Effects of Consuming Ounce-Equivalent Portions of Animal- vs. Plant-Based Protein Foods, as Defined by the Dietary Guidelines for Americans on Essential Amino Acids Bioavailability in Young and Older Adults: Two Cross-Over Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Participants
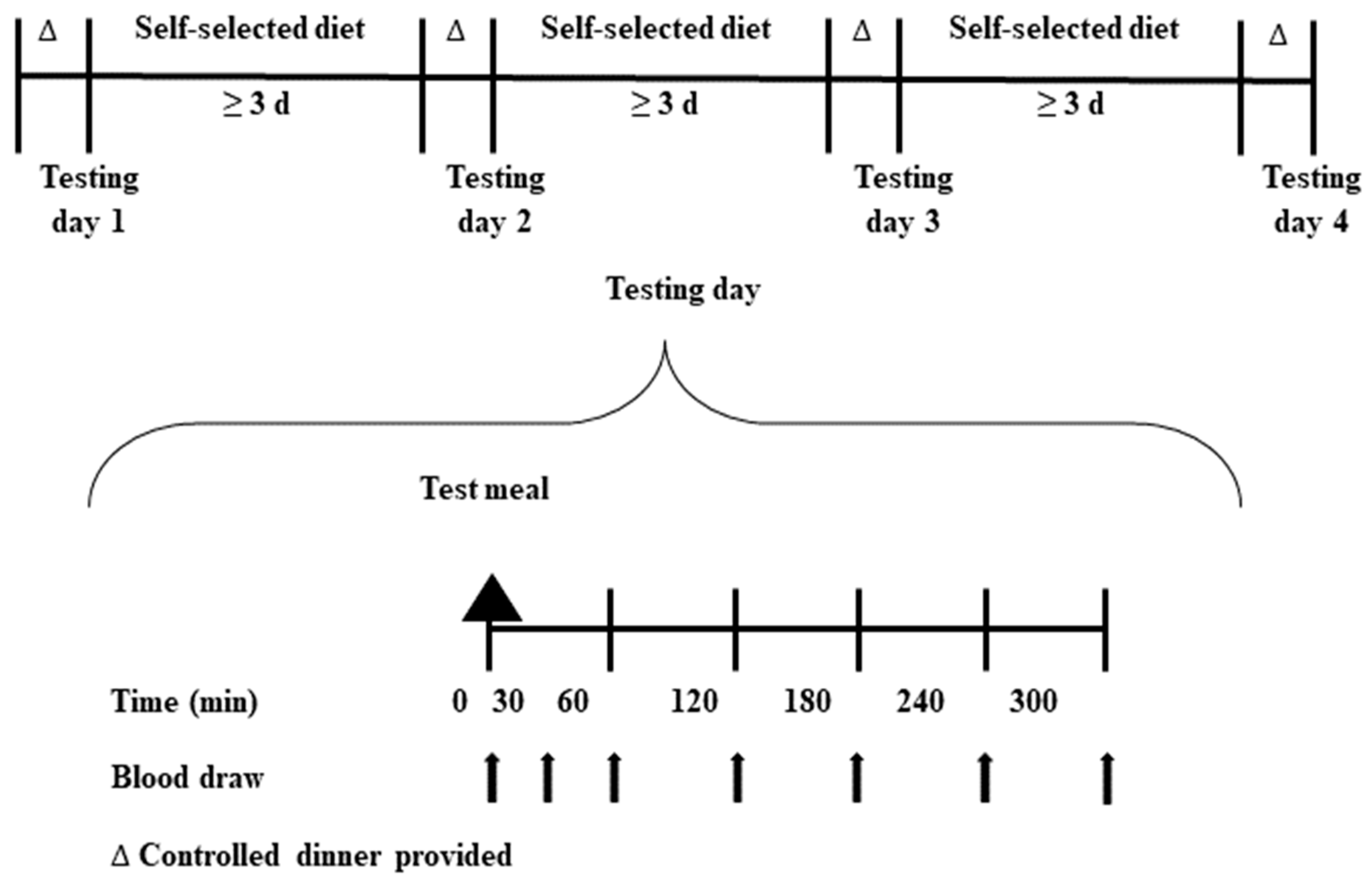
2.3. Study Design
2.4. Test Meals
2.5. Sample Collection and Biochemical Analyses
2.6. Statistical Analysis
3. Results
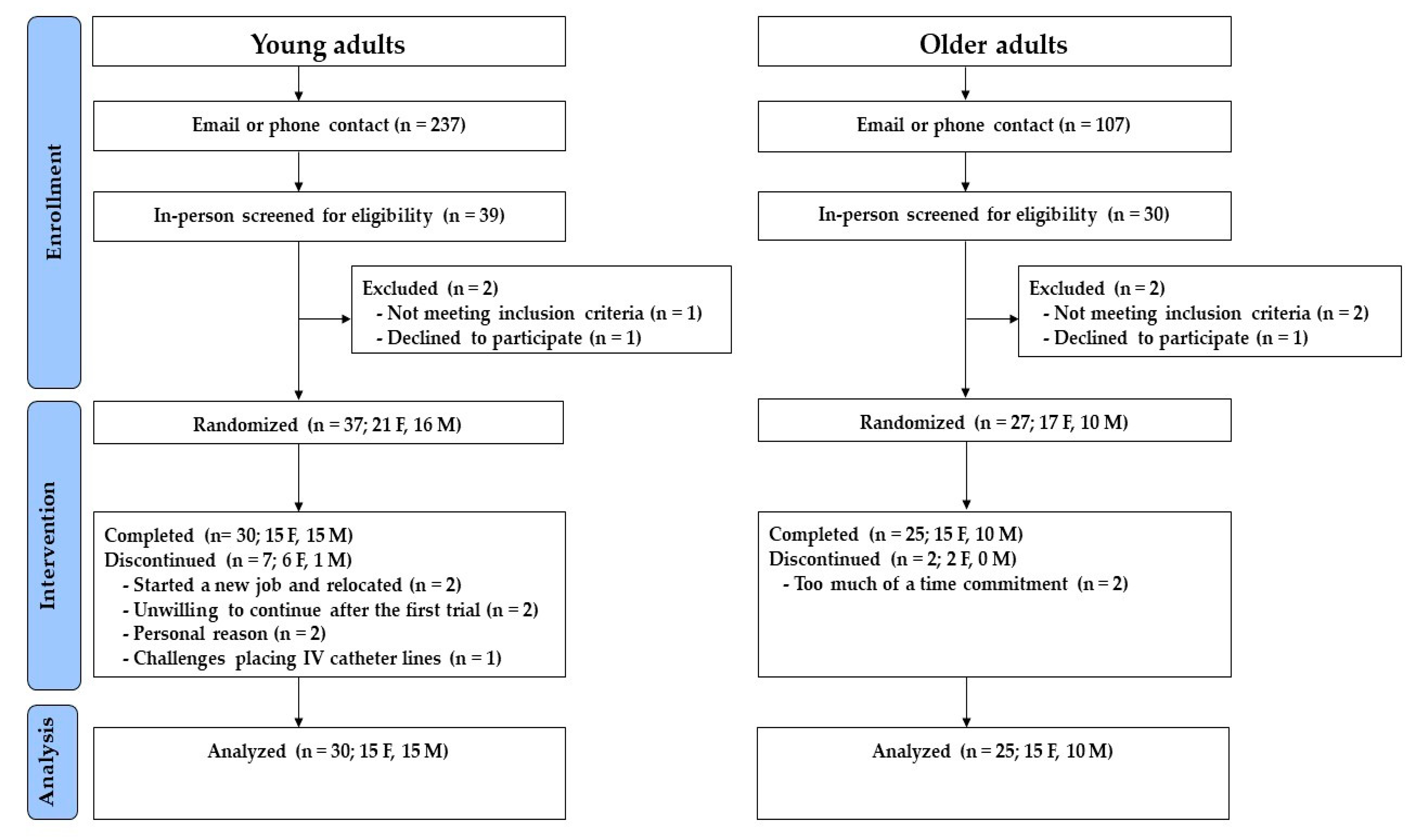
3.1. Randomization and Participant Characteristics
3.1.1. Young Adults
3.1.2. Older Adults
3.2. Baseline Fasting Blood Concentrations
3.3. Essential Amino Acids
3.3.1. Young Adults
3.3.2. Older Adults
3.3.3. Young vs. Older Adults
3.3.4. Young and Older Adults Combined
3.4. TAA, BCAA, and Leucine
3.5. Serum Glucose and Insulin
3.5.1. Young Adults
3.5.2. Older Adults
3.5.3. Young vs. Older Adults
3.5.4. Young and Older Adults Combined
3.6. Females vs. Males
3.6.1. Baseline Fasting Blood Concentrations
3.6.2. Plasma Amino Acids
3.6.3. Serum Glucose and Insulin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture and U.S. Department of Health and Human Services: Washington, DC, USA, 2020. Available online: DietaryGuidelines.gov (accessed on 23 June 2020).
- Millward, D.J.; Layman, D.K.; Tomé, D.; Schaafsma, G. Protein quality assessment: Impact of expanding understanding of protein and amino acid needs for optimal health. Am. J. Clin. Nutr. 2008, 87, 1576s–1581s. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations. Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation. In FAO Food and Nutrition Paper Number 92; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 978-92-5-107417-6. [Google Scholar]
- Schaafsma, G. Advantages and limitations of the protein digestibility-corrected amino acid score (PDCAAS) as a method for evaluating protein quality in human diets. Br. J. Nutr. 2012, 108 (Suppl. S2), S333–S336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.Y.; Shin, Y.A.; Schutzler, S.E.; Azhar, G.; Wolfe, R.R.; Ferrando, A.A. Quality of meal protein determines anabolic response in older adults. Clin. Nutr. 2018, 37, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Church, D.D.; Schutzler, S.E.; Azhar, G.; Kim, I.Y.; Ferrando, A.A.; Wolfe, R.R. Metabolic Evaluation of the Dietary Guidelines’ Ounce Equivalents of Protein Food Sources in Young Adults: A Randomized Controlled Trial. J. Nutr. 2021, 151, 1190–1196. [Google Scholar] [CrossRef]
- Church, D.D.; Hirsch, K.R.; Park, S.; Kim, I.-Y.; Gwin, J.A.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Essential Amino Acids and Protein Synthesis: Insights into Maximizing the Muscle and Whole-Body Response to Feeding. Nutrients 2020, 12, 3717. [Google Scholar] [CrossRef]
- Wang, Y.; Hill, E.R.; Campbell, W.W.; O’Connor, L.E. Plant- and Animal-Based Protein-Rich Foods and Cardiovascular Health. Curr. Atheroscler. Rep. 2022, 24, 197–213. [Google Scholar] [CrossRef]
- Hudson, J.L.; Wang, Y.; Bergia Iii, R.E.; Campbell, W.W. Protein Intake Greater than the RDA Differentially Influences Whole-Body Lean Mass Responses to Purposeful Catabolic and Anabolic Stressors: A Systematic Review and Meta-analysis. Adv. Nutr. 2020, 11, 548–558. [Google Scholar] [CrossRef]
- Ferrari, L.; Panaite, S.-A.; Bertazzo, A.; Visioli, F. Animal- and Plant-Based Protein Sources: A Scoping Review of Human Health Outcomes and Environmental Impact. Nutrients 2022, 14, 5115. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health (NIH) Nutrition Research Task Force. 2020–2030 Strategic Plan for NIH Nutrition Research; National Institutes of Health (NIH) Nutrition Research Task Force: Bethesda, MD, USA, 2020. Available online: https://dpcpsi.nih.gov/sites/default/files/2020NutritionStrategicPlan_508.pdf. (accessed on 10 August 2022).
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Armstrong, C.L.; Campbell, W.W. Effects of Dietary Protein Source and Quantity during Weight Loss on Appetite, Energy Expenditure, and Cardio-Metabolic Responses. Nutrients 2016, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Gratzfeld-Huesgen, A. Sensitive and Reliable Amino AcidAnalysis in Protein Hydrolysates Using the Agilent 1100 Series HPLC; Publication number 5968-5658EN; Technical Note Agilent Technologies, Inc.: Santa Clara, CA, USA, 1999. [Google Scholar]
- Wolever, T.M.; Jenkins, D.J. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am. J. Clin. Nutr. 1986, 43, 167–172. [Google Scholar] [CrossRef]
- Phillips, S.M.; Fulgoni, V.L., III; Heaney, R.P.; Nicklas, T.A.; Slavin, J.L.; Weaver, C.M. Commonly consumed protein foods contribute to nutrient intake, diet quality, and nutrient adequacy. Am. J. Clin. Nutr. 2015, 101, 1346S–1352S. [Google Scholar] [CrossRef] [Green Version]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 422–424. [Google Scholar] [CrossRef] [Green Version]
- Volpi, E.; Mittendorfer, B.; Rasmussen, B.B.; Wolfe, R.R. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J. Clin. Endocrinol. Metab. 2000, 85, 4481–4490. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Selby, A.; Rankin, D.; Patel, R.; Atherton, P.; Hildebrandt, W.; Williams, J.; Smith, K.; Seynnes, O.; Hiscock, N.; et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J. Physiol. 2009, 587, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Sheffield-Moore, M.; Zhang, X.-J.; Volpi, E.; Wolf, S.E.; Aarsland, A.; Ferrando, A.A.; Wolfe, R.R. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am. J. Physiol. -Endocrinol. Metab. 2004, 286, E321–E328. [Google Scholar] [CrossRef] [PubMed]
- Markofski, M.M.; Dickinson, J.M.; Drummond, M.J.; Fry, C.S.; Fujita, S.; Gundermann, D.M.; Glynn, E.L.; Jennings, K.; Paddon-Jones, D.; Reidy, P.T.; et al. Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp. Gerontol. 2015, 65, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Guillet, C.; Prod’homme, M.; Balage, M.; Gachon, P.; Giraudet, C.; Morin, L.; Grizard, J.; Boirie, Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004, 18, 1586–1587. [Google Scholar] [CrossRef]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005, 82, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Volpi, E.; Mittendorfer, B.; Wolf, S.E.; Wolfe, R.R. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am. J. Physiol. 1999, 277, E513–E520. [Google Scholar] [CrossRef]
- Boirie, Y.; Gachon, P.; Beaufrère, B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am. J. Clin. Nutr. 1997, 65, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Soenen, S.; Rayner, C.K.; Horowitz, M.; Jones, K.L. Gastric Emptying in the Elderly. Clin. Geriatr. Med. 2015, 31, 339–353. [Google Scholar] [CrossRef]
- Symons, T.B.; Schutzler, S.E.; Cocke, T.L.; Chinkes, D.L.; Wolfe, R.R.; Paddon-Jones, D. Aging does not impair the anabolic response to a protein-rich meal. Am. J. Clin. Nutr. 2007, 86, 451–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, W.K.; Wilkinson, D.J.; Phillips, B.E.; Lund, J.N.; Smith, K.; Atherton, P.J. Human Skeletal Muscle Protein Metabolism Responses to Amino Acid Nutrition. Adv. Nutr. 2016, 7, 828s–838s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.I.; Reeds, D.N.; Hall, A.M.; Chambers, K.T.; Finck, B.N.; Mittendorfer, B. Sexually dimorphic effect of aging on skeletal muscle protein synthesis. Biol. Sex Differ. 2012, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Jaffery, H.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. No major sex differences in muscle protein synthesis rates in the postabsorptive state and during hyperinsulinemia-hyperaminoacidemia in middle-aged adults. J. Appl. Physiol. 2009, 107, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Villareal, D.T.; Frimel, T.N.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65–80 year old men and women. PLoS ONE 2008, 3, e1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Energy (kcal) | Fat (g) | CHO (g) | Protein (g) | EAA (g) | |
|---|---|---|---|---|---|
| Test meal | 218 | 11.5 | 25.8 | 6 | 2.09 |
| Lean pork loin (2 oz-eq) | 73 | 1 | 0 | 14 | 7.36 |
| Whole eggs (2 oz-eq) | 145 | 10 | 0 | 12.5 | 5.38 |
| Black beans (2 oz-eq) | 113 | 0.5 | 20 | 7.5 | 3.02 |
| Almonds (2 oz-eq) | 161 | 14 | 6 | 6 | 1.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Connolly, G.; Hudson, J.L.; Bergia, R.E.; Davis, E.M.; Hartman, A.S.; Zhu, W.; Carroll, C.C.; Campbell, W.W. Effects of Consuming Ounce-Equivalent Portions of Animal- vs. Plant-Based Protein Foods, as Defined by the Dietary Guidelines for Americans on Essential Amino Acids Bioavailability in Young and Older Adults: Two Cross-Over Randomized Controlled Trials. Nutrients 2023, 15, 2870. https://doi.org/10.3390/nu15132870
Connolly G, Hudson JL, Bergia RE, Davis EM, Hartman AS, Zhu W, Carroll CC, Campbell WW. Effects of Consuming Ounce-Equivalent Portions of Animal- vs. Plant-Based Protein Foods, as Defined by the Dietary Guidelines for Americans on Essential Amino Acids Bioavailability in Young and Older Adults: Two Cross-Over Randomized Controlled Trials. Nutrients. 2023; 15(13):2870. https://doi.org/10.3390/nu15132870
Chicago/Turabian StyleConnolly, Gavin, Joshua L. Hudson, Robert E. Bergia, Eric M. Davis, Austin S. Hartman, Wenbin Zhu, Chad C. Carroll, and Wayne W. Campbell. 2023. "Effects of Consuming Ounce-Equivalent Portions of Animal- vs. Plant-Based Protein Foods, as Defined by the Dietary Guidelines for Americans on Essential Amino Acids Bioavailability in Young and Older Adults: Two Cross-Over Randomized Controlled Trials" Nutrients 15, no. 13: 2870. https://doi.org/10.3390/nu15132870
APA StyleConnolly, G., Hudson, J. L., Bergia, R. E., Davis, E. M., Hartman, A. S., Zhu, W., Carroll, C. C., & Campbell, W. W. (2023). Effects of Consuming Ounce-Equivalent Portions of Animal- vs. Plant-Based Protein Foods, as Defined by the Dietary Guidelines for Americans on Essential Amino Acids Bioavailability in Young and Older Adults: Two Cross-Over Randomized Controlled Trials. Nutrients, 15(13), 2870. https://doi.org/10.3390/nu15132870






