Association of Dietary Fiber, Composite Dietary Antioxidant Index and Risk of Death in Tumor Survivors: National Health and Nutrition Examination Survey 2001–2018
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
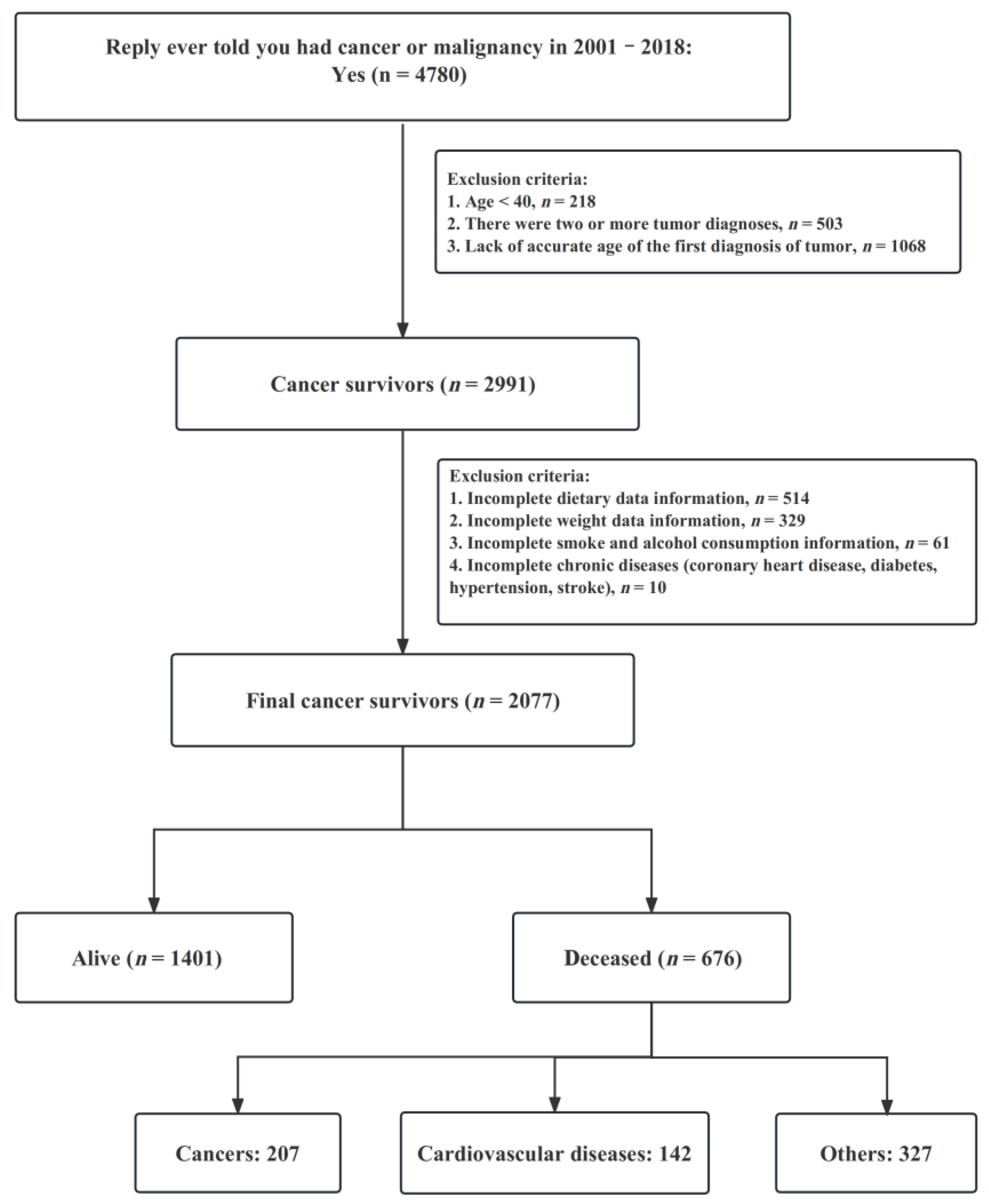
2.2. Study Population
2.3. Diet Assessment
2.4. Ascertainment of Covariates
2.5. Ascertainment of Outcomes
2.6. Statistical Analysis
3. Results
3.1. Population Characteristics
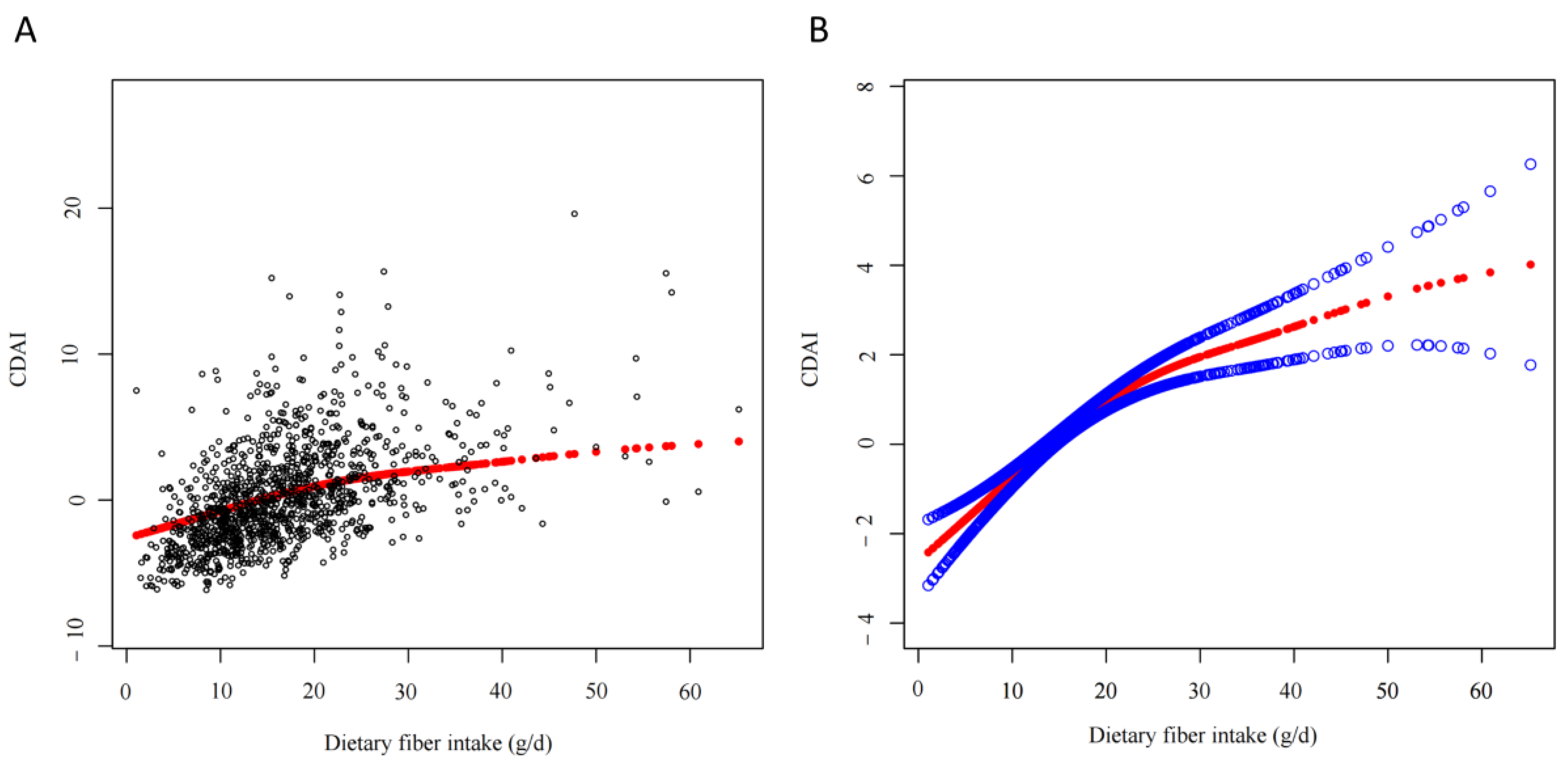
3.2. Relationship between Dietary Fiber Intake and CDAI
3.3. Association of Dietary Fiber Intake with Risks of Death in Cancer Survivors
3.4. Association of CDAI with Risks of Death in Cancer Survivors
3.5. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Dumas, J.A.; Bunn, J.Y.; Nickerson, J.; Crain, K.I.; Ebenstein, D.B.; Tarleton, E.K.; Makarewicz, J.; Poynter, M.E.; Kien, C.L. Dietary saturated fat and monounsaturated fat have reversible effects on brain function and the secretion of pro-inflammatory cytokines in young women. Metabolism 2016, 65, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.I.; Zou, X.; Ijaz, M.U.; Hussain, M.; Liu, C.; Xu, X.; Zhou, G.; Li, C. Processed Meat Protein Promoted Inflammation and Hepatic Lipogenesis by Upregulating Nrf2/Keap1 Signaling Pathway in Glrx-Deficient Mice. J. Agric. Food Chem. 2019, 67, 8794–8809. [Google Scholar] [CrossRef]
- Ferro, A.; Rosato, V.; Rota, M.; Costa, A.R.; Morais, S.; Pelucchi, C.; Johnson, K.C.; Hu, J.; Palli, D.; Ferraroni, M.; et al. Meat intake and risk of gastric cancer in the Stomach cancer Pooling (StoP) project. Int. J. Cancer 2020, 147, 45–55. [Google Scholar] [CrossRef]
- Ocvirk, S.; O’Keefe, S.J.D. Dietary fat, bile acid metabolism and colorectal cancer. Semin. Cancer Biol. 2021, 73, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Jayedi, A.; Shab-Bidar, S.; Becerra-Tomas, N.; Salas-Salvado, J. Adherence to the Mediterranean Diet in Relation to All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2019, 10, 1029–1039. [Google Scholar] [CrossRef]
- Eleftheriou, D.; Benetou, V.; Trichopoulou, A.; La Vecchia, C.; Bamia, C. Mediterranean diet and its components in relation to all-cause mortality: Meta-analysis. Br. J. Nutr. 2018, 120, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Khan, M.A.W.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 2021, 374, 1632–1640. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Marquart, L.; Slavin, J.; Kushi, L.H. Whole-grain intake and cancer: An expanded review and meta-analysis. Nutr. Cancer 1998, 30, 85–96. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef]
- Wright, M.E.; Mayne, S.T.; Stolzenberg-Solomon, R.Z.; Li, Z.; Pietinen, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am. J. Epidemiol. 2004, 160, 68–76. [Google Scholar] [CrossRef]
- Yu, Y.C.; Paragomi, P.; Wang, R.; Jin, A.; Schoen, R.E.; Sheng, L.T.; Pan, A.; Koh, W.P.; Yuan, J.M.; Luu, H.N. Composite dietary antioxidant index and the risk of colorectal cancer: Findings from the Singapore Chinese Health Study. Int. J. Cancer 2022, 150, 1599–1608. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Dwyer, J.; Terry, A.; Moshfegh, A.; Johnson, C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv. Nutr. 2016, 7, 121–134. [Google Scholar] [CrossRef]
- SSY, A.L.; Natto, Z.S.; Midle, J.B.; Gyurko, R.; O’Neill, R.; Steffensen, B. Association between time since quitting smoking and periodontitis in former smokers in the National Health and Nutrition Examination Surveys (NHANES) 2009 to 2012. J. Periodontol. 2019, 90, 16–25. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef]
- Dong, R.; Yu, Q.; Liao, W.; Liu, S.; He, Z.; Hu, X.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Composition of bound polyphenols from carrot dietary fiber and its in vivo and in vitro antioxidant activity. Food Chem. 2021, 339, 127879. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; Gonzalez-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Fuchs-Tarlovsky, V.; Rivera, M.A.; Altamirano, K.A.; Lopez-Alvarenga, J.C.; Ceballos-Reyes, G.M. Antioxidant supplementation has a positive effect on oxidative stress and hematological toxicity during oncology treatment in cervical cancer patients. Support. Care Cancer 2013, 21, 1359–1363. [Google Scholar] [CrossRef]
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef]
- Dolara, P.; Bigagli, E.; Collins, A. Antioxidant vitamins and mineral supplementation, life span expansion and cancer incidence: A critical commentary. Eur. J. Nutr. 2012, 51, 769–781. [Google Scholar] [CrossRef]
- Athreya, K.; Xavier, M.F. Antioxidants in the Treatment of Cancer. Nutr. Cancer 2017, 69, 1099–1104. [Google Scholar] [CrossRef]
- Song, M.; Wu, K.; Meyerhardt, J.A.; Ogino, S.; Wang, M.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. Fiber Intake and Survival After Colorectal Cancer Diagnosis. JAMA Oncol. 2018, 4, 71–79. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Buddington, K.K.; Donahoo, J.B.; Buddington, R.K. Dietary oligofructose and inulin protect mice from enteric and systemic pathogens and tumor inducers. J. Nutr. 2002, 132, 472–477. [Google Scholar] [CrossRef]
- Lam, K.C.; Araya, R.E.; Huang, A.; Chen, Q.; Di Modica, M.; Rodrigues, R.R.; Lopes, A.; Johnson, S.B.; Schwarz, B.; Bohrnsen, E.; et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 2021, 184, 5338–5356.e21. [Google Scholar] [CrossRef]
- He, Y.; Fu, L.; Li, Y.; Wang, W.; Gong, M.; Zhang, J.; Dong, X.; Huang, J.; Wang, Q.; Mackay, C.R.; et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 2021, 33, 988–1000.e7. [Google Scholar] [CrossRef]
- Zhang, S.L.; Mao, Y.Q.; Zhang, Z.Y.; Li, Z.M.; Kong, C.Y.; Chen, H.L.; Cai, P.R.; Han, B.; Ye, T.; Wang, L.S. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics 2021, 11, 4155–4170. [Google Scholar] [CrossRef]
- Li, Q.; Cao, L.; Tian, Y.; Zhang, P.; Ding, C.; Lu, W.; Jia, C.; Shao, C.; Liu, W.; Wang, D.; et al. Butyrate Suppresses the Proliferation of Colorectal Cancer Cells via Targeting Pyruvate Kinase M2 and Metabolic Reprogramming. Mol. Cell. Proteom. 2018, 17, 1531–1545. [Google Scholar] [CrossRef]
- Luu, H.N.; Wen, W.; Li, H.; Dai, Q.; Yang, G.; Cai, Q.; Xiang, Y.B.; Gao, Y.T.; Zheng, W.; Shu, X.O. Are dietary antioxidant intake indices correlated to oxidative stress and inflammatory marker levels? Antioxid. Redox Signal. 2015, 22, 951–959. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Z. Association of the Composite dietary antioxidant index with all-cause and cardiovascular mortality: A prospective cohort study. Front. Cardiovasc. Med. 2022, 9, 993930. [Google Scholar] [CrossRef]
- Xu, Q.; Qian, X.; Sun, F.; Liu, H.; Dou, Z.; Zhang, J. Independent and joint associations of dietary antioxidant intake with risk of post-stroke depression and all-cause mortality. J. Affect. Disord. 2023, 322, 84–90. [Google Scholar] [CrossRef]
- Albanes, D.; Heinonen, O.P.; Taylor, P.R.; Virtamo, J.; Edwards, B.K.; Rautalahti, M.; Hartman, A.M.; Palmgren, J.; Freedman, L.S.; Haapakoski, J.; et al. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: Effects of base-line characteristics and study compliance. J. Natl. Cancer Inst. 1996, 88, 1560–1570. [Google Scholar] [CrossRef]
- Yehya, A.; Baer, J.T.; Smiley, W.; Dollar, A.; Sperling, L. Hypervitaminosis A altering the lipid profile in a hypercholesterolemic patient. J. Clin. Lipidol. 2009, 3, 205–207. [Google Scholar] [CrossRef]
| Variables | Alive | Deceased | p Value |
|---|---|---|---|
| Sample size | n = 1401 | n = 676 | |
| Age | 61.97 ± 0.47 | 72.15 ± 0.51 | <0.001 |
| Energy (kcal) | 1962.67 ± 26.71 | 1857.48 ± 38.63 | 0.020 |
| Protein (g) | 76.77 ± 1.10 | 72.76 ± 1.43 | 0.019 |
| Carbohydrate (g) | 231.11 ± 3.49 | 227.47 ± 5.00 | 0.534 |
| Total sugars (g) | 100.98 ± 2.07 | 104.81 ± 3.77 | 0.385 |
| Total fat (g) | 77.44 ± 1.25 | 72.27 ± 1.86 | 0.019 |
| Cholesterol (mg) | 269.98 ± 6.70 | 266.41 ± 8.99 | 0.753 |
| Dietary fiber (g) | 17.67 ± 0.38 | 16.21 ± 0.44 | 0.005 |
| CDAI | 0.60 ± 0.15 | −0.12 ± 0.17 | <0.001 |
| Sex | |||
| Male | 651 (44.03%) | 391 (55.49%) | 0.002 |
| Female | 750 (55.97%) | 285 (44.51%) | |
| Race | 0.003 | ||
| Black | 206 (5.14%) | 94 (7.27%) | |
| White | 958 (87.33%) | 534 (88.96%) | |
| Mexican American | 85 (1.94%) | 20 (1.16%) | |
| Other | 152 (5.58%) | 28 (2.60%) | |
| Education level | <0.001 | ||
| Less than High School | 102 (3.27%) | 105 (12.03%) | |
| High School | 430 (24.45%) | 273 (37.28%) | |
| Some College or AA degree | 869 (72.28%) | 298 (50.68%) | |
| BMI | 0.050 | ||
| 18.5–25 | 332 (27.70%) | 206 (27.24%) | |
| <18.5 | 14 (0.96%) | 16 (2.86%) | |
| ≥25 | 1055 (71.34%) | 454 (69.90%) | |
| Alcohol user | <0.001 | ||
| Never | 189 (10.89%) | 91 (12.18%) | |
| Former | 305 (18.67%) | 257 (33.95%) | |
| Mild | 615 (44.01%) | 249 (41.16%) | |
| Moderate | 179 (17.05%) | 45 (6.27%) | |
| Heavy | 113 (9.38%) | 34 (6.43%) | |
| Smoke | 0.009 | ||
| Never | 666 (45.58%) | 233 (34.49%) | |
| Now | 184 (14.57%) | 100 (16.79%) | |
| Former | 551 (39.85%) | 343 (48.72%) | |
| Hypertension | <0.001 | ||
| No | 534 (43.07%) | 178 (24.08%) | |
| Yes | 867 (56.93%) | 498 (75.92%) | |
| Diabetes | <0.001 | ||
| No | 899 (70.14%) | 388 (58.58%) | |
| IFG | 73 (4.94%) | 28 (4.44%) | |
| IGT | 77 (5.23%) | 33 (4.14%) | |
| DM | 352 (19.69%) | 227 (32.84%) | |
| CHD | <0.001 | ||
| No | 1302 (93.97%) | 565 (82.78%) | |
| Yes | 99 (6.03%) | 111 (17.22%) | |
| Stroke | <0.001 | ||
| No | 1322 (96.62%) | 580 (87.68%) | |
| Yes | 79 (3.38%) | 96 (12.32%) |
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| Dietary fiber intake (g) | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p |
| 0.28 (0.17, 0.38) | <0.001 | 0.27 (0.16, 0.38) | <0.001 | 0.24 (0.08, 0.40) | 0.004 | |
| Categories | ||||||
| <25 | ref | ref | ref | ref | ref | ref |
| 25–29 | 2.9 (2.06, 3.75) | <0.001 | 2.75 (1.90, 3.60) | <0.001 | 1.2 (0.37, 2.02) | 0.004 |
| ≥29 | 4.15 (2.53, 5.78) | <0.001 | 4.06 (2.46, 5.65) | <0.001 | 1.99 (0.75, 3.24) | 0.003 |
| p for trend | <0.001 | <0.001 | <0.001 | |||
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| All-cause mortality | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| Dietary fiber intake | 0.977 (0.965, 0.990) | <0.001 | 0.983 (0.972, 0.995) | 0.006 | 0.975 (0.957, 0.994) | 0.011 |
| Categories (g) | ||||||
| <25 | ref | ref | ref | ref | ref | ref |
| 25–29 | 0.666 (0.426, 1.041) | 0.009 | 0.563 (0.366, 0.867) | 0.009 | 0.544 (0.338, 0.876) | 0.012 |
| ≥29 | 1.063 (1.050, 1.076) | 0.075 | 0.753 (0.491, 1.154) | 0.193 | 0.726 (0.453, 1.162) | 0.182 |
| p for trend | 0.022 | 0.066 | 0.086 | |||
| Cancer mortality | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| Dietary fiber intake | 0.970 (0.953, 0.988) | 0.001 | 0.979 (0.963, 0.995) | 0.009 | 0.965 (0.942, 0.988) | 0.003 |
| Categories (g) | ||||||
| <25 | ref | ref | ref | ref | ref | ref |
| 25–29 | 0.571 (0.296, 1.100) | 0.094 | 0.626 (0.311, 1.260) | 0.189 | 0.501 (0.224, 1.120) | 0.092 |
| ≥29 | 0.294 (0.113, 0.765) | 0.012 | 0.324 (0.131, 0.802) | 0.015 | 0.285 (0.108, 0.751) | 0.011 |
| p for trend | 0.005 | 0.007 | 0.007 | |||
| Cardiovascular disease mortality | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| Dietary fiber intake | 0.985 (0.949, 1.021) | 0.403 | 0.999 (0.963, 1.036) | 0.958 | 1.006 (0.969, 1.044) | 0.766 |
| Categories (g) | ||||||
| <25 | ref | ref | ref | ref | ref | ref |
| 25–29 | 0.811 (0.243, 2.704) | 0.733 | 0.858 (0.298, 2.475) | 0.777 | 0.912 (0.277, 3.005) | 0.880 |
| ≥29 | 0.932 (0.380, 2.289) | 0.878 | 1.225 (0.515, 2.914) | 0.646 | 1.356 (0.665, 2.762) | 0.402 |
| p for trend | 0.799 | 0.753 | 0.502 | |||
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| All-cause mortality | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| CDAI | 0.95 (0.92, 0.98) | <0.001 | 0.96 (0.93, 0.99) | 0.008 | 0.94 (0.91, 0.98) | 0.004 |
| Classification | ||||||
| Q1 | ref | ref | ref | ref | ref | ref |
| Q2 | 0.78 (0.56, 1.07) | 0.127 | 0.76 (0.56, 1.04) | 0.091 | 0.74 (0.53, 1.03) | 0.077 |
| Q3 | 0.70 (0.50, 0.99) | 0.043 | 0.77 (0.56, 1.05) | 0.099 | 0.72 (0.50, 1.03) | 0.071 |
| Q4 | 0.60 (0.44, 0.82) | 0.001 | 0.66 (0.49, 0.88) | 0.005 | 0.63 (0.44, 0.89) | 0.010 |
| p for trend | 0.001 | 0.008 | 0.003 | |||
| Cancer mortality | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| CDAI | 0.93 (0.87, 1.00) | 0.040 | 0.94 (0.88, 1.00) | 0.050 | 0.90 (0.83, 0.99) | 0.020 |
| Classification | ||||||
| Q1 | ref | ref | ref | ref | ref | ref |
| Q2 | 0.59 (0.29, 1.20) | 0.145 | 0.58 (0.29, 1.17) | 0.138 | 0.54 (0.25, 1.18) | 0.121 |
| Q3 | 0.67 (0.32, 1.40) | 0.286 | 0.71 (0.35, 1.45) | 0.333 | 0.60 (0.26, 1.37) | 0.228 |
| Q4 | 0.45 (0.25, 0.82) | 0.009 | 0.50 (0.29, 0.88) | 0.023 | 0.41 (0.20, 0.88) | 0.021 |
| p for trend | 0.039 | 0.042 | 0.024 | |||
| Cardiovascular disease mortality | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| CDAI | 0.95 (0.89, 1.01) | 0.114 | 0.98 (0.93, 1.04) | 0.516 | 0.98 (0.90, 1.06) | 0.567 |
| Classification | ||||||
| Q1 | ref | ref | ref | ref | ref | ref |
| Q2 | 0.76 (0.44, 1.31) | 0.325 | 0.71 (0.43, 1.16) | 0.169 | 0.70 (0.42, 1.17) | 0.169 |
| Q3 | 0.73 (0.36, 1.48) | 0.378 | 0.80 (0.40, 1.58) | 0.520 | 0.84 (0.36, 1.93) | 0.679 |
| Q4 | 0.72 (0.39, 1.32) | 0.287 | 0.88 (0.51, 1.53) | 0.645 | 0.88 (0.46, 1.67) | 0.695 |
| p for trend | 0.332 | 0.782 | 0.899 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Z.; Meng, Y.; Li, L.; Wu, Y.; Liu, C.; Dong, W.; Chen, C. Association of Dietary Fiber, Composite Dietary Antioxidant Index and Risk of Death in Tumor Survivors: National Health and Nutrition Examination Survey 2001–2018. Nutrients 2023, 15, 2968. https://doi.org/10.3390/nu15132968
Tan Z, Meng Y, Li L, Wu Y, Liu C, Dong W, Chen C. Association of Dietary Fiber, Composite Dietary Antioxidant Index and Risk of Death in Tumor Survivors: National Health and Nutrition Examination Survey 2001–2018. Nutrients. 2023; 15(13):2968. https://doi.org/10.3390/nu15132968
Chicago/Turabian StyleTan, Zongbiao, Yang Meng, Lu Li, Yanrui Wu, Chuan Liu, Weiguo Dong, and Changzheng Chen. 2023. "Association of Dietary Fiber, Composite Dietary Antioxidant Index and Risk of Death in Tumor Survivors: National Health and Nutrition Examination Survey 2001–2018" Nutrients 15, no. 13: 2968. https://doi.org/10.3390/nu15132968
APA StyleTan, Z., Meng, Y., Li, L., Wu, Y., Liu, C., Dong, W., & Chen, C. (2023). Association of Dietary Fiber, Composite Dietary Antioxidant Index and Risk of Death in Tumor Survivors: National Health and Nutrition Examination Survey 2001–2018. Nutrients, 15(13), 2968. https://doi.org/10.3390/nu15132968








