Dietary Vitamin D Intake in Italian Subjects: Validation of a Frequency Food Questionnaire (FFQ)
Abstract
1. Introduction
2. Materials and Methods
2.1. Frequency Food Questionnaire (FFQ)
2.2. Frequency Food Diary (FFD)
2.3. Statistical Analysis
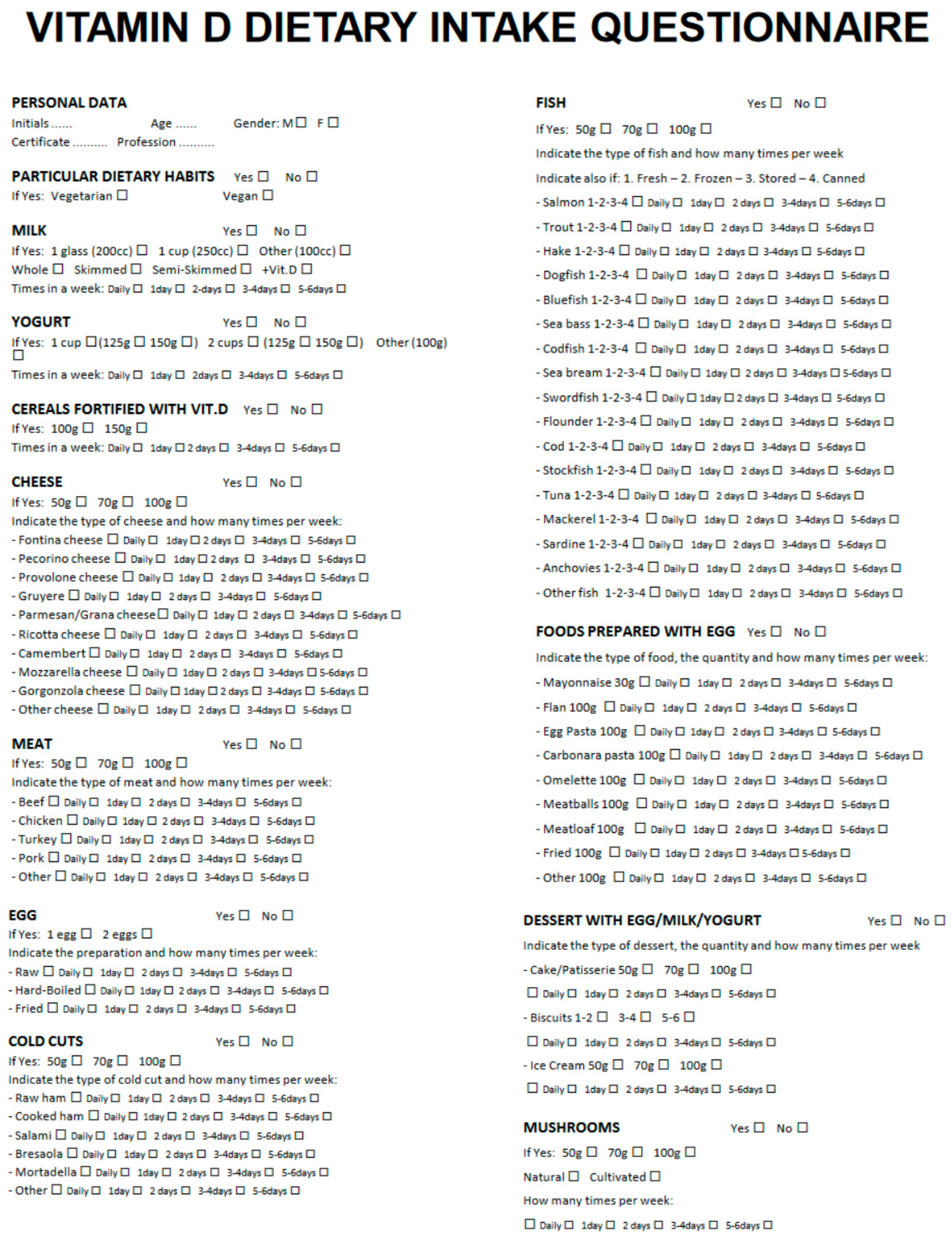
3. Results
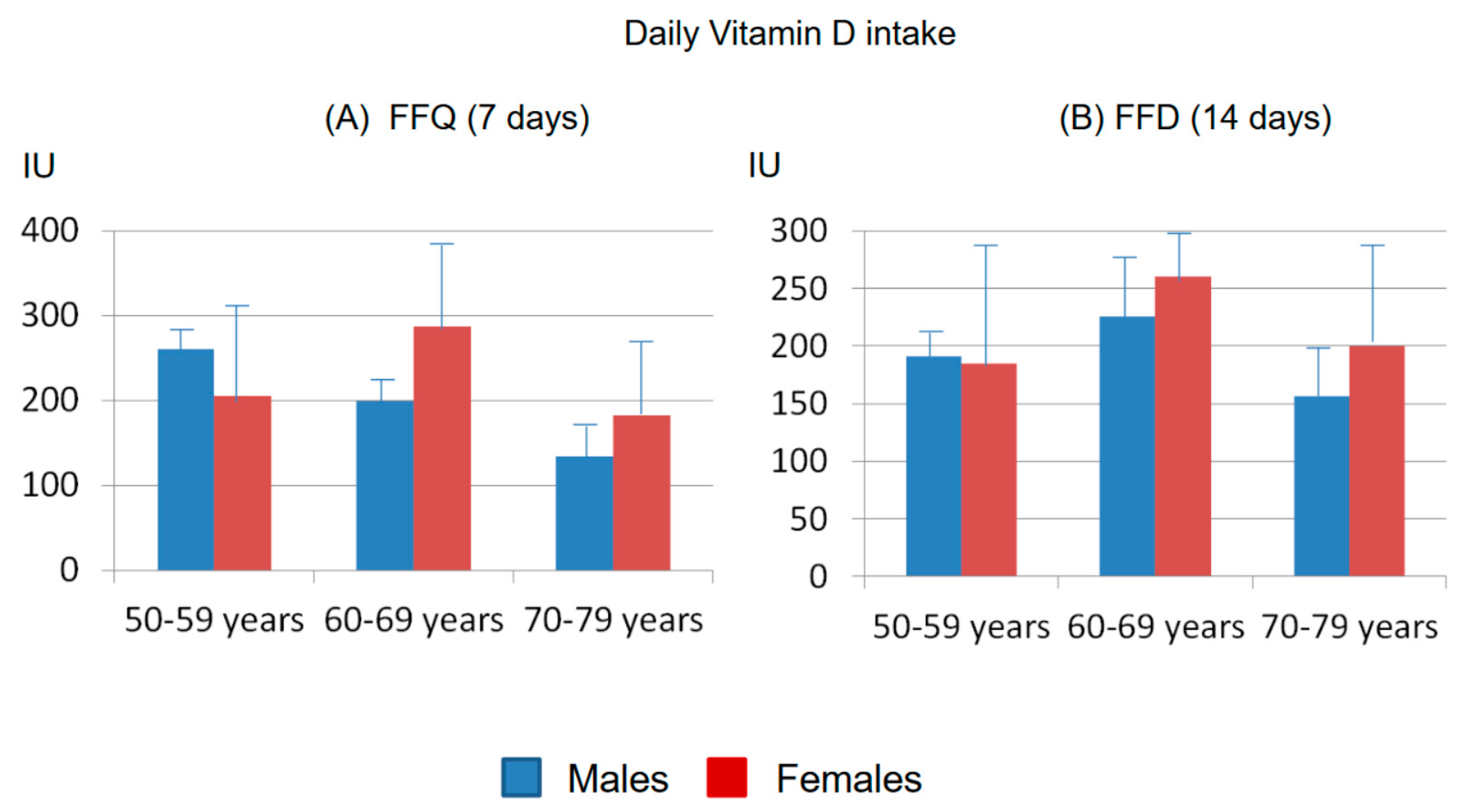
3.1. Calculation of Vitamin D Intake with the FFQ
3.2. Calculation of Vitamin D Intake with the FFD
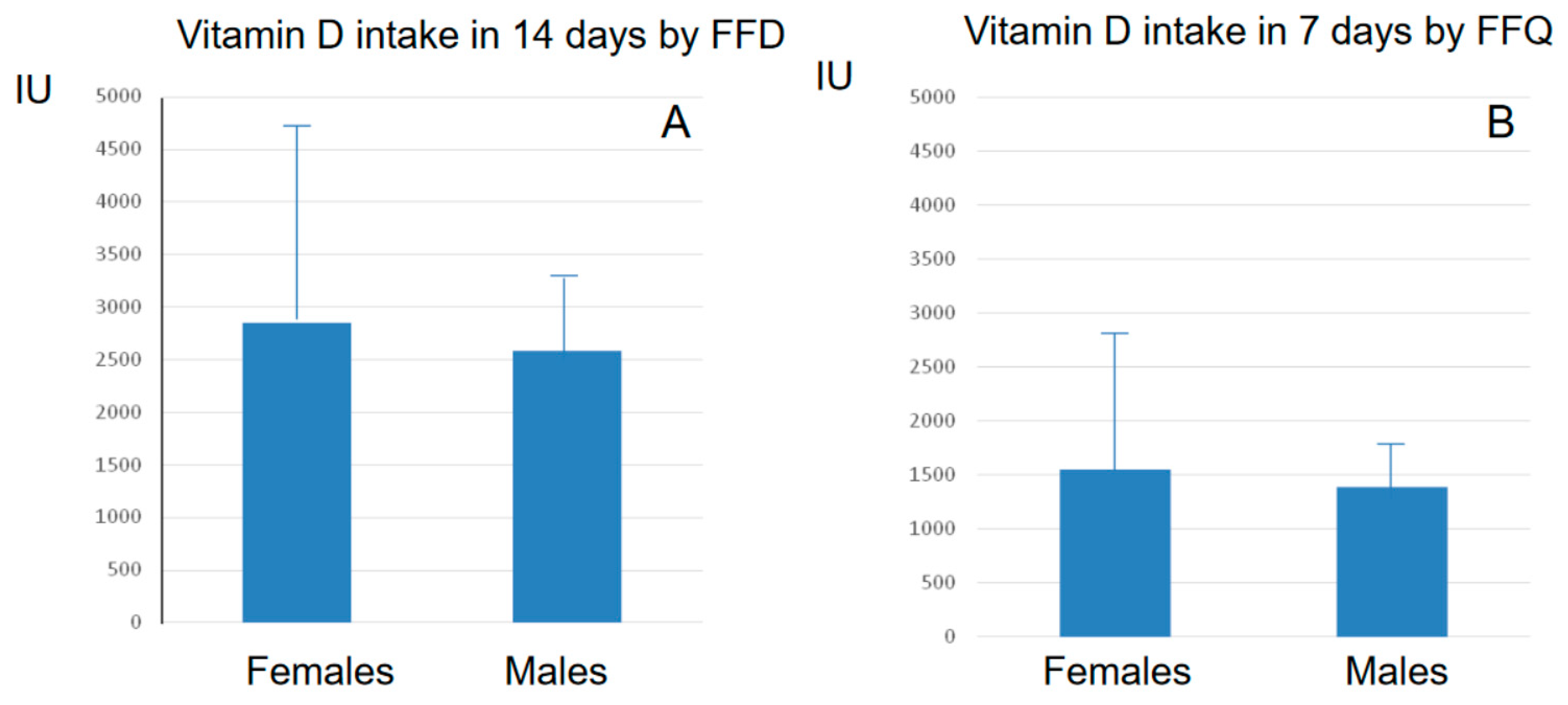
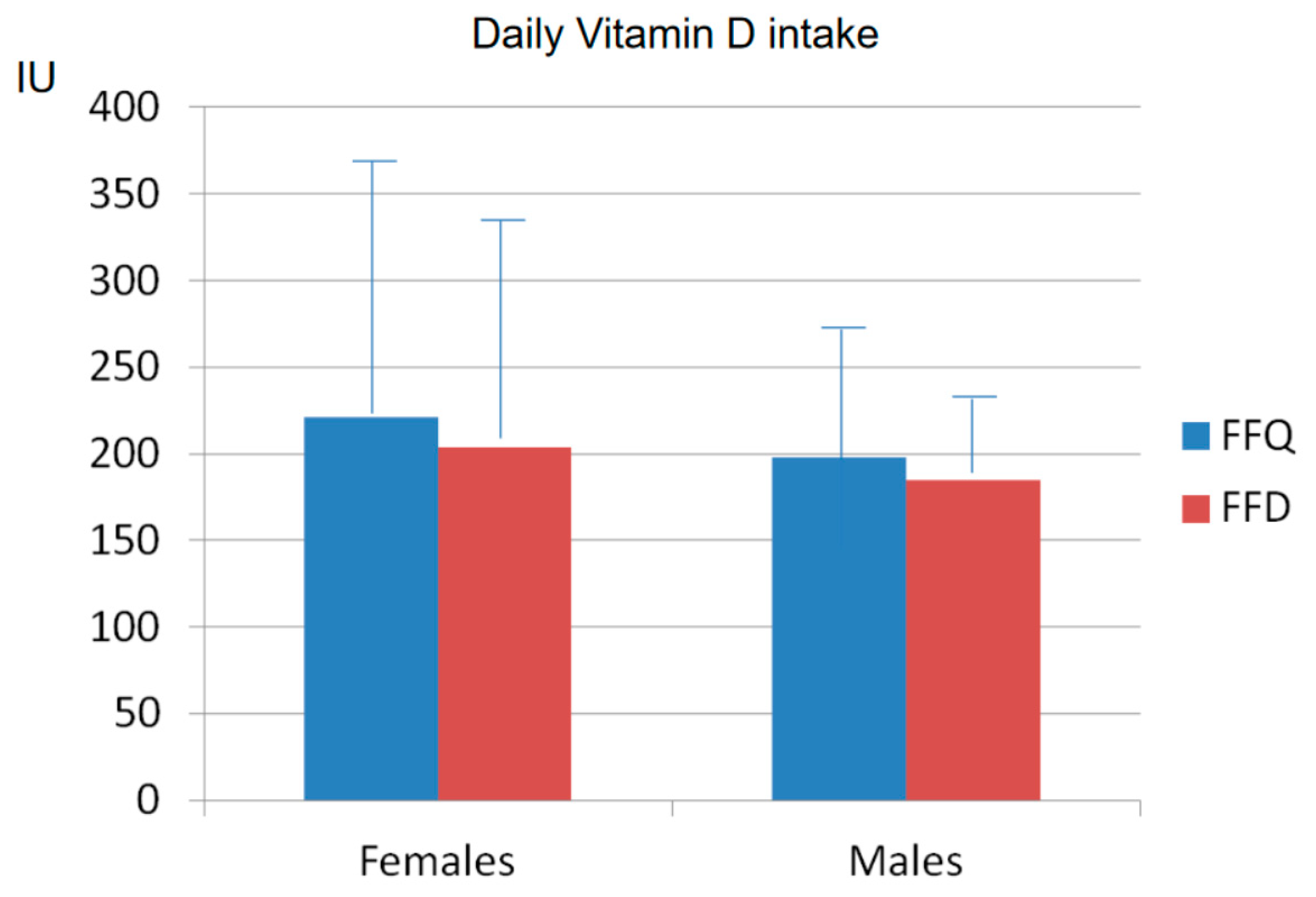
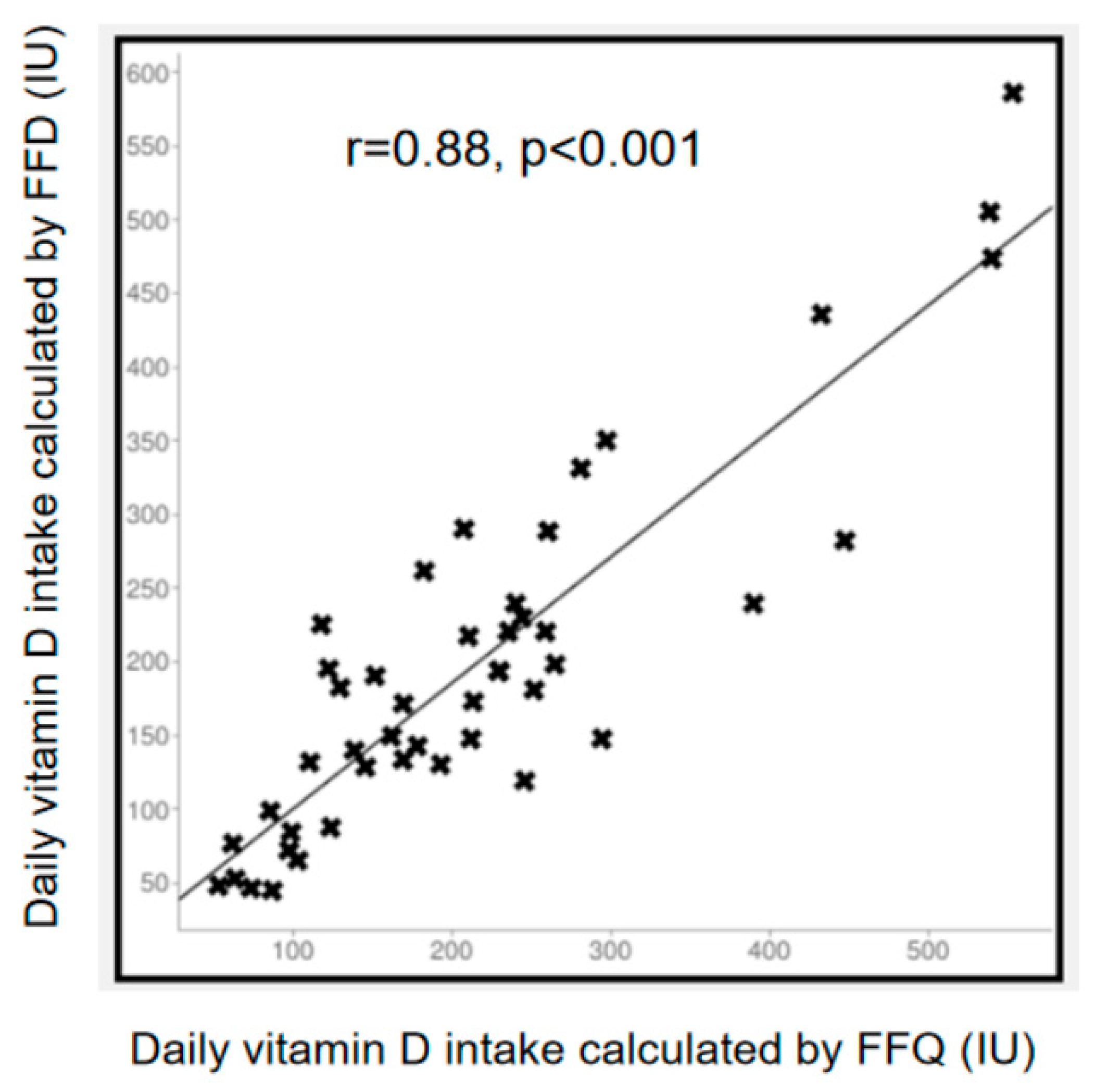
3.3. Comparison between FFQ and FFD
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouillon, R.; Carmeliet, G. Vitamin D insufficiency: Definition, diagnosis and management. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 669–684. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and vitamin D: A global perspective for health. Dermato-Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar] [CrossRef]
- Thomas, K.K.; Lloyd-Jones, D.H.; Thadhani, R.I.; Shaw, A.C.; Deraska, D.J.; Kitch, B.T.; Vamvakas, E.C.; Dick, I.M.; Prince, R.L.; Finkelstein, J.S. Hypovitaminosis D in medical inpatients. N. Engl. J. Med. 1998, 338, 777–783. [Google Scholar] [CrossRef]
- Bikle, D.D.; Adams, J.S.; Christakos, S. Vitamin D: Production, Metabolism, Action, and Clinical Requirements. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 9th ed.; Bilezikian John, P., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019. [Google Scholar]
- Thacher, T.D.; Levine, M.A. CYP2R1 mutations causing vitamin D deficiency rickets. J. Steroid Biochem. Mol. Biol. 2017, 173, 333–336. [Google Scholar] [CrossRef]
- Lips, P. Relative value of 25(OH)D and 1,25(OH)2D measurements. J. Bone Miner. Res. 2007, 22, 1668–1671. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Looker, A.C.; Johnson, C.L.; Lachner, D.A.; Pfeiffer, C.M.; Schleicher, R.L.; Sempos, C.T. Vitamin D status: United States, 2001–2006. NCHS Data Brief. 2011, 56, 1–8. [Google Scholar]
- Holick, M.F. Biologic effects of sunlight, ultraviolet radiation, visible light, infrared, and vitamin D for health. Anticancer Res. 2016, 36, 1345–1356. [Google Scholar]
- Rizzoli, R.; Biver, E.; Brennan-Speranza, T.C. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021, 9, 606–621. [Google Scholar] [CrossRef]
- Navarro, D.F.; García-Franco, A.L.; de Guzmán, E.N.; Rabassa, M.; Campos, R.Z.; Pardo-Hernández, H.; Ricci-Cabello, I.; Canelo-Aybar, C.; Meneses-Echavez, J.F.; Yepes-Nuñez, J.J.; et al. Vitamin D recommendations in clinical guidelines: A systematic review, quality evaluation and analysis of potential predictors. Int. J. Clin. Pract. 2021, 75, e14805. [Google Scholar] [CrossRef]
- McKenna, M.J. Differences in vitamin D status between countries in young adults and the elderly. Am. J. Med. 1992, 93, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Environmental factors that influence the cutaneous production of vitamin D. Am. J. Clin. Nutr. 1995, 61, 638S–645S. [Google Scholar] [CrossRef]
- Takeuchi, A.; Okano, T.; Tanda, M.; Kobayashi, T. Possible origin of extremely high contents of vitamin D3 in some kinds of fish liver. Comp. Biochem. Physiol. 1991, 100, 483–487. [Google Scholar] [CrossRef]
- Sunita Rao, D.; Raghuramulu, N. Food chain as origin of vitamin D in fish. Comp. Biochem. Physiol. A 1996, 114, 15–19. [Google Scholar] [CrossRef]
- Roseland, J.M.; Phillips, K.M.; Patterson, K.Y.; Pehrsson, P.R.; Taylor, C.L. Vitamin D in foods: An evolution of knowledge. In Vitamin D, Volume 2: Health, Disease and Therapeutics, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 41–78. ISBN 9780128099636. [Google Scholar]
- De Giuseppe, R.; Tomasinelli, C.E.; Cena, H.; Braschi, V.; Giampieri, F.; Preatoni, G.; Centofanti, D.; Princis, M.P.; Bartoletti, E.; Biino, G. Development of a Short Questionnaire for the Screening for Vitamin D Deficiency in Italian Adults: The EVIDENCe-Q Project. Nutrients 2022, 14, 1772. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, K.O.; Giambalvo, L.; Leclerc, H.L.; Cook, R.A.; Rosen, C.J. Validation of a quantitative food frequency questionnaire for rapid assessment of dietary calcium intake. J. Am. Die. Assoc. 1989, 89, 1484–1488. [Google Scholar] [CrossRef]
- Xu, L.; Porteous, J.E.; Philips, M.R.; Zheng, S. Development and validation of a calcium intake questionnaire in postmenopausal women in China. Ann. Epidemiol. 2000, 10, 169–175. [Google Scholar] [CrossRef]
- Nelson, M.; Hague, G.F.; Cooper, C.; Bunker, V.W. Calcium intake in the elderly: Validation of a dietary questionnaire. J. Hum. Nutr. Diet. 1988, 1, 115–127. [Google Scholar] [CrossRef]
- Taitano, R.T.; Novotny, R.; Davis, J.W.; Ross, P.D.; Wasnich, R.D. Validity of a food frequency questionnaire for estimating calcium intake among Japanese and white women. J. Am. Diet. Assoc. 1995, 95, 804–806. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture’s (USDA’s) FoodData Central Lists the Nutrient Content of Many Foods and Provides a Comprehensive List of Foods Containing Vitamin D Arranged by Nutrient Content. Available online: https://ods.od.nih.gov/pubs/usdandb/VitaminD-Content.pdf (accessed on 27 June 2023).
- CREA. Available online: https://www.crea.gov.it/web/alimenti-e-nutrizione/banche-dati (accessed on 27 June 2023).
- Nowson, C.A.; Margerison, C. Vitamin D intake and vitamin D status of Australians. Med. J. Aust. 2002, 177, 149–152. [Google Scholar] [CrossRef]
- Gibson, S.A.; Ashwell, M. New vitamin D values for meat and their implication for vitamin D intake in British adults. Proc. Nutr. Soc. 1997, 56, 116A. [Google Scholar]
- Moore, C.; Murphy, M.M.; Keast, D.R.; Holick, M.F. Vitamin D intake in the United States. J. Am. Diet. Assoc. 2004, 104, 980–983. [Google Scholar] [CrossRef]
- Herrick, K.A.; Storandt, R.J.; Afful, J.; Pfeiffer, C.M.; Schleicher, R.L.; Gahche, J.J.; Potischman, N. Vitamin D status in the United States, 2011–2014. Am. J. Clin. Nutr. 2019, 110, 150–157. [Google Scholar] [CrossRef]
- Ahmed, M.; Ng, A.; L’Abbe, M.R. Nutrient intakes of Canadian adults: Results from the Canadian Community Health Survey (CCHS)-2015 public use microdata file. Am. J. Clin. Nutr. 2021, 114, 1131–1140. [Google Scholar] [CrossRef]
- Page, P.; Steer, T.; Bates, B.; Cox, L.J.; Nicolson, S.; Prentice, A.; Swan, G. National Diet and Nutrition Survey Results from Years 5 and 6 (Combined) of the Rolling Programme (2012/2013–2013/2014). A Survey Carried Out on Behalf of Public Health England and the Food Standards Agency. 2016; pp. 1–29. Available online: https://www.gov.uk/government/statistics/ndns-results-from-years-5-and-6-combined (accessed on 27 June 2023).
- Scientific Advisory Committee on Nutrition. Report on Vitamin D and Health. 2016. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/537616/SACN_Vitamin_D_and_Health_report.pdf (accessed on 27 June 2023).
- Cashman, K.D. Global differences in vitamin D status and dietary intake: A review of the data. Endocr. Connect. 2022, 11, e210282. [Google Scholar] [CrossRef]
- Rippin, H.L.; Hutchinson, J.; Evans, C.E.L.; Jewell, J.; Breda, J.J.; Cade, J.E. National nutrition surveys in Europe: A review on the current status in the 53 countries of the WHO European region. Food Nutr. Res. 2018, 16, 62. [Google Scholar] [CrossRef]
- Turrini, A.; Saba, A.; Perrone, D.; Cialfa, E.; D’Amicis, A. Food consumption patterns in Italy: The INN-CA Study 1994–1996. Eur. J. Clin. Nutr. 2001, 55, 571–588. [Google Scholar] [CrossRef]
- Scalfi, L.; Brighenti, F.; Bordoni, A.; Biagi, P.; Rossi, L.; Maiani, G.; Avigliano, L.; Strazzullo, P.; Rotilio, G.; Rossi, L.; et al. LARN, Livelli di Assunzione di Riferimento di Nutrienti ed Energia per la Popolazione Italiana; IV revision; Coordinamento editoriale SINU-INRAN; SICS: Milano, Italy, 2014. [Google Scholar]
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; Le Donne, C.; Turrini, A.; On Behalf of the INRAN-SCAI 2005-06 Study Group. The Italian National Food Consumption Survey INRAN-SCAI 2005-06: Main results in terms of food consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef]
- Leclercq, C.; Piccinelli, R.; Arcella, D.; Le Donne, C. Food consumption and nutrient intake in a sample of Italian secondary school students. Results from the INRANRM-2001 food survey. Int. J. Food Sci. Nutr. 2004, 55, 265–277. [Google Scholar] [CrossRef]
- Rendina, D.; De Filippo, G.; Merlotti, D.; Di Stefano, M.; Succoio, M.; Muggianu, S.M.; Bianciardi, S.; D’Elia, L.; Coppo, E.; Faraonio, R.; et al. Vitamin D Status in Paget Disease of Bone Efficacy-Safety Profile of Cholecalciferol Treatment in Pagetic Patients with Hypovitaminosis D. Calcif. Tissue Int. 2019, 10, 412–422. [Google Scholar] [CrossRef]
- Cesareo, R.; Attanasio, R.; Caputo, M.; Castello, R.; Chiodini, I.; Falchetti, A.; Guglielmi, R.; Papini, E.; Santonati, A.; Scillitani, A.; et al. Italian Association of Clinical Endocrinologists (AME) and Italian Chapter of the American Association of Clinical Endocrinologists (AACE) Position Statement: Clinical management of Vitamin D deficiency in adults. Nutrients 2018, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, F.; Cianferotti, L.; Di Monaco, M.; Falchetti, A.; Fassio, A.; Gatti, D.; Gennari, L.; Giannini, S.; Girasole, G.; Gonnelli, S.; et al. Definition, Assessment, and Management of Vitamin D Inadequacy: Suggestions, Recommendations, and Warnings from the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS). Nutrients 2022, 14, 4148. [Google Scholar] [CrossRef] [PubMed]
- Foroni, M.Z.; Cendoroglo, M.S.; Sakane, E.N.; Marin-Mio, R.V.; Moreira, P.F.D.P.; Maeda, S.S.; Lazaretti-Castro, M. Serum 25 hydroxyvitamin D concentrations in individuals over 80 years old and their correlations with musculoskeletal and health parameters. Endocrine 2023, 79, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Adami, S.; Giannini, S.; Bianchi, G.; Sinigaglia, L.; Di Munno, O.; Fiore, C.E.; Minisola, S.; Rossini, M. Vitamin D status and response to treatment in post-menopausal osteoporosis. Osteoporos. Int. 2009, 20, 239–244. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Pittas, A.G.; Del Gobbo, L.C.; Zhang, C.; Manson, J.E.; Hu, F.B. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care. 2013, 36, 1422–1428. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diab. Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Pittas, A.G.; Kawahara, T.; Jorde, R.; Dawson-Hughes, B.; Vickery, E.M.; Angellotti, E.; Nelson, J.; Trikalinos, T.A.; Balk, E.M. Vitamin D and Risk for Type 2 Diabetes in People With Prediabetes: A Systematic Review and Meta-analysis of Individual Participant Data From 3 Randomized Clinical Trials. Ann. Intern. Med. 2023, 176, 355–363. [Google Scholar] [CrossRef]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Panagiotakos, D.B.; Chrysohoou, C.; Yannakoulia, M.; Georgousopoulou, E.N.; Tousoulis, D.; Pitsavos, C. ATTICA study Investigators. Dietary vitamin D intake, cardiovascular disease and cardiometabolic risk factors: A sex-based analysis from the ATTICA cohort study. J. Hum. Nutr. Diet. 2020, 33, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, W.; Hu, P.; Zhang, R.; Dong, X.; Zhang, D. Association of Dietary Vitamin D Intake, Serum 25(OH)D3, 25(OH)D2 with Cognitive Performance in the Elderly. Nutrients 2021, 13, 3089. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.R.; Tripkovic, L.; Hart, K.H.; Lanham-New, S.A. Vitamin D deficiency as a public health issue: Using vitamin D2 or vitamin D3 in future fortification strategies. Proc. Nutr. Soc. 2017, 76, 392–399. [Google Scholar] [CrossRef]
- Lamberg-Allardt, C. Vitamin D in foods and as supplements. Biophys. Mol. Biol. 2006, 92, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Chevalley, T.; Brandi, M.L.; Cashman, K.D.; Cavalier, E.; Harvey, N.C.; Maggi, S.; Cooper, C.; Al-Daghri, N.; Bock, O.; Bruyère, O.; et al. Role of vitamin D supplementation in the management of musculoskeletal diseases: Update from an European Society of Clinical and Economical Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) working group. Aging Clin. Exp. Res. 2022, 34, 2603–2623. [Google Scholar] [CrossRef]
- Liu, J. Vitamin D content of food and its contribution to vitamin D status: A brief overview and Australian focus. Photochem. Photobiol. Sci. 2012, 11, 1802–1807. [Google Scholar] [CrossRef]
- Holden, J.M.; Lemar, L.E. Assessing vitamin D contents in foods and supplements: Challenges and needs. Am. J. Clin. Nutr. 2008, 88, 551S–553S. [Google Scholar] [CrossRef]
- Willett, W.C. Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 1998; ISBN 9780199754038. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuti, R.; Gennari, L.; Cavati, G.; Pirrotta, F.; Gonnelli, S.; Caffarelli, C.; Tei, L.; Merlotti, D. Dietary Vitamin D Intake in Italian Subjects: Validation of a Frequency Food Questionnaire (FFQ). Nutrients 2023, 15, 2969. https://doi.org/10.3390/nu15132969
Nuti R, Gennari L, Cavati G, Pirrotta F, Gonnelli S, Caffarelli C, Tei L, Merlotti D. Dietary Vitamin D Intake in Italian Subjects: Validation of a Frequency Food Questionnaire (FFQ). Nutrients. 2023; 15(13):2969. https://doi.org/10.3390/nu15132969
Chicago/Turabian StyleNuti, Ranuccio, Luigi Gennari, Guido Cavati, Filippo Pirrotta, Stefano Gonnelli, Carla Caffarelli, Luciano Tei, and Daniela Merlotti. 2023. "Dietary Vitamin D Intake in Italian Subjects: Validation of a Frequency Food Questionnaire (FFQ)" Nutrients 15, no. 13: 2969. https://doi.org/10.3390/nu15132969
APA StyleNuti, R., Gennari, L., Cavati, G., Pirrotta, F., Gonnelli, S., Caffarelli, C., Tei, L., & Merlotti, D. (2023). Dietary Vitamin D Intake in Italian Subjects: Validation of a Frequency Food Questionnaire (FFQ). Nutrients, 15(13), 2969. https://doi.org/10.3390/nu15132969








