Relationships between Dietary Patterns and Erythropoiesis-Associated Micronutrient Deficiencies (Iron, Folate, and Vitamin B12) among Pregnant Women in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Participants
2.2. Ethics
2.3. Data Collection
2.4. Blood Biochemistry and Definition of Erythropoiesis-Related Micronutrient Deficiencies
2.5. Dietary Assessment
2.6. Statistical Analysis
3. Results
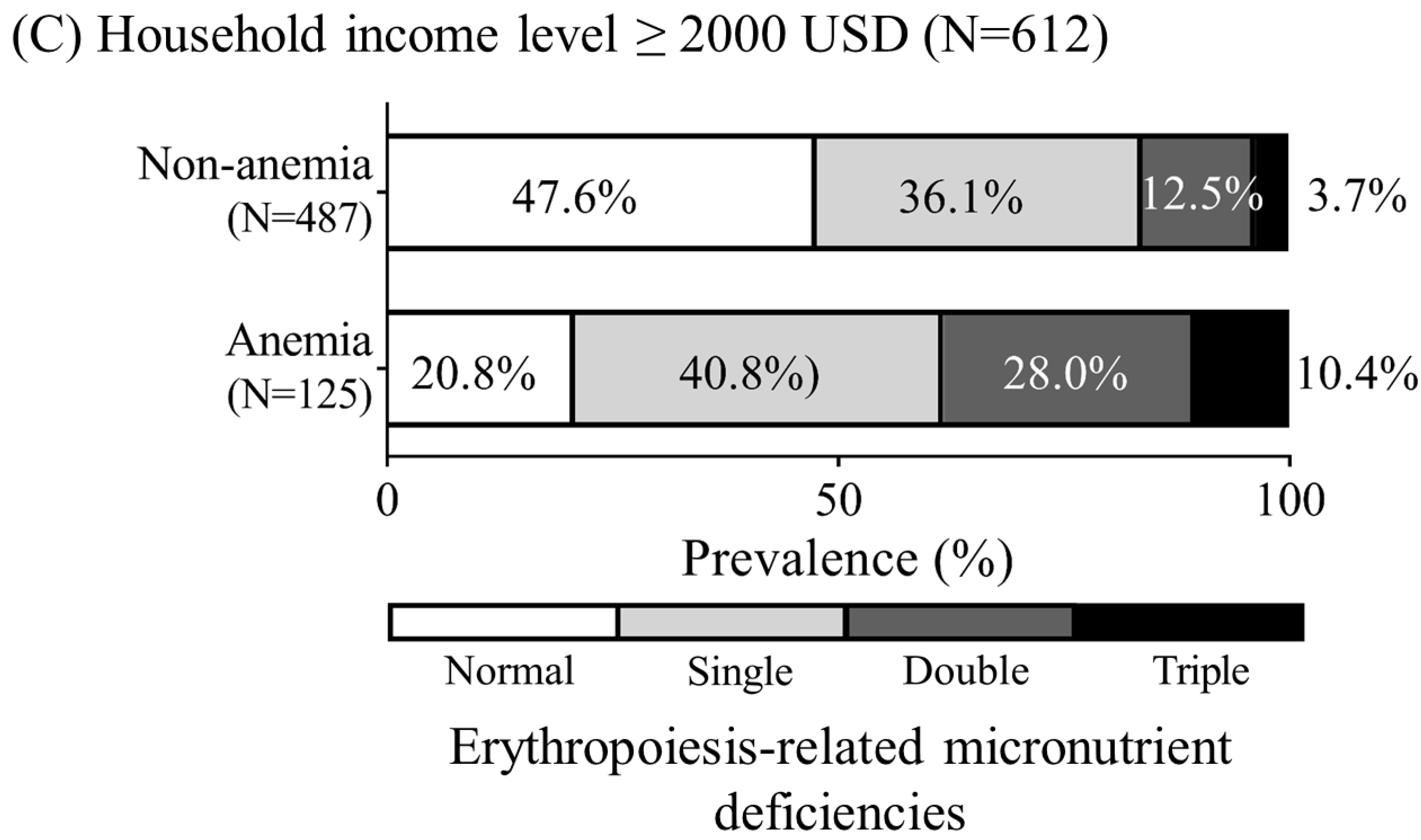
3.1. Relationships of Anemia and Household Income with Erythropoiesis-Related Micronutrient Deficiencies
3.2. Erythropoiesis-Associated Dietary Pattern Scores Using the RRR
3.3. Maternal Characteristics Stratified by Erythropoiesis-Associated Dietary Pattern Scores
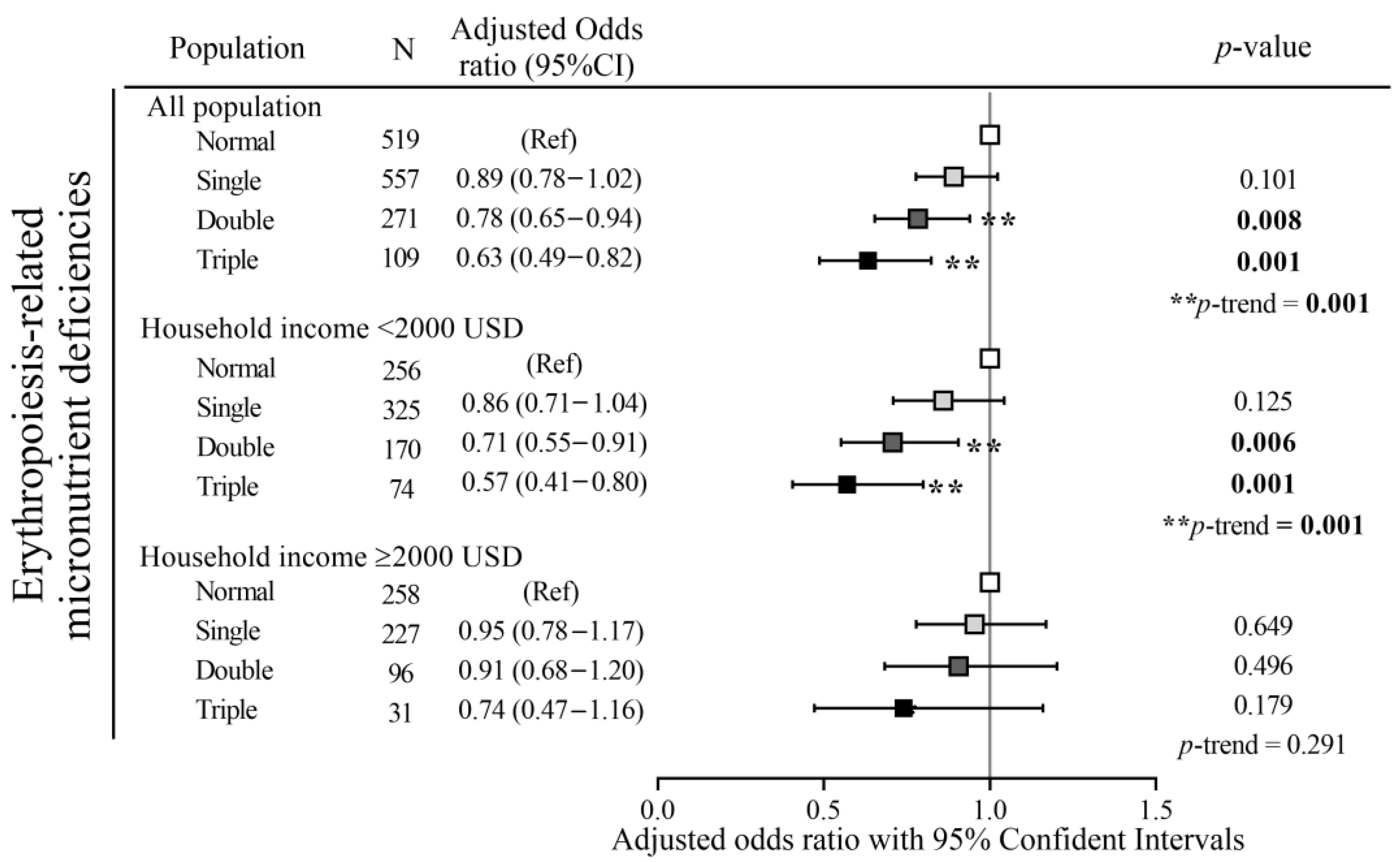
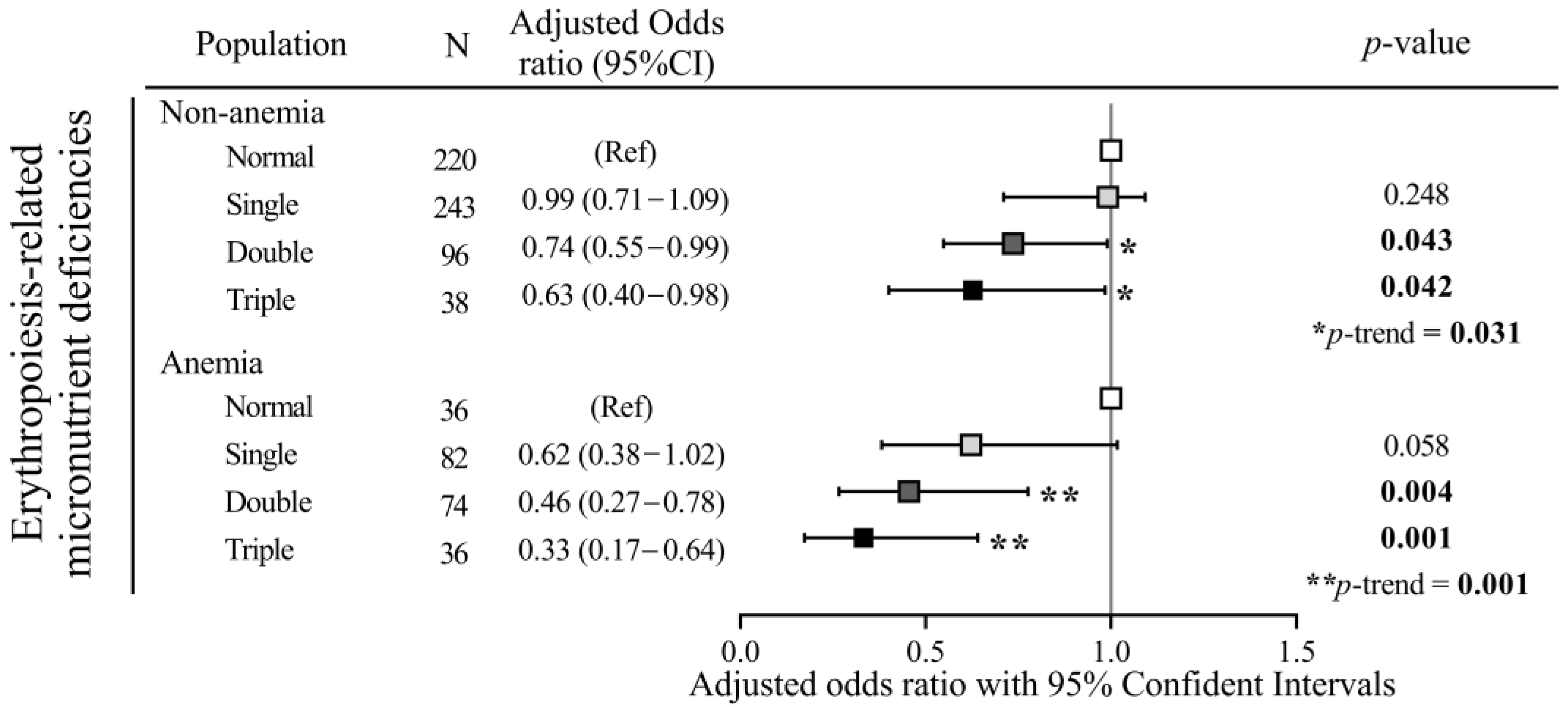
3.4. Protective Effects of Dietary Patterns on Erythropoiesis-Related Micronutrient Deficiencies in Relation to Household Income and Anemic Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization (WHO). The Global Prevalence of Anaemia in 2011; WHO: Genewa, Switzerland, 2015. [Google Scholar]
- Balarajan, Y.; Ramakrishnan, U.; Özaltin, E.; Shankar, A.H.; Subramanian, S.V. Anaemia in low-income and middle-income countries. Lancet 2011, 378, 2123–2135. [Google Scholar] [CrossRef]
- Cavill, I. Iron and erythropoiesis in normal subjects and in pregnancy. J. Perinat. Med. 1995, 23, 47–50. [Google Scholar] [CrossRef]
- Koury, M.J.; Ponka, P. NEW INSIGHTS INTO ERYTHROPOIESIS: The Roles of Folate, Vitamin B12, and Iron. Annu. Rev. Nutr. 2004, 24, 105–131. [Google Scholar] [CrossRef]
- Pathak, P.; Kapil, U.; Yajnik, C.S.; Kapoor, S.K.; Dwivedi, S.N.; Singh, R. Iron, folate, and Vitamin B12 stores among pregnant women in a rural area of Haryana State, India. Food Nutr. Bull. 2007, 28, 435–438. [Google Scholar] [CrossRef]
- Van den Broek, N. Anaemia and micronutrient deficiencies: Reducing maternal death and disability during pregnancy. Br. Med. Bull. 2003, 67, 149–160. [Google Scholar] [CrossRef]
- Mayasari, N.R.; Hu, T.-Y.; Chao, J.C.J.; Bai, C.-H.; Chen, Y.C.; Huang, Y.L.; Chang, C.-C.; Wang, F.-F.; Hadi, H.; Nurwanti, E.; et al. Associations of the pre-pregnancy weight status with anaemia and the erythropoiesis-related micronutrient status. Public Health Nutr. 2021, 24, 6247–6257. [Google Scholar] [CrossRef]
- Beckman, B.; Silberstein, P.; Aldoss, I.T. Erythropoiesis. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Smits, L.J.M.; Essed, G.G.M. Short interpregnancy intervals and unfavourable pregnancy outcome: Role of folate depletion. Lancet 2001, 358, 2074–2077. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Tomar, A.; Puri, M.; Jain, A.; Saraswathy, K.N. Trends of folate, Vitamin B12, and homocysteine levels in different trimesters of pregnancy and pregnancy outcomes. Am. J. Hum. Biol. 2020, 32, e23388. [Google Scholar] [CrossRef]
- Furness, D.; Fenech, M.; Dekker, G.; Khong, T.Y.; Roberts, C.; Hague, W. Folate, Vitamin B12, Vitamin B6 and homocysteine: Impact on pregnancy outcome. Matern. Child. Nutr. 2013, 9, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Alwan, N.A.; Hamamy, H. Maternal iron status in pregnancy and long-term health outcomes in the offspring. J. Pediatr. Genet. 2015, 4, 111–123. [Google Scholar] [PubMed]
- Hernández-Martínez, C.; Canals, J.; Aranda, N.; Ribot, B.; Escribano, J.; Arija, V. Effects of iron deficiency on neonatal behavior at different stages of pregnancy. Early Hum. Dev. 2011, 87, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Talegawkar, S.A.; Merialdi, M.; Caulfield, L.E. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr. 2013, 16, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Jouanne, M.; Oddoux, S.; Noël, A.; Voisin-Chiret, A.S. Nutrient Requirements during Pregnancy and Lactation. Nutrients 2021, 13, 692. [Google Scholar] [CrossRef]
- Karabulut, A.; Sevket, O.; Acun, A. Iron, folate and Vitamin B12 levels in first trimester pregnancies in the Southwest region of Turkey. J. Turk. Ger. Gynecol. Assoc. 2011, 12, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Young, M.F.; Griffin, I.; Pressman, E.; McIntyre, A.W.; Cooper, E.; McNanley, T.; Harris, Z.L.; Westerman, M.; O'Brien, K.O. Utilization of Iron from an Animal-Based Iron Source Is Greater Than That of Ferrous Sulfate in Pregnant and Nonpregnant Women. J. Nutr. 2010, 140, 2162–2166. [Google Scholar] [CrossRef]
- Li, Y.; Bahadur, R.; Ahuja, J.; Pehrsson, P.; Harnly, J. Macro-and micronutrients in raw plant foods: The similarities of foods and implication for dietary diversification. J. Food Compost. Anal. 2021, 102, 103993. [Google Scholar] [CrossRef]
- Mayasari, N.R.; Bai, C.-H.; Hu, T.-Y.; Chao, J.C.-J.; Chen, Y.C.; Huang, Y.L.; Wang, F.-F.; Tinkov, A.A.; Skalny, A.V.; Chang, J.-S. Associations of Food and Nutrient Intake with Serum Hepcidin and the Risk of Gestational Iron-Deficiency Anemia among Pregnant Women: A Population-Based Study. Nutrients 2021, 13, 3501. [Google Scholar] [CrossRef]
- Bowman, S. Low economic status is associated with suboptimal intakes of nutritious foods by adults in the National Health and Nutrition Examination Survey 1999–2002. Nutr. Res. 2007, 27, 515–523. [Google Scholar] [CrossRef]
- Novaković, R.; Cavelaars, A.; Geelen, A.; Nikolić, M.; Altaba, I.I.; Viñas, B.R.; Ngo, J.; Golsorkhi, M.; Medina, M.W.; Brzozowska, A.; et al. Socio-economic determinants of micronutrient intake and status in Europe: A systematic review. Public Health Nutr. 2014, 17, 1031–1045. [Google Scholar] [CrossRef]
- Torheim, L.E.; Ferguson, E.L.; Penrose, K.; Arimond, M. Women in Resource-Poor Settings Are at Risk of Inadequate Intakes of Multiple Micronutrients. J. Nutr. 2010, 140, 2051S–2058S. [Google Scholar] [CrossRef]
- de Castro, M.B.T.; Freitas Vilela, A.A.; Oliveira, A.S.D.d.; Cabral, M.; Souza, R.A.G.d.; Kac, G.; Sichieri, R. Sociodemographic characteristics determine dietary pattern adherence during pregnancy. Public Health Nutr. 2016, 19, 1245–1251. [Google Scholar] [CrossRef]
- Gao, H.; Stiller, C.K.; Scherbaum, V.; Biesalski, H.K.; Wang, Q.; Hormann, E.; Bellows, A.C. Dietary Intake and Food Habits of Pregnant Women Residing in Urban and Rural Areas of Deyang City, Sichuan Province, China. Nutrients 2013, 5, 2933–2954. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, D.; Mao, X.; Xia, Y.; Baker, P.N.; Zhang, H. Maternal Dietary Patterns and Pregnancy Outcome. Nutrients 2016, 8, 351. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Gao, Q.; Zhao, H.; Chen, S.; Huang, L.; Wang, W.; Wang, T. A review of statistical methods for dietary pattern analysis. Nutr. J. 2021, 20, 37. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S, discussion 1229S–1231S. [Google Scholar] [CrossRef]
- Bird, J.K.; Murphy, R.A.; Ciappio, E.D.; McBurney, M.I. Risk of Deficiency in Multiple Concurrent Micronutrients in Children and Adults in the United States. Nutrients 2017, 9, 655. [Google Scholar] [CrossRef]
- Marshall, N.E.; Abrams, B.; Barbour, L.A.; Catalano, P.; Christian, P.; Friedman, J.E.; Hay, W.W., Jr.; Hernandez, T.L.; Krebs, N.F.; Oken, E.; et al. The importance of nutrition in pregnancy and lactation: Lifelong consequences. Am. J. Obstet. Gynecol. 2022, 226, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.J.; Pan, W.H.; Shaw, N.S.; Huang, R.F.; Lin, B.F. Association between dietary folate-rich food intake and folate status of elderly Taiwanese. Asia Pac. J. Clin. Nutr. 2005, 14, 244–249. [Google Scholar] [PubMed]
- Gille, D.; Schmid, A. Vitamin B12 in meat and dairy products. Nutr. Rev. 2015, 73, 106–115. [Google Scholar] [CrossRef]
- Shiraseb, F.; Hosseininasab, D.; Mirzababaei, A.; Bagheri, R.; Wong, A.; Suzuki, K.; Mirzaei, K. Red, white, and processed meat consumption related to inflammatory and metabolic biomarkers among overweight and obese women. Front. Nutr. 2022, 9, 1015566. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Köse, S.; Sözlü, S.; Bölükbaşi, H.; Ünsal, N.; Gezmen-Karadağ, M. Obesity is associated with folate metabolism. Int. J. Vitam. Nutr. Res. 2020, 90, 353–364. [Google Scholar] [CrossRef]
- Abdul-Fatah, B.N.; Murshid, R.M.; Ahmed, T.E. Assessment of iron deficiency anemia (IDA) and dietary pattern among pregnant women in Baghdad city, Iraq. J. Pharm. Sci. Res. 2018, 10, 2279–2284. [Google Scholar]
- Ghose, B.; Yaya, S. Fruit and vegetable consumption and anemia among adult non-pregnant women: Ghana demographic and health survey. PeerJ 2018, 6, e4414. [Google Scholar] [CrossRef]
- Paramastri, R.; Hsu, C.Y.; Lee, H.A.; Lin, L.Y.; Kurniawan, A.L.; Chao, J.C. Association between Dietary Pattern, Lifestyle, Anthropometric Status, and Anemia-Related Biomarkers among Adults: A Population-Based Study from 2001 to 2015. Int. J. Environ. Res. Public Health 2021, 18, 3438. [Google Scholar] [CrossRef]
- Kurniawan, A.L.; Hsu, C.Y.; Rau, H.H.; Lin, L.Y.; Chao, J.C. Inflammatory Dietary Pattern Predicts Dyslipidemia and Anemia in Middle-Aged and Older Taiwanese Adults with Declined Kidney Function: A Cross-Sectional Population Study from 2008 to 2010. Nutrients 2019, 11, 2052. [Google Scholar] [CrossRef]
- Robinson, S.; Godfrey, K.; Osmond, C.; Cox, V.; Barker, D. Evaluation of a food frequency questionnaire used to assess nutrient intakes in pregnant women. Eur. J. Clin. Nutr. 1996, 50, 302–308. [Google Scholar] [PubMed]
- Crozier, S.R.; Inskip, H.M.; Godfrey, K.M.; Robinson, S.M. Dietary patterns in pregnant women: A comparison of food-frequency questionnaires and 4 d prospective diaries. Br. J. Nutr. 2008, 99, 869–875. [Google Scholar] [CrossRef]
- Thompson, W.G.; Meola, T.; Lipkin, M., Jr.; Freedman, M.L. Red Cell Distribution Width, Mean Corpuscular Volume, and Transferrin Saturation in the Diagnosis of Iron Deficiency. Arch. Intern. Med. 1988, 148, 2128–2130. [Google Scholar] [CrossRef]

| Explained Variation (%) | Factor Loading * | |
|---|---|---|
| Food groups | ||
| Positive association | ||
| 29.33 | 0.48 |
| 23.22 | 0.43 |
| 12.64 | 0.31 |
| 9.50 | 0.27 |
| 7.28 | 0.24 |
| 6.57 | 0.23 |
| Negative association | ||
| 10.57 | −0.29 |
| 5.02 | −0.20 |
| Total explained variation | 104.13 |
| Variable | Dietary Pattern Scores # | p for Trend | ||
|---|---|---|---|---|
| T1 (N = 479) | T2 (N = 479) | T3 (N = 479) | ||
| Basic characteristic | ||||
| Age (years) | 31.63 ± 4.93 | 32.54 ± 4.56 | 33.51 ± 4.38 | <0.001 |
| pBMI (kg/m2) | 22.87 ± 4.38 | 22.78 ± 4.14 | 22.25 ± 3.35 | 0.016 |
| Underweight (n, %) | 51 (10.7) | 44 (9.2) | 45 (9.4) | 0.129 |
| Normal weight (n, %) | 282 (59.2) | 284 (59.5) | 312 (65.3) | |
| Overweight (n, %) | 73 (15.3) | 77 (16.1) | 73 (15.3) | |
| Obesity (n, %) | 70 (14.7) | 72 (15.1) | 48 (10.0) | |
| Trimester | ||||
| First (n, %) | 129 (26.9) | 118 (24.6) | 111 (23.2) | 0.198 |
| Second (n, %) | 151 (31.5) | 157 (32.8) | 155 (32.4) | |
| Third (n, %) | 199 (41.5) | 204 (42.6) | 213 (44.5) | |
| Educational level | ||||
| Less than undergraduate (n, %) | 114 (23.9) | 67 (14.0) | 35 (7.3) | <0.001 |
| Undergraduate or above (n, %) | 362 (76.1) | 410 (86.0) | 443 (92.7) | |
| Household income level (USD) | ||||
| <2000 (n, %) | 333 (69.5) | 260 (54.3) | 232 (48.4) | <0.001 |
| ≥2000 (n, %) | 146 (30.5) | 219 (45.7) | 247 (51.6) | |
| Blood biomarkers | ||||
| Serum hepcidin (ng/mL) | 23.03 ± 32.56 | 24.84 ± 32.43 | 24.21 ± 32.14 | 0.573 |
| Serum iron (µg/dL) | 67.23 ± 37.13 | 75.08 ± 43.06 | 74.37 ± 39.37 | 0.006 |
| Log TS (%) | 15.48 ± 9.61 | 17.39 ± 10.38 | 16.84 ± 9.87 | 0.034 |
| Hemoglobin (g/dL) | 11.69 ± 2.01 | 11.82 ± 2.07 | 11.72 ± 1.74 | 0.813 |
| Serum ferritin (ng/mL) | 22.92 ± 27.99 | 24.86 ± 26.32 | 21.96 ± 22.07 | 0.564 |
| Folic acid (ng/mL) | 10.92 ± 6.84 | 13.07 ± 7.42 | 14.19 ± 6.98 | <0.001 |
| Log vit. B12 (pg/mL) | 309.59 ± 242.63 | 316.39 ± 207.02 | 316.95 ± 138.02 | 0.031 |
| Erythropoiesis-related nutritional deficiencies | ||||
| Normal (n, %) | 139 (29.0) | 174 (36.3) | 201 (42.0) | <0.001 |
| Single (n, %) | 178 (37.2) | 198 (41.3) | 176 (36.7) | |
| Double (n, %) | 103 (21.5) | 78 (16.3) | 85 (17.7) | |
| Triple (n, %) | 59 (12.3) | 29 (6.1) | 17 (3.5) | |
| Reported use of prenatal dietary supplements | ||||
| Total supplement use (n, %) | 364 (77.4) | 409 (86.1) | 427 (90.1) | <0.001 |
| Multivitamin/mineral (n, %) | 250 (53.2) | 311 (65.8) | 325 (69.1) | <0.001 |
| Vit. B (n, %) | 88 (18.8) | 84 (18.1) | 87 (18.4) | 0.872 |
| Folate (n, %) | 217 (46.0) | 221 (47.3) | 214 (45.6) | 0.860 |
| Iron (n, %) | 46 (9.8) | 42 (9.0) | 63 (13.4) | 0.073 |
| Adjusted nutrients intake status | ||||
| Carbohydrates (g/day) | 124.15 ± 25.14 | 124.67 ± 24.56 | 126.29 ± 23.28 | 0.174 |
| Protein (g/day) | 37.62 ± 9.61 | 38.10 ± 8.88 | 39.03 ± 9.47 | 0.019 |
| Fat (g/day) | 40.27 ± 9.98 | 40.01 ± 10.21 | 39.09 ± 9.49 | 0.065 |
| Log dietary Fe (mg/day) | 5.75 ± 4.54 | 5.99 ± 3.83 | 6.02 ± 2.60 | 0.001 |
| Log dietary folate (mg/day) | 113.27 ± 69.05 | 117.65 ± 53.71 | 119.77 ± 48.03 | 0.006 |
| Log dietary vit. B12 (µg/day) | 2.80 ± 5.12 | 2.99 ± 4.49 | 3.41 ± 7.02 | 0.02 |
| Log dietary vit. C (mg/day) | 54.00 ± 66.04 | 63.56 ± 67.75 | 78.09 ± 83.41 | <0.001 |
| Log dietary vit. A (µg RE/day) | 404.17 ± 805.96 | 436.52 ± 692.95 | 508.50 ± 577.59 | <0.001 |
| Under RDA of protein (n, %) | 195 (41.0) | 199 (41.6) | 158 (33.0) | 0.011 |
| Under RDA of Fe (n, %) | 441 (92.6) | 433 (90.6) | 440 (91.9) | 0.660 |
| Under RDA of folate (n, %) | 459 (96.4) | 457 (95.6) | 459 (95.8) | 0.637 |
| Under RDA of vit. B12 (n, %) | 231 (48.5) | 209 (43.7) | 186 (38.8) | 0.003 |
| Under RDA of vit. C (n, %) | 356 (74.8) | 317 (66.3) | 252 (52.6) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayasari, N.R.; Bai, C.-H.; Chao, J.C.-J.; Chen, Y.-C.; Huang, Y.-L.; Wang, F.-F.; Wiratama, B.S.; Chang, J.-S. Relationships between Dietary Patterns and Erythropoiesis-Associated Micronutrient Deficiencies (Iron, Folate, and Vitamin B12) among Pregnant Women in Taiwan. Nutrients 2023, 15, 2311. https://doi.org/10.3390/nu15102311
Mayasari NR, Bai C-H, Chao JC-J, Chen Y-C, Huang Y-L, Wang F-F, Wiratama BS, Chang J-S. Relationships between Dietary Patterns and Erythropoiesis-Associated Micronutrient Deficiencies (Iron, Folate, and Vitamin B12) among Pregnant Women in Taiwan. Nutrients. 2023; 15(10):2311. https://doi.org/10.3390/nu15102311
Chicago/Turabian StyleMayasari, Noor Rohmah, Chyi-Huey Bai, Jane C.-J. Chao, Yi-Chun Chen, Ya-Li Huang, Fan-Fen Wang, Bayu Satria Wiratama, and Jung-Su Chang. 2023. "Relationships between Dietary Patterns and Erythropoiesis-Associated Micronutrient Deficiencies (Iron, Folate, and Vitamin B12) among Pregnant Women in Taiwan" Nutrients 15, no. 10: 2311. https://doi.org/10.3390/nu15102311
APA StyleMayasari, N. R., Bai, C.-H., Chao, J. C.-J., Chen, Y.-C., Huang, Y.-L., Wang, F.-F., Wiratama, B. S., & Chang, J.-S. (2023). Relationships between Dietary Patterns and Erythropoiesis-Associated Micronutrient Deficiencies (Iron, Folate, and Vitamin B12) among Pregnant Women in Taiwan. Nutrients, 15(10), 2311. https://doi.org/10.3390/nu15102311







