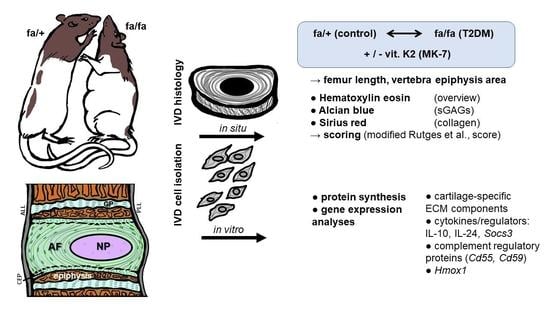
Does Vitamin K2 Influence the Interplay between Diabetes Mellitus and Intervertebral Disc Degeneration in a Rat Model?
Abstract
1. Introduction
1.1. Intervertebral Discs
1.2. Low Back Pain, Intervertebral Disc Degeneration and Diabetes Mellitus Type 2
1.3. Interrelation between Vitamin K, Diabetes Mellitus Type 2 and Intervertebral Disc Degeneration
2. Materials and Methods
2.1. Study Design
Rat ZDF Diabetic Model
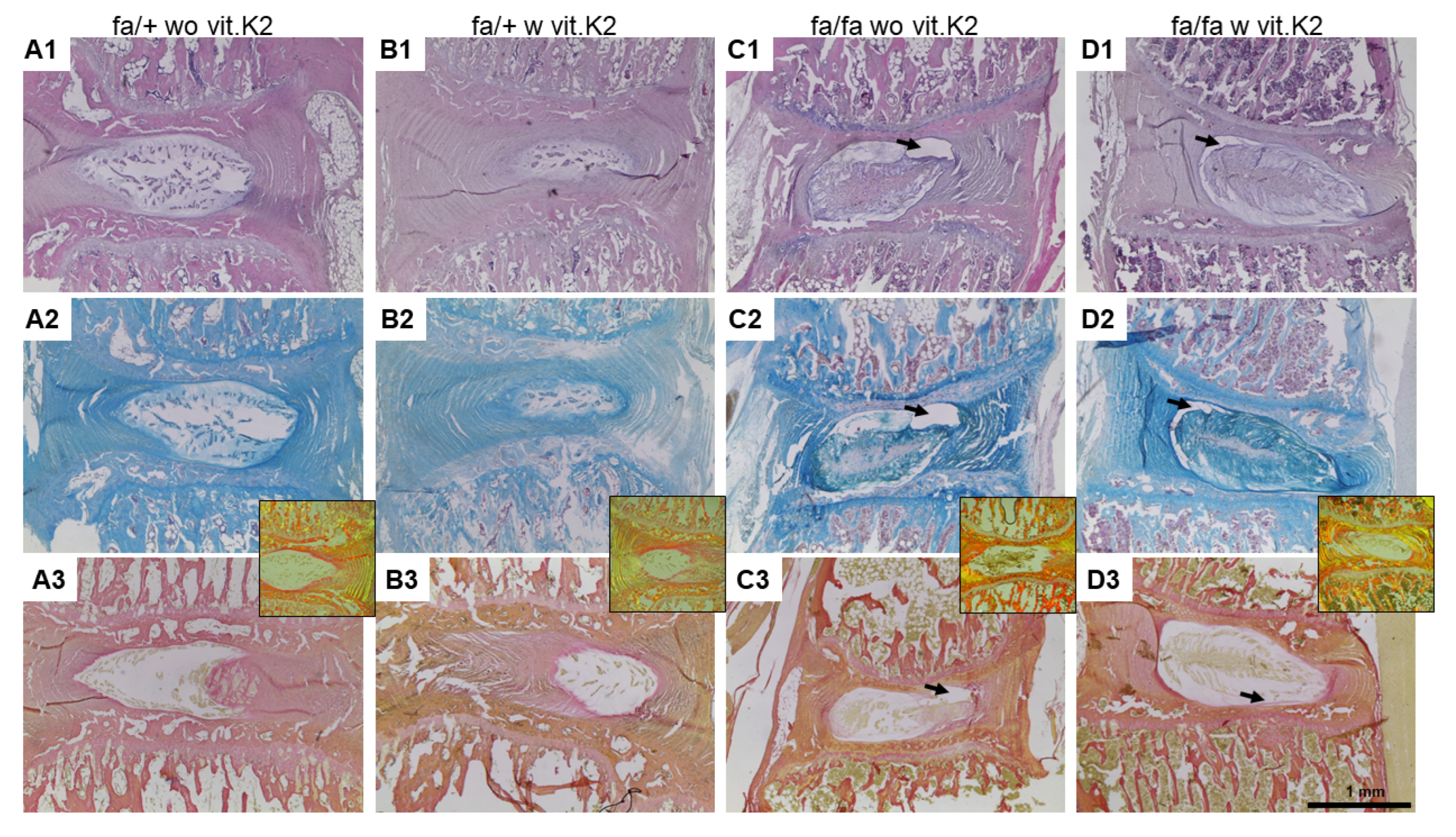
2.2. Preparation and Staining of Histological Samples
2.2.1. Hematoxylin–Eosin (HE) Stain
2.2.2. Alcian Blue (AB) Stain
2.2.3. Sirius Red (SR) Stain
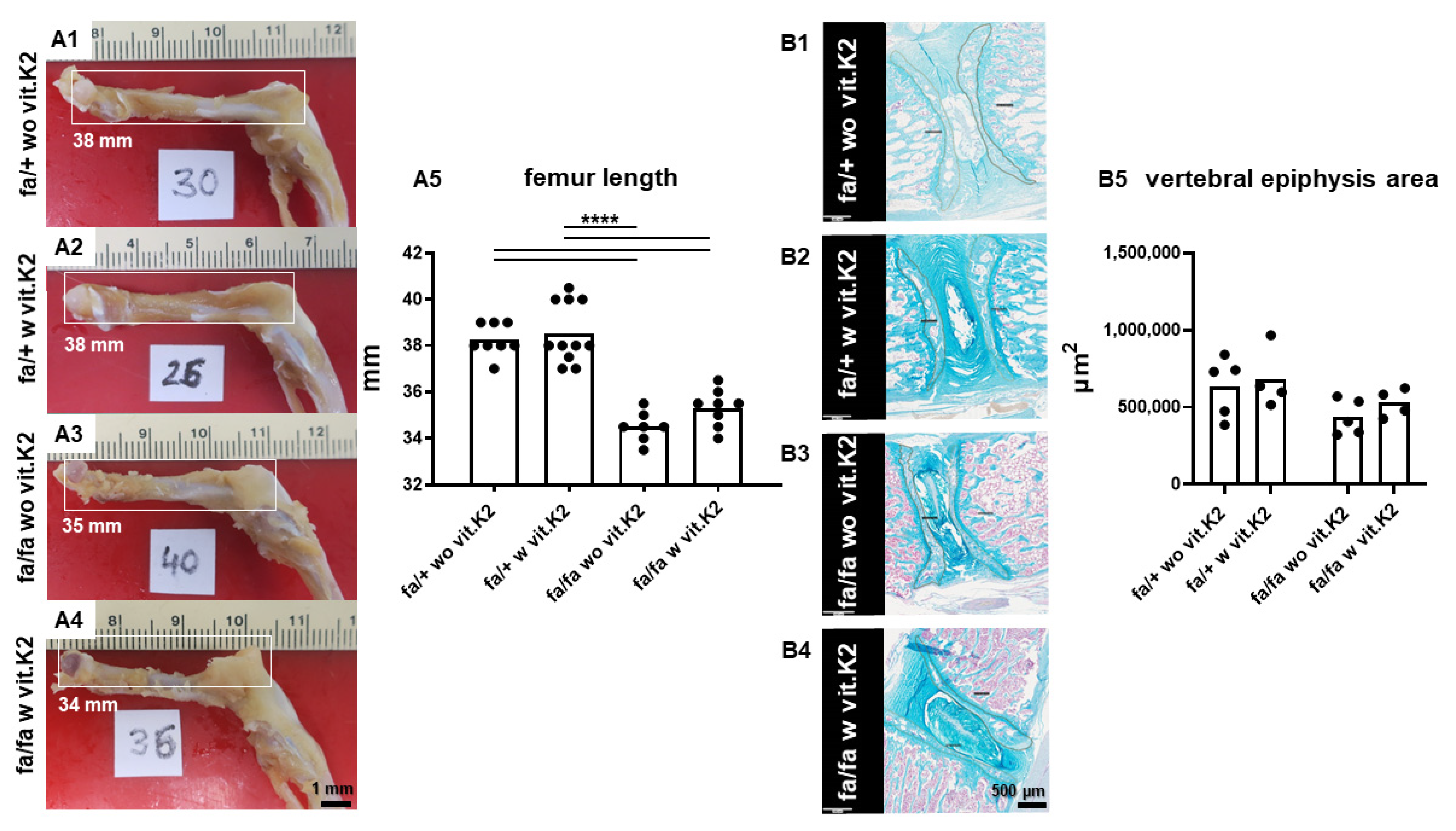
2.2.4. Measurement of Epiphyseal Cross-Section Areas
2.2.5. Histopathological Scoring System to Assess IVD Degeneration
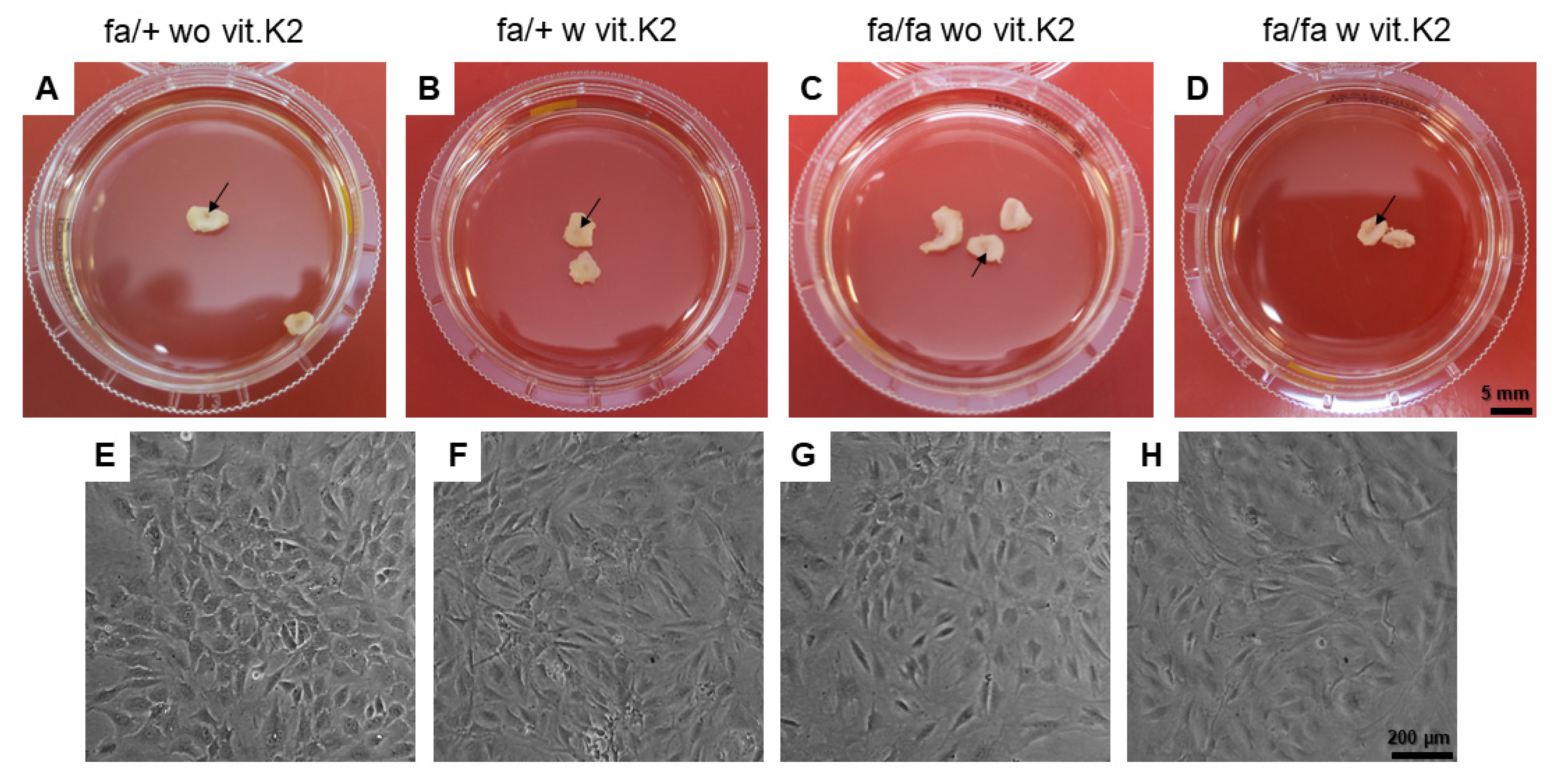
2.3. Rat IVD Cell Isolation and Cultivation
2.4. Immunofluorescence Labeling for Protein Expression Analysis in IVD Fibrochondrocytes
2.5. RNA Isolation from Fibrochondrocytes
2.6. Quantitative Realtime PCR Analysis
2.7. Statistics
3. Results
3.1. Overall Performance and Behavior of the Rats
3.2. Incidental/Coincidental Macroscopic Findings
3.3. IVDD Evaluation and Scoring
3.4. Cell Isolation from the IVDs
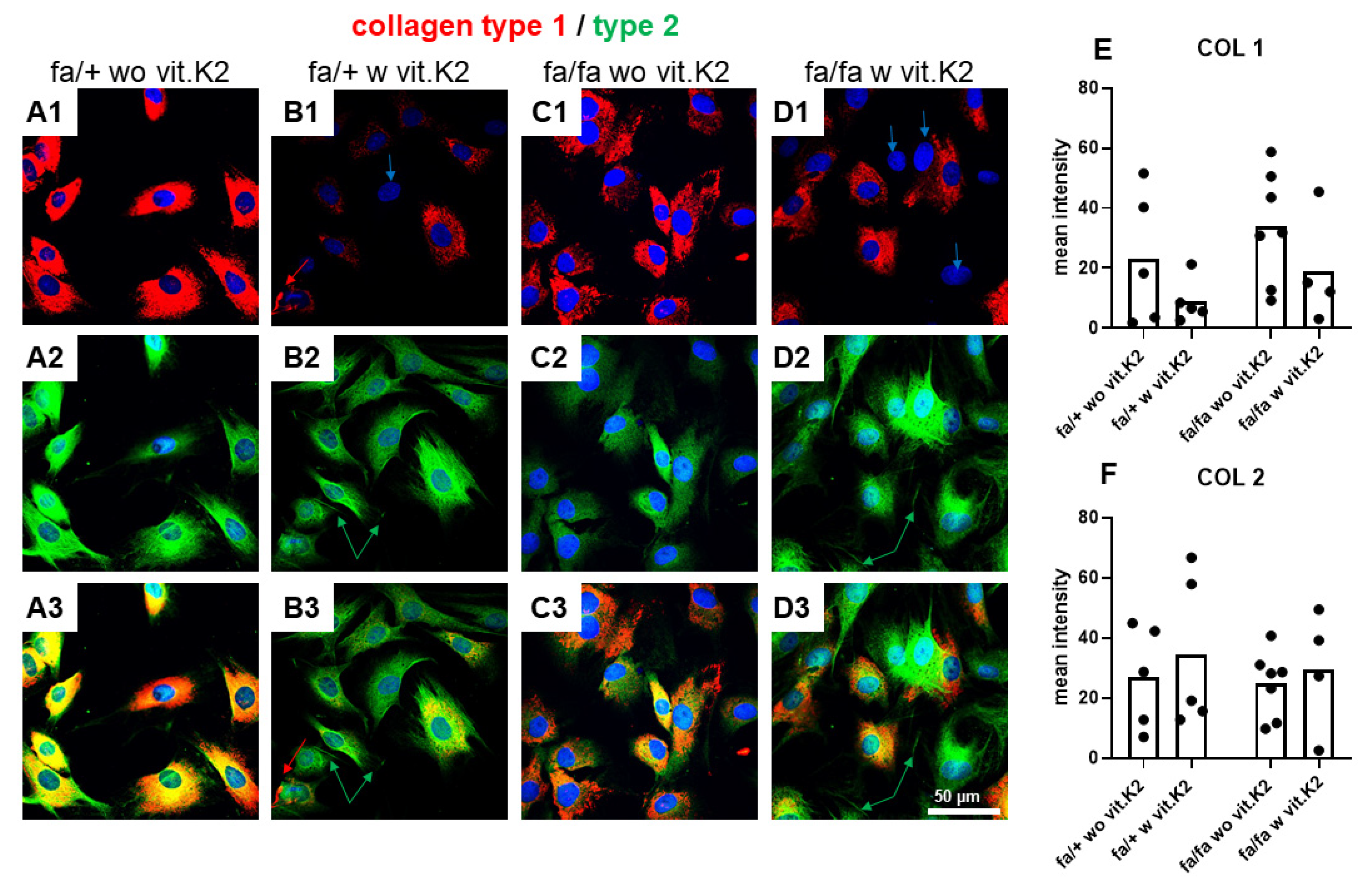
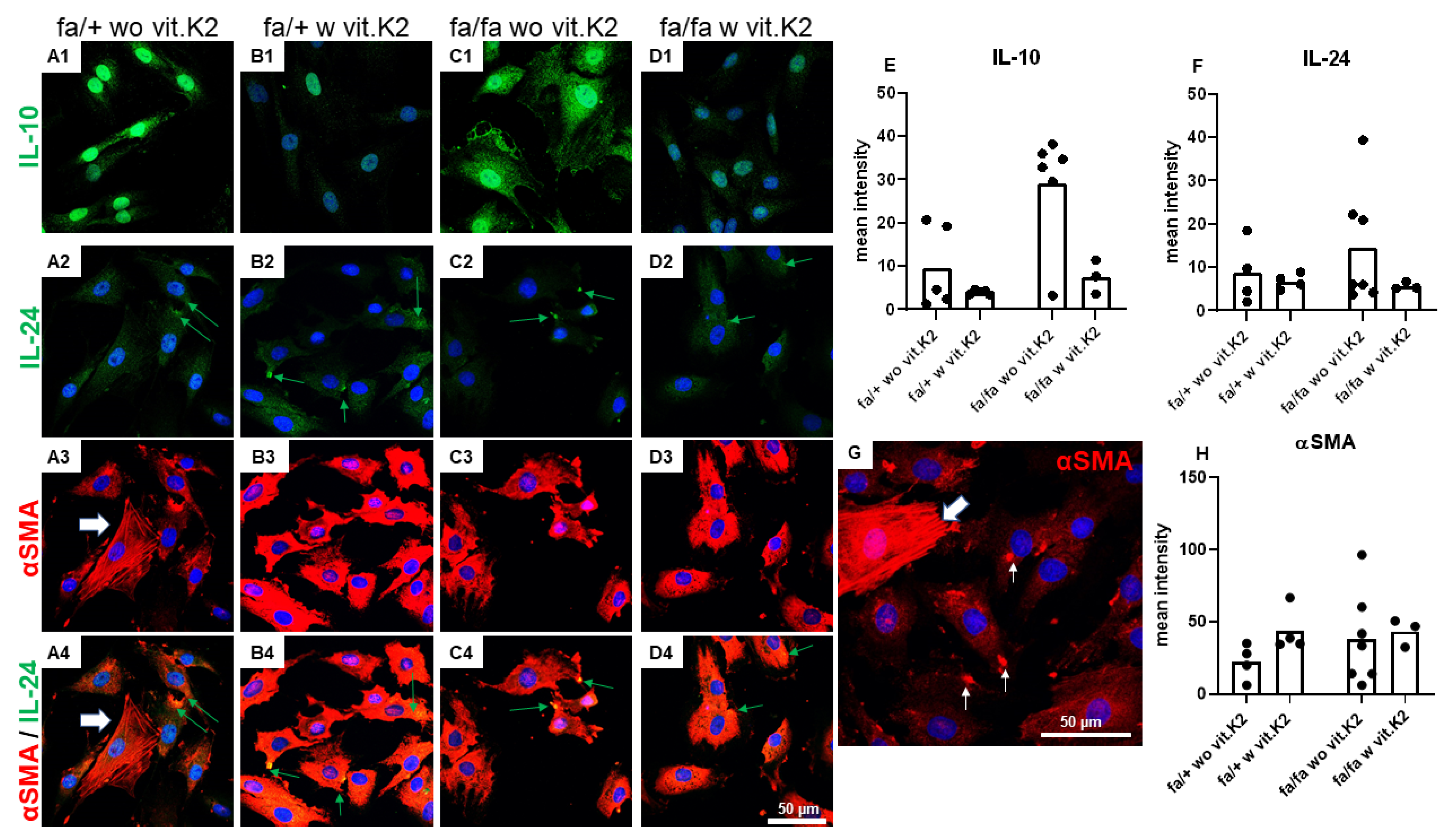
3.5. IVD-Derived Fibrochondrocyte-Related Protein Synthesis
3.6. Fibrochondrocytes Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frost, B.A.; Camarero-Espinosa, S.; Foster, E.J. Materials for the Spine: Anatomy, Problems, and Solutions. Materials 2019, 12, 253. [Google Scholar] [CrossRef]
- Cannata, F.; Vadalà, G.; Ambrosio, L.; Fallucca, S.; Napoli, N.; Papalia, R.; Pozzilli, P.; Denaro, V. Intervertebral disc degeneration: A focus on obesity and type 2 diabetes. Diabetes/Metab. Res. Rev. 2020, 36, e3224. [Google Scholar] [CrossRef]
- Newell, N.; Little, J.; Christou, A.; Adams, M.; Adam, C.; Masouros, S. Biomechanics of the human intervertebral disc: A review of testing techniques and results. J. Mech. Behav. Biomed. Mater. 2017, 69, 420–434. [Google Scholar] [CrossRef]
- Ferguson, S.J.; Steffen, T. Biomechanics of the aging spine. Eur. Spine J. 2003, 12 (Suppl. S2), S97–S103. [Google Scholar] [CrossRef]
- Walter, B.A.; Torre, O.M.; Laudier, D.; Naidich, T.P.; Hecht, A.C.; Iatridis, J.C. Form and function of the intervertebral disc in health and disease: A morphological and stain comparison study. J. Anat. 2015, 227, 707–716. [Google Scholar] [CrossRef]
- Disney, C.M.; Madi, K.; Bodey, A.J.; Lee, P.D.; Hoyland, J.A.; Sherratt, M.J. Visualising the 3D microstructure of stained and native intervertebral discs using X-ray microtomography. Sci. Rep. 2017, 7, 16279. [Google Scholar] [CrossRef]
- Zhang, Y.; Lenart, B.A.; Lee, J.K.; Chen, D.; Shi, P.; Ren, J.; Muehleman, C.; Chen, D.; An, H.S. Histological features of endplates of the mammalian spine: From mice to men. Spine 2014, 39, E312–E317. [Google Scholar] [CrossRef]
- Kim, H.S.; Wu, P.H.; Jang, I.-T. Lumbar Degenerative Disease Part 1: Anatomy and Pathophysiology of Intervertebral Discogenic Pain and Radiofrequency Ablation of Basivertebral and Sinuvertebral Nerve Treatment for Chronic Discogenic Back Pain: A Prospective Case Series and Review of Literature. Int. J. Mol. Sci. 2020, 21, 1483. [Google Scholar] [CrossRef]
- Guzik, G. Early developed ASD (adjacent segmental disease) in patients after surgical treatment of the spine due to cancer metastases. J. Orthop. Surg. Res. 2017, 12, 70. [Google Scholar] [CrossRef]
- Liu, X.; Pan, F.; Ba, Z.; Wang, S.; Wu, D. The potential effect of type 2 diabetes mellitus on lumbar disc degeneration: A retrospective single-center study. J. Orthop. Surg. Res. 2018, 13, 52. [Google Scholar] [CrossRef]
- Park, C.-H.; Min, K.-B.; Min, J.-Y.; Kim, D.H.; Seo, K.M.; Kim, D.-K. Strong association of type 2 diabetes with degenerative lumbar spine disorders. Sci. Rep. 2021, 11, 16472. [Google Scholar] [CrossRef]
- Maruf, M.H.; Suzuki, A.; Hayashi, K.; Habibi, H.; Salimi, H.; Terai, H.; Tamai, K.; Hoshino, M.; Toyoda, H.; Yamada, K.; et al. Increased advanced glycation end products in hypertrophied ligamentum flavum of diabetes mellitus patients. Spine J. 2019, 19, 1739–1745. [Google Scholar] [CrossRef]
- Glaeser, J.D.; Ju, D.; Tawackoli, W.; Yang, J.H.; Salehi, K.; Stefanovic, T.; Kanim, L.E.A.; Avalos, P.; Kaneda, G.; Stephan, S.; et al. Advanced Glycation End Product Inhibitor Pyridoxamine Attenuates IVD Degeneration in Type 2 Diabetic Rats. Int. J. Mol. Sci. 2020, 21, 9709. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, P.; Yang, C.; Wu, X. Amyloid protein aggregation in diabetes mellitus accelerate intervertebral disc degeneration. Med. Hypotheses 2020, 141, 109739. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; Kokozidou, M.; Auffarth, A.; Schulze-Tanzil, G. The Relationship between Diabetes Mellitus Type II and Intervertebral Disc Degeneration in Diabetic Rodent Models: A Systematic and Comprehensive Review. Cells 2020, 9, 2208. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-M.; Hu, F.; Wang, X.-Y.; Tong, S.-L. Relationship Between Apoptosis of Endplate Microvasculature and Degeneration of the Intervertebral Disk. World Neurosurg. 2019, 125, e392–e397. [Google Scholar] [CrossRef]
- Wang, W.; Li, P.; Xu, J.; Wu, X.; Guo, Z.; Fan, L.; Song, R.; Wang, J.; Wei, L.; Teng, H. Resveratrol attenuates high glucose-induced nucleus pulposus cell apoptosis and senescence through activating the ROS-mediated PI3K/Akt pathway. Biosci. Rep. 2018, 38, BSR20171454. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.Q.; Yong, Z.; Kuhn, A.; Riegger, J.; Goncalves, R.M.; Ruf, M.; Mauer, U.M.; Huber-Lang, M.; Ignatius, A.; Brenner, R.E.; et al. Interleukin-1beta and cathepsin D modulate formation of the terminal complement complex in cultured human disc tissue. Eur. Spine J. 2021, 30, 2247–2256. [Google Scholar] [CrossRef]
- Hanaei, S.; Abdollahzade, S.; Sadr, M.; Mirbolouk, M.H.; Khoshnevisan, A.; Rezaei, N. Association of IL10 and TGFB single nucleotide polymorphisms with intervertebral disc degeneration in Iranian population: A case control study. BMC Med. Genet. 2018, 19, 59. [Google Scholar] [CrossRef]
- Lin, W.; Lin, J.; Chen, X.; Wu, C.; Zhang, L.; Huang, Z.; Lai, J. Interleukin-10 promoter polymorphisms associated with susceptibility to lumbar disc degeneration in a Chinese cohort. Genet. Mol. Res. 2011, 10, 1719–1727. [Google Scholar] [CrossRef]
- Huang, X.; Chen, F.; Zhao, J.; Wang, D.; Jing, S.; Li, H.; Meng, C. Interleukin 6 (IL-6) and IL-10 Promoter Region Polymorphisms Are Associated with Risk of Lumbar Disc Herniation in a Northern Chinese Han Population. Genet. Test. Mol. Biomark. 2017, 21, 17–23. [Google Scholar] [CrossRef]
- Sudhir, G.; Balasubramaniam, S.; Jayabalan, V.; Sundaram, S.; Kumar, V.; Kailash, K. Does Type II Diabetes Induce Early Senescence and Degeneration in Human Intervertebral Discs? A Tissue Biomarker Evaluation. Int. J. Spine Surg. 2020, 14, 341–346. [Google Scholar] [CrossRef]
- Fernández, P.; Guillén, M.I.; Gomar, F.; Alcaraz, M.J. Expression of heme oxygenase-1 and regulation by cytokines in human osteoarthritic chondrocytes. Biochem. Pharmacol. 2003, 66, 2049–2052. [Google Scholar] [CrossRef]
- Zhu, C.; Jiang, W.; Cheng, Q.; Hu, Z.; Hao, J. Hemeoxygenase-1 Suppresses IL-1beta-Induced Apoptosis Through the NF-kappaB Pathway in Human Degenerative Nucleus Pulposus Cells. Cell Physiol. Biochem. 2018, 46, 644–653. [Google Scholar] [CrossRef]
- Hu, B.; Shi, C.; Xu, C.; Cao, P.; Tian, Y.; Zhang, Y.; Deng, L.; Chen, H.; Yuan, W. Heme oxygenase-1 attenuates IL-1beta induced alteration of anabolic and catabolic activities in intervertebral disc degeneration. Sci. Rep. 2016, 6, 21190. [Google Scholar] [CrossRef]
- Guillén, M.; Megías, J.; Gomar, F.; Alcaraz, M. Haem oxygenase-1 regulates catabolic and anabolic processes in osteoarthritic chondrocytes. J. Pathol. 2008, 214, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Isa, I.L.M.; Rooney, P.; Pandit, A. Bioengineered three-dimensional diseased intervertebral disc model revealed inflammatory crosstalk. Biomaterials 2017, 123, 127–141. [Google Scholar] [CrossRef]
- Müller, R.D.; John, T.; Kohl, B.; Oberholzer, A.; Gust, T.; Hostmann, A.; Hellmuth, M.; LaFace, D.; Hutchins, B.; Laube, G.; et al. IL-10 overexpression differentially affects cartilage matrix gene expression in response to TNF-α in human articular chondrocytes in vitro. Cytokine 2008, 44, 377–385. [Google Scholar] [CrossRef] [PubMed]
- John, T.; Müller, R.; Oberholzer, A.; Zreiqat, H.; Kohl, B.; Ertel, W.; Hostmann, A.; Tschoeke, S.; Schulze-Tanzil, G. Interleukin-10 modulates pro-apoptotic effects of TNF-α in human articular chondrocytes in vitro. Cytokine 2007, 40, 226–234. [Google Scholar] [CrossRef]
- Akbari, S.; Rasouli-Ghahroudi, A.A. Vitamin K and Bone Metabolism: A Review of the Latest Evidence in Preclinical Studies. BioMed Res. Int. 2018, 2018, 4629383. [Google Scholar] [CrossRef] [PubMed]
- Popa, D.-S.; Bigman, G.; Rusu, M.E. The Role of Vitamin K in Humans: Implication in Aging and Age-Associated Diseases. Antioxidants 2021, 10, 566. [Google Scholar] [CrossRef]
- Hey, H.; Brasen, C.L. Vitamin K2 influences several diseases. Ugeskr. laeger 2015, 177, V12140700. [Google Scholar]
- Valdes-Ramos, R.; Ana Laura, G.L.; Beatriz Elina, M.C.; Alejandra Donaji, B.A. Vitamins and type 2 diabetes mellitus. Endocr. Metab. Immune Disord.-Drug Targets 2015, 15, 54–63. [Google Scholar] [CrossRef]
- Ho, H.-J.; Komai, M.; Shirakawa, H. Beneficial Effects of Vitamin K Status on Glycemic Regulation and Diabetes Mellitus: A Mini-Review. Nutrients 2020, 12, 2485. [Google Scholar] [CrossRef]
- Karamzad, N.; Maleki, V.; Carson-Chahhoud, K.; Azizi, S.; Sahebkar, A.; Gargari, B.P. A systematic review on the mechanisms of vitamin K effects on the complications of diabetes and pre-diabetes. Biofactors 2020, 46, 21–37. [Google Scholar] [CrossRef]
- Zwakenberg, S.R.; Remmelzwaal, S.; Beulens, J.W.; Booth, S.L.; Burgess, S.; Dashti, H.S.; Imamura, F.; Feskens, E.J.M.; van der Schouw, Y.T.; Sluijs, I. Circulating Phylloquinone Concentrations and Risk of Type 2 Diabetes: A Mendelian Randomization Study. Diabetes 2019, 68, 220–225. [Google Scholar] [CrossRef]
- Aguayo-Ruiz, J.I.; García-Cobián, T.A.; Pascoe-González, S.; Sánchez-Enríquez, S.; Llamas-Covarrubias, I.M.; García-Iglesias, T.; López-Quintero, A.; Trujillo-Quiroz, J.; Rivera-Leon, E.A. Effect of supplementation with vitamins D3 and K2 on undercarboxylated osteocalcin and insulin serum levels in patients with type 2 diabetes mellitus: A randomized, double-blind, clinical trial. Diabetol. Metab. Syndr. 2020, 12, 73. [Google Scholar] [CrossRef]
- Beulens, J.W.J.; van der A, D.L.; Grobbee, D.E.; Sluijs, I.; Spijkerman, A.M.W.; van der Schouw, Y. Dietary Phylloquinone and Menaquinones Intakes and Risk of Type 2 Diabetes. Diabetes Care 2010, 33, 1699–1705. [Google Scholar] [CrossRef]
- Słowik-Borowiec, M.; Potocki, L.; Oklejewicz, B.; Broda, D.; Podbielska, M.; Szpyrka, E. Preparation of Vitamin K2 Mk-7 in a Process of Fermentation of Different Seeds and Cereals by Bacteria Bacillus Subtilis. Acta Univ. Cibiniensis. Ser. E Food Technol. 2021, 25, 93–104. [Google Scholar] [CrossRef]
- Asrar, F.A.A.M. Evolving role of vitamin K2-7(Menaquinone) in Osteoporosis & cardiovascular health. International. J. Pharmacol. Res. 2014, 4, 43–48. [Google Scholar]
- Akbulut, A.C.; Pavlic, A.; Petsophonsakul, P.; Halder, M.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K2 Needs an RDI Separate from Vitamin K1. Nutrients 2020, 12, 1852. [Google Scholar] [CrossRef]
- Sato, T.; Inaba, N.; Yamashita, T. MK-7 and Its Effects on Bone Quality and Strength. Nutrients 2020, 12, 965. [Google Scholar] [CrossRef]
- Huang, Z.; Godkin, O.; Schulze-Tanzil, G. The Challenge in Using Mesenchymal Stromal Cells for Recellularization of Decellularized Cartilage. Stem Cell Rev. Rep. 2017, 13, 50–67. [Google Scholar] [CrossRef]
- Gundula Schulze-Tanzil, M.L.; Meier, C.; Ertel, W.; Kohl, B.; Huang, Z.; Muschik, M.; Markart, M.; Hoyer, M.; Arens, S. Characterization of Human Anulus Fibrosus– and Nucleus Pulposus–Derived Cells During Monolayer Expansion and in Hydrogel Cultures. Bone Tissue Regen. Insights 2014, 5, 15–23. [Google Scholar] [CrossRef]
- Le Maitre, C.L.; Dahia, C.L.; Giers, M.; Illien-Junger, S.; Cicione, C.; Samartzis, D.; Vadala, G.; Fields, A.; Lotz, J. Development of a standardized histopathology scoring system for human intervertebral disc degeneration: An Orthopaedic Research Society Spine Section Initiative. JOR Spine 2021, 4, e1167. [Google Scholar] [CrossRef]
- Rutges, J.; Duit, R.; Kummer, J.; Bekkers, J.; Oner, F.; Castelein, R.; Dhert, W.; Creemers, L. A validated new histological classification for intervertebral disc degeneration. Osteoarthr. Cartil. 2013, 21, 2039–2047. [Google Scholar] [CrossRef]
- Paul, C.P.L.; de Graaf, M.; Bisschop, A.; Holewijn, R.M.; van de Ven, P.M.; van Royen, B.J.; Mullender, M.G.; Smit, T.H.; Helder, M.N. Static axial overloading primes lumbar caprine intervertebral discs for posterior herniation. PLoS ONE 2017, 12, e0174278. [Google Scholar] [CrossRef]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaiser, H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel “gene expression’s CT difference” formula. J. Mol. Med. 2006, 84, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Fakhree, N.K.; Mhaibes, S.H.; Khalil, H.H. Review article—Impact of Vitamin K on Human Health. Iraqi J. Pharm. Sci. 2021, 30, 1–13. [Google Scholar] [CrossRef]
- Booth, S.L. Vitamin K: Food composition and dietary intakes. Food Nutr. Res. 2012, 56, 5505. [Google Scholar] [CrossRef] [PubMed]
- Mladenka, P.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Mrštná, K.; Carazo, A.; Protti, M.; Remião, F.; Nováková, L. Vitamin K—Sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr. Rev. 2022, 80, 677–698. [Google Scholar] [CrossRef]
- Rasmussen, S.E.; Andersen, N.L.; Dragsted, L.O.; Larsen, J.C. A safe strategy for addition of vitamins and minerals to foods. Eur. J. Nutr. 2006, 45, 123–135. [Google Scholar] [CrossRef]
- Hallett, S.A.; Ono, W.; Ono, N. Growth Plate Chondrocytes: Skeletal Development, Growth and Beyond. Int. J. Mol. Sci. 2019, 20, 6009. [Google Scholar] [CrossRef]
- Hunziker, E.B. Elongation of the Long Bones in Humans by the Growth Plates. In Recent Research in Nutrition and Growth; Karger Publishers: Basel, Switzerland, 2018; Volume 89, pp. 13–23. [Google Scholar] [CrossRef]
- Roselló-Díez, A.; Joyner, A.L. Regulation of Long Bone Growth in Vertebrates; It Is Time to Catch Up. Endocr. Rev. 2015, 36, 646–680. [Google Scholar] [CrossRef]
- Duffy, S.; Lutz, T.A.; Boyle, C.N. Rodent models of leptin receptor deficiency are less sensitive to amylin. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R856–R865. [Google Scholar] [CrossRef]
- Reinwald, S.; Peterson, R.G.; Allen, M.R.; Burr, D.B.; Hamann, C.; Goettsch, C.; Mettelsiefen, J.; Henkenjohann, V.; Rauner, M.; Hempel, U.; et al. Skeletal changes associated with the onset of type 2 diabetes in the ZDF and ZDSD rodent models. Am. J. Physiol. Metab. 2009, 296, E765–E774. [Google Scholar] [CrossRef] [PubMed]
- Ağırdil, Y. The growth plate: A physiologic overview. EFORT Open Rev. 2020, 5, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Hough, S.; Russell, J.E.; Teitelbaum, S.; Avioli, L.V. Regulation of epiphyseal cartilage metabolism and morphology in the chronic diabetic rat. Calcif. Tissue Int. 1983, 35, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Morini, S.; Pannarale, L.; Franchitto, A.; Donati, S.; Gaudio, E. Microvascular features and ossification process in the femoral head of growing rats. J. Anat. 1999, 195 Pt 2, 225–233. [Google Scholar] [CrossRef]
- Zhang, Y.; Drapeau, S.; An, H.S.; Markova, D.; Lenart, B.A.; Anderson, D.G. Histological Features of the Degenerating Intervertebral Disc in a Goat Disc-Injury Model. Spine 2011, 36, 1519–1527. [Google Scholar] [CrossRef]
- Lintz, M.; Walk, R.E.; Tang, S.Y.; Bonassar, L.J. The degenerative impact of hyperglycemia on the structure and mechanics of developing murine intervertebral discs. JOR Spine 2022, 5, e1191. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Dong, H.; Cui, Y.; Du, Q.; Wang, X.; Li, L.; Zhang, H. Telmisartan Mitigates TNF-α-Induced Type II Collagen Reduction by Upregulating SOX-9. ACS Omega 2021, 6, 11756–11761. [Google Scholar] [CrossRef]
- Ng, L.-J.; Wheatley, S.; Muscat, G.; Conway-Campbell, J.; Bowles, J.; Wright, E.; Bell, D.; Tam, P.P.; Cheah, K.S.; Koopman, P. SOX9 Binds DNA, Activates Transcription, and Coexpresses with Type II Collagen during Chondrogenesis in the Mouse. Dev. Biol. 1997, 183, 108–121. [Google Scholar] [CrossRef]
- Sekiya, I.; Tsuji, K.; Koopman, P.; Watanabe, H.; Yamada, Y.; Shinomiya, K.; Nifuji, A.; Noda, M. SOX9 Enhances Aggrecan Gene Promoter/Enhancer Activity and Is Up-regulated by Retinoic Acid in a Cartilage-derived Cell Line, TC6. J. Biol. Chem. 2000, 275, 10738–10744. [Google Scholar] [CrossRef]
- Song, H.; Park, K.-H. Regulation and function of SOX9 during cartilage development and regeneration. Semin. Cancer Biol. 2020, 67, 12–23. [Google Scholar] [CrossRef]
- Couchman, J.R.; Pataki, C.A. An Introduction to Proteoglycans and Their Localization. J. Histochem. Cytochem. 2012, 60, 885–897. [Google Scholar] [CrossRef]
- Sivan, S.S.; Wachtel, E.; Roughley, P. Structure, function, aging and turnover of aggrecan in the intervertebral disc. Biochim. Biophys. Acta 2014, 1840, 3181–3189. [Google Scholar] [CrossRef] [PubMed]
- Kiani, C.; Chen, L.; Wu, Y.J.; Yee, A.J.; Yang, B.B. Structure and function of aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ni, B.; Zhang, Z.; Liu, Q.; Wang, L.; Ding, Y.; Hu, Y. Polyol Pathway Mediates Enhanced Degradation of Extracellular Matrix Via p38 MAPK Activation in Intervertebral Disc of Diabetic Rats. Connect. Tissue Res. 2013, 54, 118–122. [Google Scholar] [CrossRef]
- Mohan, R.R.; Tovey, J.C.; Gupta, R.; Sharma, A.; Tandon, A. Decorin Biology, Expression, Function and Therapy in the Cornea. Curr. Mol. Med. 2011, 11, 110–128. [Google Scholar] [CrossRef] [PubMed]
- Clerigues, V.; Guillén, M.I.; Gomar, F.; Alcaraz, M.J. Haem oxygenase-1 counteracts the effects of interleukin-1beta on inflammatory and senescence markers in cartilage-subchondral bone explants from osteoarthritic patients. Clin. Sci. 2012, 122, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Lei, H.; Shen, J.; Liu, X.; Zhang, X.; Wu, L.; Hao, J.; Jiang, W.; Hu, Z. HO-1 induced autophagy protects against IL-1 beta-mediated apoptosis in human nucleus pulposus cells by inhibiting NF-kappaB. Aging 2020, 12, 2440–2452. [Google Scholar] [CrossRef] [PubMed]
- Osuka, K.; Usuda, N.; Aoyama, M.; Yamahata, H.; Takeuchi, M.; Yasuda, M.; Takayasu, M. Expression of the JAK/STAT3/SOCS3 signaling pathway in herniated lumbar discs. Neurosci. Lett. 2014, 569, 55–58. [Google Scholar] [CrossRef]
- van Helvoort, E.; van der Heijden, E.; van Roon, J.; Eijkelkamp, N.; Mastbergen, S. The Role of Interleukin-4 and Interleukin-10 in Osteoarthritic Joint Disease: A Systematic Narrative Review. Cartilage 2022, 13, 19476035221098167. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, C.; Xiao, J.; Wu, Y.; Zhang, Z.; Zhou, Y.; Tian, N.; Wu, Y.; Wang, X.; Zhang, X. The Potential Role of Cytokines in Diabetic Intervertebral Disc Degeneration. Aging Dis. 2022, 13, 1323. [Google Scholar] [CrossRef]
- Ge, J.; Yan, Q.; Wang, Y.; Cheng, X.; Song, D.; Wu, C.; Yu, H.; Yang, H.; Zou, J. IL-10 delays the degeneration of intervertebral discs by suppressing the p38 MAPK signaling pathway. Free. Radic. Biol. Med. 2020, 147, 262–270. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, L.; Zhang, W.; Tang, Q.; Zhong, Y. IL-10 alleviates radicular pain by inhibiting TNF-alpha/p65 dependent Nav1.7 up-regulation in DRG neurons of rats. Brain Res. 2022, 1791, 147997. [Google Scholar] [CrossRef]
- Persaud, L.; De Jesus, D.; Brannigan, O.; Richiez-Paredes, M.; Huaman, J.; Alvarado, G.; Riker, L.; Mendez, G.; Dejoie, J.; Sauane, M. Mechanism of Action and Applications of Interleukin 24 in Immunotherapy. Int. J. Mol. Sci. 2016, 17, 869. [Google Scholar] [CrossRef]
- Cherng, S.; Young, J.; Ma, H. Alpha-Smooth Muscle Actin (α-SMA). J. Am. Sci. 2008, 4, 7–9. [Google Scholar]
- Castro, A.L.; Ribeiro-Machado, C.; Oliveira, C.M.; Teixeira, G.Q.; Neidlinger-Wilke, C.; Pereira, P.; Vaz, R.; Barbosa, M.A.; Gonçalves, R.M. Fibrotic alterations in human annulus fibrosus correlate with progression of intervertebral disc herniation. Thromb. Haemost. 2022, 24, 25. [Google Scholar] [CrossRef]
- Cucchiarini, M.; Schetting, S.; Terwilliger, E.F.; Kohn, D.; Madry, H. rAAV-mediated overexpression of FGF-2 promotes cell proliferation, survival, and alpha-SMA expression in human meniscal lesions. Gene Ther. 2009, 16, 1363–1372. [Google Scholar] [CrossRef] [PubMed]




| Non-Diabetic Rats | Diabetic Rats | ||
|---|---|---|---|
| fa/+ without vit. K2 (fa/+ wo vit.K2) | fa/+ with vit. K2 (fa/+ w vit.K2) | fa/fa without vit. K2 (fa/fa wo vit.K2) | fa/fa with vit. K2 (fa/fa w vit.K2) |
| 9 rats | 11 rats | 7 rats | 9 rats |
| Item and Grade | 0 | 1 | 2 | |
|---|---|---|---|---|
| AF | Normal lamellar pattern | Slight lamellar disorganization | Loss of lamellar organization | |
| AF/NP-border | Normal | Minimal interruption, distinct space/bleb formation between both | Loss of distinction, severe space/bleb formation between both | |
| NP cellularity | Normal cellularity and arrangement | Mild cell density or distribution changes, few cell clusters | Profound cell density/distribution changes, prominent cell clusters | |
| NP ECM distribution | Normal gelatinous appearance | Mild condensation, disorganization | Severe condensation, disorganization | |
| NP morphology | NP shape | Oval | Oval/round, mild distortion | Irregular shape |
| NP area | NP constitutes more than 50% of disc area | NP constitutes between 25–50% of disc area | NP constitutes less than 25% of disc area | |
| CEP | No bony sclerosis and regular CEP | Mild bony sclerosis with mildly thickened CEP | Severely thickened CEP with severe sclerosis | |
| Maximal number of points (severe degeneration): 14 | ||||
| Target | Primary Antibody | Dilution | Secondary Antibody | Dilution |
|---|---|---|---|---|
| Aggrecan | mouse-anti-human, R&D systems, Minneapolis, MN, USA | 1:30 | donkey-anti-mouse; Cy3, Invitrogen, Carlsbad, CA, USA | 1:200 |
| Collagen type 1 | goat-anti-human, (COL1A1 chain), Abcam, Cambridge, UK | 1:50 | donkey-anti-goat; Cy3, Invitrogen, Carlsbad, CA, USA | 1:200 |
| Collagen type 2 | rabbit-anti-human, Acris Laboratories, Hiddenhausen, Germany | 1:50 | donkey-anti-rabbit, Alexa Fluor 488, Invitrogen, Carlsbad, CA, USA | 1:200 |
| Decorin | rabbit-anti-human, OriGene Rockville, MD, USA | 1:50 | donkey-anti-rabbit; Alexa-Fluor488, Invitrogen, Carlsbad, CA, USA | 1:200 |
| IL-10 | rabbit-anti-human, PeproTech, Cranbury, NJ, USA | 1:30 | donkey-anti-rabbit, Alexa Fluor 488, Invitrogen, Carlsbad, CA, USA | 1:200 |
| IL-24 | rabbit-anti-human, Invitrogen, Carlsbad, CA, USA | 1:30 | donkey-anti-rabbit; Alexa-Fluor488, Invitrogen, Carlsbad, CA, USA | 1:200 |
| IL-24 | mouse-anti-human, R&D systems, Minneapolis, MN, USA | 1:30 | donkey-anti-mouse; Cy3, Invitrogen, Carlsbad, CA, USA | 1:200 |
| Phalloidin Alexa488 | Santa Cruz Biotechnologies, Santa Cruz, CA, USA | 1:100 | none | |
| Proteoglycans | mouse-anti-human, Chemicon International, Temecula, CA, USA | 1:70 | donkey-anti-mouse; Cy3, Invitrogen, Carlsbad, CA, USA | 1:200 |
| SOX9 | rabbit-anti-human, Merck, Darmstadt, Germany | 1:100 | donkey-anti-rabbit, Alexa Fluor 488, Invitrogen, Carlsbad, CA, USA | 1:200 |
| αsmooth muscle actin | mouse-anti-human, Sigma-Aldrich (A5228), Munich, Germany | 1:50 | donkey-anti-mouse; Cy3, Invitrogen, Carlsbad, CA, USA | 1:200 |
| Gene Symbol | Gene Name | Assay ID | Amplicon Length (bp) | Efficiency | NCBI Gene Reference |
|---|---|---|---|---|---|
| Acan * | Aggrecan | Rn00573424_m1 | 74 | 2.04 | NM_022190.1 |
| Cd55 | Decay accelerating factor | Rn00709472_m1 | 92 | 1.94 | NM_022269.2 |
| Cd59 | Protectin/MAC inhibitory protein | Rn00563929_m1 | 80 | 2.05 | NM_012925.1 |
| Col1a1 | Collagen type 1, alpha 1 | Rn01463848_m1 | 115 | 2.01 | NM_053304.1 |
| COL2A1 ** | Collagen type 2, alpha 1 | Ec03467411_m1 | 81 | 2.17 | NM_001081764.1 |
| Hmox1 | Heme oxygenase-1 | Rn00561387_m1 | 132 | 2.25 | NM_012580.2 |
| Hprt1 | Hypoxanthine-Guanine-phosphoribosyltransferase | Rn01527840_m1 | 64 | 1.98 | NM_012583.2 |
| Socs3 | Suppressor of Cytokine Signaling 3 | Rn00585674_s1 | 73 | 1.90 | NM_053565.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, M.; Kokozidou, M.; Gögele, C.; Werner, C.; Auffarth, A.; Kohl, B.; Mrosewski, I.; Schulze-Tanzil, G.G. Does Vitamin K2 Influence the Interplay between Diabetes Mellitus and Intervertebral Disc Degeneration in a Rat Model? Nutrients 2023, 15, 2872. https://doi.org/10.3390/nu15132872
Mahmoud M, Kokozidou M, Gögele C, Werner C, Auffarth A, Kohl B, Mrosewski I, Schulze-Tanzil GG. Does Vitamin K2 Influence the Interplay between Diabetes Mellitus and Intervertebral Disc Degeneration in a Rat Model? Nutrients. 2023; 15(13):2872. https://doi.org/10.3390/nu15132872
Chicago/Turabian StyleMahmoud, Mohamed, Maria Kokozidou, Clemens Gögele, Christian Werner, Alexander Auffarth, Benjamin Kohl, Ingo Mrosewski, and Gundula Gesine Schulze-Tanzil. 2023. "Does Vitamin K2 Influence the Interplay between Diabetes Mellitus and Intervertebral Disc Degeneration in a Rat Model?" Nutrients 15, no. 13: 2872. https://doi.org/10.3390/nu15132872
APA StyleMahmoud, M., Kokozidou, M., Gögele, C., Werner, C., Auffarth, A., Kohl, B., Mrosewski, I., & Schulze-Tanzil, G. G. (2023). Does Vitamin K2 Influence the Interplay between Diabetes Mellitus and Intervertebral Disc Degeneration in a Rat Model? Nutrients, 15(13), 2872. https://doi.org/10.3390/nu15132872