Formononetin, a Beer Polyphenol with Catabolic Effects on Chondrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Design
2.2.1. Forty-Eight-Hour Experiments
2.2.2. Cell Differentiation
2.3. Cell Treatments
2.4. Cell Viability Assay
2.5. Nitrite Assay
2.6. RNA Extraction and Quantitative Real-Time PCR
2.7. Docking Analysis
2.8. Statistical Analysis
3. Results
3.1. FNT Does Not Alter Chondrocyte Viability
3.2. FNT Has Moderate Anti-Inflammatory Activity Only at Low Concentrations
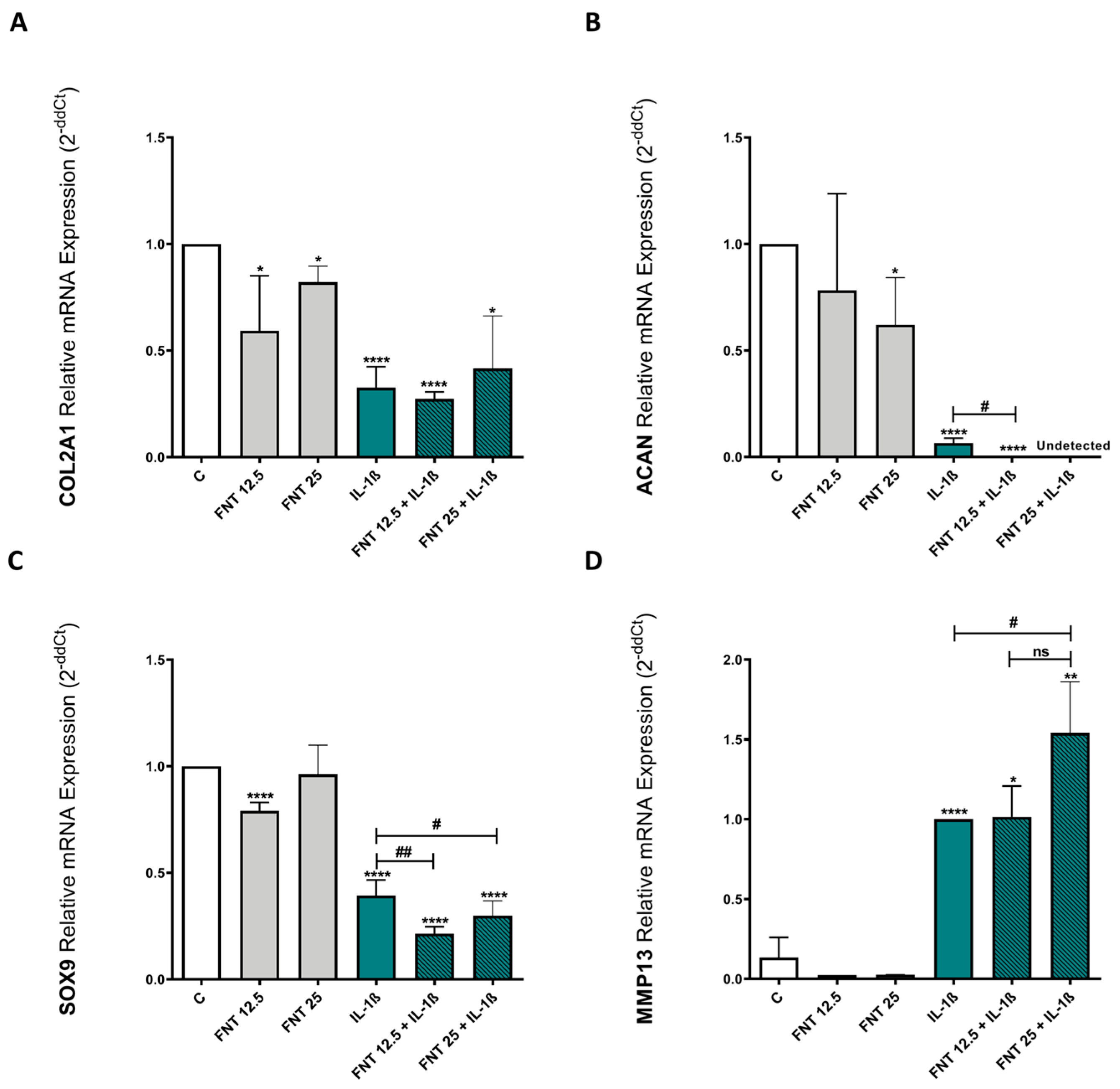
3.3. FNT Promotes Chondrocyte Catabolic Responses
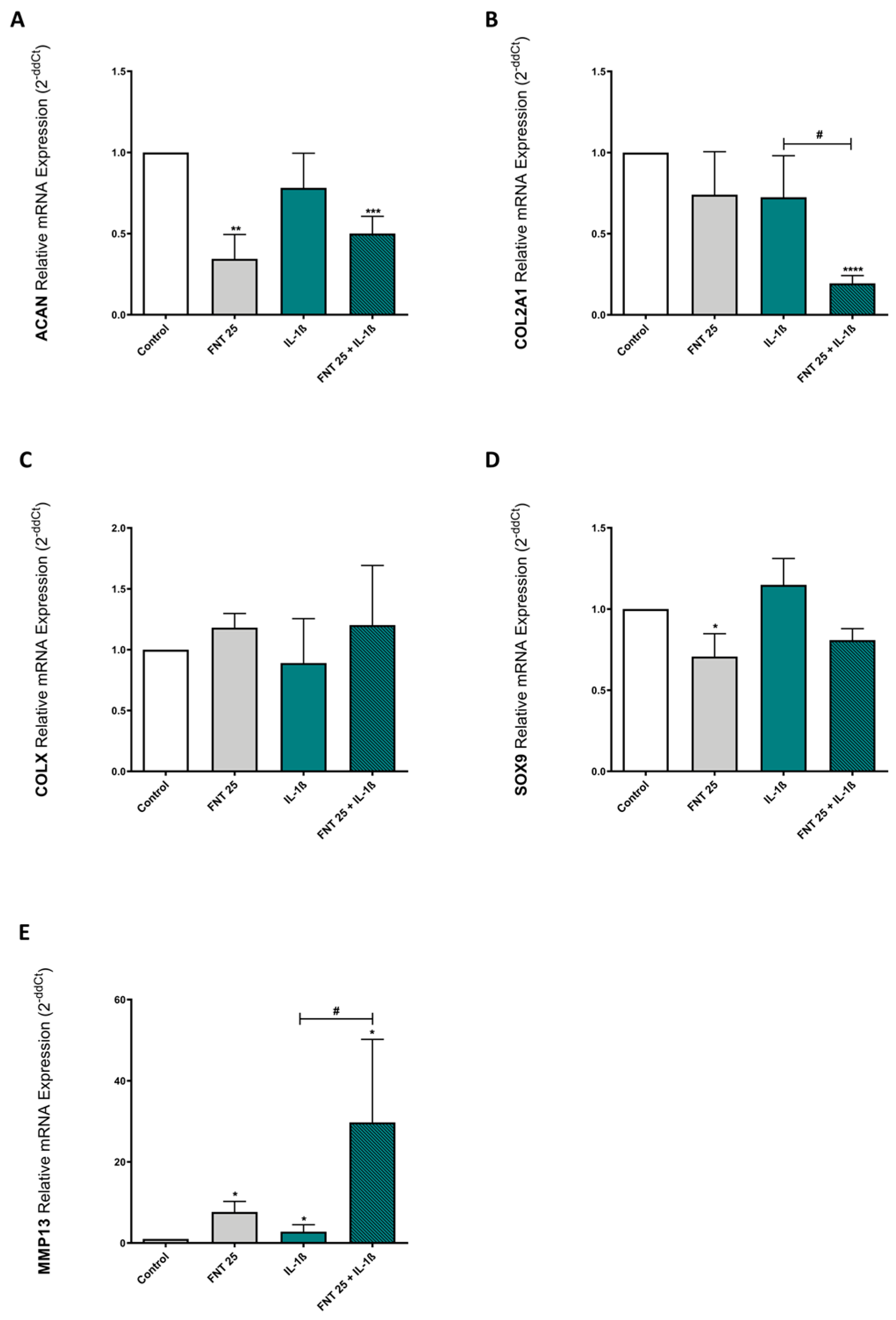
3.4. FNT Negatively Impacts Chondrocyte Differentiation
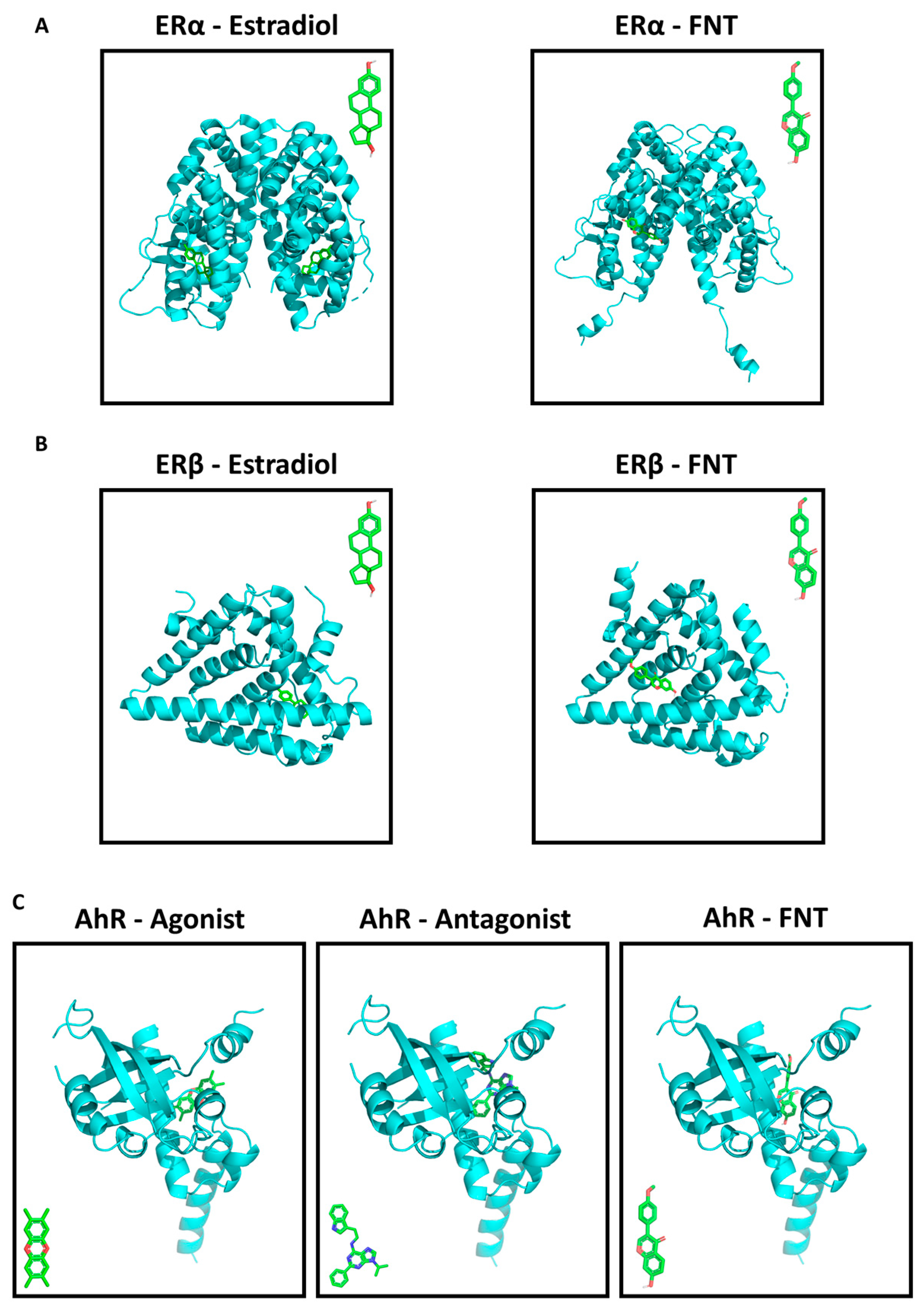
3.5. FNT Has a Predicted Strong Binding Affinity to ERs and AhR
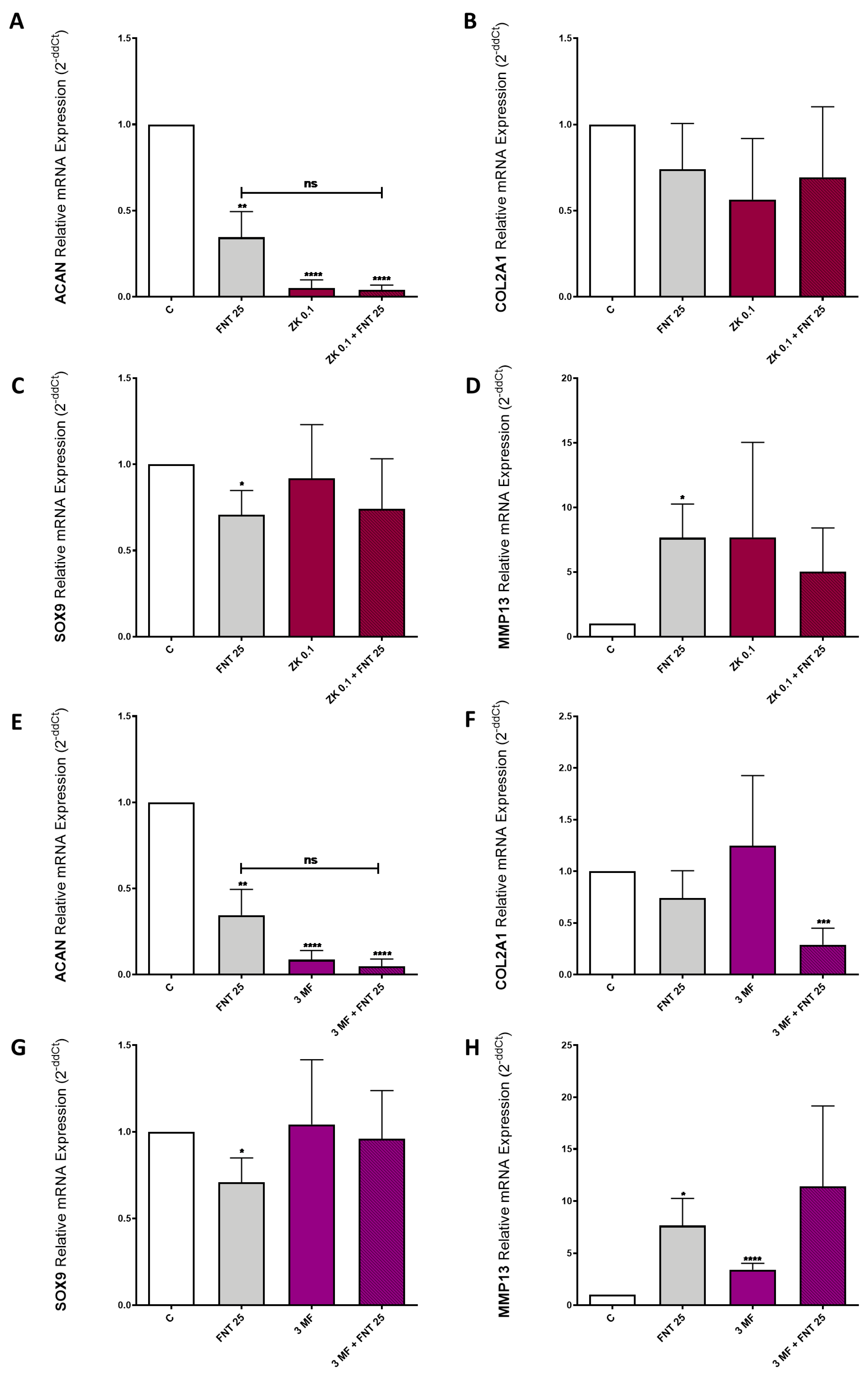
3.6. FNT Effects on Chondrocyte Differentiation Are Not Mediated by ERs or AhR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guillán-Fresco, M.; Franco-Trepat, E.; Alonso-Pérez, A.; Jorge-Mora, A.; López-Fagúndez, M.; Pazos-Pérez, A.; Gualillo, O.; Gómez, R. Caffeine, a Risk Factor for Osteoarthritis and Longitudinal Bone Growth Inhibition. J. Clin. Med. 2020, 9, 1163. [Google Scholar] [CrossRef]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Konttinen, Y.T.; Sillat, T.; Barreto, G.; Ainola, M.; Nordström, D.C.E. Osteoarthritis as an autoinflammatory disease caused by chondrocyte-mediated inflammatory responses. Arthritis Rheum. 2012, 64, 613–616. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, T.W.; McCabe, P.S.; McBeth, J. Update on the epidemiology, risk factors and disease outcomes of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2018, 32, 312–326. [Google Scholar] [CrossRef]
- Gómez, R.; Villalvilla, A.; Largo, R.; Gualillo, O.; Herrero-Beaumont, G. TLR4 signalling in osteoarthritis-finding targets for candidate DMOADs. Nat. Rev. Rheumatol. 2015, 11, 159–170. [Google Scholar] [CrossRef]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef] [PubMed]
- Muthuri, S.G.; Zhang, W.; Maciewicz, R.A.; Muir, K.; Doherty, M. Beer and wine consumption and risk of knee or hip osteoarthritis: A case control study. Arthritis Res. Ther. 2015, 17, 23. [Google Scholar] [CrossRef]
- Pedrera-Zamorano, J.D.; Lavado-Garcia, J.M.; Roncero-Martin, R.; Calderon-Garcia, J.F.; Rodriguez-Dominguez, T.; Canal-Macias, M.L. Effect of beer drinking on ultrasound bone mass in women. Nutrition 2009, 25, 1057–1063. [Google Scholar] [CrossRef]
- Singh, K.B.; Dixit, M.; Dev, K.; Maurya, R.; Singh, D. Formononetin, a methoxy isoflavone, enhances bone regeneration in a mouse model of cortical bone defect. Br. J. Nutr. 2017, 117, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Compston, J.E.; Day, N.E.; Dowsett, M.; Bingham, S.A. The effects of phytoestrogen isoflavones on bone density in women: A double-blind, randomized, placebo-controlled trial 1–3. Am. J. Clin. Nutr. 2004, 79, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Lapcík, O.; Hill, M.; Hampl, R.; Wähälä, K.; Adlercreutz, H. Identification of isoflavonoids in beer. Steroids 1998, 63, 14–20. [Google Scholar] [CrossRef]
- Lin, C.C.; Tsai, Y.L.; Ho, C.T.; Teng, S.C. Determination of the differential estrogenicity of isoflavonoids by E2-ER-ERE-dependent gene expression in recombinant yeast and MCF-7 human breast cancer cells. Food Chem. 2008, 108, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Vishnuvathan, V.J.; Lakshmi, K.S.; Srividya, A.R. Medicinal Uses of Formononetin—A review. J. Ethnobiol. Tradit. Med. 2016, 126, 1197–1209. [Google Scholar]
- Alves, R.C.; Almeida, I.M.C.; Casal, S.; Oliveira, M.B.P.P. Isoflavones in coffee: Influence of species, roast degree, and brewing method. J. Agric. Food Chem. 2010, 58, 3002–3007. [Google Scholar] [CrossRef]
- Caprioli, G.; Navarini, L.; Cortese, M.; Ricciutelli, M.; Torregiani, E.; Vittori, S.; Sagratini, G. Quantification of isoflavones in coffee by using solid phase extraction (SPE) and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). J. Mass Spectrom. 2016, 51, 698–703. [Google Scholar] [CrossRef]
- Mazur, W.; Wahala, K.; Rasku, S.; Makkonen, A.; Hase, T.; Adlercreutz, H. Lignans and isoflavonoid polyphenols in tea and coffee. J. Med. Food 1999, 2, 199–202. [Google Scholar] [CrossRef]
- Surh, J.; Kim, M.J.; Koh, E.; Kim, Y.K.L.; Kwon, H. Estimated intakes of isoflavones and coumestrol in Korean population. Int. J. Food Sci. Nutr. 2006, 57, 325–344. [Google Scholar] [CrossRef]
- Chun, O.K.; Chung, S.J.; Song, W.O. Urinary isoflavones and their metabolites validate the dietary isoflavone intakes in US adults. J. Am. Diet. Assoc. 2009, 109, 245–254. [Google Scholar] [CrossRef]
- Tay, K.-C.; Tan, L.T.-H.; Chan, C.K.; Hong, S.L.; Chan, K.-G.; Yap, W.H.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Formononetin: A Review of Its Anticancer Potentials and Mechanisms. Front. Pharmacol. 2019, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Zorita, S.; González-Arceo, M.; Fernández-Quintela, A.; Eseberri, I.; Trepiana, J.; Portillo, M.P. Scientific Evidence Supporting the Beneficial Effects of Isoflavones on Human Health. Nutrients 2020, 12, 3853. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, G.; Ding, M.; Zong, G.; Hu, F.B.; Willett, W.C.; Rimm, E.B.; Manson, J.A.E.; Sun, Q. Isoflavone Intake and the Risk of Coronary Heart Disease in US Men and Women: Results from 3 Prospective Cohort Studies. Circulation 2020, 141, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Naudhani, M.; Thakur, K.; Ni, Z.J.; Zhang, J.G.; Wei, Z.J. Formononetin reshapes the gut microbiota, prevents progression of obesity and improves host metabolism. Food Funct. 2021, 12, 12303–12324. [Google Scholar] [CrossRef]
- Jia, C.; Hu, F.; Lu, D.; Jin, H.; Lu, H.; Xue, E.; Wu, D. Formononetin inhibits IL-1β-induced inflammation in human chondrocytes and slows the progression of osteoarthritis in rat model via the regulation of PTEN/AKT/NF-κB pathway. Int. Immunopharmacol. 2022, 113, 109309. [Google Scholar] [CrossRef]
- Cho, I.A.; Kim, T.H.; Lim, H.I.; Park, J.H.; Kang, K.R.; Lee, S.Y.; Kim, C.S.; Kim, D.K.; Kim, H.J.; Yu, S.K.; et al. Formononetin Antagonizes the Interleukin-1β-Induced Catabolic Effects through Suppressing Inflammation in Primary Rat Chondrocytes. Inflammation 2019, 42, 1426–1440. [Google Scholar] [CrossRef]
- Xiong, W.; Lan, Q.; Liang, X.; Zhao, J.; Huang, H.; Zhan, Y.; Qin, Z.; Jiang, X.; Zheng, L. Cartilage-targeting poly(ethylene glycol) (PEG)-formononetin (FMN) nanodrug for the treatment of osteoarthritis. J. Nanobiotechnology 2021, 19, 197. [Google Scholar] [CrossRef]
- nan Ni, K.; Ye, L.; Zhang, Y.J.; Fang, J.W.; Yang, T.; Pan, W.Z.; Hu, X.Y.; Lai, H.H.; Pan, B.; Lou, C.; et al. Formononetin improves the inflammatory response and bone destruction in knee joint lesions by regulating the NF-kB and MAPK signaling pathways. Phytother. Res. 2023. [Google Scholar] [CrossRef]
- Irvine, C.H.G.; Fitzpatrick, M.G.; Alexander, S.L. Phytoestrogens in soy-based infant foods: Concentrations, daily intake, and possible biological effects. Proc. Soc. Exp. Biol. Med. 1998, 217, 247–253. [Google Scholar] [CrossRef]
- Franke, A.A.; Custer, L.J.; Tanaka, Y. Isoflavones in human breast milk and other biological fluids. Am. J. Clin. Nutr. 1998, 68, 1466S–1473S. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, H.; Wang, Q.; Zhou, X.; Zhang, Y.; Wu, W.; Wang, Y.; Ren, Z.; Li, H.; Ling, Y.; et al. Simultaneous determination of formononetin, biochanin A and their active metabolites in human breast milk, saliva and urine using salting-out assisted liquid-liquid extraction and ultra high performance liquid chromatography-electrospray ionization tandem mass spectrum. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1145, 122108. [Google Scholar] [CrossRef]
- Foster, W.G.; Chan, S.; Platt, L.; Hughes, C.L. Detection of phytoestrogens in samples of second trimester human amniotic fluid. Toxicol. Lett. 2002, 129, 199–205. [Google Scholar] [CrossRef]
- Knight, D.C.; Eden, J.A.; Huang, J.L.; Waring, M.A. Isoflavone content of infant foods and formulas. J. Paediatr. Child Health 1998, 34, 135–138. [Google Scholar] [CrossRef]
- Kuhnle, G.G.C.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen content of foods of animal origin: Dairy products, eggs, meat, fish, and seafood. J. Agric. Food Chem. 2008, 56, 10099–10104. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.H.; Tang, F.Y.; Wang, Y.; Huang, W.J.; Tian, J.; Lu, H.L.; Xin, M.; Chen, J. Low concentration of formononetin promotes proliferation of estrogen receptor-positive cells through an ERα-miR-375-PTENERK1/2-bcl-2 pathway. Oncotarget 2017, 8, 100045–100055. [Google Scholar] [CrossRef] [PubMed]
- Morito, K.; Hirose, T.; Kinjo, J.; Hirakawa, T.; Okawa, M.; Nohara, T.; Ogawa, S.; Inoue, S.; Muramatsu, M.; Masamune, Y. Interaction of phytoestrogens with estrogen receptors α and β. Biol. Pharm. Bull. 2001, 24, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dang, Y.; Zhou, X.; Huang, B.; Huang, X.; Zhang, Z.; Kwan, Y.W.; Chan, S.W.; Leung, G.P.H.; Lee, S.M.Y.; et al. Formononetin promotes angiogenesis through the estrogen receptor alpha-enhanced ROCK pathway. Sci. Rep. 2015, 5, 16815. [Google Scholar] [CrossRef]
- Kung, M.H.; Yukata, K.; O’Keefe, R.J.; Zuscik, M.J. Aryl hydrocarbon receptor-mediated impairment of chondrogenesis and fracture healing by cigarette smoke and benzo(α)pyrene. J. Cell. Physiol. 2012, 227, 1062–1070. [Google Scholar] [CrossRef]
- Cedervall, T.; Lind, P.M.; Sävendahl, L. Expression of the aryl hydrocarbon receptor in growth plate cartilage and the impact of its local modulation on longitudinal bone growth. Int. J. Mol. Sci. 2015, 16, 8059–8069. [Google Scholar] [CrossRef]
- Safe, S.; Wormke, M.; Samudio, I. Mechanisms of inhibitory aryl hydrocarbon receptor-estrogen receptor crosstalk in human breast cancer cells. J. Mammary Gland Biol. Neoplasia 2000, 5, 295–306. [Google Scholar] [CrossRef]
- Medjakovic, S.; Jungbauer, A. Red clover isoflavones biochanin A and formononetin are potent ligands of the human aryl hydrocarbon receptor. J. Steroid Biochem. Mol. Biol. 2008, 108, 171–177. [Google Scholar] [CrossRef]
- Atsumi, T.; Ikawa, Y.; Miwa, Y.; Kimata, K. A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell Differ. Dev. 1990, 30, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Altaf, F.M.; Hering, T.M.; Kazmi, N.H.; Yoo, J.U.; Johnstone, B. Ascorbate-enhanced chondrogenesis of ATDC5 cells. Eur. Cell. Mater. 2006, 12, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, A.; Yamaguchi, M.; Fujiwara, A.; Shimizu, M.; Takahashi, A.; Sone, H.; Kamiyama, S. Genistein inhibits chondrogenic differentiation and mineralization of ATDC5 cells. Biochem. Biophys. Res. Commun. 2021, 566, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Hodax, J.K.; Quintos, J.B.; Gruppuso, P.A.; Chen, Q.; Desai, S.; Jayasuriya, C.T. Aggrecan is required for chondrocyte differentiation in ATDC5 chondroprogenitor cells. PLoS ONE 2019, 14, e0218399. [Google Scholar] [CrossRef] [PubMed]
- Iacono, A.; Gómez, R.; Sperry, J.; Conde, J.; Bianco, G.; Meli, R.; Gómez-Reino, J.J.; Smith, A.B.; Gualillo, O. Effect of oleocanthal and its derivatives on inflammatory response induced by lipopolysaccharide in a murine chondrocyte cell line. Arthritis Rheum. 2010, 62, 1675–1682. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Bhikadiya, C.; Bi, C.; Chen, L.; Di Costanzo, L.; Christie, C.; Dalenberg, K.; Duarte, J.M.; Dutta, S.; et al. RCSB Protein Data Bank: Biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2019, 47, D464–D474. [Google Scholar] [CrossRef]
- DeLano, W.L. PyMOL|pymol.org. Available online: https://pymol.org/2/ (accessed on 15 June 2022).
- Mehana, E.-S.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef] [PubMed]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef]
- Mueller, M.B.; Tuan, R.S. Anabolic/Catabolic balance in pathogenesis of osteoarthritis: Identifying molecular targets. PMR 2011, 3, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Umlauf, D.; Frank, S.; Pap, T.; Bertrand, J. Cartilage biology, pathology, and repair. Cell. Mol. Life Sci. 2010, 67, 4197–4211. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.R.; Kojima, T.; Yasuda, T.; Mwale, F.; Kobayashi, M.; Laverty, S. Composition and structure of articular cartilage: A template for tissue repair. Clin. Orthop. Relat. Res. 2001, 391, S26–S33. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Wang, W.; Tu, B.; Zhu, Y.; Fan, C.; Li, Y. Overexpression of SOX9 alleviates the progression of human osteoarthritis in vitro and in vivo. Drug Des. Devel. Ther. 2019, 13, 2833–2842. [Google Scholar] [CrossRef]
- Zhang, Q.; Ji, Q.; Wang, X.; Kang, L.; Fu, Y.; Yin, Y.; Li, Z.; Liu, Y.; Xu, X.; Wang, Y. SOX9 is a regulator of ADAMTSs-induced cartilage degeneration at the early stage of human osteoarthritis. Osteoarthr. Cartil. 2015, 23, 2259–2268. [Google Scholar] [CrossRef]
- Long, F.; Ornitz, D.M. Development of the endochondral skeleton. Cold Spring Harb. Perspect. Biol. 2013, 5, a008334. [Google Scholar] [CrossRef]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef]
- Ghimire, S.; Miramini, S.; Edwards, G.; Rotne, R.; Xu, J.; Ebeling, P.; Zhang, L. The investigation of bone fracture healing under intramembranous and endochondral ossification. Bone Rep. 2021, 14, 100740. [Google Scholar] [CrossRef]
- Miadoková, E. Isoflavonoids—An overview of their biological activities and potential health benefits. Interdiscip. Toxicol. 2009, 2, 211–218. [Google Scholar] [CrossRef]
- Kong, X.; Wang, F.; Niu, Y.; Wu, X.; Pan, Y. A comparative study on the effect of promoting the osteogenic function of osteoblasts using isoflavones from Radix Astragalus. Phytother. Res. 2018, 32, 115–124. [Google Scholar] [CrossRef]
- Huh, J.E.; Seo, D.M.; Baek, Y.H.; Choi, D.Y.; Park, D.S.; Lee, J.D. Biphasic positive effect of formononetin on metabolic activity of human normal and osteoarthritic subchondral osteoblasts. Int. Immunopharmacol. 2010, 10, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, L. Formononetin-induced apoptosis by activation of Ras/p38 mitogen-activated protein kinase in estrogen receptor-positive human breast cancer cells. Horm. Metab. Res. 2012, 44, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, X.; Ye, Y.; Wang, Y.; Tian, J. Estrogen receptor beta-mediated proliferative inhibition and apoptosis in human breast cancer by calycosin and formononetin. Cell. Physiol. Biochem. 2013, 32, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Franchin, M.; Colón, D.F.; Da Cunha, M.G.; Castanheira, F.V.S.; Saraiva, A.L.L.; Bueno-Silva, B.; Alencar, S.M.; Cunha, T.M.; Rosalen, P.L. Neovestitol, an isoflavonoid isolated from Brazilian red propolis, reduces acute and chronic inflammation: Involvement of nitric oxide and IL-6. Sci. Rep. 2016, 6, 36401. [Google Scholar] [CrossRef]
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin β1−SMAD1 interaction. Bone Res. 2019, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.M.J.; Janssen, M.P.F.; Peeters, L.; Haudenschild, D.R.; Cremers, A.; Surtel, D.A.M.; van Rhijn, L.W.; Emans, P.J.; Welting, T.J.M. Aggrecan and COMP Improve Periosteal Chondrogenesis by Delaying Chondrocyte Hypertrophic Maturation. Front. Bioeng. Biotechnol. 2020, 8, 1036. [Google Scholar] [CrossRef]
- Domowicz, M.S.; Cortes, M.; Henry, J.G.; Schwartz, N.B. Aggrecan modulation of growth plate morphogenesis. Dev. Biol. 2009, 329, 242. [Google Scholar] [CrossRef]
- Inada, M.; Wang, Y.; Byrne, M.H.; Rahman, M.U.; Miyaura, C.; López-Otín, C.; Krane, S.M. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc. Natl. Acad. Sci. USA 2004, 101, 17192–17197. [Google Scholar] [CrossRef]
- Ahmad, N.; Chen, S.; Wang, W.; Kapila, S. 17β-estradiol Induces MMP-9 and MMP-13 in TMJ Fibrochondrocytes via Estrogen Receptor α. J. Dent. Res. 2018, 97, 1023–1030. [Google Scholar] [CrossRef]
- Wilhelm, D.; Kempf, H.; Bianchi, A.; Vincourt, J.B. ATDC5 cells as a model of cartilage extracellular matrix neosynthesis, maturation and assembly. J. Proteomics 2020, 219, 103718. [Google Scholar] [CrossRef] [PubMed]
- Claassen, H.; Briese, V.; Manapov, F.; Nebe, B.; Schünke, M.; Kurz, B. The phytoestrogens daidzein and genistein enhance the insulin-stimulated sulfate uptake in articular chondrocytes. Cell Tissue Res. 2008, 333, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Tabata, A.; Tomoyasu, T.; Ueno, T.; Uchiyama, S.; Yuasa, K.; Tsuji, A.; Nagamune, H. Estrogen stimuli promote osteoblastic differentiation via the subtilisin-like proprotein convertase PACE4 in MC3T3-E1 cells. J. Bone Miner. Metab. 2015, 33, 30–39. [Google Scholar] [CrossRef]
- Gautam, J.; Khedgikar, V.; Kushwaha, P.; Choudhary, D.; Nagar, G.K.; Dev, K.; Dixit, P.; Singh, D.; Maurya, R.; Trivedi, R. Formononetin, an isoflavone, activates AMP-activated protein kinase/β-catenin signalling to inhibit adipogenesis and rescues C57BL/6 mice from high-fat diet-induced obesity and bone loss. Br. J. Nutr. 2017, 117, 645–661. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Sequence (5′-3′) | Sequence (5′-3′) | |
| IL-6 | AAGAAATGATGGATGCTACC | GAGTTTCTGTATCTCTCTGAAG |
| LCN2 | ATATGCACAGGTATCCTCAG | GAAACGTTCCTTCAGTTCAG |
| CCL2 | CAAGATGATCCCAATGAGTAG | TTGGTGACAAAAACTACAGC |
| COL2A1 | GCGATGACATTATCTGTGAAG | TATCTCTGATATCTCCAGGTTC |
| ACAN | CTACCTTGGAGATCCAGAAC | TGGAACACAATACCTTTCAC |
| SOX9 | CTCATTACCATTTTGAGGGG | AAAATACTCTGGTTGCAAGG |
| MMP13 | CTTTAGAGGGAGAAAATTCTGG | CATCATCATAACTCCACACG |
| COLX | TCATGGGATGTTTTATGCTG | TCTTACTGGAATCCCTTTACTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillán-Fresco, M.; Franco-Trepat, E.; Alonso-Pérez, A.; Jorge-Mora, A.; López-López, V.; Pazos-Pérez, A.; Piñeiro-Ramil, M.; Gómez, R. Formononetin, a Beer Polyphenol with Catabolic Effects on Chondrocytes. Nutrients 2023, 15, 2959. https://doi.org/10.3390/nu15132959
Guillán-Fresco M, Franco-Trepat E, Alonso-Pérez A, Jorge-Mora A, López-López V, Pazos-Pérez A, Piñeiro-Ramil M, Gómez R. Formononetin, a Beer Polyphenol with Catabolic Effects on Chondrocytes. Nutrients. 2023; 15(13):2959. https://doi.org/10.3390/nu15132959
Chicago/Turabian StyleGuillán-Fresco, María, Eloi Franco-Trepat, Ana Alonso-Pérez, Alberto Jorge-Mora, Verónica López-López, Andrés Pazos-Pérez, María Piñeiro-Ramil, and Rodolfo Gómez. 2023. "Formononetin, a Beer Polyphenol with Catabolic Effects on Chondrocytes" Nutrients 15, no. 13: 2959. https://doi.org/10.3390/nu15132959
APA StyleGuillán-Fresco, M., Franco-Trepat, E., Alonso-Pérez, A., Jorge-Mora, A., López-López, V., Pazos-Pérez, A., Piñeiro-Ramil, M., & Gómez, R. (2023). Formononetin, a Beer Polyphenol with Catabolic Effects on Chondrocytes. Nutrients, 15(13), 2959. https://doi.org/10.3390/nu15132959







