Combination of Chemically Characterized Pomegranate Extract and Hydrophilic Vitamins against Prolonged Fatigue: A Monocentric, Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Characterization of Pomegranate Extract with UHPLC-HRMS Analysis
2.2. Food Supplement Based on Pomegranate Extract and Hydro-Soluble Vitamins
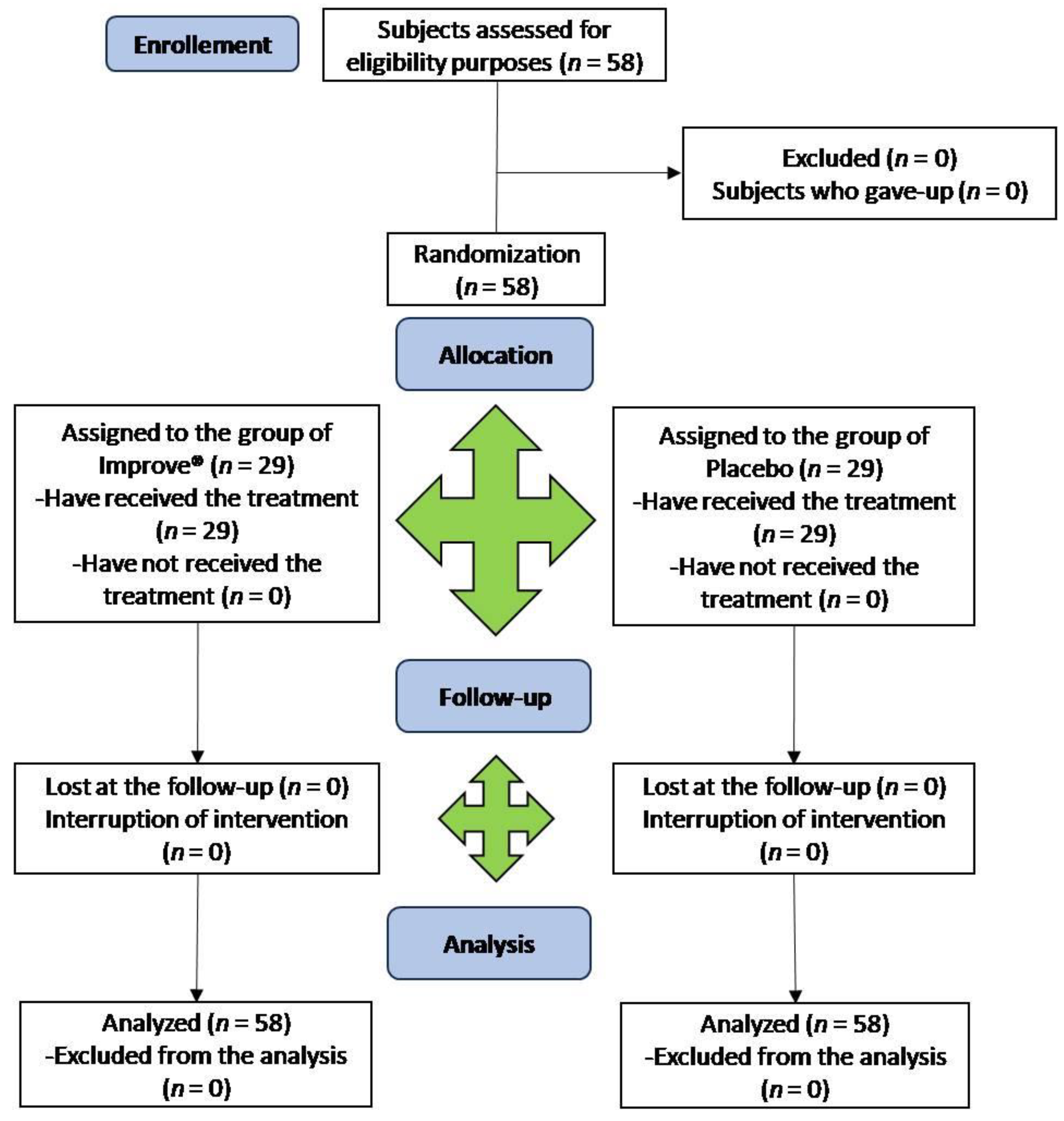
2.3. Clinical Trial Design
2.4. Participants and Recruiting
2.5. Outcome of the Study
2.6. Safety
2.7. Statistical Analysis
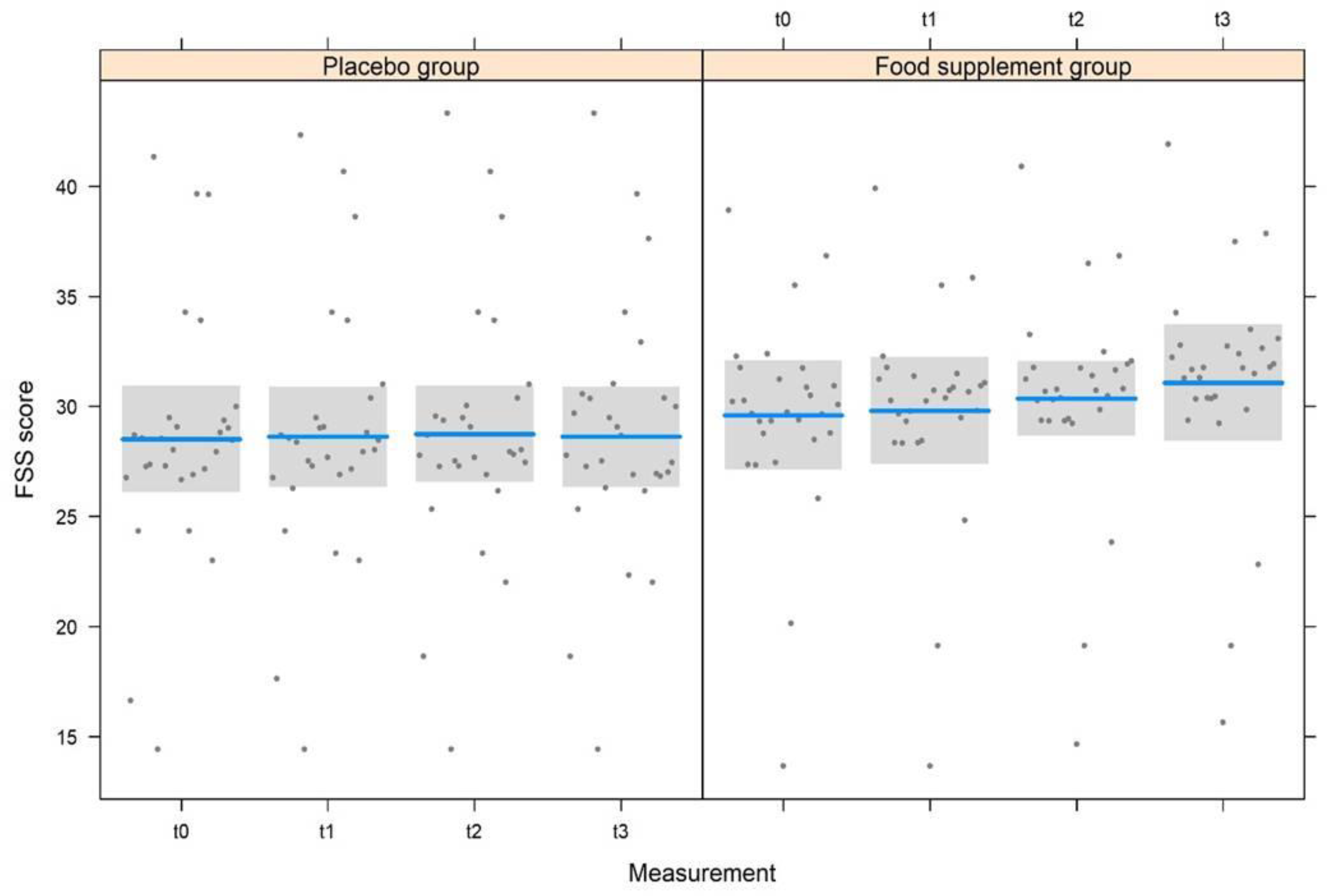
3. Results
3.1. RP-UHPLC-HRMS Analysis of Pomegranate Extract
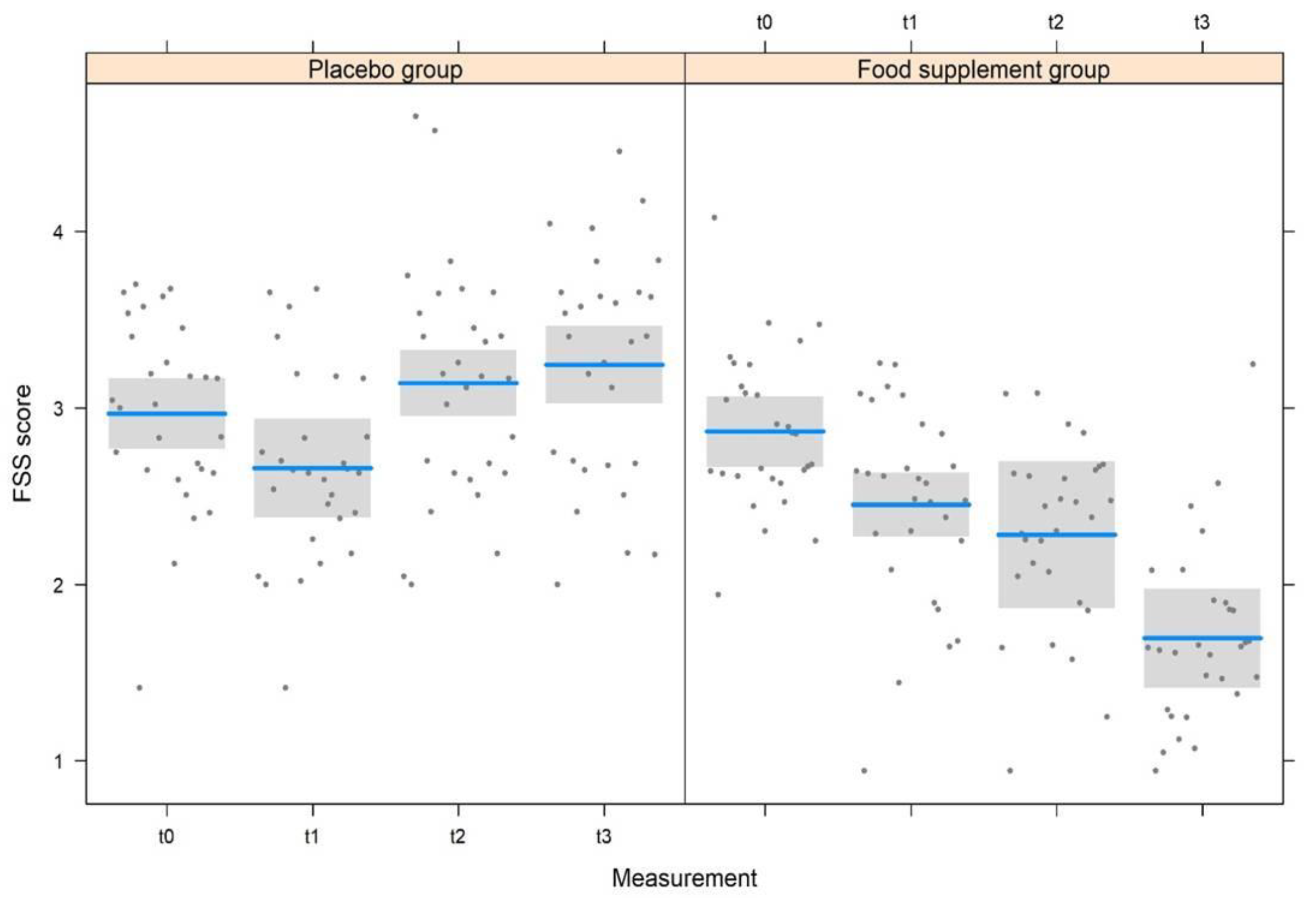
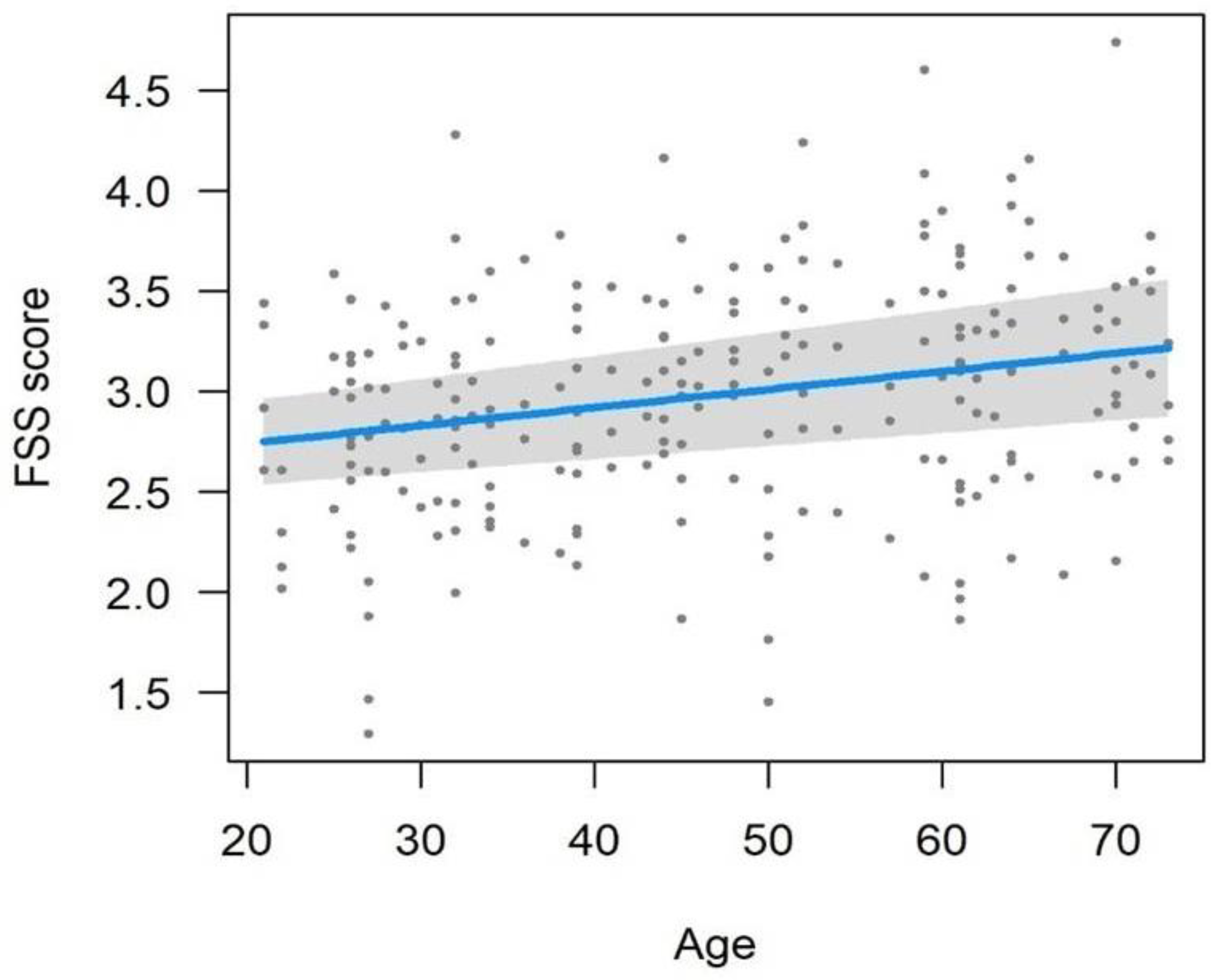
3.2. Clinical Trial Design
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phillips, R.O. A review of definitions of fatigue–and a step towards a whole definition. Transp. Res. PartF Traffic Psychol. Behav. 2015, 29, 48–56. [Google Scholar] [CrossRef]
- Kamal, M.; Rahman, M.M. Advances in fatigue life modeling: A review. Renew. Sust. Energ. Rev. 2018, 82, 940–949. [Google Scholar] [CrossRef]
- Gawron, V.J.; French, J.; Funke, D. An overview of fatigue. Stress, workload, and fatigue. In Stress, Workload, and Fatigue; Hancock, P.A., Desmond, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 581–595. [Google Scholar]
- Kluger, B.M.; Krupp, L.B.; Enoka, R.M. Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology 2013, 80, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Santarcangelo, C.; Di Minno, A.; Sacchi, R.; Sommella, E.; De Lellis, L.F.; De Pasquale, M.A.; Montarolo, F.; Campiglia, P.; Baldi, A.; et al. Chemical characterization and preliminary evaluation of the efficacy and tolerability of a food supplement based on pomegranate extract, B vitamins, and vitamin C against prolonged fatigue in healthy consumers. Processes 2022, 10, 208. [Google Scholar] [CrossRef]
- Davis, M.P.; Walsh, D. Mechanisms of fatigue. J. Support. Oncol. 2010, 8, 164–174. [Google Scholar]
- Kant, I.J.; Bültmann, U.; Schröer, K.A.P.; Beurskens, A.J.H.M.; Van Amelsvoort, L.G.P.M.; Swaen, G.M.H. An epidemiological approach to study fatigue in the working population: The Maastricht cohort study. Occup. Environ. Med. 2003, 60, i32–i39. [Google Scholar] [CrossRef]
- Tardy, A.L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and minerals for energy, fatigue and cognition: A narrative review of the biochemical and clinical evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef]
- Swaen, G.M.H.; Van Amelsvoort, L.G.P.M.; Bültmann, U.; Kant, I.J. Fatigue as a risk factor for being injured in an occupational accident: Results from the Maastricht cohort study. Occup. Environ. Med. 2003, 60, i88–i92. [Google Scholar] [CrossRef]
- Yancey, J.R.; Thomas, S.M. Chronic fatigue syndrome: Diagnosis and treatment. Am. Fam. Physician 2012, 86, 741–746. [Google Scholar]
- Muecke, M.; Cuhls, H.; Peuckmann-Post, V.; Minton, O.; Stone, P.; Radbruch, L. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst. Rev. 2015, 2015, CD006788. [Google Scholar]
- da Silva, J.A.T.; Rana, T.S.; Narzary, D.; Verma, N.; Meshram, D.T.; Ranade, S.A. Pomegranate biology and biotechnology: A review. Sci. Hortic. 2013, 160, 85–107. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Pomegranate and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.A.N.U.E.L.; Pérez-Álvarez, J.A.; Sendra, E.; Fernández-López, J.U.A.N.A. In vitro antioxidant properties of pomegranate (Punica granatum) peel powder extract obtained as coproduct in the juice extraction process. J. Food Process. Preserv. 2013, 37, 772–776. [Google Scholar] [CrossRef]
- Urbaniak, A.; Skarpańska-Stejnborn, A. Effect of pomegranate fruit supplementation on performance and various markers in athletes and active subjects: A systematic review. Int. J. Vitam. Nutr. Res. 2019, 91, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Bailey, S.J.; Chtourou, H.; Trabelsi, K.; Turki, M.; Hökelmann, A.; Souissi, N. Effects of pomegranate supplementation on exercise performance and post-exercise recovery in healthy adults: A systematic review. Br. J. Nutr. 2018, 120, 1201–1216. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Q.; Hou, H.; Liu, Z.; Wang, L.; Rasekhmagham, R.; Kord-Varkaneh, H.; Santos, H.O.; Yao, G. The effects of pomegranate supplementation on biomarkers of inflammation and endothelial dysfunction: A meta-analysis and systematic review. Complement. Ther. Med. 2020, 49, 102358. [Google Scholar] [CrossRef] [PubMed]
- Bellows, L.; Moore, R.; Anderson, J.; Young, L. Water-Soluble Vitamins: B-Complex and Vitamin C. Food and Nutrition Series. Health; No. 9.312. 2012. Available online: https://extension.colostate.edu/docs/foodnut/09312.pdf (accessed on 21 March 2023).
- Chakraborthy, A.; Ramani, P.; Sherlin, H.J.; Premkumar, P.; Natesan, A. Antioxidant and pro-oxidant activity of Vitamin C in oral environment. Indian J. Dent. Res. 2014, 25, 499. [Google Scholar] [CrossRef]
- Ueland, P.M.; McCann, A.; Midttun, Ø.; Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Aspects Med. 2017, 53, 10–27. [Google Scholar] [CrossRef]
- Peterson, C.T.; Rodionov, D.A.; Osterman, A.L.; Peterson, S.N. B vitamins and their role in immune regulation and cancer. Nutrients 2020, 12, 3380. [Google Scholar] [CrossRef]
- Rodrigo, R.; Prieto, J.C.; Aguayo, R.; Ramos, C.; Puentes, Á.; Gajardo, A.; Panieri, E.; Rojas-Solé, C.; Lillo-Moya, J.; Saso, L. Joint cardioprotective effect of vitamin C and other antioxidants against reperfusion injury in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Molecules 2021, 26, 5702. [Google Scholar] [CrossRef]
- Monacelli, F.; Acquarone, E.; Giannotti, C.; Borghi, R.; Nencioni, A. Vitamin C, aging and Alzheimer’sdisease. Nutrients 2017, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Ragheb, S.R.; El Wakeel, L.M.; Nasr, M.S.; Sabri, N.A. Impact of rutin and vitamin C combination on oxidative stress and glycemic control in patients with type 2 diabetes. Clin. Nutr. ESPEN 2020, 35, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Aljaadi, A.M.; Devlin, A.M.; Green, T.J. Riboflavin intake and status and relationship to anemia. Nutr. Rev. 2023, 81, 114–132. [Google Scholar] [CrossRef] [PubMed]
- Werbach, M.R. Nutritional strategies for treating chronic fatigue syndrome. Altern. Med. Rev. 2000, 5, 93–108. [Google Scholar]
- Barnish, M.; Sheikh, M.; Scholey, A. Nutrient therapy for the improvement of fatigue symptoms. Nutrients 2023, 15, 2154. [Google Scholar] [CrossRef]
- Ullah, H.; Sommella, E.; Santarcangelo, C.; D’Avino, D.; Rossi, A.; Dacrema, M.; Di Minno, A.; Di Matteo, G.; Mannina, L.; Campiglia, P.; et al. Hydroethanolic extract of Prunus domestica L.: Metabolite profiling and in vitro modulation of molecular mechanisms associated to cardiometabolic diseases. Nutrients 2022, 14, 340. [Google Scholar] [CrossRef]
- Cohen, H.A.; Varsano, I.; Kahan, E.; Sarrell, E.M.; Uziel, Y. Effectiveness of an herbal preparation containing echinacea, propolis, and vitamin C in preventing respiratory tract infections in children: A randomized, double-blind, placebo-controlled, multicenter study. Arch. Pediatr. Adolesc. Med. 2004, 158, 217–221. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.r-project.org/index.html (accessed on 23 October 2022).
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Calvert, M.; Blazeby, J.; Altman, D.G.; Revicki, D.A.; Moher, D.; Brundage, M.D.; CONSORT PRO Group, F.T. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA 2013, 309, 814–822. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Antimicrobial potential of pomegranate peel: A review. Int. J. Food Sci. Technol. 2019, 54, 959–965. [Google Scholar] [CrossRef]
- Bassiri-Jahromi, S. Punica granatum (Pomegranate) activity in health promotion and cancer prevention. Oncol. Rev. 2018, 12, 345. [Google Scholar] [CrossRef]
- Khomich, L.M.; Perova, I.B.; Eller, K.I. Pomegranatejuicenutritionalprofile. Vopr. Pitan. 2019, 88, 80–92. [Google Scholar]
- Benchagra, L.; Berrougui, H.; Islam, M.O.; Ramchoun, M.; Boulbaroud, S.; Hajjaji, A.; Fulop, T.; Ferretti, G.; Khalil, A. Antioxidant effect of moroccan pomegranate (Punica granatum L. sefri variety) extracts rich in punicalagin against the oxidative stress process. Foods 2021, 10, 2219. [Google Scholar] [CrossRef] [PubMed]
- Directive, E.U. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. Off. J. Eur. Communities Legis. 2002, 45, 51–57. [Google Scholar]
- Norberg, E.B.; Boman, K.; Löfgren, B. Impact of fatigue on everyday life among older people with chronic heart failure. Aust. Occup. Ther. J. 2010, 57, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, Ş.U.; Şendemir-Ürkmez, A.; Türker, K.S. Effect of gender, age, fatigue and contraction level on electromechanical delay. Clin. Neurophysiol. 2010, 121, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.S.L.; Naveen, S.; Singsit, D.; Naika, M.; Khanum, F. Anti-fatigue effects of polyphenols extracted from pomegranate peel. Int. J. Integr. Biol. 2011, 11, 69–72. [Google Scholar]
- Trexler, E.T.; Smith-Ryan, A.E.; Melvin, M.N.; Roelofs, E.J.; Wingfield, H.L. Effects of pomegranate extract on blood flow and running time to exhaustion. Appl. Physiol. Nutr. Metab. 2014, 39, 1038–1042. [Google Scholar] [CrossRef]
- Suzuki, M.; Itokawa, Y. Effects of thiamine supplementation on exercise-induced fatigue. Metab. Brain Dis. 1996, 11, 95–106. [Google Scholar] [CrossRef]
- Huck, C.J.; Johnston, C.S.; Beezhold, B.L.; Swan, P.D. Vitamin C status and perception of effort during exercise in obese adults adhering to a calorie-reduced diet. Nutrition 2013, 29, 42–45. [Google Scholar] [CrossRef]
- Yeom, C.H.; Jung, G.C.; Shin, S.W.; Kim, S.; Choi, J.; Lee, W.; Kang, J.; Song, K. Changes in worker fatigue after vitamin C administration. J. Orthomol. Med. 2008, 23, 205. [Google Scholar]
- Grabež, M.; Škrbić, R.; Stojiljković, M.P.; Vučić, V.; Grujić, V.R.; Jakovljević, V.; Djuric, D.M.; Suručić, R.; Šavikin, K.; Bigović, D.; et al. A prospective, randomized, double-blind, placebo-controlled trial of polyphenols on the outcomes of inflammatory factors and oxidative stress in patients with type 2 diabetes mellitus. Rev. Cardiovasc. Med. 2022, 23, 57. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Saedisomeolia, A.; Wood, L.G.; Yaseri, M.; Tavasoli, S. Effects of pomegranate extract supplementation on inflammation in overweight and obese individuals: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2016, 22, 44–50. [Google Scholar] [CrossRef]
- Jafarirad, S.; Goodarzi, R.; Mohammadtaghvaei, N.; Dastoorpoor, M.; Alavinejad, P. Effectiveness of the pomegranate extract in improving hepatokines and serum biomarkers of non-alcoholic fatty liver disease: A randomized double blind clinical trial. Diabetes. Metab. Syndr. 2023, 17, 102693. [Google Scholar] [CrossRef]
- Jafari, T.; Fallah, A.A.; Reyhanian, A.; Sarmast, E. Effects of pomegranate peel extract and vitamin E on the inflammatory status and endothelial function in hemodialysis patients: A randomized controlled clinical trial. Food Funct. 2020, 11, 7987–7993. [Google Scholar] [CrossRef]
- Urbaniak, A.; Basta, P.; Ast, K.; Wołoszyn, A.; Kuriańska–Wołoszyn, J.; Latour, E.; Skarpańska–Stejnborn, A. The impact of supplementation with pomegranate fruit (Punica granatum L.) juice on selected antioxidant parameters and markers of iron metabolism in rowers. J. Int. Soc. Sports Nutr. 2018, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Trombold, J.R.; Barnes, J.N.; Critchley, L.; Coyle, E.F. Ellagitannin consumption improves strength recovery 2-3 d after eccentric exercise. Med. Sci. Sports Exerc. 2010, 42, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.L.; Ranchordas, M.K.; Johnson, E.; Denning, J.; Downing, F.; Lynn, A. No effect of tart cherry juice or pomegranate juice on recovery from exercise-induced muscle damage in non-resistance trained men. Nutrients 2019, 11, 1593. [Google Scholar] [CrossRef]
- O’Doherty, M.G.; Gilchrist, S.E.C.M.; Young, I.S.; McKinley, M.C.; Yarnell, J.W.G.; Gey, K.F.; Evans, A.; Skidmore, P.M.L.; Woodside, J.V. Effect of supplementation with B vitamins and antioxidants on levels of asymmetric dimethylarginine (ADMA) and C-reactive protein (CRP): A double-blind, randomised, factorial design, placebo-controlled trial. Eur. J. Nutr. 2010, 49, 483–492. [Google Scholar] [CrossRef]
- Lu, X.Y.; Han, B.; Deng, X.; Deng, S.Y.; Zhang, Y.Y.; Shen, P.X.; Hui, T.; Chen, R.H.; Li, X.; Zhang, Y. Pomegranate peel extract ameliorates the severity of experimental autoimmune encephalomyelitis via modulation of gut microbiota. Gut Microbes 2020, 12, 1857515. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, J.R.; Wettenhall, R.E.; Scanlon, D.; Gooley, P.R.; Lewis, D.P.; Mcgregor, N.; Stapleton, D.I.; Butt, H.L.; De Meirleir, K.L. Increased d-lactic acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo 2009, 23, 621–628. [Google Scholar] [PubMed]
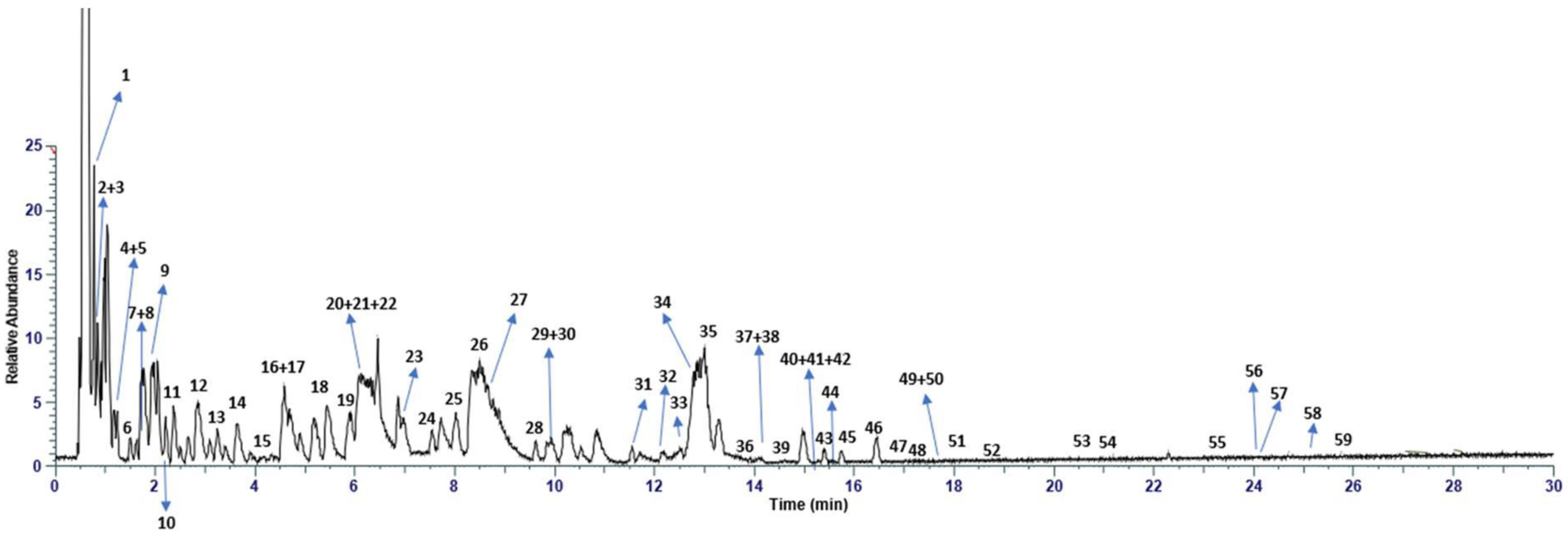
| Peak | Tr | Compound | Molecular Formula | [M-H]-/[M-2H]2- | [MS/MS] | Error (ppm) | Intensity |
|---|---|---|---|---|---|---|---|
| 1 | 0.65 | Citric acid | C6H8O7 | 191.0194 | 111, 173 | 2.01 | 2.08 × 1011 |
| 2 | 0.92 | Galloyl-hexoside | C13H15O10 | 331.0577 | 211, 169 | 1.54 | 1.23 × 108 |
| 3 | 0.99 | HHDP-hexose | C20H18O14 | 481.0693 | 300, 275 | 6.9 | 1.28 × 109 |
| 4 | 1.08 | Gallic acid | C7H6O5 | 169.0133 | 125 | 0.99 | 2.60 × 1010 |
| 5 | 1.16 | Galloyl-hexoside_I | C13H15O10 | 331.0577 | 211, 169 | 1.52 | 2.29 × 107 |
| 6 | 1.44 | HHDP galloyl hexose | C27H22O18 | 633.0739 | 275, 300 | 0.49 | 3.78× 108 |
| 7 | 1.77 | Punicalin β | C34H22O22 | 781.0593 | 721, 601, 575, 392, 298, 273 | 0.1 | 6.04 × 108 |
| 8 | 1.87 | Protocatecuic acid | C7H6O4 | 153.0184 | 109 | 0.97 | 5.50 × 108 |
| 9 | 1.98 | Punicalin β_I | C34H22O22 | 781.0593 | 721, 601, 575, 392, 298, 273, | 0.11 | 6.27 × 108 |
| 10 | 2.38 | HHDP galloylhexose_I | C27H22O18 | 633.0739 | 275, 300 | 0.5 | 7.92 × 109 |
| 11 | 2.52 | Epigallocatechin | C15H14O7 | 305.0673 | 261, 219, 179 | 0.58 | 1.54 × 108 |
| 12 | 2.84 | Pedunculagin (di-HHDP-hexose) | C34H24O22 | 783.0629 | 481, 300 275, 249 | 2.75 | 2.77 × 108 |
| 13 | 3.24 | HHDP galloylhexose_II | C27H22O18 | 633.0740 | 275, 300 | 0.47 | 5.38 × 109 |
| 14 | 3.69 | Punicalagin | C48H28O30 | 541.0262 ** | 301, 601, 275 | 2.49 | 8.36 × 107 |
| 15 | 4.16 | HHDP galloylhexose_III | C27H22O18 | 633.0738 | 275, 300 | 0.51 | 1.92 × 108 |
| 16 | 4.57 | Hamamelitannin | C20H20O14 | 483.0773 | 169, 271, 313 | 0.76 | 5.17 × 108 |
| 17 | 4.59 | Punicalagin_I | C48H28O30 | 541.0262 ** | 301, 601, 275 | 2.49 | 1.85 × 108 |
| 18 | 5.44 | Pedunculagin (di-HHDP-hexose)_I | C34H24O22 | 783.0629 | 481, 300 275, 249 | 2.75 | 2.77 × 108 |
| 19 | 5.98 | HHDP galloylhexose_IV | C27H22O18 | 633.0740 | 275, 300 | 0.47 | 2.22 × 109 |
| 20 | 6.10 | Punicalagin_II | C48H28O30 | 541.0266 ** | 301, 601, 275 | 2.49 | 2.44 × 108 |
| 21 | 6.12 | 2-hydroxycinnamic acid | C9H8O3 | 163.0393 | 119 | 0.79 | 6.10 × 107 |
| 22 | 6.15 | p-Coumaric acid hexoside | C15H18O8 | 325.0929 | 163, 119 | 0.12 | 3.17 × 108 |
| 23 | 6.96 | Pedunculagin (di-HHDP-hexose)_II | C34H24O22 | 783.0629 | 481, 300 275, 249 | 2.75 | 2.27 × 108 |
| 24 | 7.92 | 1,3,6-tri-O-galloylhexose | C27H24O18 | 635.0881 | 483, 465, 169 | 3.65 | 1.42 × 107 |
| 25 | 8.14 | Eriodictyol-7-O-hexoside | C21H22O11 | 449.1094 | 287, 259 | 2.47 | 2.99 × 108 |
| 26 | 8.47 | Punicalagin | C48H28O30 | 541.0266** | 301, 601, 275 | 2.49 | 2.83 × 108 |
| 27 | 8.77 | HHDP galloylhexose_V | C27H22O18 | 633.0740 | 275, 300 | 0.47 | 4.13 × 108 |
| 28 | 9.25 | 1,3,6-tri-O-galloylhexose_I | C27H24O18 | 635.0881 | 483, 465, 169 | 3.65 | 1.65 × 107 |
| 29 | 9.62 | 1,3,6-tri-O-galloylhexose_II | C27H24O18 | 635.0880 | 483, 465, 169 | 3.63 | 6.02 × 106 |
| 30 | 9.93 | 1,3,6-tri-O-galloylhexose_III | C27H24O18 | 635.0882 | 483, 465, 169 | 3.61 | 5.35 × 106 |
| 31 | 11.22 | 1,3,6-tri-O-galloylhexose_IV | C27H24O18 | 635.0883 | 483, 465, 169 | 3.61 | 1.11 × 107 |
| 32 | 11.56 | Myricetin-3-O-beta-L-galactopyranoside | C21H20O13 | 479.0844 | 316 | 2.58 | 2.05 × 107 |
| 33 | 11.72 | 1,3,6-tri-O-galloylhexose_V | C27H24O18 | 635.0883 | 483, 465, 169 | 3.61 | 2.19 × 107 |
| 34 | 12.73 | Quercetin-3-O-glucuronide | C21H18O13 | 477.0669 | 301 | 1.17 | 1.17 × 107 |
| 35 | 13.01 | Ellagic acid | C14H6O8 | 300.9987 | 229 | 3.75 | 6.98 × 108 |
| 36 | 13.54 | Quercetin-3-O-rutinoside | C27H30O16 | 609.1458 | 301, 463 | 3.52 | 4.34 × 107 |
| 37 | 13.89 | Naringenin-7-O-hexoside | C21H22O10 | 433.1143 | 271, 313 | 3.36 | 3.71 × 106 |
| 38 | 13.91 | Quercetin-3-O-hexoside | C21H20O12 | 463.0895 | 301 | 4.54 | 5.22 × 107 |
| 39 | 14.59 | Quercetin-3-O-glucuronide | C21H18O13 | 477.0669 | 301 | 1.17 | 1.08 × 107 |
| 40 | 15.24 | quercetin-3-arabinoside | C20H18O11 | 433.0780 | 301 | 3.95 | 5.47 × 107 |
| 41 | 15.27 | Eryodictol | C15H12O6 | 287.0568 | 259, 125 | 4.39 | 3.20 × 106 |
| 42 | 15.38 | Kaempferol-7-O-hexoside | C21H20O11 | 447.0943 | 285, 299 | 3.56 | 1.59 × 107 |
| 43 | 15.40 | Kaempferol-3-O-glucorhamnoside | C27H30O15 | 593.1523 | 285 | 3.27 | 1.09 × 108 |
| 44 | 15.62 | quercetin-3-arabinoside_I | C20H18O11 | 433.0780 | 301 | 3.95 | 3.86 × 105 |
| 45 | 15.75 | Kaempferol-7-O-hexoside_I | C21H20O11 | 447.0943 | 285, 299 | 3.56 | 9.14 × 107 |
| 46 | 16.41 | 1,2,3,6-tetragalloylglucose | C34H28O22 | 787.1018 | 617, 465, 169 | 1.7 | 4.69 × 105 |
| 47 | 16.63 | Myricetin | C15H10O8 | 317.0309 | 178, 151 | 3.50 | 3.17 × 106 |
| 48 | 16.99 | Kaempferol-3-alpha-L-arabinopyranoside | C20H18O10 | 417.0833 | 284 | 2.88 | 1.11 × 107 |
| 49 | 17.31 | Apigenin-8-C-hexoside | C21H20O10 | 431.0996 | 269 | 3.58 | 3.52 × 107 |
| 50 | 17.35 | Kaempferol-3-alpha-L-arabinopyranoside_I | C20H18O10 | 417.0833 | 284 | 2.88 | 9.85 × 106 |
| 51 | 17.57 | Phloridzin | C21H24O10 | 435.1304 | 273, 167 | 3.64 | 1.67 × 107 |
| 52 | 19.11 | Hesperidin | C28H34O15 | 609.1816 | 301 | 3.11 | 5.37 × 106 |
| 53 | 20.50 | Quercetin | C15H10O7 | 301.0359 | 178, 151 | 1.29 | 1.03 × 107 |
| 54 | 20.90 | Kaempferol | C15H10O6 | 285.0402 | 151, 133 | 4.72 | 5.31 × 106 |
| 55 | 23.58 | Naringenin | C15H12O5 | 271.0613 | 177, 151, 119 | 5.01 | 1.38 × 106 |
| 56 | 23.99 | Apigenin | C15H10O5 | 269.0453 | 187, 119 | 3.5 | 1.65 × 106 |
| 57 | 24.06 | Luteolin | C15H10O6 | 285.0402 | 133, 151 | 4.83 | 5.57 × 106 |
| 58 | 25.48 | Isorhamnetin | C16H12O7 | 315.0517 | 300 | 4.25 | 1.48 × 106 |
| 59 | 25.80 | Syringetin | C17H14O8 | 345.0612 | 315, 330 | 3.87 | 4.72 × 106 |
| Characteristics of Enrolled Subjects | Group 1 (n = 29) Placebo | Group 2 (n = 29) Treated |
|---|---|---|
| Mean age (years): | 49 ± 16 | 45 ± 15 |
| ○ Males (years) | 46 ± 12 | 50 ± 16 |
| ○ Female (years) | 44 ± 17 | 45 ± 16 |
| Gender: | ||
| ○ Males | 12 | 10 |
| ○ Females | 17 | 19 |
| Ethnicity: | ||
| ○ European | 29 | 29 |
| Placebo | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| t0 | t1 | t2 | t3 | t0 | t1 | t2 | t3 | |
| FSS | 3.0 ± 0.8 | 2.7 ± 0.9 | 3.1 ± 1.0 | 3.2 ± 0.8 | 2.9 ± 0.8 | 2.4 ± 0.9 | 2.3 ± 0.8 | 1.7 ± 0.7 |
| 2–4 | 1–4 | 1–5 | 2–5 | 2–4 | 1–4 | 1–4 | 1–3 | |
| SF-12 | 27.6 ± 9.5 | 27.7 ± 9.7 | 27.8 ± 9.8 | 27.7 ± 9.8 | 30.5 ± 8.1 | 30.7 ± 8.1 | 31.2 ± 8.1 | 32 ± 8.2 |
| 13–44 | 14–45 | 13–45 | 13–45 | 13–43 | 12–44 | 12–45 | 12–46 | |
| Model | Gdlnum | Gdl Den | F | p |
|---|---|---|---|---|
| FSS | ||||
| Measurement | 3 | 176 | 6.104 | <0.001 |
| Treatment | 1 | 206 | 30.39 | <0.001 |
| Gender | 1 | 158 | 0.968 | 0.33 |
| Age | 1 | 194 | 4.568 | 0.034 |
| Measurement × Treatment | 3 | 176 | 17.73 | <0.001 |
| SF-12 | ||||
| Measurement | 3 | 179 | 0.219 | 0.88 |
| Treatment | 1 | 221 | 1.631 | 0.20 |
| Gender | 1 | 198 | 0.495 | 0.48 |
| Age | 1 | 217 | 1.983 | 0.16 |
| Measurement × Treatment | 3 | 179 | 0.171 | 0.92 |
| Biochemical Markers | Placebo | Treatment | ||
|---|---|---|---|---|
| t0 | t2 | t0 | t2 | |
| CRP (mg/L) | 4.5 ± 1.8 (2–7) | 3.1 ± 2.3 (0–7) | 4.0 ± 1.7 (2–7) | 3.6 ± 1.4 (2–6) |
| Cortisol (μg/dL) | 15.2 ± 5.0 (7–25) | 15.3 ± 5.6 (8–25) | 17.3 ± 5.7 (8–25) | 16.4 ± 6.2 (7–25) |
| IL-6 (pg/mL) | 3.4 ± 1.4 (1–5.5) | 2.9 ± 1.1 (1.3–4.8) | 3.8 ± 1.4 (1.2–5.7) | 3.4 ± 1.3 (1.1–5.1) |
| Mg++ (mEq/L) | 2.0 ± 0.2 (1.7–2.2) | 1.9 ± 0.2 (1.7–2.2) | 1.9 ± 0.1 (1.7–2.2) | 2.0 ± 0.1 (1.7–2.2) |
| K+ (mEq/L) | 4.5 ± 0.4 (3.8–5.1) | 4.5 ± 0.3 (3.8–5.1) | 4.5 ± 0.4 (3.8–5.1) | 4.4 ± 0.4 (3.8–5.1) |
| Ca++ (mEq/L) | 9.2 ± 0.5 (8.6–10.2) | 9.4 ± 0.5 (8.6–10.2) | 9.3 ± 0.5 (8.7–10.3) | 9.5 ± 0.5 (8.7–10.2) |
| CPK (U/L) | 73.3 ± 34.5 (22–142) | 90.7 ± 34.7 (31–145) | 84.5 ± 33.8 (34–140) | 91.6 ± 35.5 (21–143) |
| Vitamin B12 (pg/mL) | 524.4 ± 189.4 (197–880) | 559.6 ± 191.7 (242–869) | 603.1 ± 188.6 (277–888) | 583.4 ± 198.7 (192–840) |
| Folic acid (ng/mL) | 15.8 ± 7.1 (4.1–26.3) | 15.6 ± 7.0 (4.3–26.5) | 15.5 ± 5.9 (4.3–25.3) | 14 ± 6.9 (4–26.9) |
| Vitamin D (ng/mL)* | 48.4 ± 24.9 (11–98) | 48.2 ± 26.5 (12–92) | 50.6 ± 26.4 (14–98) | 60.6 ± 28.0 (13–96) |
| Model | Dfnum | Df Den | F | p |
|---|---|---|---|---|
| CRP | ||||
| Measurement | 1 | 110 | 6.796 | 0.011 |
| Treatment | 1 | 110 | 0.002 | 0.97 |
| Gender | 1 | 110 | 1.172 | 0.28 |
| Age | 1 | 110 | 0.072 | 0.79 |
| Measurement × Treatment | 1 | 110 | 2.511 | 0.12 |
| Cortisol | ||||
| Measurement | 1 | 80 | 0.130 | 0.72 |
| Treatment | 1 | 88 | 2.234 | 0.14 |
| Gender | 1 | 65 | 0.436 | 0.51 |
| Age | 1 | 83 | 0.049 | 0.82 |
| Measurement × Treatment | 1 | 80 | 0.210 | 0.65 |
| IL-6 | ||||
| Measurement | 1 | 110 | 3.921 | 0.050 |
| Treatment | 1 | 110 | 3.775 | 0.050 |
| Gender | 1 | 110 | 3.112 | 0.08 |
| Age | 1 | 110 | 0.692 | 0.41 |
| Measurement × Treatment | 1 | 110 | 0.028 | 0.87 |
| Mg++ | ||||
| Measurement | 1 | 59 | 0.505 | 0.48 |
| Treatment | 1 | 87 | 0.067 | 0.80 |
| Gender | 1 | 57 | 0.049 | 0.82 |
| Age | 1 | 79 | 0.013 | 0.91 |
| Measurement × Treatment | 1 | 59 | 2.825 | 0.10 |
| K+ | ||||
| Measurement | 1 | 65 | 0.000 | 0.99 |
| Treatment | 1 | 83 | 0.093 | 0.76 |
| Gender | 1 | 54 | 0.956 | 0.33 |
| Age | 1 | 76 | 0.222 | 0.64 |
| Measurement × Treatment | 1 | 65 | 0.115 | 0.74 |
| Ca++ | ||||
| Measurement | 1 | 110 | 4.873 | 0.029 |
| Treatment | 1 | 110 | 1.140 | 0.29 |
| Gender | 1 | 110 | 0.026 | 0.87 |
| Age | 1 | 110 | 0.601 | 0.44 |
| Measurement × Treatment | 1 | 110 | 0.078 | 0.78 |
| CPK | ||||
| Measurement | 1 | 79 | 3.998 | 0.049 |
| Treatment | 1 | 92 | 0.766 | 0.38 |
| Gender | 1 | 69 | 0.184 | 0.67 |
| Age | 1 | 87 | 3.413 | 0.07 |
| Measurement × Treatment | 1 | 79 | 0.695 | 0.41 |
| Vitamin B12 | ||||
| Measurement | 1 | 110 | 0.048 | 0.83 |
| Treatment | 1 | 110 | 2.417 | 0.12 |
| Gender | 1 | 110 | 3.593 | 0.06 |
| Age | 1 | 110 | 0.068 | 0.79 |
| Measurement × Treatment | 1 | 110 | 0.600 | 0.44 |
| Folic acid | ||||
| Measurement | 1 | 110 | 0.461 | 0.50 |
| Treatment | 1 | 110 | 0.644 | 0.42 |
| Gender | 1 | 110 | 2.783 | 0.10 |
| Age | 1 | 110 | 0.196 | 0.66 |
| Measurement × Treatment | 1 | 110 | 0.271 | 0.60 |
| Vitamin D | ||||
| Measurement | 1 | 79 | 0.989 | 0.32 |
| Treatment | 1 | 83 | 2.350 | 0.13 |
| Gender | 1 | 60 | 1.093 | 0.30 |
| Age | 1 | 78 | 0.015 | 0.90 |
| Measurement × Treatment | 1 | 79 | 1.089 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, H.; Sommella, E.; Minno, A.D.; Piccinocchi, R.; Buccato, D.G.; Lellis, L.F.D.; Riccioni, C.; Baldi, A.; El-Seedi, H.R.; Khalifa, S.A.M.; et al. Combination of Chemically Characterized Pomegranate Extract and Hydrophilic Vitamins against Prolonged Fatigue: A Monocentric, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 2883. https://doi.org/10.3390/nu15132883
Ullah H, Sommella E, Minno AD, Piccinocchi R, Buccato DG, Lellis LFD, Riccioni C, Baldi A, El-Seedi HR, Khalifa SAM, et al. Combination of Chemically Characterized Pomegranate Extract and Hydrophilic Vitamins against Prolonged Fatigue: A Monocentric, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients. 2023; 15(13):2883. https://doi.org/10.3390/nu15132883
Chicago/Turabian StyleUllah, Hammad, Eduardo Sommella, Alessandro Di Minno, Roberto Piccinocchi, Daniele Giuseppe Buccato, Lorenza Francesca De Lellis, Costanza Riccioni, Alessandra Baldi, Hesham R. El-Seedi, Shaden A. M. Khalifa, and et al. 2023. "Combination of Chemically Characterized Pomegranate Extract and Hydrophilic Vitamins against Prolonged Fatigue: A Monocentric, Randomized, Double-Blind, Placebo-Controlled Clinical Trial" Nutrients 15, no. 13: 2883. https://doi.org/10.3390/nu15132883
APA StyleUllah, H., Sommella, E., Minno, A. D., Piccinocchi, R., Buccato, D. G., Lellis, L. F. D., Riccioni, C., Baldi, A., El-Seedi, H. R., Khalifa, S. A. M., Piccinocchi, G., Campiglia, P., Sacchi, R., & Daglia, M. (2023). Combination of Chemically Characterized Pomegranate Extract and Hydrophilic Vitamins against Prolonged Fatigue: A Monocentric, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients, 15(13), 2883. https://doi.org/10.3390/nu15132883












