Nutrient Supplementation during the Prenatal Period in Substance-Using Mothers: A Narrative Review of the Effects on Offspring Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
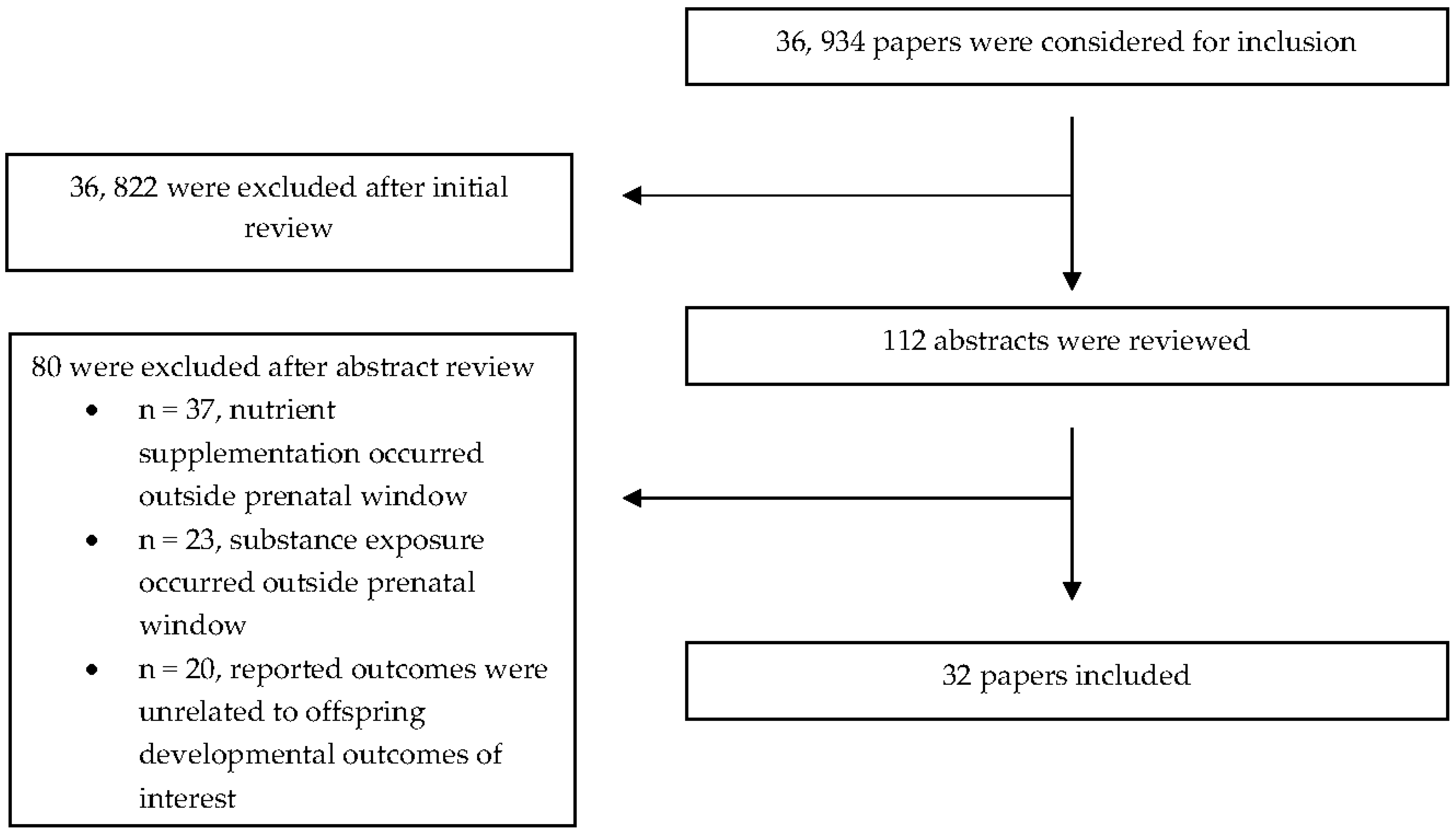
3. Results
3.1. General Study Characteristics
3.2. Animal Studies
3.2.1. Effects of Choline on Maternal Ethanol Consumption and Offspring Developmental Outcomes
3.2.2. Effects of Zinc on Maternal Ethanol Consumption and Offspring Developmental Outcomes
3.2.3. Effects of Vitamin E on Maternal Ethanol Consumption and Offspring Developmental Outcomes
3.2.4. Effects of Fatty Acids on Maternal Ethanol Consumption and Offspring Developmental Outcomes
3.2.5. Effects of Iron on Maternal Ethanol Consumption and Offspring Developmental Outcomes
3.3. Human Studies
3.3.1. Effects of Choline on Maternal Alcohol Consumption and Offspring Developmental Outcomes
3.3.2. Effects of Vitamin C on Maternal Nicotine Consumption and Offspring Developmental Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Tran, E.L.; England, L.J.; Park, Y.; Denny, C.H.; Kim, S.Y. Systematic Review: Polysubstance Prevalence Estimates Reported during Pregnancy, US, 2009–2020. Matern. Child Health J. 2023, 27, 426–458. [Google Scholar] [CrossRef] [PubMed]
- Gosdin, L.K.; Deputy, N.P.; Kim, S.Y.; Dang, E.P.; Denny, C.H. Alcohol Consumption and Binge Drinking during Pregnancy among Adults Aged 18–49 Years—United States, 2018–2020. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Substance Abuse and Mental Health Services Administration. 2020 NSDUH Detailed Tables. National Survey on Drug Use and Health. 2022. Available online: https://www.samhsa.gov/data/report/2020-nsduh-detailed-tables (accessed on 30 March 2023).
- Schneider, M.L.; Moore, C.F.; Adkins, M.M. The effects of prenatal alcohol exposure on behavior: Rodent and primate studies. Neuropsychol. Rev. 2011, 21, 186–203. [Google Scholar] [CrossRef] [PubMed]
- Larkby, C.; Day, N. The effects of prenatal alcohol exposure. Alcohol. Health Res. World 1997, 21, 192–198. [Google Scholar]
- Streissguth, A.P.; Aase, J.M.; Clarren, S.K.; Randels, S.P.; LaDue, R.A.; Smith, D.F. Fetal Alcohol Syndrome in Adolescents and Adults. JAMA 1991, 265, 1961–1967. [Google Scholar] [CrossRef]
- Day, N.L. Research on the effects of prenatal alcohol exposure—A new direction. Am. J. Public Health 1995, 85, 1614–1615. [Google Scholar] [CrossRef]
- Riley, E.P.; Mattson, S.N.; Sowell, E.R.; Jernigan, T.L.; Sobel, D.F.; Jones, K.L. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol. Clin. Exp. Res. 1995, 19, 1198–1202. [Google Scholar] [CrossRef]
- Berman, R.F.; Hannigan, J.H. Effects of prenatal alcohol exposure on the hippocampus: Spatial behavior, electrophysiology, and neuroanatomy. Hippocampus 2000, 10, 94–110. [Google Scholar] [CrossRef]
- Denny, L.; Coles, S.; Blitz, R. Fetal Alcohol Syndrome and Fetal Alcohol Spectrum Disorders. Am. Fam. Physician 2017, 96, 515–522. [Google Scholar]
- Banderali, G.; Martelli, A.; Landi, M.; Moretti, F.; Betti, F.; Radaelli, G.; Lassandro, C.; Verduci, E. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: A descriptive review. J. Transl. Med. 2015, 13, 327. [Google Scholar] [CrossRef]
- Ko, T.J.; Tsai, L.Y.; Chu, L.C.; Yeh, S.J.; Leung, C.; Chen, C.Y.; Chou, H.C.; Tsao, P.N.; Chen, P.C.; Hsieh, W.S. Parental smoking during pregnancy and its association with low birth weight, small for gestational age, and preterm birth offspring: A birth cohort study. Pediatr. Neonatol. 2014, 55, 20–27. [Google Scholar] [CrossRef]
- Ekblad, M.; Korkeila, J.; Lehtonen, L. Smoking during pregnancy affects foetal brain development. Acta Paediatr. 2015, 104, 12–18. [Google Scholar] [CrossRef]
- Ino, T. Maternal smoking during pregnancy and offspring obesity: Meta-analysis. Pediatr. Int. 2010, 52, 94–99. [Google Scholar] [CrossRef]
- Bruin, J.E.; Gerstein, H.C.; Holloway, A.C. Long-term consequences of fetal and neonatal nicotine exposure: A critical review. Toxicol. Sci. 2010, 116, 364–374. [Google Scholar] [CrossRef]
- Oken, E.; Huh, S.Y.; Taveras, E.M.; Rich-Edwards, J.W.; Gillman, M.W. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes. Res. 2005, 13, 2021–2028. [Google Scholar] [CrossRef]
- Cheraghi, M.; Salvi, S. Environmental tobacco smoke (ETS) and respiratory health in children. Eur. J. Pediatr. 2009, 168, 897–905. [Google Scholar] [CrossRef]
- Chang, J.C.; Tarr, J.A.; Holland, C.L.; De Genna, N.M.; Richardson, G.A.; Rodriguez, K.L.; Sheeder, J.; Kraemer, K.L.; Day, N.L.; Rubio, D.; et al. Beliefs and attitudes regarding prenatal marijuana use: Perspectives of pregnant women who report use. Drug Alcohol. Depend. 2019, 196, 14–20. [Google Scholar] [CrossRef]
- Gunn, J.K.; Rosales, C.B.; Center, K.E.; Nuñez, A.; Gibson, S.J.; Christ, C.; Ehiri, J.E. Prenatal exposure to cannabis and maternal and child health outcomes: A systematic review and meta-analysis. BMJ Open 2016, 6, e009986. [Google Scholar] [CrossRef]
- Wu, C.S.; Jew, C.P.; Lu, H.C. Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol. 2011, 6, 459–480. [Google Scholar] [CrossRef]
- Fried, P.A.; Watkinson, B.; Gray, R. Neurocognitive consequences of marihuana—A comparison with pre-drug performance. Neurotoxicol. Teratol. 2005, 27, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Noland, J.S.; Singer, L.T.; Short, E.J.; Minnes, S.; Arendt, R.E.; Kirchner, H.L.; Bearer, C. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol. Teratol. 2005, 27, 429–438. [Google Scholar] [CrossRef]
- Finer, L.B.; Zolna, M.R. Declines in Unintended Pregnancy in the United States, 2008–2011. N. Engl. J. Med. 2016, 374, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.J.; Graham, D.L.; Money, K.M.; Stanwood, G.D. Developmental consequences of fetal exposure to drugs: What we know and what we still must learn. Neuropsychopharmacology 2015, 40, 61–87. [Google Scholar] [CrossRef]
- Eiden, R.D.; Homish, G.G.; Colder, C.R.; Schuetze, P.; Gray, T.R.; Huestis, M.A. Changes in smoking patterns during pregnancy. Subst. Use Misuse 2013, 48, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Forray, A.; Merry, B.; Lin, H.; Ruger, J.P.; Yonkers, K.A. Perinatal substance use: A prospective evaluation of abstinence and relapse. Drug Alcohol. Depend. 2015, 150, 147–155. [Google Scholar] [CrossRef]
- Radziejewska, A.; Chmurzynska, A. Folate and choline absorption and uptake: Their role in fetal development. Biochimie 2019, 158, 10–19. [Google Scholar] [CrossRef]
- Gower-Winter, S.D.; Levenson, C.W. Zinc in the central nervous system: From molecules to behavior. Biofactors 2012, 38, 186–193. [Google Scholar] [CrossRef]
- Georgieff, M.K. Iron deficiency in pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef]
- Mahmood, N.; Hameed, A.; Hussain, T. Vitamin E and Selenium Treatment Alleviates Saline Environment-Induced Oxidative Stress through Enhanced Antioxidants and Growth Performance in Suckling Kids of Beetal Goats. Oxidative Med. Cell. Longev. 2020, 2020, 4960507. [Google Scholar] [CrossRef]
- Tveden-Nyborg, P.; Johansen, L.K.; Raida, Z.; Villumsen, C.K.; Larsen, J.O.; Lykkesfeldt, J. Vitamin C deficiency in early postnatal life impairs spatial memory and reduces the number of hippocampal neurons in guinea pigs. Am. J. Clin. Nutr. 2009, 90, 540–546. [Google Scholar] [CrossRef]
- Birch, E.E.; Garfield, S.; Castañeda, Y.; Hughbanks-Wheaton, D.; Uauy, R.; Hoffman, D. Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum. Dev. 2007, 83, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Balaraman, S.; Idrus, N.M.; Miranda, R.C.; Thomas, J.D. Postnatal choline supplementation selectively attenuates hippocampal microRNA alterations associated with developmental alcohol exposure. Alcohol 2017, 60, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Bearer, C.F.; Wellmann, K.A.; Tang, N.; He, M.; Mooney, S.M. Choline Ameliorates Deficits in Balance Caused by Acute Neonatal Ethanol Exposure. Cerebellum 2015, 14, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Birch, S.M.; Lenox, M.W.; Kornegay, J.N.; Paniagua, B.; Styner, M.A.; Goodlett, C.R.; Cudd, T.A.; Washburn, S.E. Maternal choline supplementation in a sheep model of first trimester binge alcohol fails to protect against brain volume reductions in peripubertal lambs. Alcohol 2016, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bottom, R.T.; Abbott, C.W., 3rd; Huffman, K.J. Rescue of ethanol-induced FASD-like phenotypes via prenatal co-administration of choline. Neuropharmacology 2020, 168, 107990. [Google Scholar] [CrossRef]
- Carugati, M.; Goodlett, C.R.; Cudd, T.A.; Washburn, S.E. The effects of gestational choline supplementation on cerebellar Purkinje cell number in the sheep model of binge alcohol exposure during the first trimester-equivalent. Alcohol 2022, 100, 11–21. [Google Scholar] [CrossRef]
- Goeke, C.M.; Roberts, M.L.; Hashimoto, J.G.; Finn, D.A.; Guizzetti, M. Neonatal Ethanol and Choline Treatments Alter the Morphology of Developing Rat Hippocampal Pyramidal Neurons in Opposite Directions. Neuroscience 2018, 374, 13–24. [Google Scholar] [CrossRef]
- Hunt, P.S.; Jacobson, S.E.; Kim, S. Supplemental choline does not attenuate the effects of neonatal ethanol administration on habituation of the heart rate orienting response in rats. Neurotoxicol. Teratol. 2014, 44, 121–125. [Google Scholar] [CrossRef]
- Kwan, S.T.C.; Ricketts, D.K.; Presswood, B.H.; Smith, S.M.; Mooney, S.M. Prenatal choline supplementation during mouse pregnancy has differential effects in alcohol-exposed fetal organs. Alcohol. Clin. Exp. Res. 2021, 45, 2471–2484. [Google Scholar] [CrossRef]
- Monk, B.R.; Leslie, F.M.; Thomas, J.D. The effects of perinatal choline supplementation on hippocampal cholinergic development in rats exposed to alcohol during the brain growth spurt. Hippocampus 2012, 22, 1750–1757. [Google Scholar] [CrossRef]
- Otero, N.K.; Thomas, J.D.; Saski, C.A.; Xia, X.; Kelly, S.J. Choline supplementation and DNA methylation in the hippocampus and prefrontal cortex of rats exposed to alcohol during development. Alcohol. Clin. Exp. Res. 2012, 36, 1701–1709. [Google Scholar] [CrossRef]
- Sawant, O.B.; Birch, S.M.; Goodlett, C.R.; Cudd, T.A.; Washburn, S.E. Maternal choline supplementation mitigates alcohol-induced fetal cranio-facial abnormalities detected using an ultrasonographic examination in a sheep model. Alcohol 2019, 81, 31–38. [Google Scholar] [CrossRef]
- Steane, S.E.; Fielding, A.M.; Kent, N.L.; Andersen, I.; Browne, D.J.; Tejo, E.N.; Gardebjer, E.M.; Kalisch-Smith, J.I.; Sullivan, M.A.; Moritz, K.M.; et al. Maternal choline supplementation in a rat model of periconceptional alcohol exposure: Impacts on the fetus and placenta. Alcohol. Clin. Exp. Res. 2021, 45, 2130–2146. [Google Scholar] [CrossRef]
- Thomas, J.D.; La Fiette, M.H.; Quinn, V.R.; Riley, E.P. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol. Teratol. 2000, 22, 703–711. [Google Scholar] [CrossRef]
- Thomas, J.D.; O'Neill, T.M.; Dominguez, H.D. Perinatal choline supplementation does not mitigate motor coordination deficits associated with neonatal alcohol exposure in rats. Neurotoxicol. Teratol. 2004, 26, 223–229. [Google Scholar] [CrossRef]
- Thomas, J.D.; Garrison, M.; O’Neill, T.M. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol. Teratol. 2004, 26, 35–45. [Google Scholar] [CrossRef]
- Thomas, J.D.; Abou, E.J.; Dominguez, H.D. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol. Teratol. 2009, 31, 303–311. [Google Scholar] [CrossRef]
- Thomas, J.D.; Idrus, N.M.; Monk, B.R.; Dominguez, H.D. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res. A Clin. Mol. Teratol. 2010, 88, 827–837. [Google Scholar] [CrossRef]
- Wagner, A.F.; Hunt, P.S. Impaired trace fear conditioning following neonatal ethanol: Reversal by choline. Behav. Neurosci. 2006, 120, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Summers, B.L.; Rofe, A.M.; Coyle, P. Prenatal zinc treatment at the time of acute ethanol exposure limits spatial memory impairments in mouse offspring. Pediatr. Res. 2006, 59, 66–71. [Google Scholar] [CrossRef]
- Summers, B.L.; Henry, C.M.; Rofe, A.M.; Coyle, P. Dietary zinc supplementation during pregnancy prevents spatial and object recognition memory impairments caused by early prenatal ethanol exposure. Behav. Brain Res. 2008, 186, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Summers, B.L.; Rofe, A.M.; Coyle, P. Dietary zinc supplementation throughout pregnancy protects against fetal dysmorphology and improves postnatal survival after prenatal ethanol exposure in mice. Alcohol. Clin. Exp. Res. 2009, 33, 591–600. [Google Scholar] [CrossRef]
- Marino, M.D.; Aksenov, M.Y.; Kelly, S.J. Vitamin E protects against alcohol-induced cell loss and oxidative stress in the neonatal rat hippocampus. Int. J. Dev. Neurosci. 2004, 22, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Jackson, H.D.; Horn, K.H.; Goodlett, C.R. Vitamin E does not protect against neonatal ethanol-induced cerebellar damage or deficits in eyeblink classical conditioning in rats. Alcohol. Clin. Exp. Res. 2005, 29, 117–129. [Google Scholar] [CrossRef]
- Abel, E.L.; Reddy, P.P. Prenatal high saturated fat diet modifies behavioral effects of prenatal alcohol exposure in rats. Alcohol 1997, 14, 25–29. [Google Scholar] [CrossRef]
- Wainwright, P.; Ward, G.R.; Molnar, J.D. gamma-Linolenic acid fails to prevent the effects of prenatal ethanol exposure on brain and behavioral development in B6D2F2 mice. Alcohol. Clin. Exp. Res. 1985, 9, 377–383. [Google Scholar] [CrossRef]
- Helfrich, K.K.; Saini, N.; Kwan, S.T.C.; Rivera, O.C.; Hodges, R.; Smith, S.M. Gestational Iron Supplementation Improves Fetal Outcomes in a Rat Model of Prenatal Alcohol Exposure. Nutrients 2022, 14, 1653. [Google Scholar] [CrossRef] [PubMed]
- Coles, C.D.; Kable, J.A.; Keen, C.L.; Jones, K.L.; Wertelecki, W.; Granovska, I.V.; Pashtepa, A.O.; Chambers, C.B.; CIFSAD. Dose and Timing of Prenatal Alcohol Exposure and Maternal Nutritional Supplements: Developmental Effects on 6-Month-Old Infants. Matern. Child Health J. 2015, 19, 2605–2614. [Google Scholar] [CrossRef]
- Jacobson, S.W.; Carter, R.C.; Molteno, C.D.; Stanton, M.E.; Herbert, J.S.; Lindinger, N.M.; Lewis, C.E.; Dodge, N.C.; Hoyme, H.E.; Zeisel, S.H.; et al. Efficacy of Maternal Choline Supplementation during Pregnancy in Mitigating Adverse Effects of Prenatal Alcohol Exposure on Growth and Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Alcohol. Clin. Exp. Res. 2018, 42, 1327–1341. [Google Scholar] [CrossRef]
- Kable, J.A.; Coles, C.D.; Keen, C.L.; Uriu-Adams, J.Y.; Jones, K.L.; Yevtushok, L.; Kulikovsky, Y.; Wertelecki, W.; Pederson, T.L.; Chambers, C.D.; et al. The impact of micronutrient supplementation in alcohol-exposed pregnancies on information processing skills in Ukrainian infants. Alcohol 2015, 49, 647–656. [Google Scholar] [CrossRef]
- Kable, J.A.; Coles, C.D.; Keen, C.L.; Uriu-Adams, J.Y.; Jones, K.L.; Yevtushok, L.; Zymak-Zakutnya, N.; Dubchak, I.; Akhmedzhanova, D.; Wertelecki, W.; et al. The impact of micronutrient supplementation in alcohol-exposed pregnancies on reaction time responses of preschoolers in Ukraine. Alcohol 2022, 99, 49–58. [Google Scholar] [CrossRef]
- Warton, F.L.; Molteno, C.D.; Warton, C.M.R.; Wintermark, P.; Lindinger, N.M.; Dodge, N.C.; Zollei, L.; van der Kouwe, A.J.W.; Carter, R.C.; Jacobson, J.L.; et al. Maternal choline supplementation mitigates alcohol exposure effects on neonatal brain volumes. Alcohol. Clin. Exp. Res. 2021, 45, 1762–1774. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Schilling, D.; Clay, N.; Jackson, K.; Go, M.D.; Spitale, P.; Bunten, C.; Leiva, M.; Gonzales, D.; Hollister-Smith, J.; et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: A randomized clinical trial. JAMA 2014, 311, 2074–2082. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Shorey-Kendrick, L.E.; Milner, K.; Schilling, D.; Tiller, C.; Vuylsteke, B.; Scherman, A.; Jackson, K.; Haas, D.M.; Harris, J.; et al. Oral Vitamin C (500 mg/d) to Pregnant Smokers Improves Infant Airway Function at 3 Months (VCSIP). A Randomized Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 1139–1147. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Shorey-Kendrick, L.E.; Milner, K.; Schilling, D.; Tiller, C.; Vuylsteke, B.; Scherman, A.; Jackson, K.; Haas, D.M.; Harris, J.; et al. Vitamin C to Pregnant Smokers Persistently Improves Infant Airway Function to 12 Months of Age: A Randomised Trial. Eur. Respir. J. 2020, 56, 1902208. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Shorey-Kendrick, L.E.; Milner, K.; Harris, J.; Vuylsteke, B.; Cunningham, M.; Tiller, C.; Stewart, J.; Schilling, D.; Brownsberger, J.; et al. Effect of Vitamin C Supplementation for Pregnant Smokers on Offspring Airway Function and Wheeze at Age 5 Years: Follow-up of a Randomized Clinical Trial. JAMA Pediatr. 2023, 177, 16–24. [Google Scholar] [CrossRef]
- Patten, A.R.; Fontaine, C.J.; Christie, B.R. A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Front. Pediatr. 2014, 2, 93. [Google Scholar] [CrossRef]
- Gupta, K.K.; Gupta, V.K.; Shirasaka, T. An Update on Fetal Alcohol Syndrome-Pathogenesis, Risks, and Treatment. Alcohol. Clin. Exp. Res. 2016, 40, 1594–1602. [Google Scholar] [CrossRef]
- Pappalardo-Carter, D.L.; Balaraman, S.; Sathyan, P.; Carter, E.S.; Chen, W.J.; Miranda, R.C. Suppression and epigenetic regulation of MiR-9 contributes to ethanol teratology: Evidence from zebrafish and murine fetal neural stem cell models. Alcohol. Clin. Exp. Res. 2013, 37, 1657–1667. [Google Scholar] [CrossRef]
- Wozniak, J.R.; Fuglestad, A.J.; Eckerle, J.K.; Kroupina, M.G.; Miller, N.C.; Boys, C.J.; Brearley, A.M.; Fink, B.A.; Hoecker, H.L.; Zeisel, S.H.; et al. Choline supplementation in children with fetal alcohol spectrum disorders has high feasibility and tolerability. Nutr. Res. 2013, 33, 897–904. [Google Scholar] [CrossRef]
- Meck, W.H.; Smith, R.A.; Williams, C.L. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav. Neurosci. 1989, 103, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, A.; Sekizawa, A.; Koide, K.; Hasegawa, J.; Satoh, K.; Arakaki, T.; Takenaka, S.; Matsuoka, R. Vitamin C Induces the Reduction of Oxidative Stress and Paradoxically Stimulates the Apoptotic Gene Expression in Extravillous Trophoblasts Derived from First-Trimester Tissue. Reprod. Sci. 2015, 22, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Kuzawa, C.W. Adipose tissue in human infancy and childhood: An evolutionary perspective. Am. J. Phys. Anthropol. 1998, 27, 177–209. [Google Scholar] [CrossRef]
- Donangelo, C.M.; King, J.C. Maternal zinc intakes and homeostatic adjustments during pregnancy and lactation. Nutrients 2012, 4, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Barnett, E.R.; Knight, E.; Herman, R.J.; Amarakaran, K.; Jankowski, M.K. Difficult binds: A systematic review of facilitators and barriers to treatment among mothers with substance use disorders. J. Subst. Abuse Treat. 2021, 126, 108341. [Google Scholar] [CrossRef]
- Garg, M.; Garrison, L.; Leeman, L.; Hamidovic, A.; Borrego, M.; Rayburn, W.F. Validity of Self-Reported Drug Use Information among Pregnant Women. Matern. Child Health J. 2016, 20, 41–47. [Google Scholar] [CrossRef]
- Borland, T.; Babayan, A.; Irfan, S.; Schwartz, R. Exploring the adequacy of smoking cessation support for pregnant and postpartum women. BMC Public Health 2013, 13, 472. [Google Scholar] [CrossRef]
- El-Mohandes, A.; Herman, A.A.; Nabil El-Khorazaty, M.; Katta, P.S.; White, D.; Grylack, L. Prenatal care reduces the impact of illicit drug use on perinatal outcomes. J. Perinatol. 2003, 23, 354–360. [Google Scholar] [CrossRef]
- Roberts, S.C.; Pies, C. Complex calculations: How drug use during pregnancy becomes a barrier to prenatal care. Matern. Child Health J. 2011, 15, 333–341. [Google Scholar] [CrossRef]
- England, L.J.; Bennett, C.; Denny, C.H.; Honein, M.A.; Gilboa, S.M.; Kim, S.Y.; Guy, G.P., Jr.; Tran, E.L.; Rose, C.E.; Bohm, M.K.; et al. Alcohol Use and Co-Use of Other Substances among Pregnant Females Aged 12–44 Years—United States, 2015–2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1009–1014. [Google Scholar] [CrossRef]
| a. Effects of choline on maternal ethanol consumption and offspring developmental outcomes. | ||||
| Reference and Study Population | Prenatal Substance Use (PSU) | Prenatal Nutrition | Offspring Development Outcomes | Results |
| Balaraman et al. (2017) [33] Sprague Dawley rats (n = 48, male and female pups) | Intervention group: From PDs 4–9, received 5.25 g/kg ethanol each day in milk feeding Control group: From PDs 4–9, received sham intubations (i.e., milk feeding without ethanol) | Timeframe delivered: From PDs 4–21, 100 mg/kg choline or saline control per day Assignment: Randomly assigned to one of the following eight groups: (1) ethanol + saline in males, (2) ethanol + choline in males, (3) sham + saline in males, (4) sham + choline in males, (5) ethanol + saline in females, (6) ethanol + choline in females, (7) sham + saline in females, and (8) sham + choline in females | Age at assessment: PDs 4–9 Outcomes assessed: Body weight | Choline supplementation did not protect against deficits to body growth in ethanol-exposed subjects |
| Bearer et al. (2015) [34] C57B16/J mice (n = 48, male and female pups) | Intervention group: On PD 5, received 6.0 g/kg ethanol each day Control group: On PD 5, received Intralipid® sham intubations | Timeframe delivered: From PDs 1–20, 10 μL of 18.8 mg/mL choline or saline Assignment: Pseudorandomly assigned to one of the following eight groups: (1) saline from PD 1–5 + ethanol + saline from PD 6–20, (2) saline from PD 1–5 + ethanol + choline PD 6–20, (3) choline from PD 1–5 + ethanol + saline from PD 6–20, (4) choline from PD 1–5 + ethanol + choline from PD 6–20, (5) saline from PD 1–5 + sham + saline from PD 6–20, (6) saline from PD 1–5 + sham + choline from PD 6–20, (7) choline from PD 1–5 + sham + saline from PD 6–20, and (8) choline from PD 1–5 + sham + choline from PD 6–20 | Age at assessment: PD 30 Outcomes assessed: Body weight Balance and coordination, as measured using the Dowel Test | Choline supplementation had no effect on body weight For both sexes, choline supplementation before and after ethanol exposure increased balance and coordination (p < 0.001) In males, choline supplementation prior to ethanol exposure increased balance and coordination (p = 0.009) Choline supplementation after ethanol exposure did not significantly increase performance on balance and coordination in males (p = 0.522) In females, choline supplementation prior to ethanol did not reach statistical significance for balance and coordination performance (p = 0.056) Choline supplementation post ethanol exposure had no effect on balance and coordination in females |
| Birch et al. (2016) [35] Suffolk ewes (n = 44, and their male and female lambs) | Intervention group: From GDs 4–41, received 2.5 g/kg ethanol on three consecutive days per week, followed by four days without treatment Control group: From GDs 4–41, received isotonic saline (0.9%) equal in volume to the ethanol infusions | Timeframe delivered: From GD 4 to term, 10 mg/kg/day choline Assignment: Randomly assigned to one of the following five groups: (1) normal untreated control, (2) saline control, (3) saline control + choline, (4) ethanol, and (5) ethanol + choline | Age at assessment: 6 months Outcomes assessed: Body weight at birth and 6 months Whole brain, cerebellar, and pituitary volumes | Choline supplementation had no protective effects on reductions in body weight and whole brain, cerebellar, and pituitary volumes in ethanol-exposed subjects |
| Bottom et al. (2020) [36] CD1 mice (n = 98? (authors did not provide the total number), male and female pups) | Intervention group: Throughout gestation, drank water with 25% ethanol Control group: Throughout gestation, drank water | Timeframe delivered: Throughout gestation, 642 mg/L choline Assignment: Randomly assigned to one of the following four groups: (1) water (control), (2) 25% ethanol in water, (3) 25% ethanol in water + choline, and (4) choline in water | Age at assessment: PD 0 and PD 20 Outcomes assessed: Body and brain weights at PD 0 Cortical length Ability to integrate sensory inputs and motor outputs, as measured using the Ledge test at PD 20 Anxiety-like behaviors, as measured using the Suok test at PD 20 | Choline supplementation prevented reductions in body weight (p < 0.01), brain weight (p < 0.01), and cortical length (p = 0.049) Choline supplementation fully prevented deficits in motor function; choline-supplemented subjects took fewer missteps (p = 0.0002) and fewer falls (p = 0.036) compared to ethanol-exposed subjects Choline supplementation partially ameliorated anxiety-like behaviors in ethanol-exposed subjects (latency to leave center: H = 8.196, p = 0.042; directed exploration: H = 16.61, p = 0.001; rearing/grooming: H = 15.33, p = 0.002) |
| Carugati et al. (2022) [37] Suffolk ewes (n = 56, and their male and female lambs) | Intervention group: From GDs 4–41, received either 1.75 or 2.5 g/kg ethanol Control group: From GDs 4–41, received isotonic saline (0.9% w/v) | Timeframe: Throughout gestation, 10 mg/kg of oral choline Assignment: Randomly assigned to one of the following seven treatment groups: (1) normal control group, (2) saline control + placebo, (3) saline control + choline, (4) 1.75 g/kg ethanol + placebo, (5) 1.75 g/kg ethanol + choline, (6) 2.5 g/kg ethanol + placebo, and (7) 2.5 g/kg ethanol + choline | Age at assessment: At birth and 6 months Outcomes assessed: Birth and brain weights | Choline supplementation did not significantly affect birth or brain weights in ethanol-exposed subjects |
| Goeke et al. (2018) [38] Sprague Dawley rats (n = 31, male and female pups) | Intervention group: From PDs 4–9, received 5 g/kg/day ethanol Control group: From PDs 4–9, received sham intragastric intubations | Timeframe delivered: From PDs 4–9, 100 mg/kg choline or saline Assignment: Randomly assigned to one of the following five groups: (1) sham intubation + saline, (2) sham intubation + choline, (3) ethanol intubation + saline, (4) ethanol intubation + choline, and (5) untreated control | Age at assessment: PD 9 Outcomes assessed: Body weight | No significant differences in body weight across all treatment groups on PD 4; animals in all groups gained weight during the treatment window from PD 4 to PD 9 |
| Hunt et al. (2014) [39] Sprague Dawley rats (n = 9 treatment litters and 10 control litters with 8–10 pups per litter; offspring sex was not reported) | Intervention group: From PDs 4–9, received 5.0 g/kg/day ethanol Control group: From PDs 4–9, sham controls received the tube-insertion procedure, but were not given any fluid | Timeframe delivered: From PDs 4–20, 18.8 mg/mL choline or saline Assignment: Assigned to one of the following four groups: (1) ethanol + choline, (2) ethanol + saline, (3) sham + choline, and (4) sham + saline | Age at assessment: PDs 4–9 and 20 for body weight, PD 23 for heart-rate-orienting response and response habituation Outcomes assessed: Body weight Form and magnitude of heart-rate-orienting response Habituation of orienting response | Choline supplementation did not protect against observed reductions in body weight in ethanol-exposed subjects No effect of choline supplementation on the form or magnitude of the heart-rate-orienting response or on habituation of orienting response |
| Kwan et al. (2021) [40] C57BL/6J mice (n = 32 litters, male and female fetuses) | Intervention group: From EDs 8.5–17.5, received 3.0 g/kg/day ethanol Control group: From EDs 8.5–17.5, received a single 4.20 g/kg gavage of maltodextrin | Timeframe delivered: From EDs 8.5–17.5, 100 mg/kg choline or saline Assignment: Randomly assigned to one of the following four groups: (1) sham, (2) ethanol, (3) sham + choline, and (4) ethanol + choline | Age at assessment: ED 17.5 Outcomes assessed: Body, brain, and liver weights Fetal brain-to-body weight ratio Fetal liver-to-body weight ratio Brain-to-liver-weight ratio | In males, choline supplementation did not have significant effects on body weight (p = 0.353), brain weight (p = 0.653), fetal brain-to-body weight ratio (0.497), liver weight (p = 0.973), fetal liver-to-body weight ratio (p = 0.282), or fetal brain-to-liver weight ratio (p = 0.536) In females, choline supplementation did not have significant effects on body weight (p = 0.489), brain weight (p = 0.673), brain-to-body weight ratio (0.066), or fetal liver-to-body weight ratio (p = 0.078) In females, choline supplementation mitigated reductions in liver weights such that the choline-supplemented group did not differ from the control (p = 0.31) or control + choline groups (p = 0.17) Choline supplementation mitigated high brain-to-liver weight ratio (p = 0.002) in females |
| Monk et al. (2012) [41] Sprague Dawley rats (n = 53, male pups only) | Intervention group: From PDs 4–9, received 5.25 g/k/day ethanol Control group: From PDs 4–9, received sham intubations | Timeframe delivered: From PDs 4–30, 100 mg/kg/day choline or saline Assignment: Randomly assigned to one of the following four groups: (1) ethanol + choline, (2) ethanol + saline, (3) control + choline, and (4) control + saline | Age at assessment: PDs 4–30 and PDs 30–33 Outcomes assessed: Body weight on PDs 4–30 Hyperactivity, as measured using the open field test on PDs 30–33 | Choline supplementation had no effect on body weight Choline supplementation reduced hyperactivity levels in ethanol-exposed subjects (p < 0.05) |
| Otero et al. (2012) [42] Long-Evans rats (n = 120, male and female pups) | Intervention group: From PDs 2–10, received 3.0 g/kg/day ethanol Control groups: From PDs 2–10, intubated without alcohol and a nontreated control group | Timeframe delivered: From PDs 2–20, 100 mg/kg choline or saline Assignment: Quasi-randomly assigned to one of the following five groups: (1) ethanol + choline, (2) ethanol + saline, (3) intubated control + choline, (4) intubated control + saline, and (5) nontreated control | Age at assessment: PD 2–21 Outcomes assessed: Body weight | Choline supplementation did not protect against deficits in growth in ethanol-exposed subjects |
| Sawant et al. (2019) [43] Suffolk ewes (n = 49, offspring sex was not reported) | Intervention group: From GDs 4–41, received either 1.75 g/kg/day or 2.25 g/kg/day ethanol Control group: From GDs 4–41, received 0.9% isotonic saline infusions intravenously | Timeframe delivered: From GD 4 until term, 10 mg/kg per day Assignment: Randomly assigned to one of the following six groups: (1) saline + placebo control, (2) saline + choline, (3) 1.75 g/kg/day ethanol + placebo, (4) 1.75 g/kg/day ethanol + choline, (5) 2.25 g/kg/day ethanol, and (6) 2.25 g/kg/day ethanol + choline | Age at assessment: GD 76 Outcomes assessed: Fetal frontothalamic distance, mean orbital diameter, interorbital distance, mean lens diameter, thalamic width, and femoral and humerus lengths | Choline supplementation protected against decreases in brain fetal frontothalamic distance (p = 0.013) Choline supplementation had no significant effect on mean orbital diameter (p > 0.05) or interorbital distance in ethanol-exposed subjects (p = 0.101) Choline supplementation significantly increased fetal mean lens diameter in ethanol-exposed subjects (p < 0.001) Choline supplementation significantly decreased fetal thalamic width (p = 0.043) There was no significant interaction between choline and ethanol use on femoral and humerus length; choline supplementation increased femoral (p = 0.002) and humerus (p = 0.011) and lengths across all groups |
| Steane et al. (2021) [44] Sprague Dawley rates (n = 57, male and female fetuses) | Intervention group: From 4 days prior to conception and 4 days after conception, received a liquid diet containing 12.5% EtOH (v/v) Control group: From 4 days prior to conception and 4 days after conception, received a control liquid diet | Timeframe delivered: From GDs 5–20, 1.6 g choline/kg or 2.6 g choline/kg with one group increased to 7.2 g choline/kg from GDs 10–20 Assignment: Randomly assigned to one of the following six groups: (1) liquid control + choline (1.6 g/kg), (2) ethanol + choline (1.6 g/kg), (3) liquid control + choline (2.6 g/kg), (4) ethanol + choline (2.6 g/kg), (5) liquid control + choline (2.6 g/kg from GDs 5–10, followed by 7.2 g/kg chow from GDs 10–20), and (6) ethanol + choline (2.6 g/kg chow from GDs 5–10, followed by 7.2 g/kg chow from GDs 10–20) | Age at assessment: GD 20 Outcomes assessed: Body, liver, and heart weights | Though not statically significant, the reduction in body weight with the 1.6 g/kg choline diet was ~8% in males and ~7% in females, compared to 2–4% with the 2.6 g/kg choline and 7.2 g/kg choline groups in ethanol-exposed males (p = 0.30) and females (p = 0.77) Choline supplementation did not have significant effects on liver weights in males (p = 0.77) or females (p = 0.85) Choline supplementation did not have significant effects on heart weights in males (p = 0.88), but there was a significant effect on heart weights in females (p = 0.01), but only for the 1.6 g/kg choline group |
| Thomas et al. (2000) [45] Sprague Dawley rats (n = 78, male and female pups from 13 dams) | Intervention group: From GDs 6–20, received a liquid diet containing 35% ethanol-derived calories Control groups: From GDs 6–20, received a liquid isocaloric maltose-dextrin and a nontreated control group was fed regular lab chow ad lib | Timeframe delivered: From PDs 2–7, 25 mg choline chloride/mL saline Assignment: Randomly assigned to one of the following nine groups: (1) ethanol + choline, (2) ethanol + saline, (3) ethanol + lab chow, (4) liquid control + choline, (5) liquid control + saline, (6) liquid control + lab chow, (7) nontreated control + choline, (8) nontreated control + saline, and (9) nontreated control + lab chow | Age at assessment: PD 4–21 and PD 45 Outcomes assessed: Body growth on PDs 4–21 Visuospatial discrimination, as measured using the T-maze task on PD 45 | Choline supplementation had no effect on body growth in ethanol-exposed subjects Choline supplementation improved visuospatial discrimination acquisition in ethanol-exposed subjects (p < 0.01) Choline supplementation produced a relatively large improvement in delayed discrimination training performance among the ethanol-treated subjects and only mild improvement in the pair-fed and liquid controls (p < 0.002) |
| Thomas et al. (2004) [46] Sprague Dawley rats (n = 82, male pups only) | Intervention group: From PDs 4–9, received 6.6 g/kg/day ethanol Control group: From PDs 4–9, received isocaloric maltose-dextrin | Timeframe delivered: From PDs 4–30, 18.8 mg choline chloride/mL or saline Assignment: Randomly assigned to one of the following six groups: (1) ethanol + choline, (2) ethanol + saline, (3) sham intubation + choline, (4) shame intubation + saline, (5) normal lactation control + choline, and (6) normal lactation control + saline | Age at assessment: PD 35–37 Outcomes assessed: Motor coordination, as measured via maximum gap successfully traversed, ratio of successful traversals to total traversals, and number of trials to first successful traversal via the Parallel Bars task | Choline supplementation did not attenuate impairments to motor coordination, measured via maximum gap successfully traversed, ratio of successful traversals to total traversals, or number of trials to first successful traversal, in ethanol-exposed subjects |
| Thomas et al. (2004) [47] Sprague Dawley rats (n = 85, male pups only) | Intervention group: From PDs 4–9, received 6.6 g/kg/day ethanol Control group: From PDs 4–9, received isocaloric maltose-dextrin | Timeframe delivered: From PDs 4–30, 18.8 mg choline chloride/mL or saline Assignment: Randomly assigned to one of the following six groups: (1) ethanol + choline, (2) ethanol + saline, (3) intubated control + choline, (4) intubated control + saline, (5) normal lactation control + choline, and (6) normal lactation control + saline | Age at assessment: PD 31–34 and 40–42 Outcomes assessed: Body weight Activity level on PDs 31–34 Spatial discrimination serial reversal learning as measured by number of trials to the first successful criterion, total number of successful discriminations achieved, and number of errors via the T-maze task, on PDs 40–42 | Choline supplementation did not have any significant effects on body weight in ethanol-exposed subjects Choline supplementation significantly reduced activity levels in ethanol-exposed subjects compared to controls (p < 0.01) There were no statistically significant effects of ethanol or choline on the number of trials to the first successful criterion or the total number of successful discriminations achieved Choline supplementation significantly reduced the number of errors committed on the spatial discrimination learning task among ethanol-treated subjects (p < 0.05) |
| Thomas et al. (2009) [48] Sprague Dawley rats (n = 72, male and female pups) | Intervention group: From GDs 5–20, received 6.0 g/kg/day in a 28.5% (v/v) ethanol solution (0.02675 mL/g body weight) Control group: From GDs 5–20, pair-fed dams received isocaloric maltose-dextrin, and ad lib control dams received a vehicle full of saline | Timeframe delivered: From GDs 5–20, 250 mg choline/kg/day or saline Assignment: Randomly assigned to one of the following six groups: (1) ethanol + choline, (2) ethanol + saline, (3) pair-fed intubation + choline, (4) pair-fed intubation + saline, (5) ad lib control + choline, and (6) ad lib control + saline | Age at assessment: PD 1–21 and PD 2–20 Outcomes assessed: Body (PDs 1–21) and brain weights Eye opening and incisor emergence Series of reflex development tasks to examine sensorimotor maturation including righting reflex, geotactic reflex, cliff avoidance, grip strength, and hindlimb coordination | Choline supplementation significantly attenuated alcohol-related birth weight reductions (p < 0.05) and brain weights (p < 0.05) Choline supplementation advanced incisor emergence across both ethanol and control groups (p < 0.05) There was no significant interaction between ethanol and choline for eye opening and grip strength Choline supplementation attenuated effects of righting reflex responses such that they were not significantly different from that observed in control groups (Fisher’s p’s < 0.05) Choline supplementation significantly mitigated deficits in the negative geotactic reflex observed in ethanol-exposed subjects (Fisher p’s < 0.05) Choline-supplemented subjects exposed to ethanol performed significantly similarly to control subjects on the behavioral measure of cliff avoidance (p < 0.01) Choline supplementation showed a tendency to reduce the deficit in decreased hindlimb coordination in ethanol-exposed subjects; no significant interaction was observed |
| Thomas et al. (2010) [49] Sprague Dawley rats (n = 71 male and female litters) | Intervention group: From GDs 5–20, received 6.0 g/kg/day (28.5% v/v) ethanol Control group: From GDs 5–20, received an isocaloric maltose-dextrin solution | Timeframe delivered: From GDs 5–20, 250 mg choline/kg/day or saline Assignment: Randomly assigned to one of the following six groups: (1) ethanol + choline, (2) ethanol + saline, (3) pair-fed isocaloric maltose-dextrin solution + choline, (4) pair-fed isocaloric maltose-dextrin solution + saline, (5) ad libitum control + choline, and (6) ad libitum control + saline | Age at assessment: PD 15–17, PD 28–32, PD 30–32, PD 39–41, PD 45–52 and PD 65–66 Outcomes assessed: Body weight Exploratory behavior, a measure of natural exploratory and foraging behavior that depends on hippocampal cholinergic functioning, as measured using T-maze spontaneous alternation behavioral task Motor coordination, as measured using success ratio and maximum width traversed via the parallel bars task Spatial learning and working memory, as measured using the Morris water maze task | Across all treatment groups, choline supplementation significantly increased body weight at PD 28 (p < 0.0001) and 45 (p < 0.05), but not at PD 30 (p = 0.13) Choline-supplemented subjects alternated at significantly higher rates; ~75% of subjects compared to ~35% of ethanol-exposed subjects not supplemented with choline during the spontaneous alteration task Choline supplementation did not affect the motor performance on the parallel bar task, including success ratio and maximum width traversed (all p-values >0.1) Choline supplementation significantly mitigated impairments to spatial working memory (p < 0.05) The interaction of choline with prenatal ethanol exposure did not reach statistical significance for the spatial learning task |
| Wagner and Hunt (2006) [50] Sprague Dawley rats (n = 85, male and female pups) | Intervention group: From PDs 4–9, received 5.25 g/kg/day ethanol Control group: From PDs 4–9, received sham intubations | Timeframe delivered: From PDs 4–20, 0.10 mL of an 18.8 mg/mL solution of choline, chloride, or saline Assignment: Randomly assigned to one of the following eight groups: (1) ethanol + choline + delay conditioning, (2) ethanol + choline + trace conditioning, (3) ethanol + saline + delay conditioning, (4) ethanol + choline + trace conditioning, (5) sham + choline + delay conditioning, (6) sham + choline + trace conditioning, (7) sham + saline + delay conditioning, and (8) sham + choline + trace conditioning | Age at assessment: PD 4–9, 15, and 20 and PD 30 Outcomes assessed: Body weight on PDs 4–9, 15, and 20 Conditioned stimulus-elicited freezing on PD 30 | Choline supplementation had no effect on body weights Choline supplementation completely reversed the deficit in conditioned stimulus freezing for the trace conditioning groups (p < 0.01) None of the groups given delayed conditioning trials differed in conditioned stimulus freezing |
| b. Effects of zinc on maternal ethanol consumption and offspring developmental outcomes. | ||||
| Reference and Study Population | Prenatal Substance Use (PSU) | Prenatal Nutrition | Offspring Development Outcomes | Results |
| Summers et al. (2006) [51] C57BL/6J mice (n = 72, male and female pups) | Intervention group: On GD 8, received 25% ethanol in 0.85% saline v/v (0.015 mL/g) intraperitoneally twice Control group: On GD 8, received saline injections | Timeframe delivered: On GD 8, 0.25 mL zinc Assignment: Assigned to one of the following three groups: (1) saline, (2) ethanol, and (3) ethanol + zinc | Age at assessment: PD 7 and 21, 56–60, and 70–71 Outcomes assessed: Body weight on PD 7, 21, and 55 Spatial learning and memory, as measured via escape latency, number of correct trials and errors via the cross-maze water escape task | Zinc supplementation increased body weights on PD 55 in males but not females (p = 0.001) Choline supplementation attenuated effects of ethanol on spatial memory on all parameters, including shorter escape latencies, more correct trials, and fewer incorrect entries (p < 0.05) |
| Summers et al. (2008) [52] C57BL/6J mice (n = 24/treatment, male and female pups) | Intervention group: On GD 8, received 25% ethanol (0.015 mL/g) injections twice Control group: On GD 8, received saline injections | Timeframe delivered: From GDs 1–18, 200 µg/g zinc-supplemented diet, or 35 µg/g zinc for the control group Assignment: Assigned to one of the following four groups: (1) saline + control diet (35 µg/g zinc), (2) ethanol + control diet (35 µg/g zinc), (3) saline + zinc -supplemented diet (200 µg/g zinc), and (4) ethanol + zinc-supplemented diet (200 µg/g zinc) | Age at assessments: PD 3, 21, 40, 60–66, 78, 105, 120, and 121 Outcomes assessed: Body weight and length on PD 3, 21, and 40 Object recognition memory, as measured by the object recognition memory tasks Spatial learning and memory impairments, as measured via escape latency, number of correct trials, and number of errors via the cross-maze water escape task | Zinc supplementation had no effect on body weight or length Zinc-supplemented subjects performed at the level of control offspring for the cross-maze water escape and object recognition memory tasks, while the ethanol only group performed worse than all other groups (p < 0.0001) Zinc-supplemented subjects performed to the level of saline-treated mice with shorter escape latencies for spatial memory and increased correct trials compared with mice treated with ethanol alone in the cross-maze water escape task (p < 0.05) |
| Summers et al. (2009) [53] C57BL⁄6J mice (n = 309, males and females) | Intervention group: On GD 8, received 25% (0.015 mL/g) ethanol injections Control group: On GD 8, received saline injections | Timeframe delivered: From GDs 1–18, 200 mg zinc⁄kg or 35 mg zinc/kg for the control group Assignment: Assigned to one of the following four groups: (1) saline + control diet (35 mg zinc⁄kg), (2) ethanol + control diet (35 mg zinc⁄kg), (3) saline + zinc-supplemented diet (200 mg zinc⁄kg), and (4) ethanol + zinc-supplemented diet (200 mg zinc⁄kg) | Age at assessment: GD 18 to PD 60 Outcomes assessed: Postnatal growth and survival Fetal dysmorphology | Cumulative postnatal mortality was significantly higher in offspring exposed to ethanol alone (35% deaths) compared to all other treatment groups (13.5 to 20.5% deaths) Zinc supplementation decreased the number of deaths from birth to PD 3 from 25 in the ethanol only group to 11 across all three other groups Zinc supplementation reduced the occurrence of stillbirths from 7 in the ethanol only group to 1 in the ethanol + zinc group, but did not reach significance Zinc supplementation reduced the incidences of physical abnormalities from 26% in the ethanol only group to 12% in the ethanol + zinc group (p < 0.05) |
| c. Effects of vitamin E on maternal ethanol consumption and offspring developmental outcomes. | ||||
| Reference and Study Population | Prenatal Substance Use (PSU) | Prenatal Nutrition | Offspring Development Outcomes | Results |
| Marino et al. (2004) [54] Long–Evans rats (n = 146, male and female pups) This study consisted of two cohorts. Cohort one outcomes were body weight and the Morris water maze. Cohort two outcomes were related to Western blot staining. We only reviewed cohort 1. | Intervention group: From PDs 7–9, received 5.25 g/kg/day ethanol in 27.8 mg/kg volume of milk Control groups: From PDs 7–9, the control was intubated without any liquids, while the nontreated control were not intubated | Timeframe Delivered: On PDs 6–9, 2.0 g/kg vitamin E in 13.9 mL/kg volume of milk Assignment: Assigned to one of the following five groups: (1) only ethanol, (2) ethanol + vitamin E, (3) intubated control, (4) intubated + vitamin E, and (5) nontreated control | Age at assessment: PD 6–30 Outcomes assessed: Body weight Spatial navigation, as measured by escape latency, duration in probe quadrant, number of probe crossings, and escape latency to reach visible platform via the Morris water maze task | Vitamin E supplementation did not mitigate reductions in body weight (p < 0.001) Vitamin E treatment did not attenuate significantly slower spatial navigation latencies in the ethanol-exposed animals (p < 0.001) No significant effect of vitamin E on the number of probes crossing in ethanol-exposed subject (p = 0.650), duration in probe quadrant (p = 0.157), or escape latency to reach visible platform (p = 0.868) |
| Tran et al. (2005) [55] Long–Evans rats (n = 44, male and female pups) | Intervention group: From PDs 4–9, received 2.625 g/kg/day ethanol, 4 intubations per day Control group: From PDs 4–9, received sham intubations | Timeframe delivered: From PDs 4–9, 12.26 mg vitamin E/kg/feeding for 4 daily feedings Assignment: Assigned to one of the following five groups: (1) ethanol + milk, (2) ethanol + vitamin E, (3) only vitamin E, (4) sham intubations, and (5) nontreated control | Age at assessment: PD 26–33 Outcomes assessed: Body weight Eyeblink classic conditioning | There were no interactive effects for body weight Vitamin E did not improve eyeblink conditioning performance (p < 0.0001) Vitamin E supplementation did not protect against reductions in eyeblink performance; mean percent conditioned responses and amplitude of conditioned responses were significantly lower than in the control groups (p < 0.05) |
| d. Effects of fatty acids on maternal ethanol consumption and offspring developmental outcomes. | ||||
| Reference and Study Population | Prenatal Substance Use (PSU) | Prenatal Nutrition | Offspring Development Outcomes | Results |
| Abel and Reddy (1997) [56] Sprague Dawley rats (n = 120–180 (authors did not provide the total number), male and female pups) | Intervention group: From GD 8–20, intubated with 5.0 or 3.0 g/kg ethanol Control group: From GD 8–20, intubated with a saline vehicle | Timeframe delivered: From GDs 8–20, diet high in saturated or polyunsaturated fat with no vitamin E or zinc (authors did not provide exact amount) Assignment: Randomly assigned to one of the following twelve groups: (1) saturated fat diet + 5.0 g/kg ethanol, (2) saturated fat diet + 3.0 g/kg ethanol, (3) saturated fat diet + saline intubation, (4) saturated fat diet + non-intubated control, (5) unsaturated fat diet (with a low dietary content of vitamin E and zinc) + 5.0 g/kg ethanol, (6) unsaturated fat diet (with a low dietary content of vitamin B and zinc) + 3.0 g/kg ethanol, (7) unsaturated fat diet (with a low dietary content of vitamin E and zinc) + saline intubation, (8) unsaturated fat diet + non-intubated control, (9) standard rodent diet + 5.0 g/kg ethanol, (10) standard rodent diet + 3.0 g/kg ethanol, (11) standard rodent diet + saline intubation, and (12) standard rodent diet + non-intubated control | Age at assessment: Birth and PD 20 Outcomes assessed: Birth weight Locomotor activity Head-dipping behavior | There were no significant interactive effects of saturated/unsaturated fat supplementation on birth weight Saturated-fat-supplemented subjects were less active than the pair-fed controls (p < 0.001) Saturated fat supplementation produced a significant decrease in head-dipping behavior, indicating a high anxiety-like state (p < 0.01) The polyunsaturated fat and low vitamin E and zinc diet did not modify ethanol’s effects significantly for locomotor activity or head-dipping behavior |
| Wainwright et al. (1985) [57] B6D2F1 mice (n = 32–48, male and female pups) | Intervention group: From GDs 7–17, received liquid diets containing 25% ethanol-derived calories (4.7 mL) Control group: From GDs 7–17, received liquid diet containing 6.25 g of isocaloric sucrose | Timeframe delivered: From GDs 7–17, either 20 mg/kg, 120 mg/kg, or 200 mg/kg gamma-linolenic acid Assignment: Randomly assigned to one of the following eight groups: (1) ethanol + gamma-linolenic acid (20 mg/kg), (2) ethanol + gamma-linolenic acid (120 mg/kg), (3) ethanol + gamma-linolenic acid (200 mg/kg), (4) ethanol + arachidonic acid control (200 mg/kg), (5) ethanol + coconut oil control, (6) ethanol + safflower oil control, (7) sucrose intubations + safflower oil, and (8) lab chow + no fatty acid supplementation | Age at assessment: PD 22, 32, and 50 Outcomes assessed: Body and brain weights Behavioral development on PD 32, including righting reflex, cliff aversion, forelimb and hindlimb grasp reflex, Vibrissa placing reflex, level screen test, vertical screen test, screen climbing test, pole grasp, forelimb and hindlimb stick grasp, opening of both eyes, visual placing reflex, auditory startle response Behavior was measured in an open field on PD 50 including the following behavioral categories: animal in quadrupedal movement, animal sniffing the air, animal sniffing an object, animal rearing, animal grooming, animal freezing | Gamma-linolenic acid supplementation did not prevent deficits in body and brain weights There were no interactive effects of gamma-linolenic acid supplementation on any behavioral development outcomes of interest |
| e. Effects of iron on maternal ethanol consumption and offspring developmental outcomes. | ||||
| Reference and Study Population | Prenatal Substance Use (PSU) | Prenatal Nutrition | Offspring Development Outcomes | Results |
| Helfrich et al. (2022) [58] Long–Evans rats (n = 140 litters, (authors did not provide the total number), male and female pups) | Intervention group: From GDs 13.5–19.5, received 5.0 g/kg/day of ethanol Control group: From GDs 13.5–19.5, received a 43.8% maltodextrin solution in water | Timeframe delivered: From GDs 12.5–19.5, received 6 mg/kg elemental iron Assignment: Randomly assigned to one of the following four groups: (1) non-intubated control + water, (2) ethanol + water, (3) non-intubated control + iron, and (4) ethanol + iron | Age at assessment: Birth and PD 20 Outcomes assessed: Birth weight Liver weight Brain weight Heart weight | Iron supplementation significantly improved absolute brain weights in male pups alone (p = 0.014) There were no interactive effects of iron supplementation on any other physical developmental outcomes of interest |
| a. Effects of choline on maternal alcohol consumption and offspring developmental outcomes. | ||||
| Reference and Study Population | Prenatal Substance Use | Prenatal Nutrition | Offspring Development Outcomes | Results |
| Coles et al. (2015) [59] Infants (n = 367, males and females) | Intervention group: “Heavy” drinkers (n = 301), defined as having at least weekly binge drinking episodes (5+ drinks), at least five episodes in which they consumed 3–4 standard drinks, or at least ten episodes in which they consumed 1–2 standard drinks either in the month around conception or the most recent month of pregnancy Control group: Nondrinking women (n = 313), meeting screening criteria (i.e., no binge episodes, minimal or no alcohol consumption in the month around conception, and no drinking in the most recent month of pregnancy) | Timeframe delivered: From the first prenatal visit (average 19 weeks) until delivery, 750 mg choline Assignment: Alcohol-using and nondrinking women were randomized to one of the following three groups: (1) no multivitamin supplement provided, but recommended (n = 176), (2) multivitamin supplement provided (n = 96), and (3) multivitamin supplement + choline provided (n = 95) | Age at assessment: Birth and 6 months of age Outcomes assessed: Birth weight and length and head circumference Bayley Scales of Infant Development 2nd Edition (BSID-II) measures current mental development (problem solving/prelinguistic development) and psychomotor development (fine/gross motor skills) yielding standardized scores (Mental Development Index: MDI; Psychomotor Development Index: PDI) Behavioral rating in the BSID-II of orientation/engagement, emotional regulation, motor quality, and total behavior quality | Supplementation (multivitamin or multivitamin with choline) did not have any effect on birth weight, length, or head circumference Choline supplementation approached significance (p = 0.10), with those taking choline having lower scores on the Psychomotor Development Index, but did not contribute significantly to differences in the Psychomotor Development Index There was no effect of the interaction of choline with alcohol exposure on the Mental Development Index (p = N.S.) The multivitamin group had significantly higher scores on the Mental Development Index, which was not observed in the multivitamin + choline group (p < 0.03) There were no interactive effects of choline supplementation on behavioral ratings on the BSID-II including orientation/engagement, emotional reactivity, motor quality, and total behavior |
| Jacobson et al. (2018) [60] Infants (n = 62, males and females) | Intervention group: Heavy drinkers (n = 35) were recruited, defined by having an average of at least 2 standard drinks (1.0 oz absolute alcohol) per day or at least one incident of binge drinking (4 or more standard drinks/occasion) Control group: Heavy drinkers (n = 35); did not receive choline supplement | Timeframe delivered: Time of enrollment (23rd week of gestation) until delivery, 2 g/day choline Assignment: Heavy drinkers were randomly assigned to one of the following two groups: (1) choline supplement and (2) placebo pill | Age at assessment: 6.5 months and 12 months Outcomes assessed: Somatic growth Recognition memory and processing speed, as measured using the Fagan Test of Infant Intelligence Eyeblink conditioning Fetal alcohol spectrum disorder (FASD) or partial fetal alcohol syndrome (PFAS) diagnosis | Choline supplementation showed significantly greater increases in weight (p = 0.009) and head circumference (p = 0.006) Choline supplementation had a significantly greater increase in percent condition eyeblink responses than the placebo group (p < 0.01) between 6.5 and 12 months of age There was a non-significant increase in the proportion of infants meeting the EBC in the choline group (p = 0.090), but when those whose mothers with poor adherence (< 20%) were excluded, the increase in the proportion meeting EBC was significant (p = 0.036) Choline-supplemented infants performed more optimally on the Fagan Test of Infant Intelligence at 12 months, with higher novelty preference scores, indicating better visual recognition memory function (d = 0.62, p < 0.05) Choline supplementation did not improve the proportion of infants diagnosed with FASD/PFAS. In the choline group, 8 infants were diagnosed with FASD and 2 were diagnosed with PFAS (32.3%), while in the placebo group, only 5 infants were diagnosed with FASD and 2 were diagnosed with PFAS (22.6%) (p = 0.393) For consideration, this study reports a high level of cigarette use (1/4 pack/day), and 4 participants reported use of methamphetamine later in pregnancy |
| Kable et al. (2015) [61] Infants (n = 168, males and females) | Intervention group: Women (n = 119) who reported at least weekly binge drinking episodes (5+ drinks), at least five episodes in which they consumed 3–4 standard drinks, or at least 10 episodes in which they consumed 1–2 standard drinks either in the month around conception or in the most recent month of pregnancy Control group: Women (n = 136) who reported no binge drinking episodes, minimal or no alcohol consumption in the month around conception, and no continued drinking during pregnancy | Timeframe delivered: From first prenatal visit until delivery, 750 mg choline Assignment: Alcohol-using and nondrinking women were randomized to one of the following three groups: (1) multivitamin supplement recommended but not provided (n = 81), (2) multivitamin supplement provided (n = 50), and (3) multivitamin supplement + choline provided (n = 37) | Age at assessment: Birth and 6–12 months Outcomes assessed: Birth weight and length Head circumference Cardiac-orienting responses during a habituation/dishabituation learning paradigm to assess neurophysiological encoding and memory of environmental events | Choline supplementation did not protect against reductions in birth weight, length, and head circumference Choline supplementation plus multivitamin did not significantly affect cardiac-orienting responses to the auditory stimuli in alcohol-exposed pregnancies There were no interactive effects of choline on latency response in the visual habituation tasks Choline supplementation resulted in a greater change in heart rate on the visual habituation task across ethanol-exposed and control groups (p < 0.001) Change in choline level from the baseline to third trimester timepoint was positively related to HR during the habitation task (p < 0.05), but not the dishabituation task or latency of the response during either task for both ethanol-exposed and control groups This study reports that those consuming alcohol had significantly higher cigarette use (p < 0.005) |
| Kable et al. (2022) [62] Infants (n = 243, males and females) | Intervention group: Women (n = 141) who reported at least weekly binge drinking episodes (5+ drinks), at least five episodes in which they consumed 3–4 standard drinks, or at least 10 episodes in which they consumed 1–2 standard drinks either in the month around conception or in the most recent month of pregnancy Control group: Women (n = 225) who reported no binge drinking episodes, minimal or no alcohol consumption in the month around conception, and no continued drinking during pregnancy | Timeframe delivered: From first prenatal visit until delivery, 750 mg choline Assignment: Alcohol-using and nondrinking women were randomized to one of the following three groups: (1) multivitamin supplement recommended but not provided (n = 114), (2) multivitamin supplement provided (n = 52), and (3) multivitamin supplement + choline provided (n = 77) | Age at assessment: Mean age 3.96 years Outcomes assessed: Reaction time involving the child making a response to a series of chromatic pictures presented on a computer screen | Prenatal choline supplementation did not improve reaction time in preschool-aged children exposed to alcohol in utero |
| Warton et al. (2021) [63] Infants (n = 50, males and females) | Intervention group: Heavy drinkers (n = 27), defined as having an average of at least 2 standard drinks (1.0 oz absolute alcohol) per day or at least one incident of binge drinking (4 or more standard drinks/occasion) Control group: Heavy drinkers (n = 23); did not receive choline supplement | Timeframe delivered: Time of enrollment (23rd week of gestation) until delivery, 2 g/day choline Assignment: Heavy drinkers were randomly assigned to one of the following two groups: (1) choline supplement and (2) placebo pill | Age at assessment: 1–7 weeks of age (median age 2.8 weeks) and 12 months of age Outcomes assessed: Brain volumes Recognition memory and processing speed, as measured using the Fagan Test of Infant Intelligence (FTII) | Choline supplementation increased brain volume in 6 of 12 regions, namely, the left (p = 0.01) and right thalamus (p = 0.05) and left (p = 0.006) and right caudate (p = 0.004), right putamen (p = 0.03), and corpus callosum (p = 0.01) The effect of choline on FTII was significant after controlling maternal supplement adherence and birth weight (p = 0.03) In regions where choline effects were observed, the presence of a larger right putamen (p = 0.003) and corpus callosum (p ≤ 0.04) were associated with higher scores for Fagan Test of Infant Intelligence More mothers in the choline group reported smoking (96% vs. 70%), and cannabis use was more common in the choline group (41% vs. 9%) |
| b. Effects of vitamin C on maternal nicotine consumption and offspring developmental outcomes. | ||||
| Reference and Study Population | Prenatal Substance Use | Prenatal Nutrition | Offspring Development Outcomes | Results |
| McEvoy et al. (2014) [64] Infants (n = 235, males and females) Stand-alone cohort | Intervention group: Current smokers (≥ 1 cigarette/day) Control group: Pregnant nonsmokers who were not given vitamin C or a placebo (n = 76) | Timeframe delivered: Randomized at ≤22 weeks gestational age to receive 500 mg/day vitamin C Assignment: Pregnant current smokers were randomly assigned to one of the following two groups: (1) vitamin C supplement (n = 89) and (2) placebo pill (n = 90) | Age at assessment: 3 days and 12 months Outcomes assessed: Newborn pulmonary function at 3 days Incidence of wheezing through 12 months and pulmonary function at 12 months | Vitamin C supplementation significantly improved pulmonary function as measured by time to peak tidal expiratory flow to expiratory time, (p =0.006), passive respiratory system compliance (p = 0.012), and total passive respiratory system compliance (p = 0.0035) at 3 days of age Vitamin C supplementation significantly decreased the occurrence of wheezing through 1 year from 40% in the placebo group to 21% in the vitamin C group (adjusted relative risk 0.56; 95% CI 0.33, 0.95; p = 0.03) |
| McEvoy et al. (2019) [65] Infants (n = 225, males and females) * VCSIP cohort | Intervention group: Current smokers (≥ 1 cigarette/day) who were given vitamin C Control group: Pregnant smokers who were given a placebo | Timeframe delivered: Randomized at ≤22 weeks gestational age to receive 500 mg/day vitamin C Assignment: Pregnant current smokers were randomly assigned to one of the following two groups: (1) vitamin C supplement (n = 125) and (2) placebo pill (n = 126) | Age at assessment: Birth and 3 months Outcomes assessed: Birth weight Delivery mode Gestational age Incidence of prematurity Forced expiratory flows at 25%, 50%, and 75% of expired volumes | Vitamin C supplementation increased (but not significantly) forced expiratory flow at 75% at 3 months of age (200.7 vs. 188.7 mL/s; adjusted 95% confidence interval (CI) for difference, −3.33 to 35.64; p = 0.10), and a significantly increased forced expiratory flow at 50% (436.7 vs. 408.5 mL/s; adjusted 95% CI, 6.10–61.30; p = 0.02) when compared with those born to smokers who were randomized to receive the placebo There were no significant (p > 0.05) effects of vitamin C supplementation on birth weight, delivery mode, gestational age, and incidence of prematurity |
| McEvoy et al. (2020) [66] Infants (n = 213, males and females) * VCSIP cohort | Intervention group: Current smokers (≥ 1 cigarette/day) Control group: Pregnant smokers who were given a placebo | Timeframe delivered: Randomized at ≤22 weeks gestational age to receive 500 mg/day vitamin C Assignment: Pregnant current smokers were randomly assigned to one of the following two groups: (1) vitamin C supplement (n = 125) and (2) placebo pill (n = 126) | Age at assessment: 3 and 12 months Outcomes assessed: Sustained changes in forced expiratory flows at 75%, 50%, and 25–75% volume, (FEF75, FEF50, FEF25–75) and forced expired volume (FEV0.5) Incidence of wheezing | Forced expiratory flows in the vitamin C-treated group increased by 16.1% for FEF75, 11.6% for FEF50, 12% for FEF25–75, and 6.8% for FEV0.5 when compared with the placebo group at 12 months of age; however, none of them reached significance (p > 0.05) Vitamin C produced a persistently significant increase in the offspring’s airway function at 12 months of age in sustained forced expiratory flows at 75%, 50%, and 25% expired volume; FEF75 of 40.2 mL/sec (adjusted 95% CI 6.6, 73.8; p = 0.0248); FEF50 of 58.3 mL/sec (10.9, 105.8; p = 0.0081)); FEF25–75 of 55.1 mL/sec (9.7, 100.5; 0.0130); and FEV0.5 of 16.3 mL (1.0, 31.6; p = 0.174) Vitamin C supplementation did not produce any significant effects on the incidence of wheezing at 12 months of age (p = 0.13) |
| McEvoy et al. (2023) [67] Infants (n = 213, males and females) * VCSIP cohort | Intervention group: Current smokers (≥ 1 cigarette/day) Control group: Pregnant smokers who were given a placebo | Timeframe delivered: Randomized at ≤22 weeks gestational age to receive 500 mg/day vitamin C Assignment: Pregnant current smokers were randomly assigned to one of the following two groups: (1) vitamin C supplement (n = 125) and (2) placebo pill (n = 126) | Age at assessment: 5 years Outcomes assessed: Forced expiratory flows at 25%, 50%, and 75% of expired volumes Forced expiratory volume in 1 s Incidence of wheezing | Vitamin C supplementation significantly increased the mean measurements of forced expiratory flow by 17.2% at 25–75% of expired volumes (1.45 (0.04) vs. 1.24 (0.04) L/s; adjusted mean difference, 0.21 (95% CI, 0.13- 0.30); p < 0.001) Vitamin C supplementation significantly increased mean measurements of forced expiratory flow by 14.1% at 50% of expired volumes (1.59 (0.04) vs. 1.39 (0.04) L/s; adjusted mean difference, 0.20 (95% CI, 0.11 to 0.30); p < 0.001) Vitamin C supplementation significantly increased mean measurements of forced expiratory flow by 25.9% at 75% of expired volumes (0.79 (0.02) vs. 0.63 (0.02) L/s; adjusted mean difference, 0.16 (95% CI, 0.11 to 0.22); p < 0.001) Supplemental vitamin C significantly increased by 4.4% for forced expiratory volume in 1 s (1.13 (0.02) vs. 1.09 (0.02) L; 0.05 (95% CI, 0.01–0.09); p = 0.02) Vitamin C supplementation improved forced expiratory flow at 25–75% of forced vital capacity by 18.4% (1.19 (0.03) vs. 1.001 (0.03); adjusted mean difference, 0.18 (95% CI, 0.10 to 0.26); p < 0.001) Supplemental vitamin C significantly decreased occurrence of wheezing between 4 to 6 years of age (28.3% vs. 47.2%; OR, 0.41 (95% CI, 0.23–0.74); p = 0.003) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serwatka, C.A.; Griebel-Thompson, A.K.; Eiden, R.D.; Kong, K.L. Nutrient Supplementation during the Prenatal Period in Substance-Using Mothers: A Narrative Review of the Effects on Offspring Development. Nutrients 2023, 15, 2990. https://doi.org/10.3390/nu15132990
Serwatka CA, Griebel-Thompson AK, Eiden RD, Kong KL. Nutrient Supplementation during the Prenatal Period in Substance-Using Mothers: A Narrative Review of the Effects on Offspring Development. Nutrients. 2023; 15(13):2990. https://doi.org/10.3390/nu15132990
Chicago/Turabian StyleSerwatka, Catherine A., Adrianne K. Griebel-Thompson, Rina D. Eiden, and Kai Ling Kong. 2023. "Nutrient Supplementation during the Prenatal Period in Substance-Using Mothers: A Narrative Review of the Effects on Offspring Development" Nutrients 15, no. 13: 2990. https://doi.org/10.3390/nu15132990
APA StyleSerwatka, C. A., Griebel-Thompson, A. K., Eiden, R. D., & Kong, K. L. (2023). Nutrient Supplementation during the Prenatal Period in Substance-Using Mothers: A Narrative Review of the Effects on Offspring Development. Nutrients, 15(13), 2990. https://doi.org/10.3390/nu15132990






