Adrenomedullin Enhances Mouse Gustatory Nerve Responses to Sugars via T1R-Independent Sweet Taste Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Immunohistochemistry
2.3. Chorda Tympani Nerve Recording
2.4. Uptake of a Fluorescent D-Glucose Derivative into Taste Bud Cells
2.5. RT-qPCR
2.6. Data Analysis
3. Results
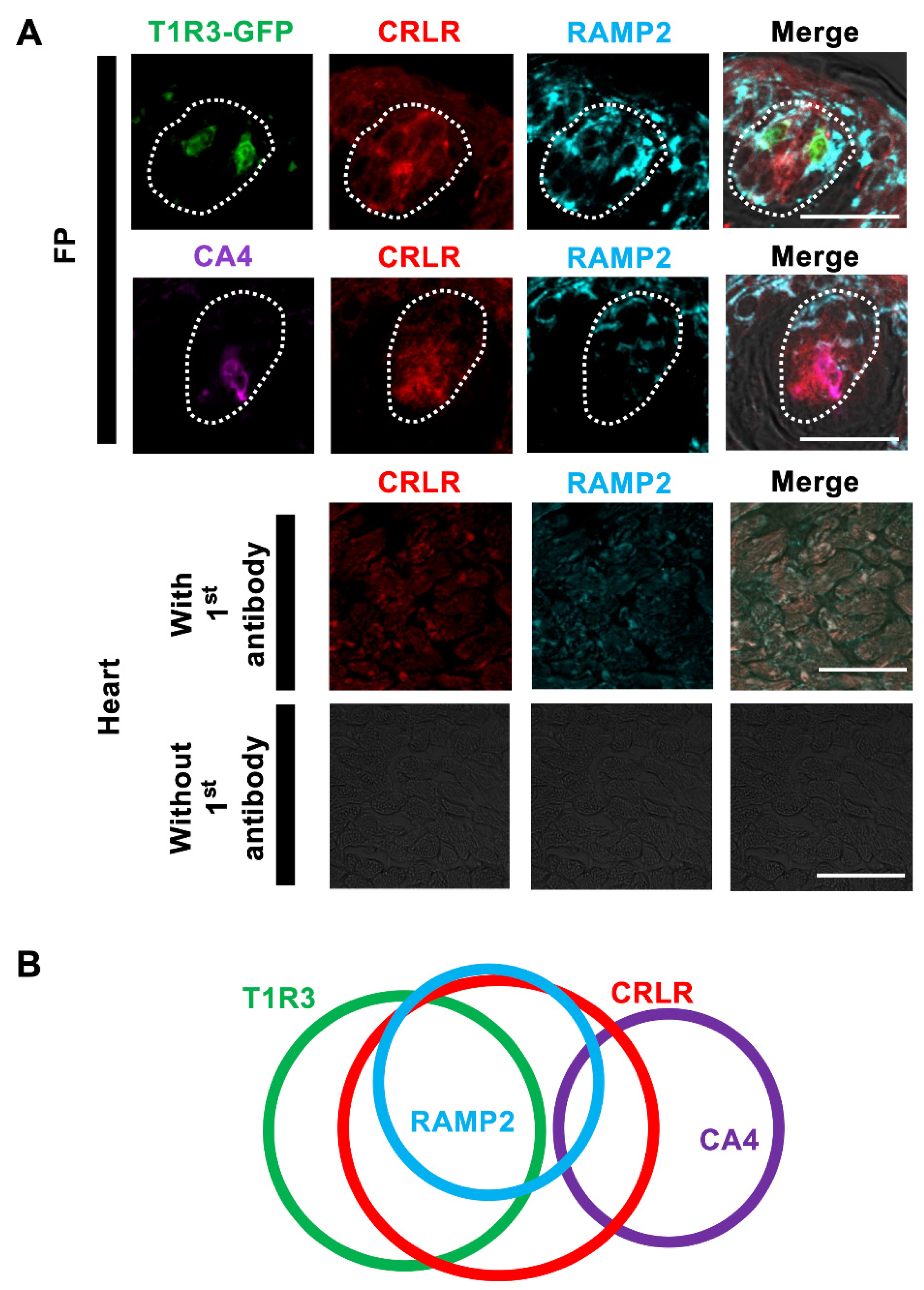
3.1. Immunohistochemistry
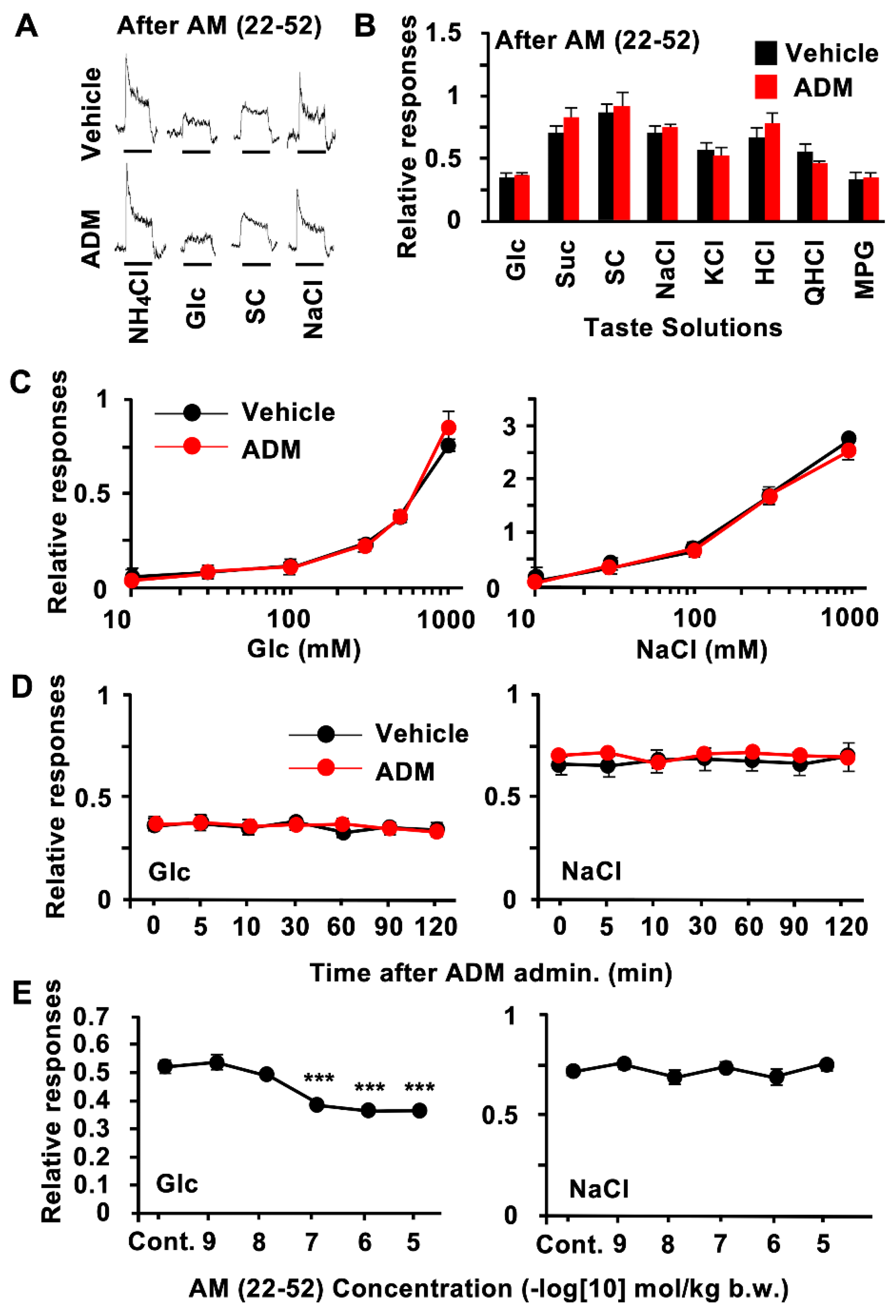
3.2. CT Nerve Recordings
3.3. 2-NBDG Uptake in Taste Cells
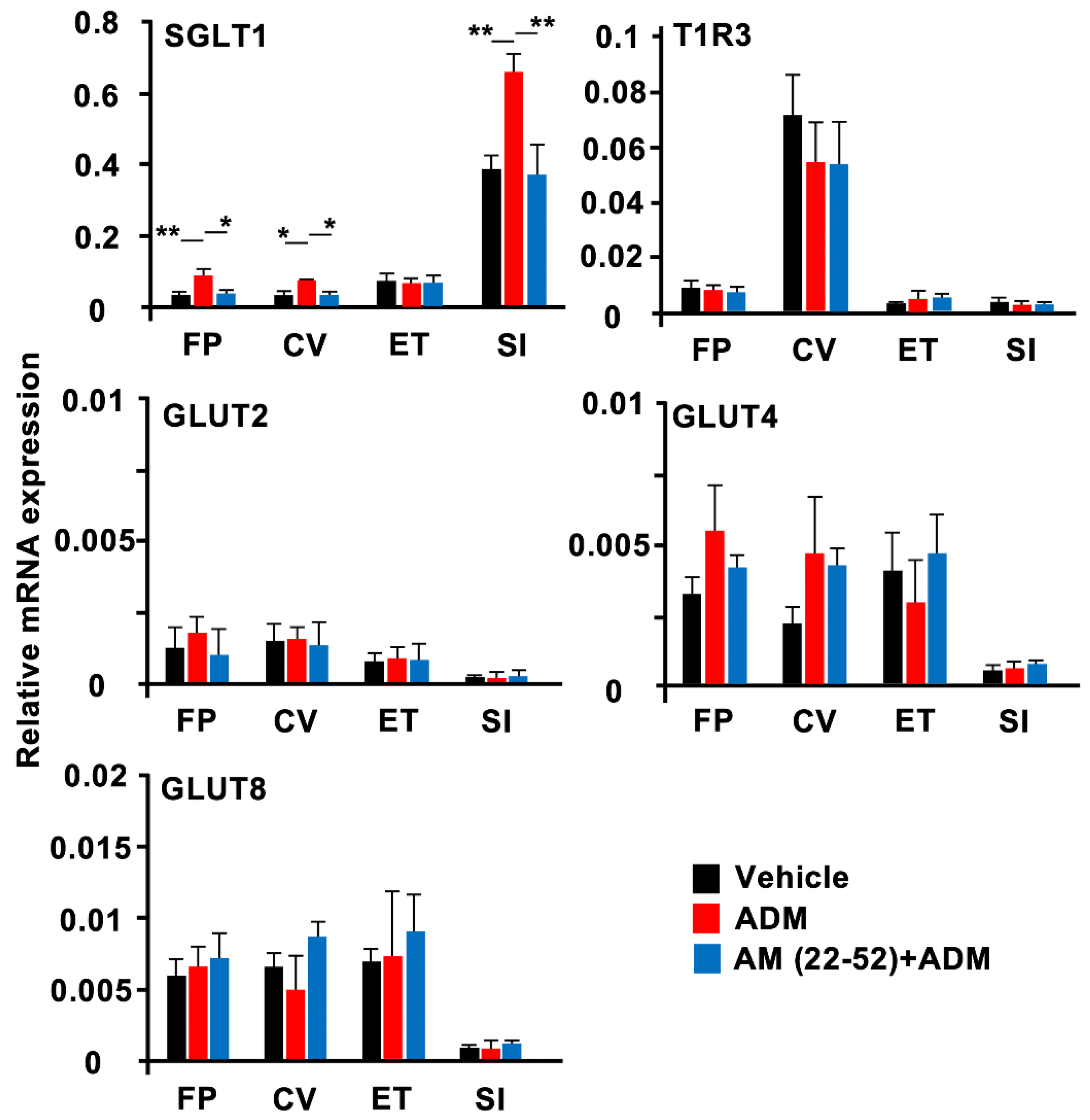
3.4. RT-qPCR
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, G.; Hoon, M.A.; Chandrashekar, J.; Zhang, Y.; Ryba, N.J.; Zuker, C.S. Mammalian Sweet Taste Receptors. Cell 2001, 106, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef] [PubMed]
- Iwata, S.; Yoshida, R.; Ninomiya, Y. Taste transductions in taste receptor cells: Basic tastes and moreover. Curr. Pharm. Des. 2014, 20, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Sigoillot, M.; Brockhoff, A.; Meyerhof, W.; Briand, L. Sweet-taste-suppressing compounds: Current knowledge and perspectives of application. Appl. Microbiol. Biotechnol. 2012, 96, 619–630. [Google Scholar] [CrossRef]
- Merigo, F.; Benati, D.; Cristofoletti, M.; Osculati, F.; Sbarbati, A. Glucose transporters are expressed in taste receptor cells. J. Anat. 2011, 219, 243–252. [Google Scholar] [CrossRef]
- Toyono, T.; Seta, Y.; Kataoka, S.; Oda, M.; Toyoshima, K. Differential expression of the glucose transporters in mouse gustatory papillae. Cell Tissue Res. 2011, 345, 243–252. [Google Scholar] [CrossRef]
- Yee, K.K.; Sukumaran, S.K.; Kotha, R.; Gilbertson, T.A.; Margolskee, R.F. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc. Natl. Acad. Sci. USA 2011, 108, 5431–5436. [Google Scholar] [CrossRef]
- Yasumatsu, K.; Ohkuri, T.; Yoshida, R.; Iwata, S.; Margolskee, R.F.; Ninomiya, Y. Sodium-glucose cotransporter 1 as a sugar taste sensor in mouse tongue. Acta Physiol. 2020, 230, e13529. [Google Scholar] [CrossRef]
- Sukumaran, S.K.; Yee, K.K.; Iwata, S.; Kotha, R.; Quezada-Calvillo, R.; Nichols, B.L.; Mohan, S.; Pinto, B.M.; Shigemura, N.; Ninomiya, Y.; et al. Taste cell-expressed α-glucosidase enzymes contribute to gustatory responses to disaccharides. Proc. Natl. Acad. Sci. USA 2016, 113, 6035–6040. [Google Scholar] [CrossRef]
- Glendinning, J.I.; Stano, S.; Holter, M.; Azenkot, T.; Goldman, O.; Margolskee, R.F.; Vasselli, J.R.; Sclafani, A. Sugar-induced cephalic-phase insulin release is mediated by a T1r2+T1r3-independent taste transduction pathway in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, 552–560. [Google Scholar] [CrossRef]
- Glendinning, J.I.; Frim, Y.G.; Hochman, A.; Lubitz, G.S.; Basile, A.J.; Sclafani, A. Glucose elicits cephalic-phase insulin release in mice by activating KATP channels in taste cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, 597–610. [Google Scholar] [CrossRef]
- Kawai, K.; Sugimoto, K.; Nakashima, K.; Miura, H.; Ninomiya, Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc. Natl. Acad. Sci. USA 2000, 97, 11044–11049. [Google Scholar] [CrossRef]
- Shigemura, N.; Ohta, R.; Kusakabe, Y.; Miura, H.; Hino, A.; Koyano, K.; Nakashima, K.; Ninomiya, Y. Leptin Modulates Behavioral Responses to Sweet Substances by Influencing Peripheral Taste Structures. Endocrinology 2004, 145, 839–847. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sanematsu, K.; Ohta, R.; Shirosaki, S.; Koyano, K.; Nonaka, K.; Shigemura, N.; Ninomiya, Y. Diurnal Variation of Human Sweet Taste Recognition Thresholds Is Correlated With Plasma Leptin Levels. Diabetes 2008, 57, 2661–2665. [Google Scholar] [CrossRef]
- Yoshida, R.; Noguchi, K.; Shigemura, N.; Jyotaki, M.; Takahashi, I.; Margolskee, R.F.; Ninomiya, Y. Leptin suppresses mouse taste cell responses to sweet compounds. Diabetes 2015, 64, 3751–3762. [Google Scholar] [CrossRef]
- Yoshida, R.; Ohkuri, T.; Jyotaki, M.; Yasuo, T.; Horio, N.; Yasumatsu, K.; Sanematsu, K.; Shigemura, N.; Yamamoto, T.; Margolskee, R.F.; et al. Endocannabinoids selectively enhance sweet taste. Proc. Natl. Acad. Sci. USA 2010, 107, 935–939. [Google Scholar] [CrossRef]
- Niki, M.; Jyotaki, M.; Yoshida, R.; Yasumatsu, K.; Shigemura, N.; DiPatrizio, N.V.; Piomelli, D.; Ninomiya, Y. Modulation of sweet taste sensitivities by endogenous leptin and endocannabinoids in mice. J. Physiol. 2015, 593, 2527–2545. [Google Scholar] [CrossRef]
- Kitamura, K.; Kangawa, K.; Kawamoto, M.; Ichiki, Y.; Nakamura, S.; Matsuo, H.; Eto, T. Adrenomedullin: A novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993, 192, 553–560. [Google Scholar] [CrossRef]
- Hinson, J.P.; Kapas, S.; Smith, D.M. Adrenomedullin, a multifunctional regulatory peptide. Endocr. Rev. 2000, 21, 138–167. [Google Scholar] [CrossRef]
- Iesato, Y.; Yuda, K.; Chong, K.T.Y.; Tan, X.; Murata, T.; Shindo, T.; Yanagi, Y. Adrenomedullin: A potential therapeutic target for retinochoroidal disease. Prog. Retin. Eye Res. 2016, 52, 112–129. [Google Scholar] [CrossRef]
- McLatchie, L.M.; Fraser, N.J.; Main, M.J.; Wise, A.; Brown, J.; Thompson, N.; Solari, R.; Lee, M.G.; Foord, S.M. RAMPS regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998, 393, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Gröschl, M.; Wendler, O.; Topf, H.G.; Bohlender, J.; Köhler, H. Significance of salivary adrenomedullin in the maintenance of oral health: Stimulation of oral cell proliferation and antibacterial properties. Regul. Pept. 2009, 154, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; MacPherson, L.J.; Parada, C.A.; Zuker, C.S.; Ryba, N.J.P. Rewiring the taste system. Nature 2017, 548, 330–333. [Google Scholar] [CrossRef]
- Sukumaran, S.K.; Lewandowski, B.C.; Qin, Y.; Kotha, R.; Bachmanov, A.A.; Margolskee, R.F. Whole transcriptome profiling of taste bud cells. Sci. Rep. 2017, 7, 7595. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Arcaya, I.; Lostao, M.P.; Martínez, A.; Berjón, A.; Barber, A. Effect of adrenomedullin and proadrenomedullin N-terminal 20 peptide on sugar transport in the rat intestine. Regul. Pept. 2005, 129, 147–154. [Google Scholar] [CrossRef]
- Damak, S.; Mosinger, B.; Margolskee, R.F. Transsynaptic transport of wheat germ agglutinin expressed in a subset of type II taste cells of transgenic mice. BMC Neurosci. 2008, 9, 96. [Google Scholar] [CrossRef]
- Damak, S.; Rong, M.; Yasumatsu, K.; Kokrashvili, Z.; Varadarajan, V.; Zou, S.; Jiang, P.; Ninomiya, Y.; Margolskee, R.F. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 2003, 301, 850–853. [Google Scholar] [CrossRef]
- Watanabe, H.; Takahashi, E.; Kobayashi, M.; Goto, M.; Krust, A.; Chambon, P.; Iguchi, T. The estrogen-responsive adrenomedullin and receptor-modifying protein 3 gene identified by DNA microarray analysis are directly regulated by estrogen receptor. J. Mol. Endocrinol. 2006, 36, 81–89. [Google Scholar] [CrossRef]
- Talavera, K.; Yasumatsu, K.; Voets, T.; Droogmans, G.; Shigemura, N.; Ninomiya, Y.; Margolskee, R.F.; Nilius, B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 2005, 438, 1022–1025. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Max, M.; Shanker, Y.G.; Huang, L.; Rong, M.; Liu, Z.; Campagne, F.; Weinstein, H.; Damak, S.; Margolskee, R.F. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat. Genet. 2001, 28, 58–63. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Yarmolinsky, D.; Buchholtz, L.; Oka, Y.; Sly, W.; Ryba, N.J.P.; Zuker, C.S. The taste of carbonation. Science 2009, 326, 443–445. [Google Scholar] [CrossRef]
- Nishikimi, T.; Kuwahara, K.; Nakagawa, Y.; Kangawa, K.; Nakao, K. Adrenomedullin in Cardiovascular Disease: A Useful Biomarker, its Pathological Roles and Therapeutic Application. Curr. Protein Pept. Sci. 2013, 14, 256–267. [Google Scholar] [CrossRef]
- Yamada, K.; Saito, M.; Matsuoka, H.; Inagaki, N. A real-time method of imaging glucose uptake in single, living mammalian cells. Nat. Protoc. 2007, 2, 753–762. [Google Scholar] [CrossRef]
- Poyner, D.R.; Sexton, P.M.; Marshall, I.; Smith, D.M.; Quirion, R.; Born, W.; Muff, R.; Fischer, J.A.; Foord, S.M. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002, 54, 233–246. [Google Scholar] [CrossRef]
- Ishida, Y.; Ugawa, S.; Ueda, T.; Murakami, S.; Shimada, S. Vanilloid receptor subtype-1 (VR1) is specifically localized to taste papillae. Mol. Brain Res. 2002, 107, 17–22. [Google Scholar] [CrossRef]
- Huang, A.Y.; Wu, S.Y. Calcitonin gene-related peptide reduces taste-evoked ATP secretion from mouse taste buds. J. Neurosci. 2015, 35, 12714–12724. [Google Scholar] [CrossRef]
- Martínez, A.; Oh, H.R.; Unsworth, E.J.; Bregonzio, C.; Saavedra, J.M.; Stevenson, W.G.S.; Cuttitta, F. Matrix metalloproteinase-2 cleavage of adrenomedullin produces a vasoconstrictor out of a vasodilator. Biochem. J. 2004, 383, 413–418. [Google Scholar] [CrossRef]
- Gardiner, S.M.; Kemp, P.A.; March, J.E.; Bennett, T. Regional haemodynamic effects of human and rat adrenomedullin in conscious rats. Br. J. Pharmacol. 1995, 114, 584–591. [Google Scholar] [CrossRef]
- Sakata, J.; Shimokubo, T.; Kitamura, K.; Nakamura, S.; Kangawa, K.; Matsuo, H.; Eto, T. Molecular cloning and biological activities of rat adrenomedullin, a hypotensive peptide. Biochem. Biophys. Res. Commun. 1993, 195, 921–927. [Google Scholar] [CrossRef]
- Bełtowski, J.; Jamroz, A. Adrenomedullin--what do we know 10 years since its discovery? Pol. J. Pharmacol. 2004, 56, 5–27. [Google Scholar] [PubMed]
- Gilbertson, T.A.; Roper, S.D.; Kinnamon, S.C. Proton currents through amiloride-sensitive Na+ channels in isolated hamster taste cells: Enhancement by vasopressin and CAMP. Neuron 1993, 10, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Abaffy, T.; Trubey, K.R.; Chaudhari, N. Adenylyl cyclase expression and modulation of cAMP in rat taste cells. Am. J. Physiol. Cell Physiol. 2003, 284, C1420–C1428. [Google Scholar] [CrossRef] [PubMed]
- Mummalaneni, S.; Qian, J.; Phan, T.H.; Rhyu, M.R.; Heck, G.L.; DeSimone, J.A.; Lyall, V. Effect of ENaC Modulators on Rat Neural Responses to NaCl. PLoS ONE 2014, 9, e98049. [Google Scholar] [CrossRef]
- Liu, F.Y.; Cogan, M.G. Angiotensin II stimulates early proximal bicarbonate absorption in the rat by decreasing cyclic adenosine monophosphate. J. Clin. Investig. 1989, 84, 83–91. [Google Scholar] [CrossRef]
- Lingueglia, E.; Renard, S.; Waldmann, R.; Voilley, N.; Champigny, G.; Plass, H.; Lazdunski, M.; Barbry, P. Different homologous subunits of the amiloride-sensitive Na+ channel are differently regulated by aldosterone. J. Biol. Chem. 1994, 269, 13736–13739. [Google Scholar] [CrossRef]
- Snyder, P.M.; Olson, D.R.; Kabra, R.; Zhou, R.; Steines, J.C. cAMP and Serum and Glucocorticoid-inducible Kinase (SGK) Regulate the Epithelial Na+ Channel through Convergent Phosphorylation of Nedd4-2. J. Biol. Chem. 2004, 279, 45753–45758. [Google Scholar] [CrossRef]
- Shigemura, N.; Iwata, S.; Yasumatsu, K.; Ohkuri, T.; Horio, N.; Sanematsu, K.; Yoshida, R.; Margolskee, R.F.; Ninomiya, Y. Angiotensin II modulates salty and sweet taste sensitivities. J. Neurosci. 2013, 33, 6267–6277. [Google Scholar] [CrossRef]
- Herness, M.S. Aldosterone increases the amiloride-sensitivity of the rat gustatory neural response to NaCl. Comp. Biochem. Physiol. Comp. Physiol. 1992, 103, 269–273. [Google Scholar] [CrossRef]
- Peng, H.; Lever, J.E. Regulation of Na+-coupled glucose transport in LLC-PK1 cells: Message stabilization induced by cyclic AMP elevation is accompanied by binding of a Mr = 48,000 protein to a uridine-rich domain in the 3′-untranslated region. J. Biol. Chem. 1995, 270, 23996–24003. [Google Scholar] [CrossRef]
- Dyer, J.; Vayro, S.; Shirazi-Beechey, S.P. Mechanism of glucose sensing in the small intestine. Biochem. Soc. Trans. 2003, 31, 1140–1142. [Google Scholar] [CrossRef]
- Moran, A.W.; Al-Rammahi, M.A.; Batchelor, D.J.; Bravo, D.M.; Shirazi-Beechey, S.P. Glucagon-Like Peptide-2 and the Enteric Nervous System Are Components of Cell-Cell Communication Pathway Regulating Intestinal Na Glucagon-Like Peptide-2 and the Enteric Nervous System Are Components of Cell-Cell Communication Pathway Regulating Intestinal Na+/Glucose Co-transport. Front. Nutr. 2018, 5, 101. [Google Scholar] [CrossRef]
- Lee, W.Y.; Loflin, P.; Clancey, C.J.; Peng, H.; Lever, J.E. Cyclic nucleotide regulation of Na+/glucose cotransporter (SGLT1) mRNA stability: Interaction of a nucleocytoplasmic protein with a regulatory domain in the 3′-untranslated region critical for stabilization. J. Biol. Chem. 2000, 275, 33998–34008. [Google Scholar] [CrossRef]
- Yoshida, R.; Margolskee, R.F.; Ninomiya, Y. Phosphatidylinositol-3 kinase mediates the sweet suppressive effect of leptin in mouse taste cells HHS Public Access. J. Neurochem. 2021, 158, 233–245. [Google Scholar] [CrossRef]
- Jung, K.M.; Mangieri, R.; Stapleton, C.; Kim, J.; Fegley, D.; Wallace, M.; Mackie, K.; Piomelli, D. Stimulation of Endocannabinoid Formation in Brain Slice Cultures through Activation of Group I Metabotropic Glutamate Receptors. Mol. Pharmacol. 2005, 68, 1196–1202. [Google Scholar] [CrossRef]
- Jung, K.M.; Astarita, G.; Zhu, C.; Wallace, M.; Mackie, K.; Piomelli, D. A Key Role for Diacylglycerol Lipase-in Metabotropic Glutamate Receptor-Dependent Endocannabinoid Mobilization. Mol. Pharmacol. 2007, 72, 612–621. [Google Scholar] [CrossRef]
- Arai, T.; Ohkuri, T.; Yasumatsu, K.; Kaga, T.; Ninomiya, Y. The role of transient receptor potential vanilloid-1 on neural responses to acids by the chorda tympani, glossopharyngeal and superior laryngeal nerves in mice. Neuroscience 2010, 165, 1476–1489. [Google Scholar] [CrossRef]
- Yasumatsu, K.; Iwata, S.; Inoue, M.; Ninomiya, Y. Fatty acid taste quality information via GPR120 in the anterior tongue of mice. Acta Physiol. 2019, 226, e13215. [Google Scholar] [CrossRef]
- Taylor, G.M.; Meeran, K.; O’Shea, D.; Smith, D.M.; Ghatei, M.A.; Bloom, S.R. Adrenomedullin Inhibits Feeding in the Rat by a Mechanism Involving Calcitonin Gene-Related Peptide Receptors. Endocrinology 1996, 137, 3260–3264. [Google Scholar] [CrossRef]
- Wang, G.; Brumfield, B.; DiCroce, M.; Nelson, L.; Newmyer, B.A.; Flower, J.; Hipskind, K.; Sharma, S.; Gilbert, E.R.; Cline, M.A. Anorexigenic effects of central adrenomedullin are associated with hypothalamic changes in juvenile Gallus gallus. Gen. Comp. Endocrinol. 2014, 204, 223–228. [Google Scholar] [CrossRef]
- Wang, J.; Webster, A.; Sims, W.; Gilbert, E.R.; Cline, M.A. The anorexigenic effect of adrenomedullin in Japanese quail (Coturnix japonica) involves increased proopiomelanocortin and cocaine- and amphetamine-regulated transcript mRNAs in the arcuate nucleus of the hypothalamus. Domest. Anim. Endocrinol. 2021, 74, 106465. [Google Scholar] [CrossRef] [PubMed]




| Forward Primer | Reverse Primer | |
|---|---|---|
| GAPDH | TGTGTCCGTCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG |
| SGLT1 | TCTGTAGTGGCAAGGGGAAG | ACAGGGCTTCTGTGTCTTGG |
| T1R3 | CAAGGCCTGCAGTGCACAA | AGGCCTTAGGTGGGCATAATAGGA |
| GLUT2 | GAGTTCCTTCCAGTTCGGCTATG | GTTCCACTGGATGACCGG |
| GLUT4 | CTGTAACTTCATTGTCGGCATGG | AGGCAGCTGAGATCTGGTCAAAC |
| GLUT8 | TCTGCATGTCAAGGGTGTGG | AGGGACAACGGTCAGTGTGAATAG |
| RAMP2/T1R3 | 68.6% (70/102, n = 26) | T1R3/RAMP2 | 87.5% (70/80, n = 26) |
| CRLR/T1R3 | 78.4% (80/102, n = 26) | T1R3/CRLR | 56.3% (80/142, n = 26) |
| ADM receptor /T1R3 | 67.6% (69/102, n = 26) | T1R3 /ADM receptor | 89.6% (69/77, n = 26) |
| RAMP2/CA4 | 13.0% (6/46, n = 29) | CA4/RAMP2 | 11.8% (6/51, n = 29) |
| CRLR/CA4 | 76.1% (35/46, n = 29) | CA4/CRLR | 36.1% (35/97, n = 29) |
| ADM receptor /CA4 | 13.0% (6/46, n = 29) | CA4 /ADM receptor | 12.2% (6/49, n = 29) |
| Figure 2B | Figure 3B | Figure 4A | Figure 4B | |
|---|---|---|---|---|
| Glc | t(12) = 5.37 p < 0.001 | t(12) = −0.507 p = 0.311 | t(23) = −4.84 p < 0.001 | t(18) = 0.951 p = 0.177 |
| Suc | t(12) = 2.52 p = 0.013 | t(12) = −1.37 p = 0.099 | t(12) = −3.21 p = 0.003 | t(12) = −1.467 p = 0.168 |
| SC | t(12) = 0.875 p = 0.199 | t(12) = −0.558 p = 0.293 | t(15) = 0.095 p = 0.463 | t(15) = −0.488 p = 0.316 |
| NaCl | t(12) = 0.097 p = 0.462 | t(12) = −0.811 p = 0.217 | t(19) = 0.034 p = 0.487 | t(15) = 1.58 p = 0.068 |
| KCl | t(12) = −0.951 p = 0.18 | t(12) = 0.37 p = 0.359 | t(20) = −0.794 p = 0.218 | t(15) = −0.074 p = 0.471 |
| HCl | t(12) = −1.88 p = 0.084 | t(12) = −1.16 p = 0.133 | t(16) = −0.866 p = 0.2 | t(13) = −0.209 p = 0.419 |
| QHCl | t(12) = −0.051 p = 0.48 | t(12) = 1.04 p = 0.16 | t(20) = 0.244 p = 0.405 | t(15) = 0.356 p = 0.363 |
| MPG | t(12) = 0.591 p = 0.283 | t(12) = −0.147 p = 0.443 | t(18) = −0.075 p = 0.471 | t(14) = −0.285 p = 0.39 |
| Treatment | Concentration | Interaction | |
|---|---|---|---|
| Glc | F(1,72) = 37.9 p < 0.001 | F(5,72) = 202 p < 0.001 | F(5,72) = 3.13 p < 0.05 |
| NaCl | F(1,60) = 2.14 p = 0.149 | F(4,60) = 273 p < 0.001 | F(4,60) = 0.625 p = 0.647 |
| Treatment | Time | Interaction | |
|---|---|---|---|
| Glc | F(1,84) = 29.9 p < 0.001 | F(6,84) = 8.15 p < 0.001 | F(6,84) = 5.78 p < 0.001 |
| NaCl | F(1,84) = 0.032 p = 0.755 | F(6,84) = 0.709 p = 0.961 | F(6,84) = 0.136 p = 0.842 |
| Treatment | Concentration | Interaction | |
|---|---|---|---|
| Glc | F(1,72) = 0.751 p = 0.389 | F(5,72) = 131 p < 0.001 | F(5,72) = 0.54 p = 0.745 |
| NaCl | F(1,60) = 0.181 p = 0.672 | F(4,60) = 340 p < 0.001 | F(4,60) = 0.376 p = 0.825 |
| Treatment | Time | Interaction | |
|---|---|---|---|
| Glc | F(1,84) = 0.032 p = 0.858 | F(6,84) = 0.709 p = 0.643 | F(6,84) = 0.136 p = 2.21 |
| NaCl | F(1,84) = 2.36 p = 0.128 | F(6,84) = 0.113 p = 0.995 | F(6,84) = 0.253 p = 0.957 |
| FP | CV | NT | SI | |
|---|---|---|---|---|
| SGLT1 | F(2,29) = 6.134 p = 0.006 | F(2,29) = 5.525 p = 0.009 | F(2,30) = 0.029 p = 0.972 | F(2,26) = 8.997 p = 0.001 |
| T1R3 | F(2,24) = 0.501 p = 0.612 | F(2,27) = 0.406 p = 0.670 | F(2,25) = 0.291 p = 0.750 | F(2,20) = 0.233 p = 0.794 |
| GLUT2 | F(2,23) = 0.322 p = 0.728 | F(2,26) = 0.031 p = 0.970 | F(2,26) = 0.016 p = 0.984 | F(2,26) = 0.099 p = 0.906 |
| GLUT4 | F(2,18) = 1.019 p = 0.381 | F(2,19) = 1.361 p = 0.280 | F(2,26) = 0.412 p = 0.667 | F(2,27) = 0.340 p = 0.715 |
| GLUT8 | F(2,17) = 0.171 p = 0.845 | F(2,16) = 1.638 p = 0.225 | F(2,26) = 0.248 p = 0.782 | F(2,32) = 0.248 p = 0.782 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwata, S.; Yoshida, R.; Takai, S.; Sanematsu, K.; Shigemura, N.; Ninomiya, Y. Adrenomedullin Enhances Mouse Gustatory Nerve Responses to Sugars via T1R-Independent Sweet Taste Pathway. Nutrients 2023, 15, 2941. https://doi.org/10.3390/nu15132941
Iwata S, Yoshida R, Takai S, Sanematsu K, Shigemura N, Ninomiya Y. Adrenomedullin Enhances Mouse Gustatory Nerve Responses to Sugars via T1R-Independent Sweet Taste Pathway. Nutrients. 2023; 15(13):2941. https://doi.org/10.3390/nu15132941
Chicago/Turabian StyleIwata, Shusuke, Ryusuke Yoshida, Shingo Takai, Keisuke Sanematsu, Noriatsu Shigemura, and Yuzo Ninomiya. 2023. "Adrenomedullin Enhances Mouse Gustatory Nerve Responses to Sugars via T1R-Independent Sweet Taste Pathway" Nutrients 15, no. 13: 2941. https://doi.org/10.3390/nu15132941
APA StyleIwata, S., Yoshida, R., Takai, S., Sanematsu, K., Shigemura, N., & Ninomiya, Y. (2023). Adrenomedullin Enhances Mouse Gustatory Nerve Responses to Sugars via T1R-Independent Sweet Taste Pathway. Nutrients, 15(13), 2941. https://doi.org/10.3390/nu15132941






