Beneficial Effects of Plant-Based Diets on Skin Health and Inflammatory Skin Diseases
Abstract
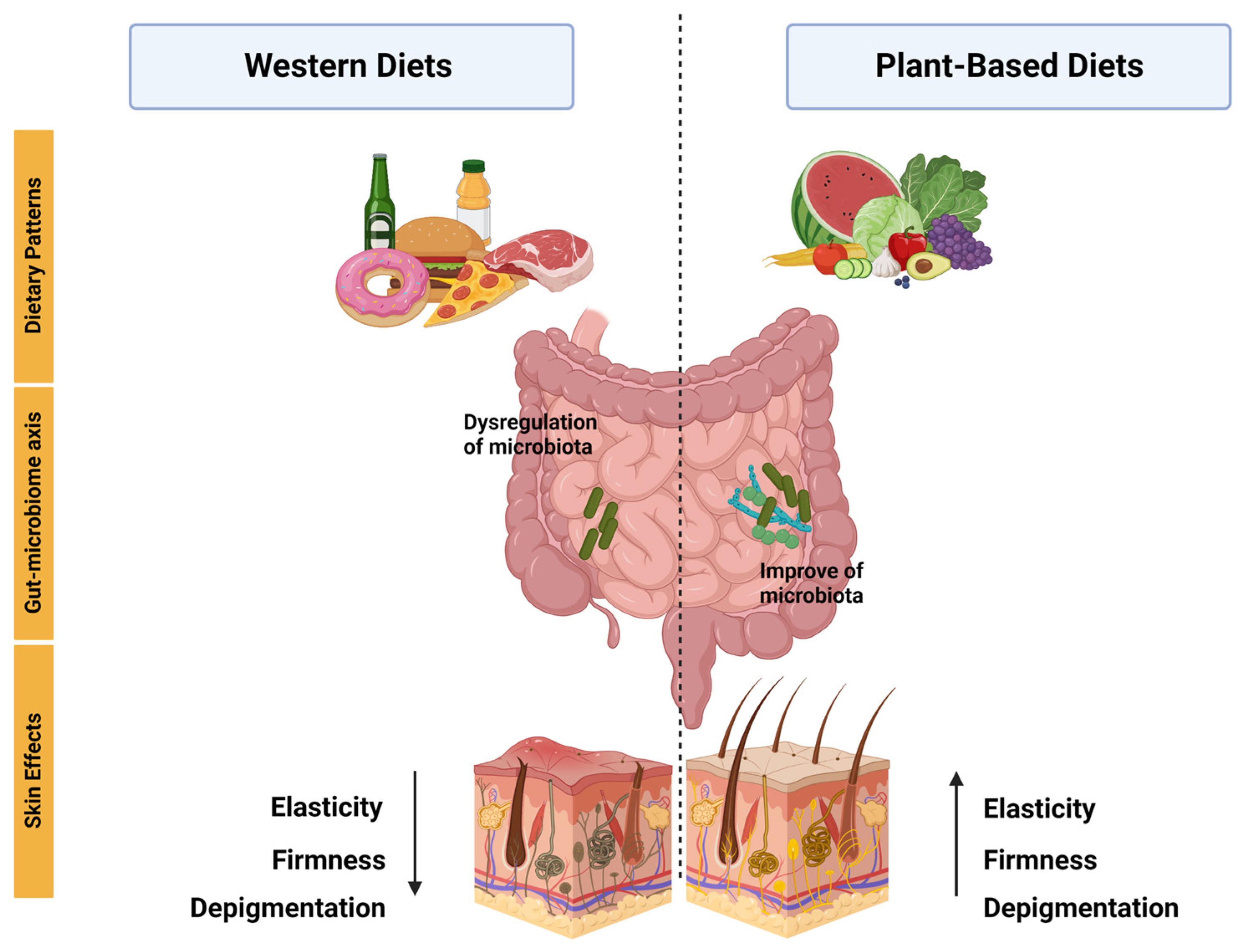
1. Introduction
2. Association between Plant-Based Diet and Psoriasis
3. Association between Plant-Based Diet and Atopic Dermatitis
4. Association between Plant-Based Diet and Acne
5. Plant-Based Diet Effects on Other Skin Conditions
6. The Role of Plant-Based Functional Foods on Skin Health
6.1. Mango
6.2. Almond
6.3. Avocado
7. Association between Plant-Based Diet Alternatives and Gut Microbiome
| Study Design | Participant Characteristics | Intervention Details | Outcomes | Ref |
|---|---|---|---|---|
| Exploratory survey study | 1206 psoriasis patients. The mean age of the sample population was 50.4, and 73% of respondents were female. | The survey contained 61 questions; the first 30 questions were from National Health and Nutrition Examination Survey; the last 31 questions focused on patient-report skin responses to dietary changes. | 72% of Pagano, 70% of vegan, and 68.9% of Paleolithic diets reported favorable skin response. The addition of fruits to the diet was protective against psoriasis, OR 0.22, p < 0.0001. | [21] |
| Assessor-blind, randomized controlled trial | 303 patients between 18 and 80 years, overweight or obese, with moderate to severe chronic plaque psoriasis. | Each participant underwent an introductory session with a dietitian. Subjects were instructed to perform sessions of continuous aerobic physical exercise for at least 40 min three times a week. Compared how psoriasis severity was affected by either a 20-week quantitative or qualitative dietary plan associated with physical exercise for weight loss. | Reduction of PASI score by 48% in the dietary intervention and 25.5% in the information-only. | [23] |
| Cross-sectional study | 56,896 participants. The mean age was 55.8, and 22,577 were female. | First, the researchers sent out a digital questionnaire focused on atopic dermatitis (AD). After collecting the responses, they administered another questionnaire to extract data on lifestyle factors, such as smoking, alcohol consumption, stress, obesity, physical activity, and diet (participants were dichotomized into vegetarian/vegan and nonvegetarian/nonvegan), and finally analyzed the association between lifestyle factors and presence of AD. | No associations were observed with abdominal obesity, physical activity, diet quality, or a vegetarian/vegan diet. | [30] |
| Controlled clinical trial | 19 patients with atopic dermatitis. The mean age was 25.5. | The patients were exposed to a low-energy diet, no pharmacotherapy. The SCORAD indices were noted by 2 dermatologists. Blood and urine samples were collected at weeks 0.4 and 8. The 24 h void urine was collected and kept frozen at −80 °C. | SCORAD significant reduction from 6.1 ± 2.8 at week 0 to 2.7 ± 2.8 at week 8. | [32] |
| Clinical trials | Twenty patients (6 males and 14 females) aged 15 to 36 years (average: 25 years) with AD varying in severity from mild to serious. All patients fulfilled the criteria for atopic dermatitis (AD) established by Hanifin and Rajka. | All patients followed the same meal plan throughout the study. The diet included a glass of fresh vegetable juice for breakfast, followed by brown rice porridge, kelp powder, tofu, and sesame paste for both lunch and dinner. In addition, a daily intake of 2.5 g of non-refined salt was included in the diet. Instead of regular water, the patients were provided with persimmon leaf tea. This treatment regimen was followed for two months. | Reduction in SCORAD index from 49.9 ± 18.6 to 27.4 ± 16.8 (p = 0.001), diminution in peripheral eosinophil count 423 ± 367 to 213 ± 267 (p = 0.01), reduction in monocyte PGE2 synthesis 2886 ± 1443 to 1390 ± 773 (p = 0.001). | [31] |
| Case-control cross-sectional questionnaire study | 57 acne vulgaris patients as cases and 57 participants as controls. Aged 14 and above and were seeking medical consultations at a private clinic. | The Comprehensive Acne Severity Scale (CASS) grading system was used to grade acne severity. Cases were defined as patients with CASS grade of two to five and controls with CASS grade 0 or 1. Thereafter, controls were recruited and matched by age, gender, and ethnicity. A self-administered questionnaire was used to collect information regarding the participants’ dietary intake and cigarette smoking habits. | No significant associations were found between dietary patterns and acne; on the other hand, they only found an association between milk and chocolate consumption and acne. | [36] |
| Case-control study | 279 acne patients and 279 controls aged 10–24 years. | Acne severity was determined by a dermatologist using the Global Acne Severity Scale. Epidemiological data were collected with a pre-structured questionnaire. The food consumption habits were recorded using a food frequency questionnaire. Investigated food included whole milk, low-fat milk, cream of milk, ice cream, cheese, chocolate, cake, potatoes, fresh fruit, fresh vegetable, meat, chicken, and egg. Consumption habit data were collected for 8 months. | The study found that consuming whole milk at least three days a week was significantly associated with moderate to severe acne (OR = 2.36, 95% CI, 1.39–4.01). The association was slightly weaker for low-fat milk (OR 1.95, 95% CI, 1.10–3.45). | [39] |
| A prospective case-control study | 460 subjects of both sexes, 230 patients with acne vulgaris, and 230 healthy volunteers were included as controls. | Acne severity in patients was classified as mild, moderate, and severe according to the classification of the American Academy of Dermatology. Two formulated tools were used in the study. First tool: interview questionnaires that included two parts (sociodemographic data, anthropometric measurements, and questions related to food habits, drug intake, and diet history). Second tool: a recommended diet regimen. | Significantly decreased consumption of vegetables was noticed among severe and moderate acne patients compared with mild ones. | [40] |
| Clinical-trial study | Healthy postmenopausal women aged 50 to 70 (N = 28). Inclusion criteria were Fitzpatrick skin type I, II, or III and a body mass index (BMI) between 18.5 and 35 kg/m2. | Participants were randomized by block design into an open-label, two-arm parallel clinical trial consuming either 85 g or 250 g of Ataulfo mango, four times per week for 16 weeks. | 0.5 cup frozen Ataulfo mango consumption decreases facial wrinkle depth and severity and photodamage induced by UVB. Serum triglycerides decreased. A higher amount of mango (1.5 cups) showed the contrary effect. | [49] |
| Randomized clinical trial | Thirty-one participants (all postmenopausal females) with a Fitzpatrick skin type 1 or 2. | Consisted of a total of five study visits following a 4-week dietary washout period: baseline, 4, 8, and 16 weeks. The patients were randomized into two intervention groups: almond group and control group. Almond group received an average of 340 kcal/day of almonds. | There were no significant differences in sebum production and transepidermal water loss between the almond and control groups. Wrinkle severity and wrinkle width were significantly decreased in the almond group compared with the control intervention. | [52] |
| Randomized- blinded | 56 postmenopausal women with a Fitzpatrick skin type I or II. | A total of 56 female participants were randomly assigned to two intervention groups: the almond group, which received almonds, and the control group, which received a calorie-matched snack. In the almond group, the dose of almonds provided accounted for 20% of the total energy in the participants’ diet. The almond snack group received approximately 2 g of sugars per day, whereas the control snack group received approximately 8 g of sugars per day that was additional to their regular food intake. 24 weeks. | Reduction in the average severity of wrinkles compared to the baseline. Additionally, intensity of facial pigmentation decreased in the almond group. | [53] |
| Randomized parallel group | 39 women with a Fitzpatrick skin type II–IV. | The patients were randomized into two intervention groups: avocado and control. The participants either consumed one avocado daily or no avocado based on randomization performed. 8 weeks. | Daily consumption of one avocado per day for 8 weeks improved firmness and elasticity and reduced the tiring of repeat stretching of the forehead skin. | [55] |
| Case-control | 30 patients with AD (according to the criteria for the diagnosis of AD established by the Japanese Dermatological Association) less than 20 years of age and age- and sex-matched control subjects (68 healthy individuals). | A questionnaire survey was conducted for 1 week, and then fecal specimens and 24 h skin secretion specimens were collected from all subjects. Fecal microflora, fecal IgA levels, and IgA concentrations in the skin were analyzed. The data for the 2 groups (i.e., patients with AD and healthy control subjects) were compared. | The counts of Bifidobacterium were significantly lower in patients with AD than in healthy control subjects (9.75 ± 0.68 vs. 10.10 ± 0.50, p < 0.05). Percentages of Bifidobacterium were significantly lower in patients with severe skin symptoms than in those with mild skin symptoms (40 ± 6% vs. 19 ± 6%, p < 0.05). In addition, the frequency of occurrence of Staphylococcus was significantly higher in patients with AD than in healthy control subjects (83% vs. 59%, p < 0.05). | [85] |
| Case-control | 21 psoriasis patients from a Brazilian referral dermatology service and 24 controls. | A stool sample was collected from each participant at the time of inclusion in the study, and the samples were analyzed by sequencing the 16S rRNA gene. | Patients with psoriasis showed higher levels of the Dialister genus and the species Prevotella copri compared to the control group. Conversely, there was a decrease in the Ruminococcus, Lachnospira, and Blautia genera, as well as the Akkermansia muciniphila species, in the psoriasis group compared to the control group. Additionally, individuals with psoriasis had lower diversity in their gut microbiota compared to the control group. | [84] |
| Case report | 63-year-old woman with ulcerations on both lower legs and diagnosed livedoid vasculopathy by biopsy. | The patient adopted a whole-food plant-based diet (WFPB) as a potential treatment with no other form of treatment. | The symptoms were initially completely resolved, but they reappeared later due to a lack of adherence to a proper diet. | [41] |
| Case report | 58-year-old female patient with pemphigus vulgaris and type 2 diabetes mellitus. | Incorporated a change in lifestyle with different approaches, including hydrotherapy, yoga, a vegetarian diet, herbal preparations, and massage. | The patient was successfully weaned off all medications and had complete remission from PV. | [42] |
| Cross-sectional | They measured skin autofluorescence (SAF) in 332 adult hemodialysis patients who were dialyzing in north central London. | SAF was measured in 332 adult dialysis patients. | SAF was lower in the twenty-seven vegetarians than it was in the non-vegetarians. | [43] |
| Observational retrospective | 21 omnivore patients, 21 vegan patients, with surgical excision of melanoma. | The assessment of postsurgical complications and the quality of scars was conducted using a modified version of the Scar Cosmesis Assessment and Rating (SCAR) scale. | Wound diastasis was more frequent in vegans (p = 0.008). Higher modified SCAR score than omnivores (p < 0.001), worst scar spread (p < 0.001), more frequent atrophic scars (p < 0.001), and worse overall impression (p < 0.001) were observed in vegans. | [29] |
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Skin. Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Mohania, D.; Chandel, S.; Kumar, P.; Verma, V.; Digvijay, K.; Tripathi, D.; Choudhury, K.; Mitten, S.K.; Shah, D. Ultraviolet Radiations: Skin Defense-Damage Mechanism. Adv. Exp. Med. Biol. 2017, 996, 71–87. [Google Scholar]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J.M.; Harris, J.E. Immunology and skin in health and disease. Cold Spring Harb. Perspect. Med. 2014, 4, a015339. [Google Scholar] [CrossRef]
- Kent, G.; Kehoe, L.; Flynn, A.; Walton, J. Plant-based diets: A review of the definitions and nutritional role in the adult diet. Proc. Nutr. Soc. 2022, 81, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Fam, V.W.; Charoenwoodhipong, P.; Sivamani, R.K.; Holt, R.R.; Keen, C.L.; Hackman, R.M. Plant-Based Foods for Skin Health: A Narrative Review. J. Acad. Nutr. Diet. 2022, 122, 614–629. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R. Position of the American Dietetic Association: Vegetarian diets. J. Am. Diet. Assoc. 2009, 109, 1266–1282. [Google Scholar]
- Solway, J.; McBride, M.; Haq, F.; Abdul, W.; Miller, R. Diet and Dermatology: The Role of a Whole-food, Plant-based Diet in Preventing and Reversing Skin Aging—A Review. J. Clin. Aesthet. Dermatol. 2020, 13, 38. [Google Scholar]
- Temple, N.J. A rational definition for functional foods: A perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.N.; Aravind, B.; Malavalli, S.S.; Sukanth, B.S.; Poornima, R.; Bharati, P.; Hefferon, K.; Kole, C.; Puppala, N. Omics Technologies to Enhance Plant Based Functional Foods: An Overview. Front. Genet. 2021, 12, 742095. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Carocho, M.; Dias, M.I.; Caleja, C.; Barros, L.; Ferreira, I.C.F.R. Wild Plant-Based Functional Foods, Drugs, and Nutraceuticals. In Wild Plants, Mushrooms and Nuts: Functional Food Properties and Applications; Wiley Online Library: New York, NY, USA, 2016; pp. 315–351. [Google Scholar]
- Kamiya, K.; Kishimoto, M.; Sugai, J.; Komine, M.; Ohtsuki, M. Risk Factors for the Development of Psoriasis. Int. J. Mol. Sci. 2019, 20, 4347. [Google Scholar] [CrossRef] [PubMed]
- Michalek, I.M.; Loring, B.; John, S.M. A systematic review of worldwide epidemiology of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Dowlatshahi, E.A.; Van Der Voort, E.A.M.; Arends, L.R.; Nijsten, T. Markers of systemic inflammation in psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2013, 169, 266–282. [Google Scholar] [CrossRef]
- Hao, Y.; Zhu, Y.J.; Zou, S.; Zhou, P.; Hu, Y.W.; Zhao, Q.X.; Gu, L.N.; Zhang, H.Z.; Wang, Z.; Li, J. Metabolic Syndrome and Psoriasis: Mechanisms and Future Directions. Front. Immunol. 2021, 12, 711060. [Google Scholar] [CrossRef]
- Jones, V.A.; Patel, P.M.; Wilson, C.; Wang, H.; Ashack, K.A. Complementary and alternative medicine treatments for common skin diseases: A systematic review and meta-analysis. JAAD Int. 2020, 2, 76–93. [Google Scholar] [CrossRef]
- Afifi, L.; Danesh, M.J.; Lee, K.M.; Beroukhim, K.; Farahnik, B.; Ahn, R.S.; Yan, D.; Singh, R.K.; Nakamura, M.; Koo, J.; et al. Dietary Behaviors in Psoriasis: Patient-Reported Outcomes from a, U.S. National Survey. Dermatol. Ther. 2017, 7, 227–242. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell. Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Naldi, L.; Conti, A.; Cazzaniga, S.; Patrizi, A.; Pazzaglia, M.; Lanzoni, A.; Veneziano, L.; Pellacani, G.; the Psoriasis Emilia Romagna Study Group. Diet and physical exercise in psoriasis: A randomized controlled trial. Br. J. Dermatol. 2014, 170, 634–642. [Google Scholar] [CrossRef]
- Lewandowska, M.; Dunbar, K.; Kassam, S. Managing Psoriatic Arthritis With a Whole Food Plant-Based Diet: A Case Study. Am. J. Lifestyle Med. 2021, 15, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, M.; Gabriel, S.; Valencia, A.; Goldhamer, A.C.; Myers, T.R. Challenging Case in Clinical Practice: Prolonged Water-Only Fasting Followed by an Exclusively Whole-Plant-Food Diet in the Management of Severe Plaque Psoriasis. Integr. Complement. Ther. 2022, 28, 85–87. [Google Scholar] [CrossRef]
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66 (Suppl. S1), 8–16. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Frazier, W.; Bhardwaj, N. Atopic Dermatitis: Diagnosis and Treatment. Am. Fam. Physician 2020, 101, 590–598. [Google Scholar]
- Fusano, M. Veganism in acne, atopic dermatitis, and psoriasis: Benefits of a plant-based diet. Clin. Dermatol. 2022. [Google Scholar] [CrossRef]
- Zhang, J.; Loman, L.; Oldhoff, M.; Schuttelaar, M.L.A. Association between moderate to severe atopic dermatitis and lifestyle factors in the Dutch general population. Clin. Exp. Dermatol. 2022, 47, 1523–1535. [Google Scholar] [CrossRef]
- Tanaka, T.; Kouda, K.; Kotani, M.; Takeuchi, A.; Tabei, T.; Masamoto, Y.; Nakamura, H.; Takigawa, M.; Suemura, M.; Takeuchi, H.; et al. Vegetarian diet ameliorates symptoms of atopic dermatitis through reduction of the number of peripheral eosinophils and of PGE2 synthesis by monocytes. J. Physiol. Anthropol. Appl. Hum. Sci. 2001, 20, 353–361. [Google Scholar] [CrossRef]
- Kouda, K.; Tanaka, T.; Kouda, M.; Takeuchi, H.; Takeuchi, A.; Nakamura, H.; Takigawa, M. Low-energy diet in atopic dermatitis patients: Clinical findings and DNA damage. J. Physiol. Anthropol. Appl. Hum. Sci. 2000, 19, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Morohashi, M. Pathogenesis of acne. Med. Electron. Microsc. 2001, 34, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Beylot, C.; Auffret, N.; Poli, F.; Claudel, J.P.; Leccia, M.T.; Del Giudice, P.; Dreno, B. Propionibacterium acnes: An update on its role in the pathogenesis of acne. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B. What is new in the pathophysiology of acne, an overview. J. Eur. Acad. Dermatol. Venereol. 2017, 31 (Suppl. S5), 8–12. [Google Scholar] [CrossRef]
- Suppiah, T.S.S.; Sundram, T.K.M.; Tan, E.S.S.; Lee, C.K.; Bustami, N.A.; Tan, C.K. Acne vulgaris and its association with dietary intake: A Malaysian perspective. Asia Pac. J. Clin. Nutr. 2018, 27, 1141–1145. [Google Scholar]
- Baldwin, H.; Tan, J. Effects of Diet on Acne and Its Response to Treatment. Am. J. Clin. Dermatol. 2021, 22, 55–65. [Google Scholar] [CrossRef]
- Stewart, T.J.; Bazergy, C. Hormonal and dietary factors in acne vulgaris versus controls. Dermato-Endocrinology 2018, 10, e1442160. [Google Scholar] [CrossRef]
- Aalemi, A.K.; Anwar, I.; Chen, H. Dairy consumption and acne: A case control study in Kabul, Afghanistan. Clin. Cosmet. Investig. Dermatol. 2019, 12, 481–487. [Google Scholar] [CrossRef]
- Youssef, E.M.K.; Youssef, M.K.E. Diet and Acne in Upper Egypt. Am. J. Dermatol. Venereol. 2014, 3, 13–22. [Google Scholar]
- Smith, M.; Wright, N.; McHugh, P.; Duncan, B. Remission of long-standing livedoid vasculopathy using a whole foods plant-based diet with symptoms recurrent on re-challenge with standard Western diet. BMJ Case Rep. 2021, 14, e237895. [Google Scholar] [CrossRef]
- Solanki, V.K.; Nair, P.M.K. Lifestyle medicine approach in managing pemphigus vulgaris: A case report. Explore, 2023; in press. [Google Scholar] [CrossRef]
- Nongnuch, A.; Davenport, A. The effect of vegetarian diet on skin autofluorescence measurements in haemodialysis patients. Br. J. Nutr. 2015, 113, 1040–1043. [Google Scholar] [CrossRef]
- Fusano, M.; Fusano, I.; Galimberti, M.G.; Bencini, M.; Bencini, P.L. Comparison of Postsurgical Scars Between Vegan and Omnivore Patients. Dermatol. Surg. 2020, 46, 1572–1576. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Bae, E.Y.; Choi, G.; Hyun, J.W.; Lee, M.Y.; Lee, H.W.; Chae, S. Protective effect of mango (Mangifera indica L.) against UVB-induced skin aging in hairless mice. Photodermatol. Photoimmunol. Photomed. 2013, 29, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Hasler, C.M.; Kundrat, S.; Wool, D. Functional foods and cardiovascular disease. Curr. Atheroscler. Rep. 2000, 2, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Lebaka, V.R.; Wee, Y.J.; Ye, W.; Korivi, M. Nutritional Composition and Bioactive Compounds in Three Different Parts of Mango Fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef]
- Fam, V.W.; Holt, R.R.; Keen, C.L.; Sivamani, R.K.; Hackman, R.M. Prospective Evaluation of Mango Fruit Intake on Facial Wrinkles and Erythema in Postmenopausal Women: A Randomized Clinical Pilot Study. Nutrients 2020, 12, 3381. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Oliver Chen, C.Y.; Blumberg, J.B.; Astiazaran-Garcia, H.; Wall-Medrano, A.; González-Aguilar, G.A. Processing “Ataulfo” Mango into Juice Preserves the Bioavailability and Antioxidant Capacity of Its Phenolic Compounds. Nutrients 2017, 9, 1082. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.L. A comprehensive review of almond clinical trials on weight measures, metabolic health biomarkers and outcomes, and the gut microbiota. Nutrients 2021, 13, 1968. [Google Scholar] [CrossRef]
- Foolad, N.; Vaughn, A.R.; Rybak, I.; Burney, W.A.; Chodur, G.M.; Newman, J.W.; Steinberg, F.M.; Sivamani, R.K. Prospective randomized controlled pilot study on the effects of almond consumption on skin lipids and wrinkles. Phytother. Res. 2019, 33, 3212–3217. [Google Scholar] [CrossRef]
- Rybak, I.; Carrington, A.E.; Dhaliwal, S.; Hasan, A.; Wu, H.; Burney, W.; Maloh, J.; Sivamani, R.K. Prospective Randomized Controlled Trial on the Effects of Almonds on Facial Wrinkles and Pigmentation. Nutrients 2021, 13, 785. [Google Scholar] [CrossRef]
- Dreher, M.L.; Davenport, A.J. Hass Avocado Composition and Potential Health Effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738. [Google Scholar] [CrossRef]
- Henning, S.M.; Guzman, J.B.; Thames, G.; Yang, J.; Tseng, C.H.; Heber, D.; Kim, J.; Li, Z. Avocado Consumption Increased Skin Elasticity and Firmness in Women—A Pilot Study. J. Cosmet. Dermatol. 2022, 21, 4028–4034. [Google Scholar] [CrossRef]
- Mann, E.A.; Bae, E.; Kostyuchek, D.; Chung, H.J.; McGee, J.S. The Gut Microbiome: Human Health and Inflammatory Skin Diseases. Ann. Dermatol. 2020, 32, 265. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef]
- Pessemier, B.; De Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Blacher, E.; Elinav, E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol. 2017, 35, 8–15. [Google Scholar] [CrossRef]
- Coates, M.; Lee, M.J.; Norton, D.; MacLeod, A.S. The Skin and Intestinal Microbiota and Their Specific Innate Immune Systems. Front. Immunol. 2019, 10, 496479. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.E.; Darby, G.; Massey, K.A.; Clarke, K.A.; Dew, T.P.; Farrar, M.D.; Bennett, S.; Watson, R.E.B.; Williamson, G.; Nicolaou, A. Oral green tea catechin metabolites are incorporated into human skin and protect against UV radiation-induced cutaneous inflammation in association with reduced production of pro-inflammatory eicosanoid 12-hydroxyeicosatetraenoic acid. Br. J. Nutr. 2013, 110, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. Polyphenol-Rich Strawberry Extract Protects Human Dermal Fibroblasts against Hydrogen Peroxide Oxidative Damage and Improves Mitochondrial Functionality. Molecules 2014, 19, 7798–7816. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, R.; Acharjee, M.; Hossain, S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Lin, G.; Ferenczi, K. The skin microbiome and the gut-skin axis. Clin. Dermatol. 2021, 39, 829–839. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Sánchez-Tapia, M.; Tovar, A.R.; Torres, N. Diet as Regulator of Gut Microbiota and its Role in Health and Disease. Arch. Med. Res. 2019, 50, 259–268. [Google Scholar] [CrossRef]
- Jefferson, A.; Adolphus, K. The Effects of Intact Cereal Grain Fibers, Including Wheat Bran on the Gut Microbiota Composition of Healthy Adults: A Systematic Review. Front. Nutr. 2019, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Pan, T.; Li, L.; Wang, H.; Zhu, J.; Zhang, H.; Zhao, J.; Chen, W.; Lu, W. Bifidobacterium longum mediated tryptophan metabolism to improve atopic dermatitis via the gut-skin axis. Gut Microbes 2022, 14, 2044723. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.D.; Satija, A.; Ivey, K.L.; Li, J.; E Wilkinson, J.; Li, R.; Baden, M.; Chan, A.T.; Huttenhower, C.; et al. Plant-Based Diet Index and Metabolic Risk in Men: Exploring the Role of the Gut Microbiome. J. Nutr. 2021, 151, 2780. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Xing, J.; Chen, G.C.; Usyk, M.; Wang, Z.; McClain, A.C.; Thyagarajan, B.; Daviglus, M.L.; Sotres-Alvarez, D.; Hu, F.B.; et al. Healthy dietary patterns are associated with the gut microbiome in the Hispanic Community Health Study/Study of Latinos. Am. J. Clin. Nutr. 2022, 117, 540–552. [Google Scholar] [CrossRef]
- Koponen, K.K.; Salosensaari, A.; Ruuskanen, M.O.; Havulinna, A.S.; Männistö, S.; Jousilahti, P.; Palmu, J.; Salido, R.; Sanders, K.; Brennan, C.; et al. Associations of healthy food choices with gut microbiota profiles. Am. J. Clin. Nutr. 2021, 114, 605–616. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.R. Western Diet and Psoriatic-Like Skin and Joint Diseases: A Potential Role for the Gut Microbiota. J. Investig. Dermatol. 2021, 141, 1630–1632. [Google Scholar] [CrossRef]
- Shi, Z.; Wu, X.; Yu, S.; Huynh, M.; Jena, P.K.; Nguyen, M.; Wan, Y.J.Y.; Hwang, S.T. Short-Term Exposure to a Western Diet Induces Psoriasiform Dermatitis by Promoting Accumulation of IL-17A–Producing γδ T Cells. J. Investig. Dermatol. 2020, 140, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Dietary intervention in acne: Attenuationof increased mTORC1 signaling promoted by Western diet. Dermatoendocrinol 2012, 4, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Seethaler, B.; Nguyen, N.K.; Basrai, M.; Kiechle, M.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Short-chain fatty acids are key mediators of the favorable effects of the Mediterranean diet on intestinal barrier integrity: Data from the randomized controlled LIBRE trial. Am. J. Clin. Nutr. 2022, 116, 928–942. [Google Scholar] [CrossRef]
- Trompette, A.; Pernot, J.; Perdijk, O.; Alqahtani, R.A.A.; Domingo, J.S.; Camacho-Muñoz, D.; Wong, N.C.; Kendall, A.C.; Wiederkehr, A.; Nicod, L.P.; et al. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal Immunol. 2022, 15, 908–926. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, X.; Yao, J.; Cao, W.; Zou, Z.; Wang, L.; Qin, H.; Zhong, D.; Li, Y.; Xue, P.; et al. The role of short-chain fatty acids in inflammatory skin diseases. Front. Microbiol. 2023, 13, 1083432. [Google Scholar] [CrossRef]
- Lazar, V.; Ditu, L.M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front. Immunol. 2018, 9, 1830. [Google Scholar] [CrossRef]
- Wang, D.D.; Nguyen, L.H.; Li, Y.; Yan, Y.; Ma, W.; Rinott, E.; Ivey, K.L.; Shai, I.; Willett, W.C.; Hu, F.B.; et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat. Med. 2021, 27, 333–343. [Google Scholar] [CrossRef]
- Asuncion, P.; Liu, C.; Castro, R.; Yon, V.; Rosas, M.; Hooshmand, S.; Kern, M.; Hong, M.Y. The effects of fresh mango consumption on gut health and microbiome—Randomized controlled trial. Food Sci. Nutr. 2023, 11, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Schade, L.; Mesa, D.; Faria, A.R.; Santamaria, J.R.; Xavier, C.A.; Ribeiro, D.; Hajar, F.N.; Azevedo, V.F. The gut microbiota profile in psoriasis: A Brazilian case-control study. Lett. Appl. Microbiol. 2022, 74, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Narisawa, Y.; Arase, S.; Okamatsu, H.; Ikenaga, T.; Tajiri, Y.; Kumemura, M. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J. Allergy Clin. Immunol. 2003, 111, 587–591. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Balderas, X.; Peña-Peña, M.; Rada, K.M.; Alvarez-Alvarez, Y.Q.; Guzmán-Martín, C.A.; Sánchez-Gloria, J.L.; Huang, F.; Ruiz-Ojeda, D.; Morán-Ramos, S.; Springall, R.; et al. Beneficial Effects of Plant-Based Diets on Skin Health and Inflammatory Skin Diseases. Nutrients 2023, 15, 2842. https://doi.org/10.3390/nu15132842
Flores-Balderas X, Peña-Peña M, Rada KM, Alvarez-Alvarez YQ, Guzmán-Martín CA, Sánchez-Gloria JL, Huang F, Ruiz-Ojeda D, Morán-Ramos S, Springall R, et al. Beneficial Effects of Plant-Based Diets on Skin Health and Inflammatory Skin Diseases. Nutrients. 2023; 15(13):2842. https://doi.org/10.3390/nu15132842
Chicago/Turabian StyleFlores-Balderas, Ximena, Mario Peña-Peña, Karla M. Rada, Yamnia Q. Alvarez-Alvarez, Carlos A. Guzmán-Martín, José L. Sánchez-Gloria, Fengyang Huang, Dayanara Ruiz-Ojeda, Sofía Morán-Ramos, Rashidi Springall, and et al. 2023. "Beneficial Effects of Plant-Based Diets on Skin Health and Inflammatory Skin Diseases" Nutrients 15, no. 13: 2842. https://doi.org/10.3390/nu15132842
APA StyleFlores-Balderas, X., Peña-Peña, M., Rada, K. M., Alvarez-Alvarez, Y. Q., Guzmán-Martín, C. A., Sánchez-Gloria, J. L., Huang, F., Ruiz-Ojeda, D., Morán-Ramos, S., Springall, R., & Sánchez-Muñoz, F. (2023). Beneficial Effects of Plant-Based Diets on Skin Health and Inflammatory Skin Diseases. Nutrients, 15(13), 2842. https://doi.org/10.3390/nu15132842






