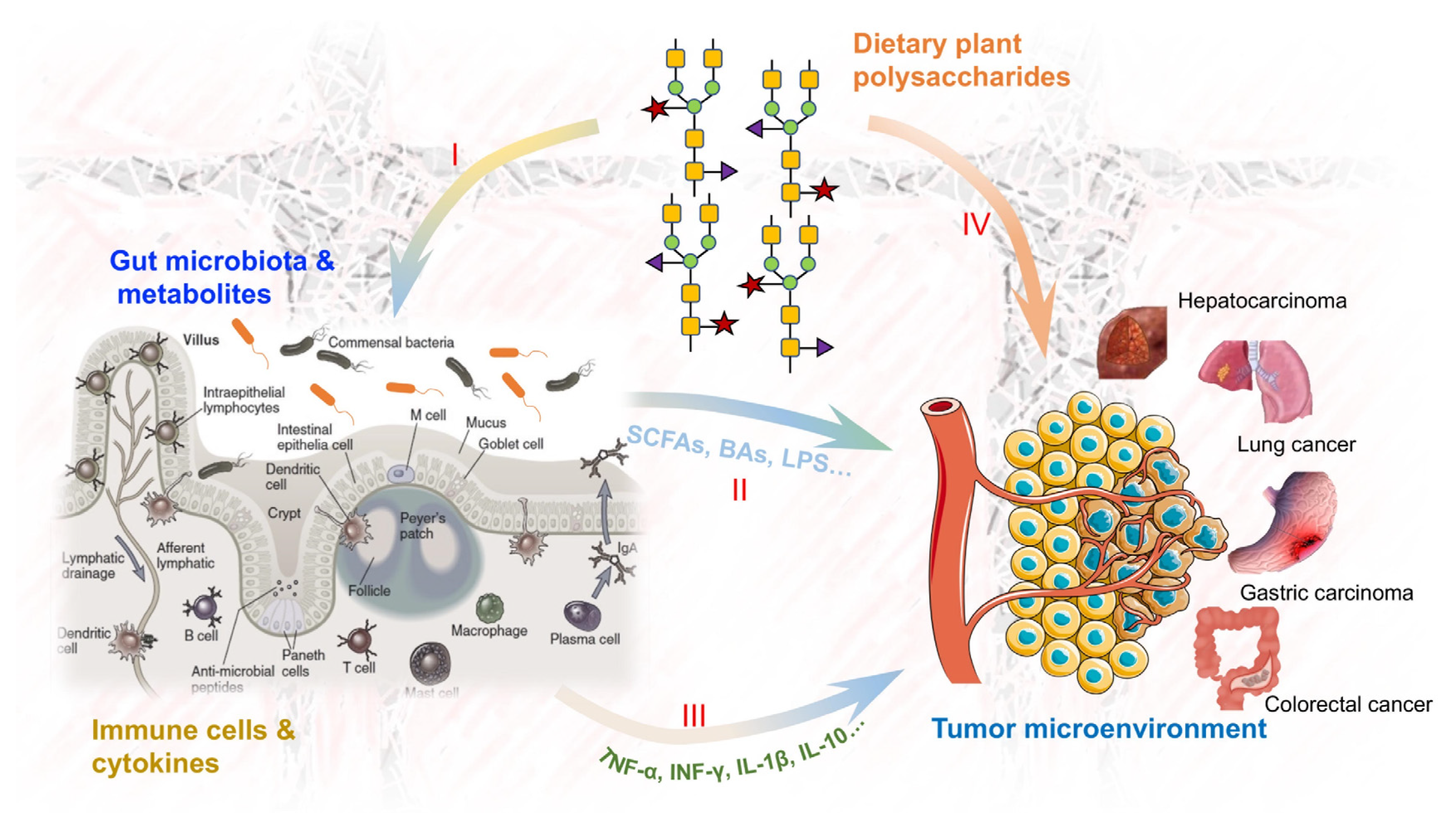
1. Introduction
2. Sources of Dietary Plant Polysaccharides with Immunoregulation
2.1. Dietary Polysaccharides from Edible Plants
2.2. Dietary Polysaccharides from Edible and Medicinal Plants
3. Effects of Chemical Types of Dietary Plant Polysaccharides on Immunoregulation
3.1. Effects of Relative Molecular Mass and Monosaccharide Composition on Immunomodulatory Activity
3.2. Effects of Glycosidic Linkage Forms on the Immunomodulatory Activities of Polysaccharides
4. The Roles of Immune Cells in Cancer Progression
5. Mechanisms of Dietary Plant Polysaccharides for Cancer Prevention and Immunoregulation
5.1. Regulation of the Immunoactivities of Monocytes and Macrophages
5.1.1. Induction of M1 Phenotype Polarization of Macrophages
5.1.2. Induction of Dendritic Cell (DC) Maturation and Activation
5.2. Regulation of the Immunoactivities of Lymphocyte Subsets
6. Relationship between Gut Microbiota and Dietary Plant Polysaccharides and Immunoregulation in In Vivo Cancer Prevention
6.1. Interactions between Gut Microbiota and Dietary Plant Polysaccharides
6.2. Interactions between Gut Microbiota and Immune System for Cancer Prevention
6.2.1. Gut Microbiota Affects Tumor Progression by Regulating Body Immunity
6.2.2. Gut Microbiota Affects Tumor Progression by Regulating Body Metabolism
7. Future Research Challenges and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, J.; Zhou, Z.W.; Sheng, H.P.; He, L.J.; Fan, X.W.; He, Z.X.; Sun, T.; Zhang, X.; Zhao, R.J.; Gu, L.; et al. An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des. Devel. Ther. 2015, 9, 33–78. [Google Scholar] [PubMed]
- Ahmad, M.F. Ganoderma lucidum: A rational pharmacological approach to surmount cancer. J. Ethnopharmacol. 2020, 260, 113047. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wan, H.; Huang, P.; Yang, J.; He, Y. A critical review of Astragalus polysaccharides: From therapeutic mechanisms to pharmaceutics. Biomed. Pharmacother. 2022, 147, 112654. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Nie, S.; Xie, M. Tumor Microenvironment as a New Target for Tumor Immunotherapy of Polysaccharides. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. S1), S85–S94. [Google Scholar] [CrossRef]
- Bian, X.; Miao, W.; Zhao, M.; Zhao, Y.; Xiao, Y.; Li, N.; Wu, J.L. Microbiota drive insoluble polysaccharides utilization via microbiome-metabolome interplay during Pu-erh tea fermentation. Food Chem. 2022, 377, 132007. [Google Scholar] [CrossRef]
- Sohretoglu, D.; Huang, S. Ganoderma lucidum Polysaccharides as An Anti-cancer Agent. Anticancer. Agents Med. Chem. 2018, 18, 667–674. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Miao, W.; Li, N.; Wu, J.L. Food-polysaccharideutilization via in vitro fermentation: Microbiota, structure, and function. Curr. Opin. Food Sci. 2022, 48, 100911. [Google Scholar] [CrossRef]
- Chen, D. Study on the Activity of Water Extract and Polysaccharides from the Flower of Camellia sinensis in Regulating Immunity and Intestinal Microorganism. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2020. [Google Scholar]
- Zeng, J.; Luan, F.; Hu, J.; Liu, Y.; Zhang, X.; Qin, T.; Zhang, X.; Liu, R.; Zeng, N. Recent research advances in polysaccharides from Undaria pinnatifida: Isolation, structures, bioactivities, and applications. Int. J. Biol. Macromol. 2022, 206, 325–354. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, P.; Zhao, S.; Nie, C.; Wang, N.; Du, X.; Zhou, Y. Characterization, antioxidant activity and immunomodulatory activity of polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2017, 95, 809–817. [Google Scholar] [CrossRef]
- Ren, Y.; Zheng, G.; You, L.; Wen, L.; Li, C.; Fu, X.; Zhou, L. Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria lemaneiformis. J. Funct. Foods. 2017, 33, 286–296. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, C.; Yu, Y.B.; Wu, L.X.; Chen, T.T.; Wang, Z.W. Physicochemical characteristics and in vitro biological activities of polysaccharides derived from raw garlic (Allium sativum L.) bulbs via three-phase partitioning combined with gradient ethanol precipitation method. Food Chem. 2021, 339, 128081. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Kim, S.M. Characterization and immunomodulatory activities of polysaccharides extracted from green alga Chlorella ellipsoidea. Int. J. Biol. Macromol. 2017, 95, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-R.; Han, A.-R.; Lim, T.-G.; Lee, E.-J.; Hong, H.-D. Isolation, purification, and characterization of novel polysaccharides from lotus (Nelumbo nucifera) leaves and their immunostimulatory effects. Int. J. Biol. Macromol. 2019, 128, 546–555. [Google Scholar] [CrossRef]
- Nie, C.; Zhu, P.; Ma, S.; Wang, M.; Hu, Y. Purification, characterization and immunomodulatory activity of polysaccharides from stem lettuce. Carbohydr. Polym. 2018, 188, 236–242. [Google Scholar] [CrossRef]
- Li, C.X.; Liu, Y.; Zhang, Y.Z.; Li, J.C.; Lai, J. Astragalus polysaccharide: A review of its immunomodulatory effect. Arch. Pharm. Res. 2022, 45, 367–389. [Google Scholar] [CrossRef]
- Xie, H.; Fang, J.; Farag, M.A.; Li, Z.; Sun, P.; Shao, P. Dendrobium officinale leaf polysaccharides regulation of immune response and gut microbiota composition in cyclophosphamide-treated mice. Food Chem. 2022, 13, 100235. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Niu, Z.; Shen, T.; Zhang, J.; Wang, X.; Hu, W.; Cho, J.Y. A review of the immunomodulatory activities of polysaccharides isolated from Panax species. J. Ginseng Res. 2022, 46, 23–32. [Google Scholar] [CrossRef]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, Structural Characterization, and Biological Functions of Lycium barbarum Polysaccharides: A Review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef]
- Xiao, Z.; Deng, Q.; Zhou, W.; Zhang, Y. Immune activities of polysaccharides isolated from Lycium barbarum L. What do we know so far? Pharmacol. Ther. 2022, 229, 107921. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Ma, P.; Huang, J.; Bai, X.; Liu, P.; Zhu, L.; Min, X. Immunomodulatory effect of polysaccharides isolated from Lonicera japonica Thunb. in cyclophosphamide-treated BALB/c mice. Heliyon 2022, 8, e11876. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, P.; Shen, H.; Zhang, Q.; Zhang, T.; Jin, X. Water-extracted Lonicera japonica polysaccharide attenuates allergic rhinitis by regulating NLRP3-IL-17 signaling axis. Carbohydr. Polym. 2022, 297, 120053. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.S.; Li, Y.X.; Jiang, S.L.; Song, A.N.; Fu, Z.; Dong, C.X.; Yao, Z.; Qiao, W. Isolation, purification, and structural characterization of polysaccharides from Atractylodis Macrocephalae Rhizoma and their immunostimulatory activity in RAW264.7 cells. Int. J. Biol. Macromol. 2020, 163, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.; Wang, Q.; Lin, R.; He, P.; Lai, F.; Zhang, M.; Wu, H. Structural characterization and immunomodulatory activity of a novel acid polysaccharide isolated from the pulp of Rosa laevigata Michx fruit. Int. J. Biol. Macromol. 2020, 145, 1080–1090. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Li, S.; Li, H.B. Dietary Natural Products for Prevention and Treatment of Liver Cancer. Nutrients 2016, 8, 156. [Google Scholar] [CrossRef]
- Ryu, M.J.; Kim, A.D.; Kang, K.A.; Chung, H.S.; Kim, H.S.; Suh, I.S.; Chang, W.Y.; Hyun, J.W. The green algae Ulva fasciata Delile extract induces apoptotic cell death in human colon cancer cells. Vitr. Cell. Dev.-An. 2013, 49, 74–81. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Jang, S.A.; Lee, A.; Cho, C.W.; Song, Y.R.; Hong, H.D.; Ha, H.; Kim, T. Polysaccharides isolated from lotus leaves (LLEP) exert anti-osteoporotic effects by inhibiting osteoclastogenesis. Int. Bio. Macro. 2020, 161, 449–456. [Google Scholar] [CrossRef]
- Li, Z.W.; Du, Z.M.; Wang, Y.W.; Feng, Y.X.; Zhang, R.; Yan, X.B. Chemical Modification, Characterization, and Activity Changes of Land Plant Polysaccharides: A Review. Polymers 2022, 14, 4161. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Zhang, L.; Song, C.; Shang, X.; Gu, Y. Study on the physical and chemical properties and in vitro immune activity of lentinus edodes fruit-body polysaccharides at different developmental stages. Mycosystema 2020, 39, 1559–1567. [Google Scholar]
- Shen, C.Y.; Jiang, J.G.; Li, M.Q.; Zheng, C.Y.; Zhu, W. Structural characterization and immunomodulatory activity of novel polysaccharides from Citrus aurantium linn. variant amara engl. J. Funct. Foods. 2017, 35, 352–362. [Google Scholar] [CrossRef]
- Jiang, Y. Comparison onphysicochemical property and immunoactivity and of different extracts from five edible mushrooms. Master’s Thesis, Hefei Polytechnic University, Hefei, China, 2013. [Google Scholar]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-Functional Activity Relationship of β-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Fang, L.; Liu, D.; Lai, C.; Zhang, Y.; Zhou, A.; Xie, J. A glucogalactomannan isolated from Agaricus bisporus induces apoptosis in macrophages through the JNK/Bim/caspase 3 pathway. Food Funct. 2018, 9, 4771–4780. [Google Scholar] [CrossRef]
- Huang, X.; Nie, S. The structure of mushroom polysaccharides and their beneficial role in health. Food Funct. 2015, 6, 3205–3217. [Google Scholar] [CrossRef] [PubMed]
- Kiyohara, H.; Uchida, T.; Takakiwa, M.; Matsuzaki, T.; Hada, N.; Takeda, T.; Shibata, T.; Yamada, H. Different contributions of side-chains in beta-D-(1-->3,6)-galactans on intestinal Peyer’s patch-immunomodulation by polysaccharides from Astragalus mongholics Bunge. Phytochemistry 2010, 71, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Jin, M.L.; Morris, G.A.; Zha, X.Q.; Chen, H.Q.; Yi, Y.; Li, J.E.; Wang, Z.J.; Gao, J.; Nie, S.P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. S1), S60–S84. [Google Scholar] [CrossRef]
- Hwang, J.; Zhang, W.; Dhananjay, Y.; An, E.K.; Kwak, M.; You, S.; Lee, P.C.; Jin, J.O. Astragalus membranaceus polysaccharides potentiate the growth-inhibitory activity of immune checkpoint inhibitors against pulmonary metastatic melanoma in mice. Int. J. Biol. Macromol. 2021, 182, 1292–1300. [Google Scholar] [CrossRef]
- Sun, S.J.; Deng, P.; Peng, C.E.; Ji, H.Y.; Mao, L.F.; Peng, L.Z. Extraction, Structure and Immunoregulatory Activity of Low Molecular Weight Polysaccharide from Dendrobium officinale. Polymers 2022, 14, 2899. [Google Scholar] [CrossRef]
- Guo, X.; Yang, M.; Wang, C.; Nie, S.; Cui, S.W.; Guo, Q. Acetyl-glucomannan from Dendrobium officinale: Structural modification and immunomodulatory activities. Front. Nutr. 2022, 9, 1016961. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, N.; Hao, M.; Zhou, J.; Xie, Y.; He, Z. Plant-Derived Polysaccharides Regulated Immune Status, Gut Health and Microbiota of Broilers: A Review. Front. Vet. Sci. 2021, 8, 791371. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- McGuirk, P.; Mills, K.H. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 2002, 23, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, Y.; Wang, N.; Zhang, X.; Tan, B.; Zhang, G.; Cheng, Y. The clinical significance of tumor-infiltrating neutrophils and neutrophil-to-CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J. Transl. Med. 2014, 12, 7. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Neutrophils in Cancer: Two Sides of the Same Coin. J. Immunol. Res. 2015, 2015, 983698. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Wong, S.L.; Martinod, K.; Gallant, M.; Cabral, J.E.; Wang, Y.; Wagner, D.D. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology 2016, 5, e1134073. [Google Scholar] [CrossRef]
- He, M.; Peng, A.; Huang, X.Z.; Shi, D.C.; Wang, J.C.; Zhao, Q.; Lin, H.; Kuang, D.M.; Ke, P.F.; Lao, X.M. Peritumoral stromal neutrophils are essential for c-Met-elicited metastasis in human hepatocellular carcinoma. Oncoimmunology 2016, 5, e1219828. [Google Scholar] [CrossRef]
- Stabile, H.; Fionda, C.; Gismondi, A.; Santoni, A. Role of Distinct Natural Killer Cell Subsets in Anticancer Response. Front. Immunol. 2017, 8, 293. [Google Scholar] [CrossRef]
- Glasner, A.; Levi, A.; Enk, J.; Isaacson, B.; Viukov, S.; Orlanski, S.; Scope, A.; Neuman, T.; Enk, C.D.; Hanna, J.H.; et al. NKp46 Receptor-Mediated Interferon-γ Production by Natural Killer Cells Increases Fibronectin 1 to Alter Tumor Architecture and Control Metastasis. Immunity 2018, 48, 107–119.e104. [Google Scholar] [CrossRef]
- Langers, I.; Renoux, V.M.; Thiry, M.; Delvenne, P.; Jacobs, N. Natural killer cells: Role in local tumor growth and metastasis. Biol. Targets Ther. 2012, 6, 73–82. [Google Scholar]
- Kyoizumi, S.; Kubo, Y.; Kajimura, J.; Yoshida, K.; Hayashi, T.; Nakachi, K.; Moore, M.A.; van den Brink, M.R.M.; Kusunoki, Y. Fate Decision Between Group 3 Innate Lymphoid and Conventional NK Cell Lineages by Notch Signaling in Human Circulating Hematopoietic Progenitors. J. Immunol. 2017, 199, 2777–2793. [Google Scholar] [CrossRef] [PubMed]
- Seillet, C.; Belz, G.T.; Huntington, N.D. Development, Homeostasis, and Heterogeneity of NK Cells and ILC1. Curr. Top. Microbiol. Immunol. 2016, 395, 37–61. [Google Scholar] [PubMed]
- Ellefsen, C.F.; Wold, C.W.; Wilkins, A.L.; Rise, F.; Samuelsen, A.B.C. Water-soluble polysaccharides from Pleurotus eryngii fruiting bodies, their activity and affinity for Toll-like receptor 2 and dectin-1. Carbohydr. Polym. 2021, 264, 117991. [Google Scholar] [CrossRef]
- Pan, G.; Xie, Z.; Huang, S.; Tai, Y.; Cai, Q.; Jiang, W.; Sun, J.; Yuan, Y. Immune-enhancing effects of polysaccharides extracted from Lilium lancifolium Thunb. Int. Immunopharmacol. 2017, 52, 119–126. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, B.; Wang, Z.; Li, M.; Zhao, W. Natural Polysaccharides with Immunomodulatory Activities. Mini Rev. Med. Chem. 2020, 20, 96–106. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in Research on Immunoregulation of Macrophages by Plant Polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Li, X.; Song, Q.; Yang, X.; Li, G.; Li, D.; Wu, H.; Li, Y. Research progress on antiviral effect and mechanism of Isatidis Radix polysaccharide. Chin. Trad. Herb. Drugs 2022, 19, 6227–6233. [Google Scholar]
- Yang, J.; Zhang, L.; Shi, B. The anti-tumor effect of polysaccharides from salvia chinensis on osteosarcoma mice and its effect on immune function through PTEN pathway. Tianjin J. Trad. Chin. Med. 2022, 39, 940–944. [Google Scholar]
- Wang, Y.; Yang, B.; Chen, Q.; Cui, N.; Zhao, W.; Wu, J. In vitro and in vivo study of coixan improving cellular immune function by regulating the JAK3/STAT5 pathway. China J. Tradi. Chin. Med. Pharm. 2021, 11, 6414–6417. [Google Scholar]
- Xu, J.; Chi, F.; Guo, T.; Punj, V.; Lee, W.N.; French, S.W.; Tsukamoto, H. NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. J. Clin. Invest. 2015, 125, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, Z.P.; Bian, Z.X.; Han, Q.B. Astragalus Polysaccharide RAP Induces Macrophage Phenotype Polarization to M1 via the Notch Signaling Pathway. Molecules 2019, 24, 2016. [Google Scholar] [CrossRef] [PubMed]
- Christopher, T.; Mahamat, O.; Chungong, M.N.; Ngwa, C.A.; Samka, P.M. Immunological Activities of Crude Polysaccharides Extracts of Climacodon pulcherrimus (Phanerochaetaceae) in Lipopolysaccharide-Induced Rat Macrophages and Neutrophils’ Responses. J. Med. Food 2021, 24, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.; Ambrozova, G.; Kratchanova, M.; Denev, P.; Kussovski, V.; Ciz, M.; Lojek, A. Effects of pectic polysaccharides isolated from leek on the production of reactive oxygen and nitrogen species by phagocytes. J. Med. Food 2013, 16, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhang, T.; Zhao, H.; Cai, Y. Effects of Portulaca oleracea L. Polysaccharides on Phenotypic and Functional Maturation of Murine Bone Marrow Derived Dendritic Cells. Nutr. Cancer 2015, 67, 987–993. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, K.; Liu, L.; Li, X.; Wu, E.; Han, L.; Shi, Z.; Deng, X. Effects of Lycium Barbarum Polysaccharides on the Metabolism of Dendritic Cells: An In Vitro Study. J. Immunol. Res. 2022, 2022, 5882136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, Z.J.; Zhou, X.W. Chinese Cordyceps: Bioactive Components, Antitumor Effects and Underlying Mechanism-A Review. Molecules 2022, 27, 6576. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The genus Cordyceps: An extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef]
- Shin, M.S.; Hwang, S.H.; Yoon, T.J.; Kim, S.H.; Shin, K.S. Polysaccharides from ginseng leaves inhibit tumor metastasis via macrophage and NK cell activation. Int. J. Biol. Macromol. 2017, 103, 1327–1333. [Google Scholar] [CrossRef]
- Huang, R.; Xie, J.; Yu, Y.; Shen, M. Recent progress in the research of yam mucilage polysaccharides: Isolation, structure and bioactivities. Int. J. Biol. Macromol. 2020, 155, 1262–1269. [Google Scholar] [CrossRef]
- Xu, Y.; Pang, H.; Li, H. Research on anti-tumor and immunomodulatory effects of yam polysaccharides on tumor mice. J. Med. Forum 2020, 41, 8–10. [Google Scholar]
- Gong, C.; Xing, Y.; Shan, J. The isolation by ion chromatography and bioactivity of polysaccharide GCpF1, extracted from Gracilaria lemaneiformis by ultra-filtration. Grains Oils 2020, 33, 91–93. [Google Scholar]
- He, X.; Li, X.; Liu, B.; Xu, L.; Zhao, H.; Lu, A. Down-regulation of Treg cells and up-regulation of TH1/TH2 cytokine ratio were induced by polysaccharide from Radix Glycyrrhizae in H22 hepatocarcinoma bearing mice. Molecules 2011, 16, 8343–8352. [Google Scholar] [CrossRef]
- Nai, J.; Zhang, C.; Shao, H.; Li, B.; Li, H.; Gao, L.; Dai, M.; Zhu, L.; Sheng, H. Extraction, structure, pharmacological activities and drug carrier applications of Angelica sinensis polysaccharide. Int. J. Biol. Macromol. 2021, 183, 2337–2353. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, H.; Zhu, J.H.; Wang, S.Y.; Zhou, S.S.; Kong, M.; Mao, Q.; Long, F.; Fang, Z.J.; Li, S.L. Efficacy of ginseng and its ingredients as adjuvants to chemotherapy in non-small cell lung cancer. Food Funct. 2021, 12, 2225–2241. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, H.; Wang, X. Effect of ginseng polysaccharides and dendritic cells on the balance of Th1/Th2 T helper cells in patients with non-small cell lung cancer. J. Tradit. Chin. Med. 2014, 34, 641–645. [Google Scholar] [CrossRef]
- Gupta, B.; Sadaria, D.; Warrier, V.U.; Kirtonia, A.; Kant, R.; Awasthi, A.; Baligar, P.; Pal, J.K.; Yuba, E.; Sethi, G.; et al. Plant lectins and their usage in preparing targeted nanovaccines for cancer immunotherapy. Semin. Cancer Biol. 2022, 80, 87–106. [Google Scholar] [CrossRef]
- Bishehsari, F.; Voigt, R.M.; Keshavarzian, A. Circadian rhythms and the gut microbiota: From the metabolic syndrome to cancer. Nat. Rev. Endocrinol. 2020, 16, 731–739. [Google Scholar] [CrossRef]
- Guo, H.; Gibson, S.A.; Ting, J.P.Y. Gut microbiota, NLR proteins, and intestinal homeostasis. J. Exp. Med. 2020, 217, e20181832. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mainali, R.; Nagpal, R.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S.; Wang, S.; Deep, G.; Kumar Mishra, S.; Yadav, H. Dietary Polysaccharides in the Amelioration of Gut Microbiome Dysbiosis and Metabolic Diseases. Obes. Control Ther. 2017, 4, 10. [Google Scholar]
- Ho Do, M.; Seo, Y.S.; Park, H.Y. Polysaccharides: Bowel health and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, H.; Xie, W. Interaction between polysaccharide and intestinal flora and its structure-effect relationship: A review. Microbiol. China 2022, 49, 2325–2346. [Google Scholar]
- Kataoka, K. The intestinal microbiota and its role in human health and disease. J. Med. Invest. 2016, 63, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, M.; Jian, C.; Wei, F.; Liu, H.; Li, K.; Qin, X. Astragalus Polysaccharide Alleviates Constipation in the Elderly Via Modification of Gut Microbiota and Fecal Metabolism. Rejuvenation Res. 2022, 25, 275–290. [Google Scholar] [CrossRef]
- Yang, C.; Du, Y.; Ren, D.; Yang, X.; Zhao, Y. Gut microbiota-dependent catabolites of tryptophan play a predominant role in the protective effects of turmeric polysaccharides against DSS-induced ulcerative colitis. Food Funct. 2021, 12, 9793–9807. [Google Scholar] [CrossRef]
- Fu, Q.; Huang, H.; Ding, A.; Yu, Z.; Huang, Y.; Fu, G.; Huang, Y.; Huang, X. Portulaca oleracea polysaccharides reduce serum lipid levels in aging rats by modulating intestinal microbiota and metabolites. Front. Nutr. 2022, 9, 965653. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, Y.; Wu, Y.; Liu, H.; Liu, Q.; Kang, Z.; Wu, M.; Yin, H.; Duan, J. A homogeneous polysaccharide from Lycium barbarum: Structural characterizations, anti-obesity effects and impacts on gut microbiota. Int. J. Biol. Macromol. 2021, 183, 2074–2087. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; Zhang, R.; Chen, Y.; Wang, F.; Zhang, M. Gut microbial fermentation promotes the intestinal anti-inflammatory activity of Chinese yam polysaccharides. Food Chem. 2023, 402, 134003. [Google Scholar] [CrossRef] [PubMed]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef] [PubMed]
- Schluter, J.; Peled, J.U.; Taylor, B.P.; Markey, K.A.; Smith, M.; Taur, Y.; Niehus, R.; Staffas, A.; Dai, A.; Fontana, E.; et al. The gut microbiota is associated with immune cell dynamics in humans. Nature 2020, 588, 303–307. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Naito, Y.; Uchiyama, K.; Takagi, T. A next-generation beneficial microbe: Akkermansia muciniphila. J. Clin. Biochem. Nutr. 2018, 63, 33–35. [Google Scholar] [CrossRef]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Xu, C.; Wang, Y.; Wang, Z.; Chen, M.; Jiang, Z.; Pan, J.; Yang, C.; Li, X.; et al. Cross-talk between the gut microbiota and monocyte-like macrophages mediates an inflammatory response to promote colitis-associated tumourigenesis. Gut 2020, 70, 1495–1506. [Google Scholar] [CrossRef]
- Guo, S.; Chen, J.; Chen, F.; Zeng, Q.; Liu, W.L.; Zhang, G. Exosomes derived from Fusobacterium nucleatum-infected colorectal cancer cells facilitate tumour metastasis by selectively carrying miR-1246/92b-3p/27a-3p and CXCL16. Gut 2020, 70, 1507–1519. [Google Scholar] [CrossRef]
- Bhattacharya, N.; Yuan, R.; Prestwood, T.R.; Penny, H.L.; DiMaio, M.A.; Reticker-Flynn, N.E.; Krois, C.R.; Kenkel, J.A.; Pham, T.D.; Carmi, Y.; et al. Normalizing Microbiota-Induced Retinoic Acid Deficiency Stimulates Protective CD8(+) T Cell-Mediated Immunity in Colorectal Cancer. Immunity 2016, 45, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Kadosh, E.; Snir-Alkalay, I.; Venkatachalam, A.; May, S.; Lasry, A.; Elyada, E.; Zinger, A.; Shaham, M.; Vaalani, G.; Mernberger, M.; et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature 2020, 586, 133–138. [Google Scholar] [CrossRef] [PubMed]
| Category | Polysaccharides from Plants | Mechanisms for Immunoregulation | Reference |
|---|---|---|---|
| Edible plants | Camellia sinensis (Linn.) O. Kuntze | Increase in TNF-α, IFN-γ, IL-1β, IL-2 and IL-6 levels in serum | [9] |
| Undaria pinnatifida Suringar | Activation of Toll-like receptors (TLRs), NF-κB and other immune-related signaling pathways | [10] | |
| Zizania latifolia (Griseb.) Stapf | Enhancement of the ability of phagocytosis and NO production of macrophages | [11] | |
| Gracilaria lemaneiformis | Activation of the expressions of iNOS, IL-6 and TNF-α mRNA; enhancement of the proliferation and pinocytosis of macrophages; promotion of the production of ROS, NO, IL-6 and TNF-α | [12] | |
| Allium sativum L. | Stimulation of NO release by macrophages | [13] | |
| Chlorella ellipsoidea | Stimulation of mouse macrophages to produce NO and various cytokines (IL-1, IL-6, IL-10 and IL-12); activation of the NF-κB and MAPK pathways | [14] | |
| Nelumbo nucifera Leaves | Activation of mRNA expression of cell proliferation and cytokine secretion; activation of MAPK and NF-κB pathways | [15] | |
| Lactuca sativa Linn. | Promotion of macrophage proliferation, phagocytosis and NO production | [16] | |
| Edible and medicinal plants | Astragalus memeranaceus | Enhancement of phagocytic function of macrophages and activity of NK cells; promotion of the function of non-specific and specific immune responses | [17] |
| Dendrobium officinale Kimura et Migo | Enhancement of proliferative activity, swallowing activity, NO release and ROS production of macrophages | [18] | |
| Panax ginseng C. A. Meyer | Promotion of the secretion of cytokines in macrophages; activation of the corresponding signaling pathways | [18,19] | |
| Lycium barbarum L. | Regulation of the production of NO, ROS, TNF-α, IL-6 and IL-1β in macrophages; promotion of the proliferation and phagocytic capacity of macrophages; elevation of the mRNA expression of iNOS; and activation of p-JNK MAPK signaling pathway | [20,21] | |
| Lonicera japonica Thunb. | Improvement of the immunosuppression index of immune organs and promotion of the proliferation and cytokine release of immune cells | [22,23] | |
| Atractylodes macrocephala Koidz. | Promotion of the proliferation and NO release in macrophages | [24] | |
| Rosa laevigata Michx. | Enhancement of phagocytosis, secretion of cytokines (TNF-α, IL-6 and NO) and mRNA expression; activation of MAPKs and NF-κB signaling pathways | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Liu, Y.; Zeng, S.; Liu, Y.; Li, W.; Wu, D.; Wu, X.; Zou, L.; Chen, H. Dietary Plant Polysaccharides for Cancer Prevention: Role of Immune Cells and Gut Microbiota, Challenges and Perspectives. Nutrients 2023, 15, 3019. https://doi.org/10.3390/nu15133019
Wang A, Liu Y, Zeng S, Liu Y, Li W, Wu D, Wu X, Zou L, Chen H. Dietary Plant Polysaccharides for Cancer Prevention: Role of Immune Cells and Gut Microbiota, Challenges and Perspectives. Nutrients. 2023; 15(13):3019. https://doi.org/10.3390/nu15133019
Chicago/Turabian StyleWang, Anqi, Ying Liu, Shan Zeng, Yuanyuan Liu, Wei Li, Dingtao Wu, Xu Wu, Liang Zou, and Huijuan Chen. 2023. "Dietary Plant Polysaccharides for Cancer Prevention: Role of Immune Cells and Gut Microbiota, Challenges and Perspectives" Nutrients 15, no. 13: 3019. https://doi.org/10.3390/nu15133019
APA StyleWang, A., Liu, Y., Zeng, S., Liu, Y., Li, W., Wu, D., Wu, X., Zou, L., & Chen, H. (2023). Dietary Plant Polysaccharides for Cancer Prevention: Role of Immune Cells and Gut Microbiota, Challenges and Perspectives. Nutrients, 15(13), 3019. https://doi.org/10.3390/nu15133019







