Abstract
Brugmansia suaveolens Bercht. & J. Presl has been widely used due to the presence of different bioactive compounds. This review summarizes the latest advances and perspectives of the B. suaveolens plant species; it is a systematic literature review on aspects of botany, traditional uses, phytochemistry, pharmacology, and toxicology as therapeutic potential. In addition, 120 compounds are described, including alkaloids, flavonoids, terpenoids, steroids, amino acids, aromatics, and aliphatics. As for the therapeutic potential, it is described in extracts and compounds in the antitumor, anti-inflammatory, antioxidant, antimicrobial, antispasmodic, anticoagulant, and analgesic aspects, as well as the effects on the central nervous system. The toxicity of the genus stands out, especially the potential for organ toxicity. Therefore, this review evidenced the knowledge related to the traditional use based on the scientific research of Brugmansia suaveolens, highlighting an overview of bioactive compounds and biological and toxicological activities in order to provide a scientific basis for future studies on the value of this species for the development of new natural products.
1. Introduction
Medicinal plants have been used as inexhaustible sources of new substances with potential therapeutic effects. Chemical and pharmacological studies of natural products have been the focus of many surveys in the scientific world, aiming at the discovery of new compounds with therapeutic activity due to the high costs of research and the elaboration of synthetic medications. There are several plant species capable of generating research and development based on the claim of a given therapeutic effect, which can become a valuable tool for the discovery of new drugs [1,2].
The Solanaceae family has approximately 150 genera and 300 cataloged species. This family holds species of great economic importance worldwide; most of its species are found in tropaneal areas such as Brazil. It is considered the third most economically important plant family and the first among vegetables. Some examples of these species are tomato (Solanum lycopersicum), with major importance in agriculture, and, for the pharmaceutical industry, Atropa belladonna stands out, among others [3,4].
The species from this family can be easily found in homes and gardens, such as the “trumpet tree” (Brugmansia suaveolens Bercht. & J. Presl), widely cultivated as an ornamental piece due to its characteristic odor and the beauty of its flowers. Within the research scope, plants belonging to the Solanaceae family are known to produce tropane alkaloids, a group of commonly toxic secondary metabolites used as plant defense [4,5].
Brugmansia suaveolens Bercht. & J. Presl is used in folk medicine for therapeutic purposes and in Peruvian religious myths to seek changes in the individual’s conscious state. Popularly, its dried flowers and leaves are used to treat strong coughs and bronchitis by inhaling its vapor. In the form of juice and/or ointment, it is applied to burns, abrasions, inflammation, hemorrhoids, arthritis, and rheumatism on the affected areas to relieve the pain generated by this trauma [6,7,8].
Previous studies show that species from this genus have tropaneal alkaloids such as scopolamine and atropine. The toxic action of this genus occurs due to the anticholinergic action of alkaloids, which are acetylcholine antagonists at muscarinic receptors, inhibiting the action of this transmitter in autonomic effectors and smooth muscles, decreasing mucous secretions, and blocking the action of the myocardial vagus nerve, providing increased heart rate [9,10,11].
Ingestion of this plant species in high doses makes the tropane alkaloids stimulate the central nervous system, causing depression of the peripheral nerves, which can cause some adverse effects such as disorientation, hallucinations, and panic. In more severe cases, the individual presents with neurological depression and cardiovascular and respiratory disorders, which may precede death [7,11].
Brazilian diversity contributes to the existence of a wide biodiversity, becoming an example of balanced ecosystems that provide a variety of still-explored plant communities. B. suaveolens Bercht. and J. Presl is still a little-reported species regarding its biological effects, which enable the elucidation of substances of natural origin, stimulating the discovery of new products with different applications. This lack of comprehensive reports enables unprecedented studies using substances of natural origin, stimulating the discovery of new products with different applications [4,8,12].
Currently, the search for new active molecules in biodiversity has intensified, and different active ingredients have been used for the development of new effective and safe technologies; however, many of these substances have an effective biological action but high toxicity. An alternative for reducing toxic substances is the development of nanopharmaceuticals in order to reduce systemic adverse effects, resulting in better adherence to the treatment. Different secondary metabolites, such as alkaloids, are oftentimes associated with different adverse effects in clinical practice due to their biological effects. Technological development represents a paradigm shift with the potential to reduce its unwanted effects by improving administration and application and minimizing toxicity [13].
This review seeks to provide an overview of the bioactive compounds from B. suaveolens Bercht. & J. Presl, aiming to support the evaluation of biological and toxic effects based on the presence of tropane alkaloids, a class of secondary metabolite that is promising in terms of its therapeutic application as a bioactive molecule.
2. Materials and Methods
This qualitative approach study reviewed the literature in question for better understanding the bioactive compounds related to the Brugmansia suaveolens plant species and their therapeutic applications. It was decided to carry out a systematic review, defined as an instrument for obtaining, identifying, analyzing, and summarizing the literature directed to the specific theme. It also allows for a broad literature review, including discussions of methods and publication results. Articles, monographs, dissertations, and books published on the subject matter were consulted in the SciELO, Science Direct, PubMed, and Medline databases.
To identify the study designs, the terms Brugmansia suaveolens, Solanaceae, and Tropane alkaloids were used. The pre-selection of the studies was based on reading the titles and/or abstracts and, when necessary, the full texts. The use of the articles was analyzed by consensus, rejecting those that did not present specific data about the research. Articles were obtained based on studies of the Solanaceae family, genus Brugmansia, Brugmansia suaveolens plant species, and the Datura suaveolens synonym. Of the 301 articles analyzed, 193 were excluded from the research because they did not present specific content for carrying out the work, and 108 presented essential data for carrying out the bibliographic survey.
The data will be reported following the recommendations set forth in the JBI Manual for Evidence Synthesis and PRISMA for Scoping Reviews (PRISMA ScR) and initially presented through a flowchart recommended by PRISMA ScR to indicate the evidence search flow. Subsequently, tables will be presented with information extracted from the articles included, taking into account the population, concept, and context. From the analysis of the tables, graphs will be prepared to present the correlations obtained in a didactic way. After presenting the data, they will be discussed in depth in order to list future research gaps and the limitations of the studies that will serve as a basis for further research focused on the analysis of this review.
3. Results and Discussion
After the selection process, 108 studies met the inclusion criteria. The study selection process is shown in a flow diagram (Figure 1), according to the PRISMA standards.
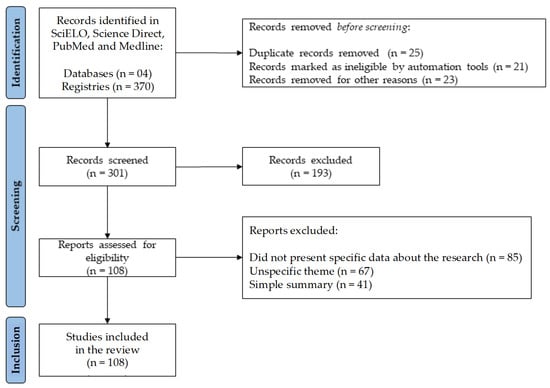
Figure 1.
Flowchart with the study selection steps adapted from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
3.1. Medicinal Plants and Their Bioactive Compounds
Currently, pharmaceutical products are considered a promising market that moves a large part of the world economy with constant product innovations. In this context, natural products have wide applicability in the pharmaceutical market, in the production of new drugs, and in other economic sectors [14]. Nature is the biggest producer of known organic substances. Natural products offer a wide variety of bioactive molecules with great diversity in their structures and biological activities [15].
Different methods can be used to synthesize secondary metabolites with therapeutic action. Plant species can be used in these processes. Many of these substances can be highly toxic and may even be carcinogenic [13,16,17].
Secondary metabolites play an important role in the interaction between the environment and its defense against invaders. Vegetation has a wide variety of secondary metabolites, which are synthesized from primary metabolites (e.g., carbohydrates, lipids, and amino acids). These compounds are necessary in the defense against herbivores, pathogens, and environmental stresses [18]. They also have characteristics that contribute to the plants’ specific odors, tastes, and colors [19].
These substances have numerous applications, such as food additives, flavors, and industrially important products such as the development of new drugs [20]. Some of the natural products derived from plants include drugs such as morphine, codeine, cocaine, pilocarpine, and steroids such as diosgenin, digoxin, and digitoxin [8,21].
Medicinal plants are considered a great source of phytochemical compounds given their therapeutic activity, which enables the development of new drugs. Most natural compounds are of plant origin, such as phenolic substances and flavonoids, which are used in cancer treatment and prevention as well as for their antioxidant activity [22].
The interest in using natural sources in the development and formulation of skin care products such as antioxidant, photoprotective, and anti-aging products is considered an alternative to conventional cosmetic products and phytomedications, contributing to the increasing interest in research and industrial application of medicinal plants [23,24,25]. The medications derived from medicinal plants used in clinical practice in recent years are presented in Table 1.

Table 1.
Medications derived from medicinal plants in clinical practice in recent years.
In the context of discovering new herbal substances, an advantageous approach is essential when applied to samples from regions marked by high biodiversity and endemism, as the chemical diversity of natural products can reflect the biodiversity of their organisms of origin [49,50].
The ethnopharmacological approach is a study where the use of traditional medicine with medicinal plants constitutes the basis for the selection of test materials and pharmacological assays. Ethnopharmacology involves the observation, description, and experimental investigation of traditionally used drugs and their bioactivities. It represents a transdisciplinary concept based on botany, chemistry, biochemistry, and pharmacology [25,51,52].
3.2. Brugmansia Suaveolens Bercht. & J. Presl
The Solanaceae family has approximately 100 genera and 2300 cataloged species. As a holder of species of world economic importance, most of its species are found in tropaneal areas such as Brazil, where it occupies a prominent place among economically important plants. Some examples of these species are tomato (Solanum lycopersicum) and, for the pharmaceutical industry, Atropa belladonna [3].
Brugmansia suaveolens Bercht. & J. Presl (Humb. & Bonpl. ex Willd.), considered a botanical synonym of Datura suaveolens (Humb. & Bonpl. ex Willd.), is a plant mainly used by indigenous peoples and credited with having originated in the Andes. It grows in Peru, Bolivia, and Ecuador in the Solanaceae family, genus Brugmansia, and species suaveolens; its growth is in the form of shrubs, reaching approximately 3–9 m in height, or even more in favorable conditions. It has white or pink flowers but presents color variations; it is aromatic, in the shape of a trombette, and can measure up to 15–50 cm, from where its popular name arises, “Trompete”, as well as other popular names such as “Saia Branca”, “Cartuchiller”, “Canudo”, or “Zabumba” [3,4].
Its leaves vary from 15–30 cm in length and approximately 10 cm in width; the best type of soil for its cultivation is in humid places, it is easily found near rivers, and the time of year for its harvest and the intensity of exposure to sunlight can significantly interfere with greater or lesser yields of tropane alkaloids [3].
The Brugmansia species is native to South America. Previously, this species was considered a subgenus of Datura; however, more recent research shows that it should be classified within a genus of its own. Its popular use and wide distribution in the Americas demonstrate its relationship with men [53].
3.3. Tropane Alkaloids
Tropane alkaloids are named after nightshades and feature a tropane ring consisting of the pyrrolidine and piperidine rings. Tropanes have a bicyclic structure called the tropane ring. Nearly 150 tropane alkaloids are known, most of which are pyrrolidine derivatives such as hygrine and cuscohygrine, and the main ones are atropine, hyoscyamine, scopolamine, and cocaine. Atropine and scopolamine are potent anticholinergic agents used in therapy in the form of sulfate salts for ophthalmic use and as gastrointestinal relaxants [54,55,56].
Among the active pharmaceutical ingredients that have the tropane portion in their structure, the most significant in terms of volume and production value are those of natural origin, including atropine, hyoscyamine, and scopolamine. It is a group of its semi-synthetic derivatives that can be obtained by a single or more chemical steps, which can result in the formation of quaternary ammonium salts or undergo other chemical modifications or substitutions of functional groups. The biggest product in terms of production of this tropane active ingredient is scopolamine butylbromide (original preparation: Buscopan®, usually in soft capsules or coated tablets of 10 mg manufactured by Boehringer Ingelheim) with indications for problems of the intestinal tract, particularly as an antispasmodic agent [57,58,59].
Drugs containing tropane alkaloids are therapeutically indicated against colics in the ureters and those caused by kidney stones, bronchial spasms, cases of bronchial asthma, spasms of the gastrointestinal tract, and gastric hypersecretion. This group of substances is also used as local anesthetics, as they act by desensitizing nerve endings [56].
The biosynthesis of alkaloids takes place through metabolic pathways that have not yet been fully biochemically delineated due to the fact that many of the enzymes involved in several steps have not yet been isolated and characterized. The formation of the heterocyclic system of alkaloids normally occurs through simple inter- or intra-molecular reactions. In general, alkaloids are formed from amino acids. The alkaloids of this group are esters of acids derived from the phenylalanine amino acid by a rearrangement process [55,56].
Brugmansia suaveolens Bercht. & J. Presl present different secondary metabolites, which include alkaloids, steroids, phenolic compounds, terpenes, triterpenes, and flavonoids, among others, related to therapeutic potential [4,60,61]. The main metabolites are the tropane alkaloids related to significant biological activities and recognized as the main secondary metabolites in medications derived from plants [62].
These alkaloids represent 40% of all compounds isolated from the genus B. suaveolens Bercht. & J. Presl, therefore also helping as chemotaxonomic markers; in this way, the compounds found in this species and in their respective organs are presented in Table 2, as they have a wide spectrum of therapeutic activities. Among the reports in the literature, Figure 2 represents the compounds described and elucidated in the literature on the species in question, including 47 tropane alkaloids; 04 pyrrolidine and indole; 03 sesquiterpenoids; 26 monoterpenoids; 07 flavonoids; 06 carotenoids; 10 benzenoid compounds; 05 aldehydes; 03 alkanes; and another 09 elucidated compounds, mainly found in calculus, roots, leaves, flowers, and seeds, demonstrating countless possibilities of new biologically active compounds, thus being considered a promising alternative for the chemistry of natural products.

Table 2.
Compounds found in Brugmansia suaveolens Bercht. & J. Presl.
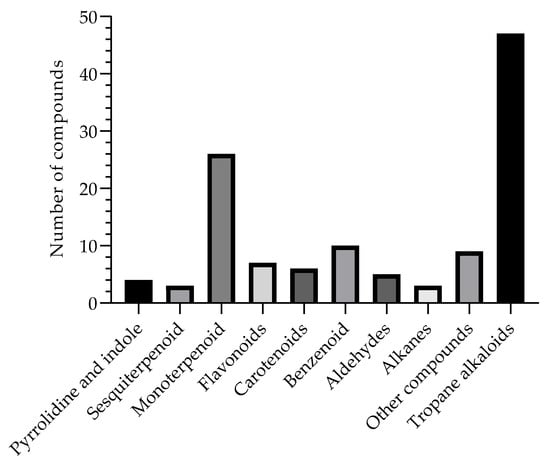
Figure 2.
Compounds isolated and described in the B. suaveolens Bercht. & J. Presl plant species obtained, compiled as source data for the review.
Based on the description of the compounds isolated from the B. suaveolens Bercht. & J. Presl plant species, Figure 3 shows the list of the main phytochemical constituents described in the literature. Thus, this review allows us to observe that Tropane alkaloids are the most frequently described compounds in the literature, followed by Monoterpenoids, respectively, corroborating data from the traditional use of the plant species.
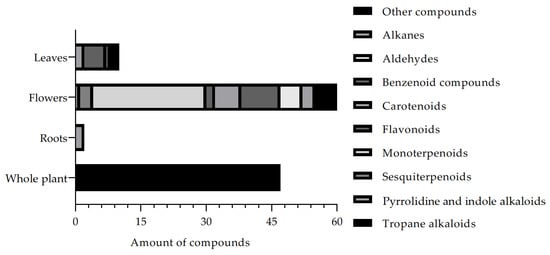
Figure 3.
Compounds isolated and described in the B. suaveolens Bercht. & J. Presl plant species obtained, compiled as source data for the review.
Another fact that can be observed through the review is the frequency of compounds isolated and described through the bibliographic survey, in which the percentage (%) is presented based on the total number of studies analyzed in this review (Figure 4). Thus, it is possible to describe that the compounds commonly isolated from the different organs of the plant and described in studies based on extraction, isolation, and biological studies are the flowers, which represent 51% of the studies, followed by the whole plant with 38% and the leaves with 8%, while the roots represent 1.6%, being respectively associated with the B. suaveolens Bercht. & J. Presl plant species. These data allow for contributing to a broad spectrum of research on flowers, such as risks in a variety of compounds, whereas for roots, further research should be encouraged to expand knowledge and related secondary metabolites.
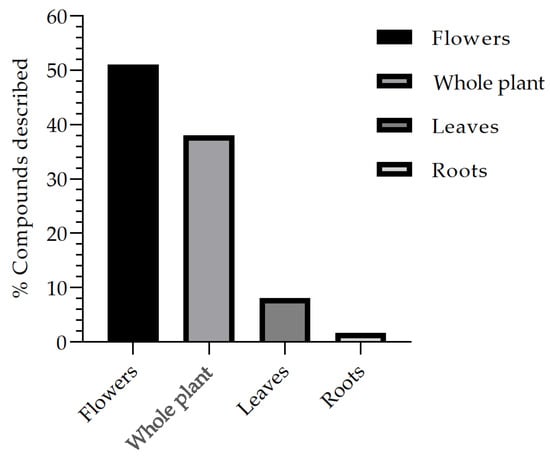
Figure 4.
Frequency of the main parts of the B. suaveolens Bercht. & J. Presl plant species used for compound isolation studies and biological assays.
3.4. Therapeutic Applications of Tropane Alkaloids from Brugmansia spp.
Plants from the genus Brugmansia, as well as other Solanaceae, have alkaloids with wide therapeutic applications. Scopolamine and its derivatives have parasympatholytic, anticholinergic, antiemetic, and sedative actions; these substances are mainly used as pre-anesthetics. Due to their action, they become mydriatic and cycloplegic agents, with a mechanism similar to atropine [1,4,12].
Brugmansia species differ in the concentration of atropine and scopolamine, both CNS depressants with sedative and tranquilizing properties, relevant ophthalmic action, and in the salivary, bronchial, and sweat glands. One of the adverse effects of scopolamine is drowsiness, which can also produce excitement and hallucinations. These effects are similar to those caused by toxic doses [4,12], although scopolamine can still be used as a heroin detoxifying agent without causing dependence [12].
Regarding pharmacodynamics, scopolamine differs only quantitatively from atropine. While atropine has almost no detectable CNS effects at clinically applicable doses, scopolamine exerts prominent CNS effects at low therapeutic doses. This difference can be explained by better penetration of scopolamine into the blood–brain barrier [4,12].
The medicinal properties of plants can be based on phytochemical effects such as antioxidant, antimicrobial, and antipyretic activity. In this way, medicinal plants can be considered potent and promising therapeutic agents for the improvement of processes such as wound healing based on their variety of active and effective components, including flavonoids, alkaloids, phenolic compounds, and terpenoids. These metabolites can be adhered to as modern therapy due to their low cost, limited adverse effects, bioavailability, and efficacy. The emergence and development of nanoscience and technology can help improve the effectiveness of different therapies. Thus, nanoformulations have advantages over conventional therapy, providing a unique opportunity to ease the treatment of skin lesions, even for chronic wounds, and providing an efficient and fast healing process, resulting in reduced hospitalization costs [81].
Traditional uses of Brugmansia and bioactive compounds have led to improved validation of the species’ therapeutic potential. These extracts have been shown to have a wide range of pharmacological properties. Table 3 shows the traditional uses and biological activities of the B. suaveolens Bercht. & J. Presl species.

Table 3.
Therapeutic potential of Brugmansia suaveolens Bercht. & J. Presl and its bioactive compounds.
3.4.1. Anti-Inflammatory Activity
Although many traditional use reports mention that the genus Brugmansia has different pharmacological properties, there is little research directing the proven biological activities to support these traditional uses. Recently, the anti-inflammatory activities of extracts from B. suaveolens flowers and leaves were evaluated through cellular electrophoretic analysis of NF-kB, p38α, TNFα and elastase. The data showed that the ethanol extract (100 μg/mL) from B. suaveolens inhibited NF-kB binding to DNA. The extract also shows inhibition of the p38α action with a value of 54.86 ± 2.82 μg/mL. These extracts directly altered elastase activity, with a value of 51.35 ± 0.69 μg/mL. In the elastase assay by human neutrophils, they showed release (65.98 ± 1.84 μg/mL) [106]. Although this study presents several in vitro tests to investigate the anti-inflammatory activity of different parts of B. suaveolens, further pharmacological and phytochemical studies are required to precisely identify which isolated compound is responsible for the anti-inflammatory effect.
3.4.2. Cytotoxic Activity
The evaluation of the cytotoxicity of extracts from B. suaveolens leaves and flowers was analyzed by colometry assay through reaction with (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). The results suggest that extracts (100 μg/mL) from the plants show cytotoxic effects with a value of 92 ± 0.7 μg/mL [106]. However, the constituents of the extracts must be elucidated by bioguided isolation to define the cytotoxic relationship. Furthermore, phytochemical studies of the ethanolic extract from B. suaveolens leaves led to the isolation of a new monoterpenoid with immunomodulator-mediated antitumor activity. The results showed that this monoterpenoid increased the secretion of IFN-γ, an immunological marker, and of IL-2 from peripheral blood mononuclear cells. They therefore observed an increase in the number of cell deaths in the A549 and MCF7 cell lines and showed an increase in ROS production and mitochondrial membrane disruption leading to apoptosis [8,80].
3.4.3. Antispasmodic Activity
The antispasmodic activity of stem and leaf extracts from B. suaveolens was evaluated on smooth muscle contraction in rabbits. The results showed that the extract (71.5 μg/mL) had a significant antispasmodic effect [107]. Together, the presence of tropane alkaloids in this species may explain the traditional use of these plants as antispasmodics. However, more research is necessary to isolate the bioactive compound with antispasmodic activity from these herbal extracts.
3.4.4. Antibacterial Activity
The antibacterial activity of methanolic extracts from B. suaveolens stem, leaves, and flowers was evaluated by means of the disk diffusion test. The result showed that extracts from the stem of the plant exerted mild antibacterial activity, while extracts from the leaves and flowers had no antibacterial activity [8,108].
3.4.5. Anti-Asthmatic Activity
Anisa et al. [109] performed an in vivo investigation of the antiasthmatic activity of the aqueous extract from the B. suaveolens leaves. Vogel’s method was used to record breathing patterns, and the aqueous extract was administered orally after being dissolved in distilled water. The results showed that the dose of 40 mg/kg of body weight of the aqueous extract exerted a significant effect when compared to salbutamol sulfate at a dose of 0.16 mg/kg of body weight. The presence of tropane alkaloids in the plant extract may explain its antiasthmatic activity and corroborate its traditional use.
3.4.6. Antinociceptive Effects
The antinociceptive effects of the B. suaveolens flower extract were investigated using hotplates, muscle contractions, a formalin assay, and tail movement experiments in murines. The extracts were dissolved and administered intraperitoneally. The extract doses (100 and 300 mg/Kg) showed an increase in latency by the hotplate method, inhibiting the abdominal constrictions induced by acetic acid and producing the hypnotic effect generated by pentobarbital in a dose-dependent manner. Furthermore, both doses inhibited the formalin test phase. Similarly, the Tail-flick test shows attenuation of the response. The results suggest that the extract from B. suaveolens flowers has antinociceptive activity related to popular reports of the plant species [110,111,112]. However, further research studies are required, seeking bioguided isolation in the identification of the bioactive compounds related to the antinociceptive activity.
3.4.7. Antiprotozoal Activity
The in vitro antileishmanial activity of extracts from B. candida and B. suaveolens species was tested against Leishmania amazonensis promastigotes. The findings showed that extracts from B. suaveolens flowers (86.2 ± 9.5 μg/mL) and leaves (33.9 ± 2.3 μg/mL) exerted antileishmanial activity, while B. candida extracts showed no antiprotozoal activity [113]. The results are in line with the traditional use of the species for the treatment of antiprotozoa, skin infections, and ulcers. However, complementary assays are necessary for better data robustness, thus seeking bioguided monitoring of the bioactive compounds present in the extracts.
3.5. Toxicity
It is known that species from the genus Brugmansia are commonly associated with toxicity due to the presence of different alkaloids, among which the presence of atropine and scopolamine in different organs stands out [75,114]. The concentrations of these alkaloids vary according to seasonality, nutritional status, and organ; thus, in the leaves, there are concentrations of atropine (0.79 ± 0.03 mg/g) and scopolamine (0.72 ± 0.05 mg/g) in dry presentation, while the scopolamine concentrations in nectar are increased in the flowers (149.80 ± 6.01 μg/mL) [75,115]. The main symptoms most related to toxicity are dry and red dermis, pupil dilation, hallucinations, headaches, hysteria, dry mouth, tachycardia, arrhythmias, fever, epilepsy, urinary incontinence, and other anticholinergic symptoms [116].
The B. suaveolens species represented one of the main ornamental plants responsible for poisoning in humans from 1992 to 2009, with the highest percentage rate (5.71%) among toxic species [117,118]. The systemic effects of poisoning by this species are similar to those caused by the atropine alkaloid from the belladonna species, which has high hallucinogenic power and causes several health problems with the possibility of leading to death [119].
According to Antony et al. (2009), the B. suaveolens flowers are also made up of essential oil, presenting a different tonality according to time alteration: before fully opening, they are yellow; at dusk, they are already fully open and white; and the next day, they are pink. The study carried out with the white flowers revealed the presence of several essential oil constituents, with 1,8-cineole (72.1%), €-nerolidol (11.7%), and α-terpineol (5.3%) identified as the main compounds. The pink flowers showed megastigmatrienone II (24.5%), nonanal (17.4%), terpinen-4-ol (10.5%), and a series of long linear hydrocarbons. These hydrocarbons were also identified in the white flowers in low concentrations, but megastigmatrienone II was not identified.
Consequently, there are numerous reports of accidental poisoning by species from the genus Brugmansia [109]. Therefore, further studies using in vivo models are required to evaluate the toxicological profile in order to determine the minimum effective dose of the extract and clarify the changes and elucidation of pharmacodynamic and pharmacokinetic models associated with newly identified and isolated compounds from this species.
4. Conclusions
This review is a study focused on the traditional uses, secondary metabolites, biological activity, and toxicity of Brugmansia suaveolens Bercht. & J. Presl, capable of dazzling the promising therapeutic potential of this species and making it possible to establish scientific grounds to subsidize future studies on the species. Thus, this paper contributes to understanding current knowledge and gaps in the bioactive compounds found in Brugmansia suaveolens. It also points the way for the design of comprehensive studies to further explore the composition of active and relevant phytochemicals in this species.
Therefore, this review evidenced knowledge related to the traditional use based on fundamental scientific research about Brugmansia suaveolens Bercht. & J. Presl, highlighting an overview of bioactive compounds and biological and toxicological activities in order to provide the scientific grounds for future studies on the value of this species for the development of new therapeutic agents.
Author Contributions
S.P.d.C.: methodology, writing, and editing; R.A.S.-R.: writing and editing; V.d.S.C.: formal analysis and review; S.S.V.: writing and editing; A.B.V.: formal analysis and review; E.R.-J.: conceptualization, methodology, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by: Carlos Chagas Filho Foundation for Research Support of Rio de Janeiro State (Fundação do Amparo à Pesquisa do Estado de Rio de Janeiro, FAPERJ)—Cientista do Nosso Estado (CNE) [Grant number: E-26/201.077/2021], Support for Stricto Sensu Graduate Programs and Courses in the State of Rio de Janeiro [Grant number: E-26/210.136/2021], and Rede NanoSaúde [Grant number: E-26/010.000981/2019]; National Council for Scientific and Technological Development (CNPq)—Research Productivity Scholarship—PQ2 [Grant number: 309,522/2020-0]. This paper was also supported with a scholarship granted by the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, (CAPES).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing interest in this research. The authors declare no conflict of financial or non-financial interests that are directly or indirectly related to the paper submitted for publication.
References
- Elisabetsky, E. From indigenous disease concepts to laboratory working hypothesis: The case of “Nerve Tonics” from the Brazilian Amazon. Intern. Found. Sci. Prov. Rep. Series 1987, 19, S11438. [Google Scholar]
- Singh, M.; Govindarajan, R.; Nath, V.; Rawat, A.K.; Mehrotra, S. Antimicrobial, wound healing and antioxidant activity of Plagiochasma ppendiculatum Lehm. et Lind. J. Ethnopharmacol. 2006, 107, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Tanksley, S.D. Chromosomal evolution in the plant Family Solanaceae. BMC Genom. 2010, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, S.P.; Schuenck-Rodrigues, R.A.; Da Cardoso, V.S.; Valverde, S.S.; Vermelho, A.B.; Ricci-Júnior, E. Antimicrobial activity of endophytic fungi isolated from Brugmansia suaveolens Bercht. & J. Presl. Res. Soc. Dev. 2021, 10, e113101421646. [Google Scholar] [CrossRef]
- Saroya, A.S.; Singh, J. Psychoactive Medicinal Plants and Fungal Neurotoxins; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Marín-Saez, J.; Romero-Gonzalez, R.; Frenich, A.G. Effect of tea making and boiling processes on the degradation of tropane alkaloids in tea and pasta samples contaminated with Solanaceae seeds and coca leaf. Food Chem. 2019, 287, 265–272. [Google Scholar] [CrossRef]
- Anthony, S.J.; Zuchowski, W.; Setzer, W.N. Composition of the floral essential oil of Brugmansia suaveolens. J. Chem. Inf. Model. 2009, 3, 76. [Google Scholar] [CrossRef]
- Da Costa, S.P.; Schuenck-Rodrigues, R.A.; Cardoso, V.S.; Valverde, S.S.; Vermelho, A.B.; Ricci-Júnior, E. Phytochemical analysis of Brugmansia suaveolens Bercht. & J. Presl and its therapeutic potential for topical use. Nat. Prod. Res. 2022, 1–7. [Google Scholar] [CrossRef]
- Carlini, E.A.; Lucas, O.M. Plant and fungal hallucinogens as toxic and therapeutic agents. In Plant Toxins, Toxinology; Gopalakrishnakone, P., Carlini, C.R., Ligabue-Braun, R., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2015; pp. 1–44. [Google Scholar] [CrossRef]
- Júnior, W.S.F.; Cruz, M.P.; Albuquerque, U.P.; de Vieira, F.J. TT-son eficaces las sustancias alucinogenas en la curacion de enfermedades? [Revision]. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2010, 9, 292–301. [Google Scholar]
- Goldfarb, J.; Pesin, N.; Margolin, E. Gardening and dilated pupils: An interesting case of anisocoria from Brugmansia versicolor. Can. J. Ophthalmol. 2019, 54, e59–e61. [Google Scholar] [CrossRef]
- Geller, F.; Murillo, R.; Steinhauser, L.; Heinzmann, B.; Albert, K.; Merfort, I.; Laufer, S. Four new flavonol glycosides from the leaves of Brugmansia suaveolens. Molecules 2014, 19, 6727–6736. [Google Scholar] [CrossRef]
- Noedl, H.; Se, Y.; Schaecher, K.; Smith, B.L.; Socheat, D.; Fukuda, M.M.; Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 2008, 359, 2619–2620. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, S.J. Synthesis of Exotic Soaps in the Chemistry Laboratory Chem. Eng. News 1996, 74, 35. [Google Scholar] [CrossRef]
- Reigosa, M.; Pedrol, N. Allelopa: From Molecules to Ecosystems; Science Publishers: Plymouth, UK, 2002; p. 316. [Google Scholar]
- Masurekar, P.S. Em Biotechnology of Filamentous Fungi: Technology and Products; Finkelstein, D.B., Ball, C., Eds.; Butterworth-Heinemann: Boston, MA, USA, 1992; p. 241. [Google Scholar]
- Miller, J.D.; Trenholm, H.L. (Eds.) Mycotoxins in Grain: Compounds Other Than Aflatoxin; Eagan Press: Michigan, MI, USA, 1994; p. 552. [Google Scholar]
- Seigler, D.S. Plant Secondary Metabolism; Chapman and Hall (Kluwer Academic Publishers): Boston, MA, USA, 1998; p. 711. [Google Scholar]
- Tuteja, N.; Sopory, S.K. Chemical signaling under abiotic stress environment in plants. Plant Signal. Behav. 2008, 3, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Szakiel, A.; Paczkowski, C.; Henry, M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2011, 10, 471–491. [Google Scholar] [CrossRef]
- Venugopal, R.; Liu, R.H. Phytochemicals in diets for breast cancer prevention: The importance of resveratrol and ursolic acid. Food Sci. Hum. Wellness 2012, 1, 1–13. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109 (Suppl. S1), 69–75. [Google Scholar]
- Klayman, D.L.; Lin, A.J.; Acton, N.; Scovill, J.P.; Hoch, J.M.; Milhous, W.K.; Theoharides, A.D.; Dobek, A.S. Isolation of artemisinin (qinghaosu) from Artemisia annua growing in the United States. J. Nat. Prod. 1984, 47, 715–717. [Google Scholar] [CrossRef]
- Diallo, M.A.; Yade, M.S.; Ndiaye, Y.D.; Diallo, I.; Diongue, K.; Sy, S.A.; Sy, M.; Seck, M.C.; Ndiaye, M.; Dieye, B.; et al. Efficacy and safety of artemisinin-based combination therapy and the implications of Pfkelch13 and Pfcoronin molecular markers in treatment failure in Senegal. Sci. Rep. 2020, 10, 8907. [Google Scholar] [CrossRef] [PubMed]
- Adekenov, S.M.; Muchametzhanov, M.N.; Kagarlitskii, A.D.; Kuprianov, A.N. Arglabin—A new sesquiterpene lactone from Artemisia glabella. Chem. Nat. Compd. 1982, 18, 655–656. [Google Scholar] [CrossRef]
- He, W.; Lai, R.; Lin, Q.; Huang, Y.; Wang, L. Arglabin is a plant sesquiterpene lactone that exerts potent anticancer effects on human oral squamous cancer cells via mitochondrial apoptosis and downregulation of the mTOR/PI3K/Akt signaling pathway to inhibit tumor growth in vivo. J. Buon 2018, 23, 1679–1685. [Google Scholar] [PubMed]
- Toh, C.; Lee, T.; Kiang, A. The pharmacological actions of capsaicin and analogues. Br. J. Pharmacol. Chemother. 1955, 10, 175–182. [Google Scholar] [CrossRef]
- Ausín-Crespo, M.D.; Martín-de Castro, E.; Roldán-Cuartero, J.; de la Beldad-Diez, M.L.; Salcedo-Gámez, M.Á.; Tong, H. Capsaicin 8% Dermal Patch for Neuropathic Pain in a Pain Unit. Pain Manag. Nurs. 2022, 23, 452–457. [Google Scholar] [CrossRef]
- Dasgeb, B.; Kornreich, D.; McGuinn, K.; Okon, L.; Brownell, I.; Sackett, D.L. Colchicine: An ancient drug with novel applications. Br. J. Dermatol. 2018, 178, 350–356. [Google Scholar] [CrossRef]
- Wang, Z.; Zu, X.; Xiong, S.; Mao, R.; Qiu, Y.; Chen, B.; Zeng, Z.; Chen, M.; He, Y. The Role of Colchicine in Different Clinical Phenotypes of Behcet Disease. Clin. Ther. 2023, 45, 162–176. [Google Scholar] [CrossRef]
- Vree, T.B.; Breimer, D.D.; Van Ginneken, C.A.M.; Van Rossum, J.M. Identification in hashish of tetrahydrocannabinol, cannabidiol and cannabinol analogues with a methyl side-chain. J. Pharm. Pharmacol. 1972, 24, 7–12. [Google Scholar] [CrossRef]
- Mitchell, V.A.; Harley, J.; Casey, S.L.; Vaughan, A.C.; Winters, B.L.; Vaughan, C.W. Oral efficacy of Δ(9)-tetrahydrocannabinol and cannabidiol in a mouse neuropathic pain model. Neuropharmacology 2021, 189, 108529. [Google Scholar] [CrossRef]
- Tsakadze, D.; Abdusamatov, A.; Yunusov, S.Y. Alkaloids of Galanthus caucasicus. Chem. Nat. Compd. 1969, 5, 281–282. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. Pharmacological aspects of galantamine for the treatment of Alzheimer’s disease. EXCLI J. 2017, 16, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, J.; Evanics, F.; Berta, L.; Bartock, T. Diterpenoids from Euphorbia peplus. Planta Med. 2000, 66, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Wong, T.-W.; Lee, C.-H.; Hong, C.-H.; Chang, C.-H.; Lai, F.-J.; Lin, S.-H.; Chi, C.-C.; Lin, T.-K.; Yen, H.; et al. Efficacy and safety of topical SR-t100 gel in treating actinic keratosis in taiwan: A phase III randomized double-blind vehicle-controlled parallel trial. J. Dermatol. Sci. 2018, 90, 295–302. [Google Scholar] [CrossRef]
- Luo, J.; Chuang, T.; Cheung, J.; Quan, J.; Tsai, J.; Sullivan, C.; Hector, R.F.; Reed, M.J.; Meszaros, K.; King, S.R.; et al. Masoprocol (nordihydroguaiaretic acid): A new antihyperglycemic agent isolated from the creosote bush (Larrea tridentata). Eur. J. Pharmacol. 1998, 346, 77–79. [Google Scholar] [CrossRef]
- Pereira, J.; Miguel Castro, M.; Santos, F.; Rita Jesus, A.; Paiva, A.; Oliveira, F.; Duarte, A.R.C. Selective terpene based therapeutic deep eutectic systems against colorectal cancer. Eur. J. Pharm. Biopharm. 2022, 175, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.G.; Rogovin, S.P.; Smith, C.R., Jr. Isolation of antitumor alkaloids from Cephalotaxus harringtonia. Ind. Eng. Chem. Prod. Res. Dev. 1974, 13, 129–132. [Google Scholar] [CrossRef]
- Gandhi, V.; Plunkett, W.; Cortes, J.E. Omacetaxine: A protein translation inhibitor for treatment of chronic myelogenous leukemia. Clin. Cancer Res. 2014, 20, 1735–1740. [Google Scholar] [CrossRef]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; Mcphail, A.T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Patra, J.K.; Singh, Y.D.; Panda, M.K.; Das, G.; Adetunji, C.O.; Michael, O.S.; Sytar, O.; Polito, L.; et al. Paclitaxel: Application in Modern Oncology and Nanomedicine-Based Cancer Therapy. Oxidative Med. Cell. Longev. 2021, 2021, 3687700. [Google Scholar] [CrossRef]
- Hsu, P.; Tien, H. Studies on the components of Formosan Solanum species. Part I Alkaloids of Solanum incanum. Tai-wan Yao Hsueh. Tsa. Chih. 1974, 26, 102338t. [Google Scholar] [CrossRef]
- Liljegren, D. Glucosylation of solasodine by extracts from Solanum laciniatum. Phytochemistry 1971, 10, 3061–3064. [Google Scholar] [CrossRef]
- Burger, T.; Mokoka, T.; Fouché, G.; Steenkamp, P.; Steenkamp, V.; Cordier, W. Solamargine, a bioactive steroidal alkaloid isolated from Solanum aculeastrum induces non-selective cytotoxicity and P-glycoprotein inhibition. BMC Complement. Altern. Med. 2018, 18, 137. [Google Scholar] [CrossRef]
- Barbosa, W.L.R.; Nascimento, M.S.D.; Pinto, L.D.N.; Maia, F.L.C.; Sousa, A.J.A.; Júnior, J.O.C.S.; Melo, M.; De Oliveir, D.R. Selecting Medicinal Plants for Development of Phytomedicine and Use in Primary Health Care, Bioactive Compounds in Phytomedicine. In Rasooli; Iraj, Ed.; InTech: London, UK, 2010. [Google Scholar]
- Henrich, C.J.; Beutler, J.A. Matching the power of high throughput screening to the chemical diversity of natural products. Nat. Prod. Rep. 2013, 30, 1284–1298. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M. Ethnopharmacology in the 21st century—Grand challenges. Front. Pharmacol. 2010, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Leonti, M. The future is written: Impact of scripts on the cognition, selection, knowledge and transmission of medicinal plant use and its implications for ethnobotany and ethnopharmacology. J. Ethnopharmacol. 2011, 134, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Schultes, R.E.; Hofmann, A. Plantas de los Dioses: San Pedro de los Pinos; Solar Servicios Editoriales: Ciudad de México, México, 2000; p. 208. [Google Scholar]
- Martínez-Flórez, S.; González-Gallego, J.; Culabres, J.M.; Tuñón, M.J. Flavonoids: Properties and anti-oxidizing action. Nutr. Hosp. 2002, 17, 271–278. [Google Scholar]
- Alagille, D.; Baldwin, R.M.; Roth, B.L.; Wroblewski, J.T.; Grajkowska, E.; Tamagnan, G.D. Functionalization at position 3 of the phenyl ring of the potent mGluR5noncompetitive antagonists MPEP. Bioorganic Med. Chem. Lett. 2005, 15, 945–949. [Google Scholar] [CrossRef]
- Bruce, N.C. Alkaloids. In Biotechnology: Biotransformations I, 2nd ed.; Rehm, H.J., Reed, G., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008; Volume 8a. [Google Scholar]
- Christen, P. Tropane alkaloids: Old drugs used in modern medicine. In Studies in Natural Products Chemistry, Bioactive Natural Products, Part C; Rahman, A., Ed.; Elsevier Science and Technology: Amsterdam, The Netherlands, 2000; Volume 22, pp. 717–749. [Google Scholar] [CrossRef]
- Gyermek, L. Tropane alkaloids. In Pharmacology of Antimuscarinic Agents; Gyermek, L., Ed.; CRC Press: Boca Raton, FL, USA, 1997; pp. 47–160. [Google Scholar]
- Lounasmaa, M.; Tamminen, T. The tropane alkaloids. In The Alkaloids; Brossi, A., Ed.; Academic Press: New York, NY, USA, 1993; Volume 44, pp. 1–114. [Google Scholar] [CrossRef]
- Geller, C.F. Isolation, Structure Elucidation and Biological Investigation of Active Compounds in Cordia Americana and Brugmansia Suaveolens; University of Tübingen: Tübingen, Germany, 2010. [Google Scholar]
- Nandakumar, A.; Vaganan, M.; Sundararaju, P.; Udayakumar, R. Phytochemical analysis and nematicidal activity of ethanolic leaf extracts of Datura metel, Datura innoxia and Brugmansia suaveolens against Meloidogyne incognita. Asian J. Biol. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Li, S.; Cheng, X.; Wang, C. A review on traditional uses, phytochemistry, pharmacology, pharmacokinetics and toxicology of the genus Peganum. J. Ethnopharmacol. 2017, 203, 127–162. [Google Scholar] [CrossRef]
- Doncheva, T.; Berkov, S.; Philipov, S. Comparative study of the alkaloids in tribe Datureae and their chemosystematic significance. Biochem. Systemat. Ecol. 2006, 34, 478–488. [Google Scholar] [CrossRef]
- Mattioli, L.; Bracci, A.; Titomanlio, F.; Perfumi, M.; De Feo, V. Effects of Brugmansia arborea extract and its secondary metabolites on morphine tolerance and dependence in mice. Evid.-Based Complement. Altern. Med. 2012, 2012, 741925. [Google Scholar] [CrossRef]
- Capasso, A.; Feo, V.; De Simone, F.; De Sorrentino, L.; Farmacia, F.; Emanuele, P.V. Activity—Directed Isolation of Spasmolytic (Anti-cholinergic). Int. J. Pharmacogn. 1997, 35, 43–48. [Google Scholar] [CrossRef]
- Albuquerque, U.P.; De Medeiros, P.M.; Casas, A. Evolutionary ethnobiology. Evol. Ethnobiol. 2015, 1, 1–197. [Google Scholar] [CrossRef]
- Evans, W.C.; Lampard, J.F. Alkaloids of Datura suaveolens. Phytochemistry 1972, 11, 3293–3298. [Google Scholar] [CrossRef]
- Roses, O.E.; Lopez, C.M.; Fernandez, J.C.G. Isolation and identification of tropane alkaloids in species of the genus Brugmansia (Solanaceae). Acta Farm. Bonaerense 1987, 6, 167–174. [Google Scholar]
- Fodor, G. Chapter 5 the tropane alkaloids. Alkaloids Chem. Physiol. 1960, 6, 145–177. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Martínez, J.R. Sampling flower scent for chromatographic analysis. J. Separ. Sci. 2008, 31, 2022–2031. [Google Scholar] [CrossRef]
- Kite, G.C.; Leon, C. Volatile compounds emitted from flowers and leaves of Brugmansia × Candida (Solanaceae). Phytochemistry 1995, 40, 1093–1095. [Google Scholar] [CrossRef]
- Kim, H.G.; Ko, J.H.; Oh, H.J.; Kwon, J.H.; Oh, E.J.; Oh, S.M.; Lee, Y.G.; Lee, D.Y.; Baek, N.I. Tyrosinase inhibition activity of monoterpene glucosides from Brugmansia arborea flowers. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Shah, C.; Saoji, A. Alkaloidal estimation of aerial parts of Datura arborea Linn 1. Planta Med. 1966, 14, 465–467. [Google Scholar] [CrossRef]
- Thomas, B. The psychoactive flora of Papua New Guinea. J. Psychoact. Drugs 2003, 35, 285–293. [Google Scholar] [CrossRef]
- Kerchner, A.; Darok, J.; Bacskay, I.; Felinger, A.; Jakab, G.; Farkas, A. Protein and alkaloid patterns of the floral nectar in some solanaceous species. Acta Biol. Hung. 2015, 66, 304–315. [Google Scholar] [CrossRef]
- Bhatt, I.D.; Chang, J.I.; Hiraoka, N. In vitro propagation and storage of Brugmansia versicolor Lagerheim. Plant Biotechnol. 2004, 21, 237–241. [Google Scholar] [CrossRef]
- Zayed, R.; Wink, M. Induction of tropane alkaloid formation in transformed root cultures of Brugmansia suaveolens (Solanaceae). Z. Nat. 2004, 59, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Begum, A.S.; Sahai, M.; Fujimoto, Y.; Asai, K.; Schneider, K.; Nicholson, G.; Suessmuth, R. A new kaempferol diglycoside from Datura suaveolens Humb. & Bonpl. ex. Willd. Nat. Prod. Res. 2006, 20, 1231–1236. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, A.; Saini, R.V.; Kumar, A.; Dhar, K.L.; Mahindroo, N. Immunomodulation-mediated anticancer activity of a novel compound from Brugmansia suaveolens leaves. Bioorg. Med. Chem. 2020, 28, 115552. [Google Scholar] [CrossRef]
- Ribeiro, B.; Lopes, R.; Andrade, P.B.; Seabra, R.M.; Gonçalves, R.F.; Baptista, P.; Quelhas, I.; Valentão, P.C. Comparative study of phytochemicals and antioxidant potential of wild edible mushroom caps and stipes. Food Chem. 2008, 110, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Armijos, C.; Cota, I.; González, S. Traditional medicine applied by the Saraguro yachakkuna: A preliminary approach to the use of sacred and psychoactive plant species in the southern region of Ecuador. J. Ethnobiol. Ethnomed. 2014, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.C. Hallucinogenic plants of the Shuar and related indigenous groups in Amazonian Ecuador and Peru. Brittonia 1992, 44, 483–493. [Google Scholar] [CrossRef]
- Bennett, B.C.; Alarcon, R. Hunting and hallucinogens: The use psychoactive and other plants to improve the hunting ability of dogs. J. Ethnopharmacol. 2015, 171, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Biset, J.; Campos-de-la-Cruz, J.; Epiqui´en-Rivera, M.A.; Canigueral, S. A first survey on the medicinal plants of the Chazuta valley (Peruvian Amazon). J. Ethnopharmacol. 2009, 122, 333–362. [Google Scholar] [CrossRef] [PubMed]
- De Feo, V. Ethnomedical field study in northern Peruvian Andes with particular reference to divination practices. J. Ethnopharmacol. 2003, 85, 243–256. [Google Scholar] [CrossRef]
- De Feo, V. The ritual use of Brugmansia species in traditional andean medicine in Northern Peru. Econ. Bot. 2004, 58, 221–229. [Google Scholar] [CrossRef]
- Lucinda, N.; Inoue-Nagata, A.K.; Kitajima, E.W.; Nagata, T. Complete genome sequence of Brugmansia suaveolens mottle virus, a potyvirus from an ornamental shrub. Arch. Virol. 2010, 155, 1729–1732. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Hilgert, N.I. Phytotherapy of polish migrants in Misiones, Argentina: Legacy and acquired plant species. J. Ethnopharmacol. 2014, 153, 810–830. [Google Scholar] [CrossRef]
- DeFilipps, R.A.; Krupnick, G.A. The medicinal plants of Myanmar. PhytoKeys 2018, 102, 1–341. [Google Scholar] [CrossRef]
- Suroowan, S.; Mahomoodally, M.F. A comparative ethnopharmacological analysis of traditional medicine used against respiratory tract diseases in Mauritius. J. Ethnopharmacol. 2016, 177, 61–80. [Google Scholar] [CrossRef]
- Balangcod, T.D.; Balangcod, A.K.D. Ethnomedical knowledge of plants and healthcare practices among the Kalanguya tribe in Tinoc, Ifugao, Luzon, Philippines. Indian J. Tradit. Knowl. 2011, 10, 227–238. [Google Scholar]
- Rohman, F.; Juma, Y.; Utomo, D.H.; Lestari, S.R.; Arifah, S.N.; Putra, W.E. Plants diversity as a medicinal plants by the tengger tribe, bromo tengger semeru national park, east java, Indonesia. EurAsian J. Biosci. 2019, 13, 2293–2298. [Google Scholar]
- Batoro, J.; Siswanto, D. Ethnomedicinal survey of plants used by local society in Poncokusumo district, Malang, East Java Province, Indonesia. Asian J. Med. Biol. Res. 2017, 3, 158–167. [Google Scholar] [CrossRef]
- Nuraeni, H.; Rustaman, N.Y. Traditional knowledge of medicinal plants for health of women in Cibodas Village Lembang Subdistrict West Bandung Regency and their potency to development of biodiversity education. J. Phys. Conf. Ser. 2019, 1157, 022115. [Google Scholar] [CrossRef]
- Oktavia, A.I.; Indriani, S.; Batoro, J. Ethnobotanical study of toxic plants in ngadiwono village, tosari district, pasuruan regency, east java. J. Pembang. 2017, 8, 83–88. [Google Scholar] [CrossRef]
- Chetri, B.K.; Wangdi, P.; Penjor, T. Ethnomedicinal practices in kilikhar, mongar. Asian Plant Res. J. 2018, 1, 1–13. [Google Scholar] [CrossRef]
- Ijaz, F.; Iqbal, Z.; Rahman, I.U.; Alam, J.; Khan, S.M.; Shah, G.M.; Khan, K.; Afzal, A. Investigation of traditional medicinal floral knowledge of Sarban Hills, Abbottabad, KP, Pakistan. J. Ethnopharmacol. 2016, 179, 208–233. [Google Scholar] [CrossRef]
- Lokho, K.; Narasimhan, D. Ethnobotany of Mao-Naga Tribe of Manipur, India. Pleione 2013, 7, 314–324. [Google Scholar]
- Shankar, R.; Tripathi, A.K.; Neyaz, S.; Anku, G. Distribution and conservation of medicinal plants in, kohima mokokchung, tuenseng and zunheboto districts of Nagaland. World J. Pharmaceut. Res. 2016, 5, 1225–1237. [Google Scholar]
- Shahid, M.; Singh, M. Ethnomedicinal use of plants by bhutia tribe in Sikkim himalaya. In Proceedings of the 1st Himalayan Researchers Consortium, Dehradun, India, 26–27 April 2018; Volume 1, pp. 71–78. [Google Scholar]
- Radha, B.; Dinesh, S.; Tiwari, J.K.; Tiwari, P. Diversity and availability status of ethno-medicinal plants in the Lohba range of Kedarnath forest division, Garhwal Himalaya. Glob. J. Res. Med. Plants Indig. Med. 2013, 2, 198–212. [Google Scholar]
- Lalzarzovi, S.T.; Lalramnghinglova, H. Traditional use of medicinal plants found within Aizawl city in Mizoram, India. Pleione 2016, 10, 269–277. [Google Scholar]
- Ningthoujam, S.S.; Talukdar ADas Potsangbam, K.S.; Choudhury, M.D. Traditional uses of herbal vapour therapy in Manipur, North East India: An ethnobotanical survey. J. Ethnopharmacol. 2013, 147, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chand, G.; Sankhyan, P. Herbal folk remedies for curing various ailments in Lug valley of district Kullu, Himachal Pradesh (N. W. Himalaya). Int. J. Ayurvedic Med. 2013, 3, 1308–1314. [Google Scholar]
- Schmidt, C.; Fronza, M.; Goettert, M.; Geller, F.; Luik, S.; Flores, E.M.M.; Bittencourt, C.F.; Zanetti, G.D.; Heinzmann, B.M.; Laufer, S.; et al. Biological studies on Brazilian plants used in wound healing. J. Ethnopharmacol. 2009, 122, 523–532. [Google Scholar] [CrossRef]
- Encarnacion-Dimayuga, R.; Altamirano, L.; Aoki Maki, K. Screening of medicinal plants from Baja California Sur (Mexico) by their effects on smooth muscle contractility. Pharm. Biol. 1998, 36, 124–130. [Google Scholar] [CrossRef]
- San Luis, G.D.; Balangcod, T.D.; Abucay, J.B.; Wong, F.M.; Balangcod, K.D.; Afifi, N.I.G.; Apostol, O.G. Phytochemical and antimicrobial screening of indigenous species that have potential for revegetation of landslides in atok, benguet, Philippines. Indian J. Tradit. Knowl. 2014, 13, 56–62. [Google Scholar]
- Anisa, I.N.; Soedarmadji, A.A.; Djuliana, D. Uji efek bronkodilator ekstrak air bunga kecubung gunung (Brugmansia suaveolens bercht & presl). Kartika J. Ilm. Farm. 2016, 4, 1–4. [Google Scholar] [CrossRef]
- Parker, A.G.; Peraza, G.G.; Sena, J.; Silva, E.S.; Soares, M.C.F.; Vaz, M.R.C.; Furlong, E.B.; Muccillo-Baisch, A.L. Antinociceptive effects of the aqueous extract of Brugmansia suaveolens flowers in Mice. Biol. Res. Nurs. 2007, 8, 234–239. [Google Scholar] [CrossRef]
- Akram, M.; Asif, H.; Usmanghani, K. Anti-nociceptive activities of medicinal plants: A Review. J. Biol. Res. Appl. Sci. 2013, 4, 50–58. [Google Scholar]
- Muccillo-Baisch, A.L.; Parker, A.G.; Cardoso, G.P.; Cezar-Vaz, M.R.; Soares, M.C.F. Evaluation of the analgesic effect of aqueous extract of Brugmansia suaveolens flower in mice: Possible mechanism involved. Biol. Res. Nurs. 2010, 11, 345–350. [Google Scholar] [CrossRef]
- Monzote, L.; Jimenez, J.; Cuesta-Rubio, O.; Marquez, I.; Gutierrez, Y.; da Rocha, C.Q.; Marchi, M.; Setzer, W.N.; Vilegas, W. In vitro assessment of plants growing in Cuba belonging to solanaceae family against Leishmania amazonensis. Phyther. Res. 2016, 30, 1785–1793. [Google Scholar] [CrossRef]
- Pinto, C.F.; Salinas, S.; Flores-Prado, L.; Echeverría, J.; Niemeyer, H.M. Sequestration of tropane alkaloids from Brugmansia suaveolens (Solanaceae) by the treehopper Alchisme grossa (Hemiptera: Membracidae). Biochem. Syst. Ecol. 2016, 66, 161–165. [Google Scholar] [CrossRef]
- Reis, R.B.; Bragagnolo, F.S.; Gianeti, T.M.R.; Rodrigues, S.A.; Funari, C.S.; Gonçalves, G.G.; Ming, L.C. Brugmansia suaveolens leaf productivity and alkaloid contents under different doses of organic fertilizer. J. Agric. Sci. 2019, 11, 341. [Google Scholar] [CrossRef]
- Doan, U.V.; Wu, M.L.; Phua, D.H.; Mendez Rojas, B.; Yang, C.C. Datura and Brugmansia plants related antimuscarinic toxicity: An analysis of poisoning cases reported to the Taiwan poison control center. Clin. Toxicol. 2019, 57, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Baltar, S. Características Epidemiológicas e Clínicas das Intoxicações Provocadas por Espécies Vegetais em Seres Humanos no Estado de Pernambuco—Brasil; Tese (Doutorado em Inovação Terapêutica); Universidade Federal de Pernambuco: Recife, Brazil, 2013; p. 198. [Google Scholar]
- Baltar, S.; de Araújo, L.S.M.; Franco, E.S.; Amorim, L.P.; Pedrosa, H.; Paixão, T.N.; Pereira, R.C.; Maia, M. Aspectos botânicos e clínicos das intoxicações por plantas das famílias Araceae, Euphorbiaceae e Solanaceae no estado de Pernambuco. Revista Fitos 2017, 11, 119–249. [Google Scholar] [CrossRef]
- Dickell, O.E. Efeitos Comportamentais e Neurotóxicos em Ratos Wistar Sob Efeito do Extrato Aquoso Bruto de Flores da Brugmansia Suaveolens (Solanaceae); Dissertação (Mestrado em Fisiologia Animal Comparada); Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 2006; p. 17. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).