Resident Esophageal Microbiota Dysbiosis Correlates with Cancer Risk in Barrett’s Esophagus Patients and Is Linked to Low Adherence to WCRF/AICR Lifestyle Recommendations
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples Collection
- (i)
- Presence of other types of cancer;
- (ii)
- Taking antibiotics/probiotics within 3 months;
- (iii)
- Previous esophageal surgery.
2.2. Lifestyle and Diet Questionnaire
2.3. DNA Extraction and Sequencing
2.4. Bioinformatic Analysis
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
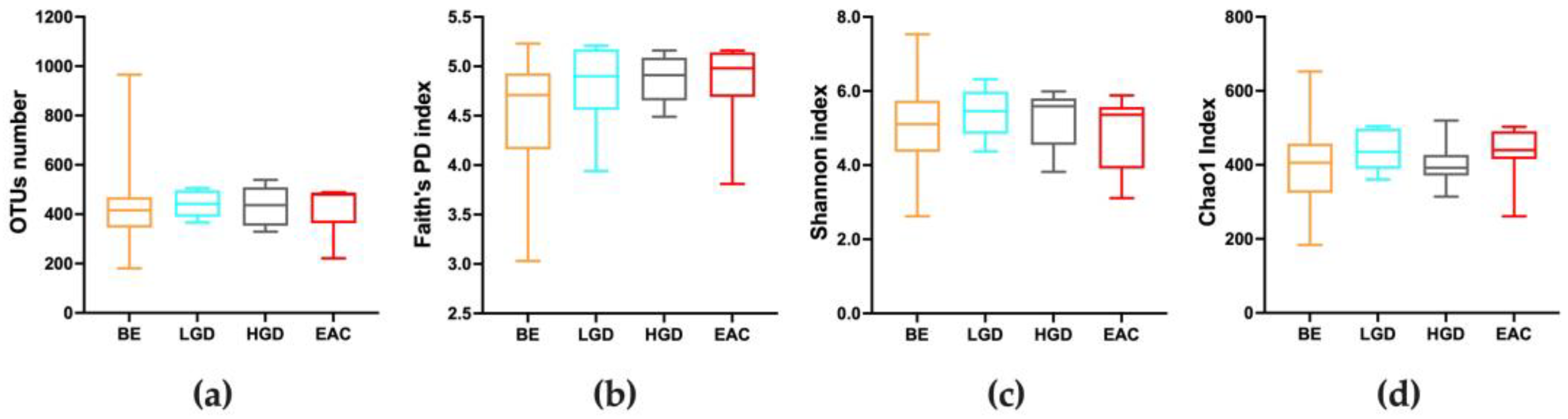
3.2. Quality of Sequencing and Diversity Measurements throughout the Disease
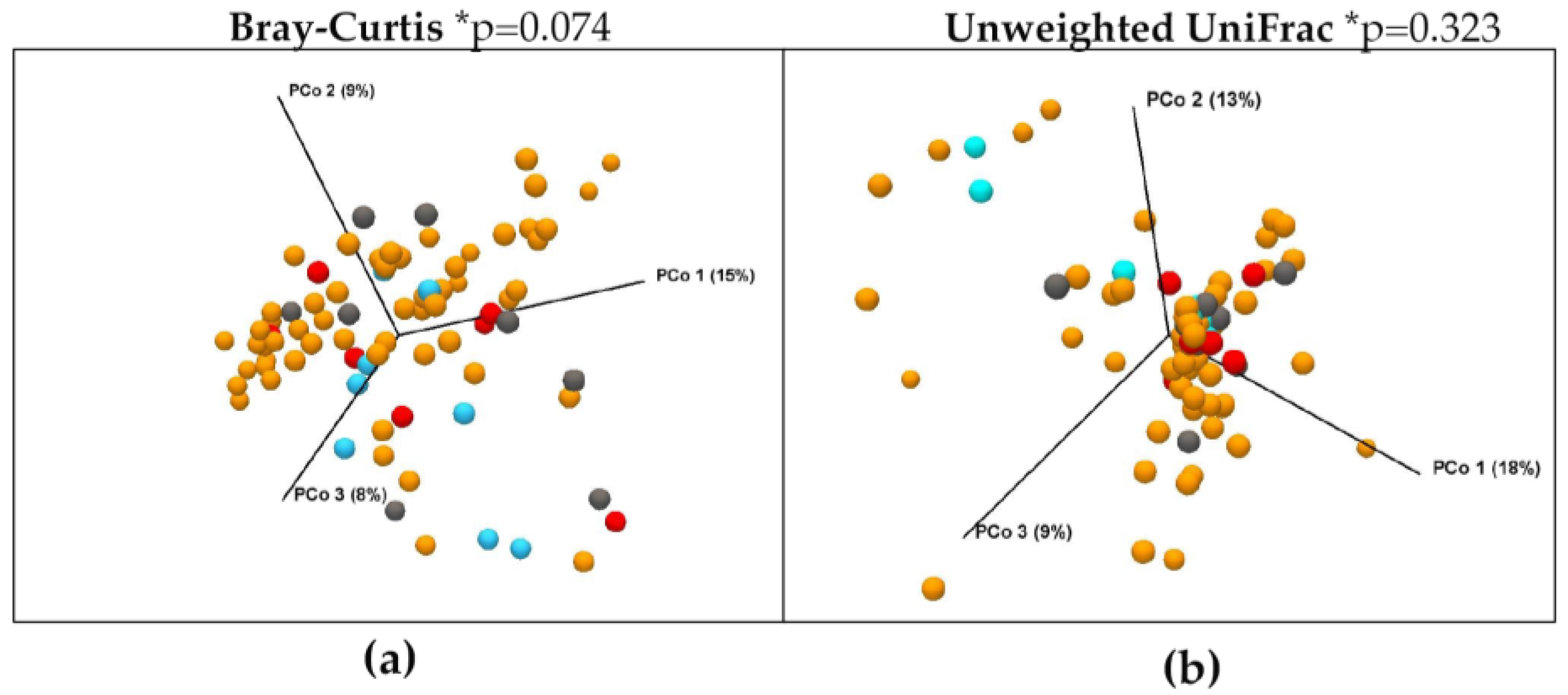
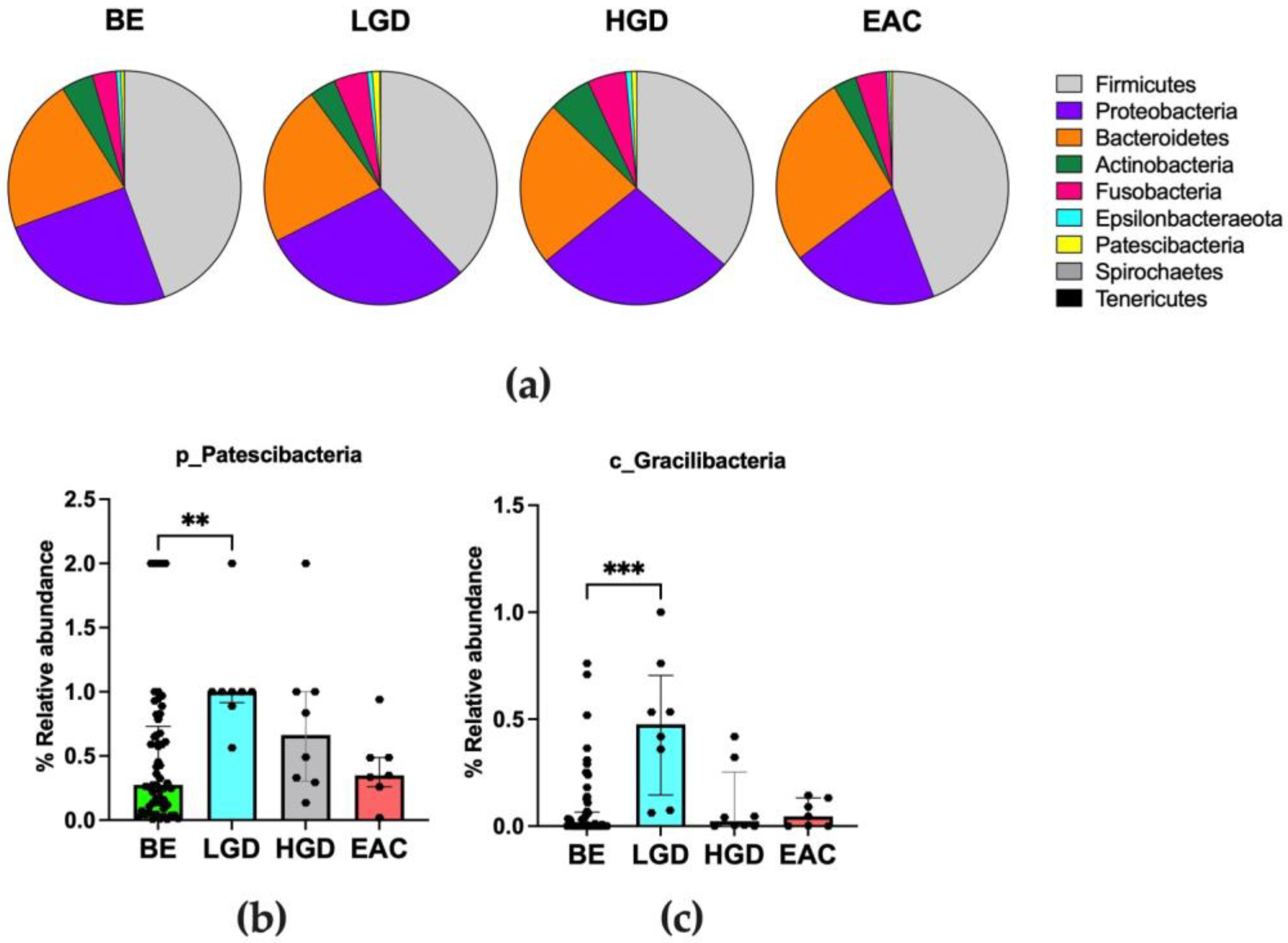
3.3. Differences in Microbiota Composition throughout the Disease
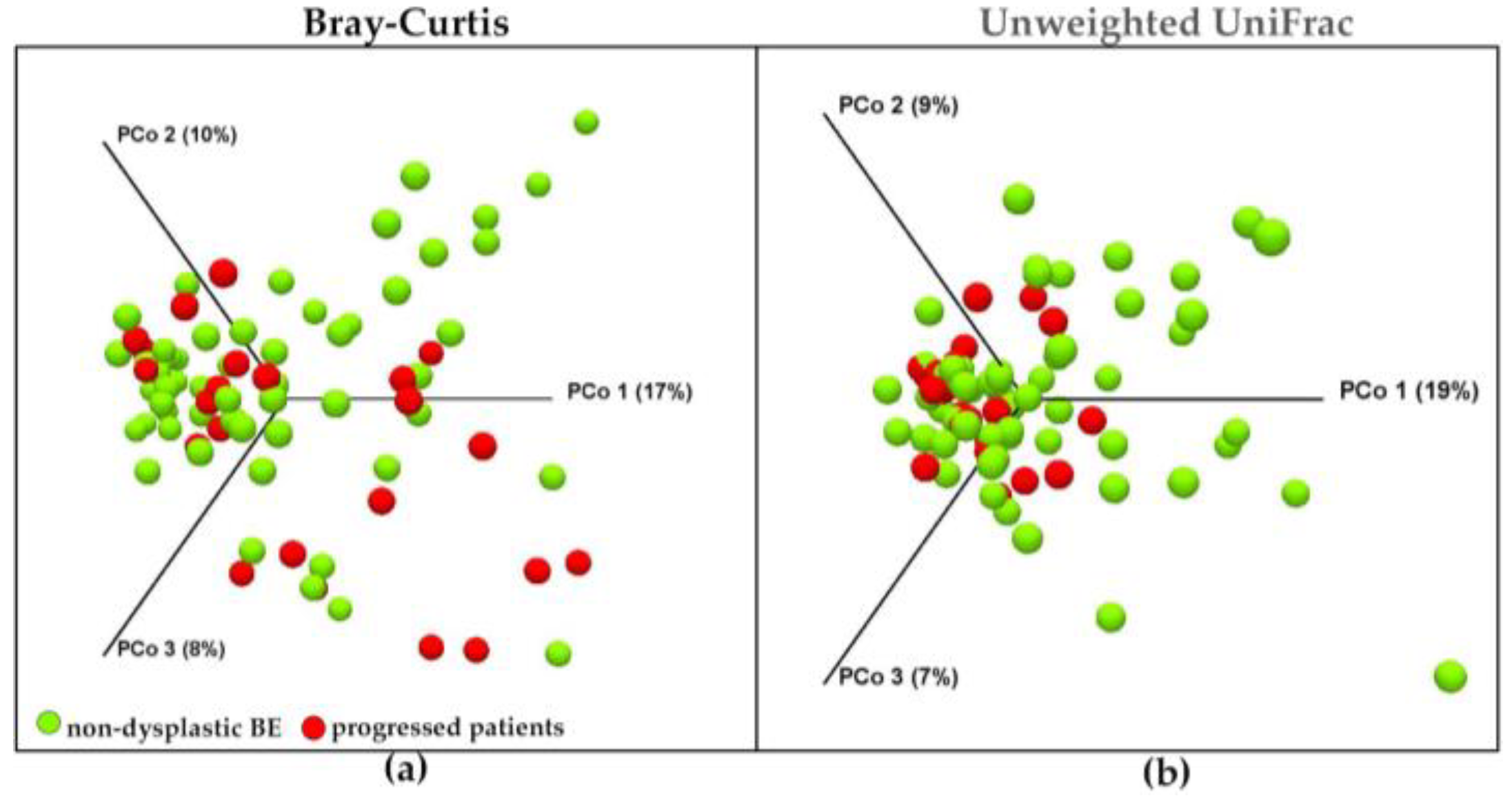
3.4. Main Distinguishing Features between Non-Dysplastic BE Patients and Patients with BE-Related Dysplasia or Cancer
3.5. Development of an Esophageal Microbiota Dysbiosis Test
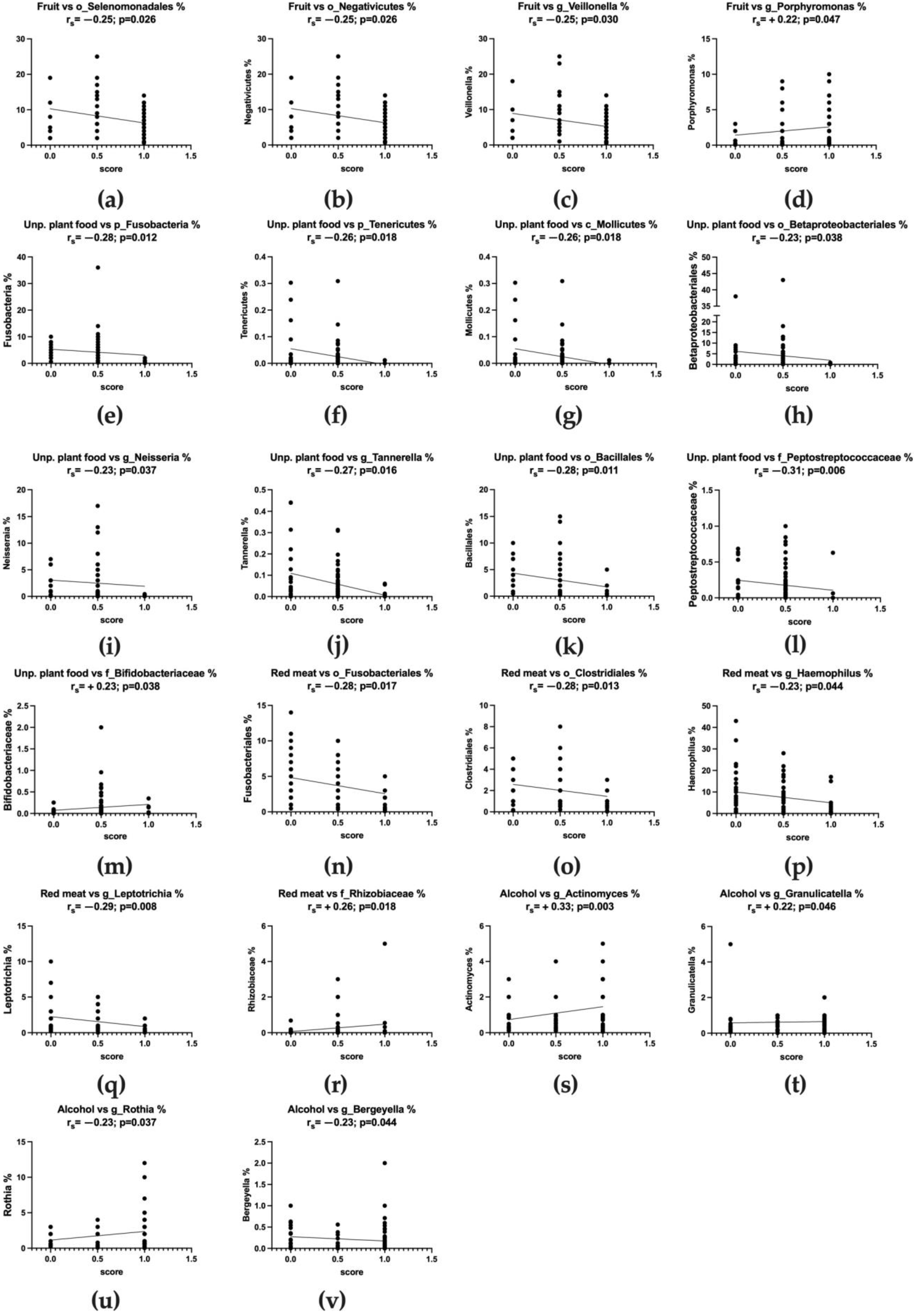
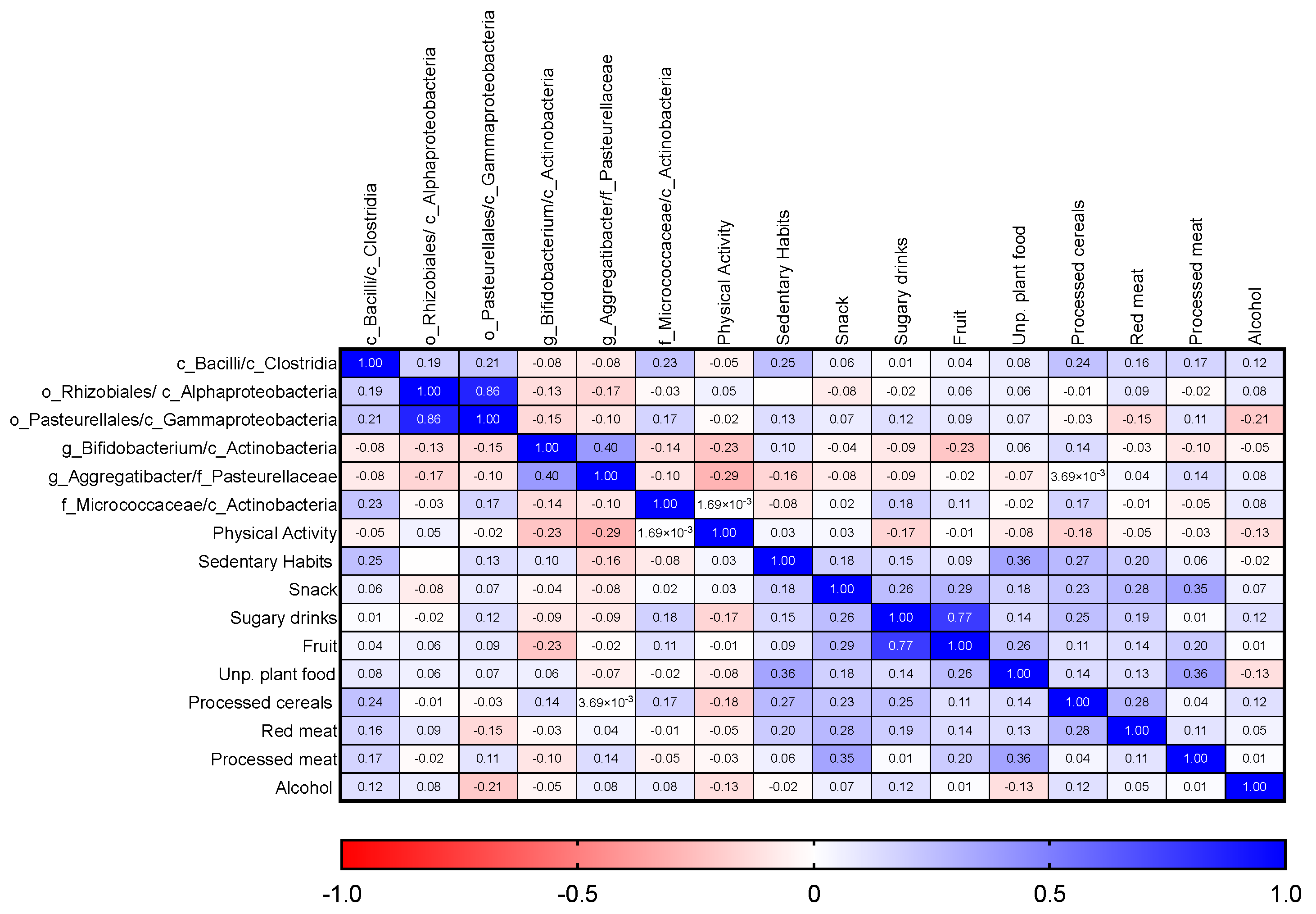
3.6. Adherence to WCRF/AICR Recommendations and Correlation with Microbiota Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Cao, S.; Dong, X.; Rao, S.; Cai, K. Understanding Esophageal Cancer: The Challenges and Opportunities for the Next Decade. Front. Oncol. 2020, 10, 1727. [Google Scholar] [CrossRef] [PubMed]
- Spechler, S.J. Barrett esophagus and risk of esophageal cancer: A clinical review. JAMA 2013, 310, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-H. From reflux esophagitis to Barrett’s esophagus and esophageal adenocarcinoma. World J. Gastroenterol. 2015, 21, 5210. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Reynolds, J. Visceral Obesity, Metabolic Syndrome, and Esophageal Adenocarcinoma. Front. Oncol. 2021, 11, 627270. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Galeone, C.; Augustin, L.S.A.; La Vecchia, C. Glycemic Index, Glycemic Load and Cancer Risk: An Updated Meta-Analysis. Nutrients 2019, 11, 2342. [Google Scholar] [CrossRef]
- Steck, S.E.; Murphy, E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer 2020, 20, 125–138. [Google Scholar] [CrossRef]
- Farvid, M.S.; Cho, E.; Chen, W.Y.; Eliassen, A.H.; Willett, W.C. Dietary protein sources in early adulthood and breast cancer incidence: Prospective cohort study. BMJ 2014, 348, g3437. [Google Scholar] [CrossRef]
- World Cancer Research Fund and American Institute for Cancer Research. Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective; World Cancer Research Fund and American Institute for Cancer Research: Washington, DC, USA, 2007. [Google Scholar]
- Vergnaud, A.C.; Romaguera, D.; Peeters, P.H.; van Gils, C.H.; Chan, D.S.; Romieu, I.; Freisling, H.; Ferrari, P.; Clavel-Chapelon, F.; Fagherazzi, G.; et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research guidelines and risk of death in Europe: Results from the European Prospective Investigation into Nutrition and Cancer cohort study1-5. Am. J. Clin. Nutr. 2013, 97, 1107–1120. [Google Scholar] [CrossRef]
- Makarem, N.; Lin, Y.; Bandera, E.V.; Jacques, P.F.; Parekh, N. Concordance with World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) guidelines for cancer prevention and obesity-related cancer risk in the Framingham Offspring cohort (1991–2008). Cancer Causes Control 2015, 26, 277–286. [Google Scholar] [CrossRef]
- Realdon, S.; Antonello, A.; Arcidiacono, D.; Dassie, E.; Cavallin, F.; Fassan, M.; Nardi, M.T.; Alberti, A.; Rugge, M.; Battaglia, G. Adherence to WCRF/AICR lifestyle recommendations for cancer prevention and the risk of Barrett’s esophagus onset and evolution to esophageal adenocarcinoma: Results from a pilot study in a high-risk population. Eur. J. Nutr. 2016, 55, 1563–1571. [Google Scholar] [CrossRef]
- Sharma, T.; Gupta, A.; Chauhan, R.; Bhat, A.A.; Nisar, S.; Hashem, S.; Akhtar, S.; Ahmad, A.; Haris, M.; Singh, M.; et al. Cross-talk between the microbiome and chronic inflammation in esophageal cancer: Potential driver of oncogenesis. Cancer Metastasis Rev. 2022, 41, 281–299. [Google Scholar] [CrossRef]
- Macfarlane, S.; Furrie, E.; Macfarlane, G.T.; Dillon, J.F. Microbial Colonization of the Upper Gastrointestinal Tract in Patients with Barrett’s Esophagus. Clin. Infect. Dis. 2007, 45, 29–38. [Google Scholar] [CrossRef]
- Yang, L.; Lu, X.; Nossa, C.W.; Francois, F.; Peek, R.M.; Pei, Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 2009, 137, 588–597. [Google Scholar] [CrossRef]
- Snider, E.J.; Compres, G.; Freedberg, D.E.; Khiabanian, H.; Nobel, Y.R.; Stump, S.; Uhlemann, A.; Lightdale, C.J.; Abrams, J.A. Alterations to the Esophageal Microbiome Associated with Progression from Barrett’s Esophagus to Esophageal Adenocarcinoma. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1687–1693. [Google Scholar] [CrossRef]
- Kennedy, M.S.; Chang, E.B. The microbiome: Composition and locations. Prog. Mol. Biol. Transl. Sci. 2020, 176, 1–42. [Google Scholar] [CrossRef]
- Pei, Z.; Bini, E.J.; Yang, L.; Zhou, M.; Francois, F.; Blaser, M.J. Bacterial biota in the human distal esophagus. Proc. Natl. Acad. Sci. USA 2004, 101, 4250–4255. [Google Scholar] [CrossRef]
- Fillon, S.A.; Harris, J.K.; Wagner, B.D.; Wagner, B.D.; Kelly, C.J.; Stevens, M.J.; Moore, W.; Fang, R.; Schroeder, S.; Masterson, J.C.; et al. Novel Device to Sample the Esophageal Microbiome—The Esophageal String Test. PLoS ONE 2012, 7, e42938. [Google Scholar] [CrossRef]
- Deshpande, N.P.; Riordan, S.M.; Castaño-Rodríguez, N.; Wilkins, M.R.; Kaakoush, N.O. Signatures within the esophageal microbiome are associated with host genetics, age, and disease. Microbiome 2018, 6, 227. [Google Scholar] [CrossRef]
- Amir, I.; Konikoff, F.M.; Oppenheim, M.; Gophna, U.; Half, E.E. Gastric microbiota is altered in oesophagitis and Barrett’s oesophagus and further modified by proton pump inhibitors. Environ. Microbiol. 2014, 16, 2905–2914. [Google Scholar] [CrossRef]
- Nobel, Y.R.; Snider, E.J.; Compres, G.; Freedberg, D.E.; Khiabanian, H.; Lightdale, C.J.; Toussaint, N.C.; Abrams, J.A. Increasing Dietary Fiber Intake Is Associated with a Distinct Esophageal Microbiome. Clin. Transl. Gastroenterol. 2018, 9, e199. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Institute for Cancer. Continuous Update Project Report: Diet, Nutrition, Physical Activity and Stomach Cancer 2016. Revised 2018. wcrf.org/sites/default/files/Stomach-Cancer-2016-Report.pdf. Accessed. 2018. Available online: https://www.wcrf.org/dietandcancer/stomach-cancer (accessed on 16 July 2020).
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.D.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R.; Poulsen, S.K.; Larsen, T.M.; Bahl, M.I. Microbial Enterotypes, Inferred by the Prevotella-to-Bacteroides Ratio, Remained Stable during a 6-Month Randomized Controlled Diet Intervention with the New Nordic Diet. Appl. Environ. Microbiol. 2014, 80, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F. Gut Microbiota Parameters Potentially Useful in Clinical Perspective. Microorganisms 2021, 9, 2402. [Google Scholar] [CrossRef] [PubMed]
- Vogtmann, E.; Goedert, J.J. Epidemiologic studies of the human microbiome and cancer. Br. J. Cancer 2016, 114, 237–242. [Google Scholar] [CrossRef]
- Moreira, C.; Figueiredo, C.; Ferreira, R.M. The Role of the Microbiota in Esophageal Cancer. Cancers 2023, 15, 2576. [Google Scholar] [CrossRef]
- Münch, N.S.; Fang, H.-Y.; Ingermann, J.; Maurer, H.C.; Anand, A.; Kellner, V.; Sahm, V.; Wiethaler, M.; Baumeister, T.; Wein, F.; et al. High-Fat Diet Accelerates Carcinogenesis in a Mouse Model of Barrett’s Esophagus via Interleukin 8 and Alterations to the Gut Microbiome. Gastroenterology 2019, 157, 492–506.e2. [Google Scholar] [CrossRef]
- Zou, Q.; Feng, L.; Cai, X.; Qian, Y.; Xu, L. Esophageal microflora in esophageal diseases. Front. Cell. Infect. Microbiol. 2023, 13, 1145791. [Google Scholar] [CrossRef]
- Elliott, D.R.F.; Walker, A.W.; O’Donovan, M.; Parkhill, J.; Fitzgerald, R.C. A non-endoscopic device to sample the oesophageal microbiota: A case-control study. Lancet Gastroenterol. Hepatol. 2017, 2, 32–42. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Severgnini, M.; Pecere, S.; Ponziani, F.R.; Boskoski, I.; Larghi, A.; Quaranta, G.; Masucci, L.; Ianiro, G.; Carboni, T.; et al. Esophageal microbiome signature in patients with Barrett’s esophagus and esophageal adenocarcinoma. PLoS ONE 2020, 15, e0231789. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Z.; Lin, Y.; Chen, Y.; Peng, X.; He, F.; Liu, S.; Yan, S.; Huang, L.; Lu, W.; et al. Streptococcus and Prevotella are associated with the prognosis of oesophageal squamous cell carcinoma. J. Med. Microbiol. 2018, 67, 1058–1068. [Google Scholar] [CrossRef]
- Wong, S.H.; Kwong, T.N.Y.; Chow, T.C.; Luk, A.K.C.; Dai, R.Z.W.; Nakatsu, G.; Lam, T.Y.T.; Zhang, L.; Wu, J.C.Y.; Chan, F.K.L.; et al. Quantitation of faecal Fusobacterium improves faecal immunochemical test in detecting advanced colorectal neoplasia. Gut 2017, 66, 1441–1448. [Google Scholar] [CrossRef]
- Smet, A.; Kupcinskas, J.; Link, A.; Hold, G.L.; Bornschein, J. The Role of Microbiota in Gastrointestinal Cancer and Cancer Treatment: Chance or Curse? Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 857–874. [Google Scholar] [CrossRef]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. Lancet Oncol. 2017, 18, e457–e471. [Google Scholar] [CrossRef]
- Arcidiacono, D.; Zaramella, A.; Fabris, F.; Sánchez-Rodríguez, R.; Nucci, D.; Fassan, M.; Nardi, M.; Benna, C.; Cristofori, C.; Morbin, T.; et al. Insulin/IGF-1 Signaling Is Downregulated in Barrett’s Esophagus Patients Undergoing a Moderate Calorie and Protein Restriction Program: A Randomized 2-Year Trial. Nutrients 2021, 13, 3638. [Google Scholar] [CrossRef]
- Cheng, J.; Ouwehand, A.C. Gastroesophageal Reflux Disease and Probiotics: A Systematic Review. Nutrients 2020, 12, 132. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, S.; Luan, S.; Luan, S.; Xiao, X.; Yang, Y.; Mao, C.; Chen, L.; Zeng, X.; Zhang, Y.; et al. Gut Microbiota for Esophageal Cancer: Role in Carcinogenesis and Clinical Implications. Front. Oncol. 2021, 11, 717242. [Google Scholar] [CrossRef]
- Liu, C.; Yang, J.; Dong, W.; Yuan, J. Effects of probiotics on gastrointestinal complications and nutritional status of postoperative patients with esophageal cancer. Medicine 2021, 100, e25138. [Google Scholar] [CrossRef]
- Indrio, F.; Riezzo, G.; Raimondi, F.; Filannino, A.; Bisceglia, M.; Cavallo, L.; Francavilla, R. 77 Lactobacillus Reuterii Accelerates Gastric Emptying and Improves Regurgitation in Infants. Pediatr. Res. 2010, 68, 42. [Google Scholar] [CrossRef]
- Zhang, K.; Dai, H.; Liang, W.; Zhang, L.; Deng, Z. Fermented dairy foods intake and risk of cancer. Int. J. Cancer 2019, 144, 2099–2108. [Google Scholar] [CrossRef]




| WCRF/AICR Recommendation | Sub-Recommendations | Operationalization | Scoring |
|---|---|---|---|
| Body fatness: be as lean as possible without becoming underweight | (a) Ensure that body weight throughout childhood and adolescent growth projects toward the lower end of normal BMI range at age 21 | Due to the already established role of both excessive BMI and waist circumference as independent risk factors for BE and EAC, this recommendation was not included in the questionnaire | NA |
| (b) Maintain body weight within normal range from the age of 21 | |||
| (c) Avoid weight gain and increase in waist circumference throughout adulthood | |||
| Physical activity: be physically active as part of your everyday life | (a) Be moderately physically active, equivalent of brisk walking ≥30 min every day | Heavy manual job, or ≥2 h/week of vigorous physical activity, or ≥30 min of brisk walking every day | 1 |
| 15–30 min/day of brisk walking | 0.5 | ||
| Less than 15 min/day of brisk walking | 0 | ||
| (c) Limit sedentary habits such as watching television | ≤1 h/day of sedentary activities | 1 | |
| Between 1 and 3 h/day of sedentary activities | 0.5 | ||
| ≥3 h/day of sedentary activities | 0 | ||
| Foods and drinks that promote weight gain: limit consumption of energy-dense foods and avoid sugary drinks | (a) Consume energy-dense foods sparingly | Less than one serving per week | 1 |
| Between one and two servings per week | 0.5 | ||
| More than two servings per week | 0 | ||
| (b) Avoid sugary drinks | Less than 250 g of sugary drinks per week | 1 | |
| Between 250 g and 500 g of sugary drinks per week | 0.5 | ||
| More than 500 g of sugary drinks per week | 0 | ||
| Plant foods: eat mostly foods of plant origin | (a) Eat ≥5 servings of non-starchy vegetables and/or fruits every day | ≥5 servings per day | 1 |
| Between 1 and 5 servings per day | 0.5 | ||
| ≤1 serving per day/not at all | 0 | ||
| (b) Eat relatively unprocessed cereals and pulses with every meal | >1 serving per day | 1 | |
| 1 serving per day | 0.5 | ||
| <1 serving per day/not at all | 0 | ||
| (c) Limit refined starchy foods | <1 serving per day/not at all | 1 | |
| 1 serving per day | 0.5 | ||
| >1 serving per day | 0 | ||
| Animal foods: limit intake of red meat and avoid processed meat | (a) People who eat red meat should consume <500 g/wk and very few, if any, processed meats | No red meat intake | 1 |
| Red meat intake <500 g/wk | 0.5 | ||
| Red meat intake ≥500 g/wk | 0 | ||
| No processed meat intake | 1 | ||
| <50 g/day processed meat intake | 0.5 | ||
| ≥50 g/day processed meat intake | 0 | ||
| Alcoholic drinks: limit alcoholic drinks | (a) If alcoholic drinks are consumed, limit consumption to ≤2 drinks/d for men and 1 drink/d for women | <2 drink/d (men) or <1 drink/d (women) | 1 |
| 2 drink/d (men) or 1 drink/d (women) | 0.5 | ||
| ≥2 drink/d (men) or ≥1 drink/d (women) | 0 | ||
| Preservation, processing, preparation: limit consumption of salt, avoid moldy cereals or pulses | (a) Avoid salt-preserved, or salty foods; preserve foods without using salt | Insufficient data available | NA |
| (b) Limit consumption of processed foods with salt to ensure an intake <6 g/d of salt | Insufficient data available | NA | |
| (c) Do not eat moldy cereals or pulses | Not applicable to this population | NA | |
| Dietary supplements: aim to meet nutritional needs through diet alone | (a) Dietary supplements are not recommended for cancer prevention | Insufficient data available | NA |
| BE | LGD | HGD | EAC | p Value | |
|---|---|---|---|---|---|
| n | 57 | 8 | 8 | 7 | n/a |
| AGE, mean (±SD) | 58.3 (±10.7) | 57.9 (±7.8) | 64.0 (±6.5) | 62.3 (±12.6) | 0.375 * |
| SEX: female, n (%) | 8 (14) | 0 (0) | 1 (12) | 0 (0) | n/a |
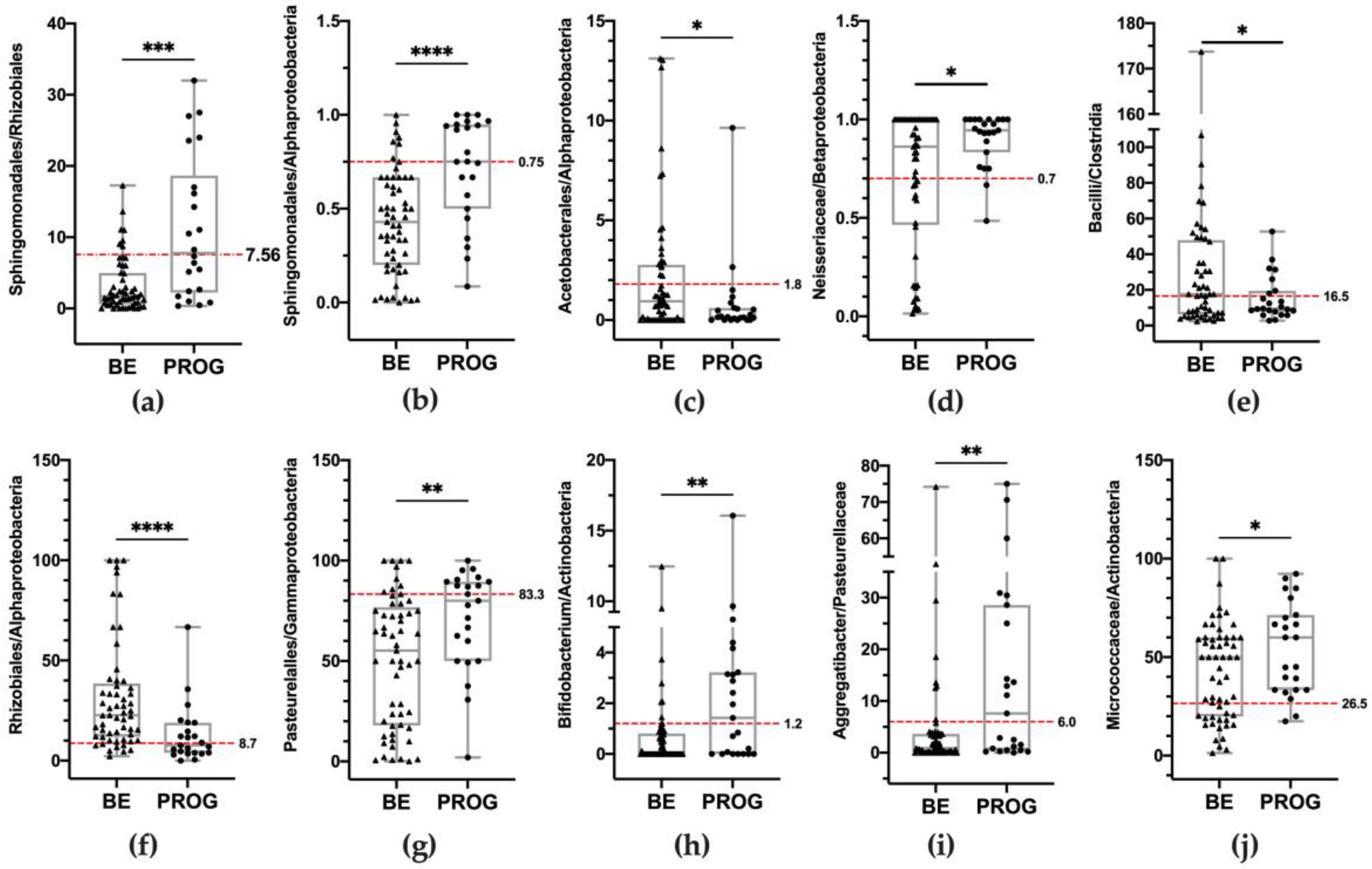
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Risk Conditions | BE n = 57 | PROG n = 23 | OR | 95% CI | p | p | 95% CI |
| Sphingomondales/Rhizobiales ≥7.56 (Q3) | 8 (14.0) | 12 (52.2) | 6.68 | 2.21 to 20.24 | 0.001 | n.s. | / |
| Sphingomonadales/ Alphaproteobacteria ≥0.75 (Q3) | 7 (12.3) | 12 (52.2) | 7.79 | 2.50 to 24.32 | <0.001 | n.s. | / |
| Acetobacterales/Alphaproteobacteria ≤1.8 (Q3) | 39 (68.4) | 21 (91.3) | 4.85 | 1.02 to 22.93 | 0.046 | n.s. | / |
| Neisseriaceae/Betaproteobacteriales ≥0.70 (Q3) | 38 (66.7) | 22 (95.6) | 11.00 | 1.38 to 87.90 | 0.024 | n.s. | / |
| Bacilli/Clostridia ≤16.5 (Q2) | 25 (43.9) | 16 (69.6) | 2.92 | 1.04 to 8.22 | 0.041 | 0.023 | 0.030 to 0.397 |
| Rhizobiales/Alphaproteobacteria ≤8.7 (Q1) | 8 (14.0) | 12 (52.2) | 6.68 | 2.21 to 20.24 | 0.001 | 0.003 | 0.106 to 0.491 |
| Pasteurellales/Gammaproteobacteria ≥83.3 (Q3) | 10 (17.5) | 11 (47.8) | 4.22 | 1.45 to 12.25 | 0.008 | 0.021 | 0.037 to 0.427 |
| Bifidobacterium/Actinobacteria ≥1.2 (Q3) | 8 (14.0) | 12 (52.2) | 6.68 | 2.21 to 20.24 | 0.001 | <0.001 | 0.174 to 0.537 |
| Aggregatibacter/Pasteurellaceae ≥6.0 (Q3) | 8 (14.0) | 12 (52.2) | 6.68 | 2.21 to 20.24 | 0.001 | 0.037 | 0.012 to 0.387 |
| Micrococcaceae/Actinobacteria ≥26.5 (Q1) | 39 (68.4) | 21 (91.3) | 4.85 | 1.02 to 22.93 | 0.047 | 0.002 | 0.094 to 0.407 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaramella, A.; Arcidiacono, D.; Nucci, D.; Fabris, F.; Benna, C.; Pucciarelli, S.; Fassan, M.; Fantin, A.; De Re, V.; Cannizzaro, R.; et al. Resident Esophageal Microbiota Dysbiosis Correlates with Cancer Risk in Barrett’s Esophagus Patients and Is Linked to Low Adherence to WCRF/AICR Lifestyle Recommendations. Nutrients 2023, 15, 2885. https://doi.org/10.3390/nu15132885
Zaramella A, Arcidiacono D, Nucci D, Fabris F, Benna C, Pucciarelli S, Fassan M, Fantin A, De Re V, Cannizzaro R, et al. Resident Esophageal Microbiota Dysbiosis Correlates with Cancer Risk in Barrett’s Esophagus Patients and Is Linked to Low Adherence to WCRF/AICR Lifestyle Recommendations. Nutrients. 2023; 15(13):2885. https://doi.org/10.3390/nu15132885
Chicago/Turabian StyleZaramella, Alice, Diletta Arcidiacono, Daniele Nucci, Federico Fabris, Clara Benna, Salvatore Pucciarelli, Matteo Fassan, Alberto Fantin, Vallì De Re, Renato Cannizzaro, and et al. 2023. "Resident Esophageal Microbiota Dysbiosis Correlates with Cancer Risk in Barrett’s Esophagus Patients and Is Linked to Low Adherence to WCRF/AICR Lifestyle Recommendations" Nutrients 15, no. 13: 2885. https://doi.org/10.3390/nu15132885
APA StyleZaramella, A., Arcidiacono, D., Nucci, D., Fabris, F., Benna, C., Pucciarelli, S., Fassan, M., Fantin, A., De Re, V., Cannizzaro, R., & Realdon, S. (2023). Resident Esophageal Microbiota Dysbiosis Correlates with Cancer Risk in Barrett’s Esophagus Patients and Is Linked to Low Adherence to WCRF/AICR Lifestyle Recommendations. Nutrients, 15(13), 2885. https://doi.org/10.3390/nu15132885









