Role of Gut Microbiota in Overweight Susceptibility in an Adult Population in Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol and Participants
2.2. Laboratory Measurements
2.3. Faecal Sample Collection, DNA Extraction and 16S rRNA Sequencing
2.4. Statistical Analyses
3. Results
3.1. Study Cohort
3.2. Gut Microbiota Composition of the Study Cohort
3.3. Relationship between the Gut Microbiota Composition and Overweight/Obesity
3.4. Univariable and Multivariable Regression Analyses of the Relationship between the F/B Ratio and BMI
4. Discussion
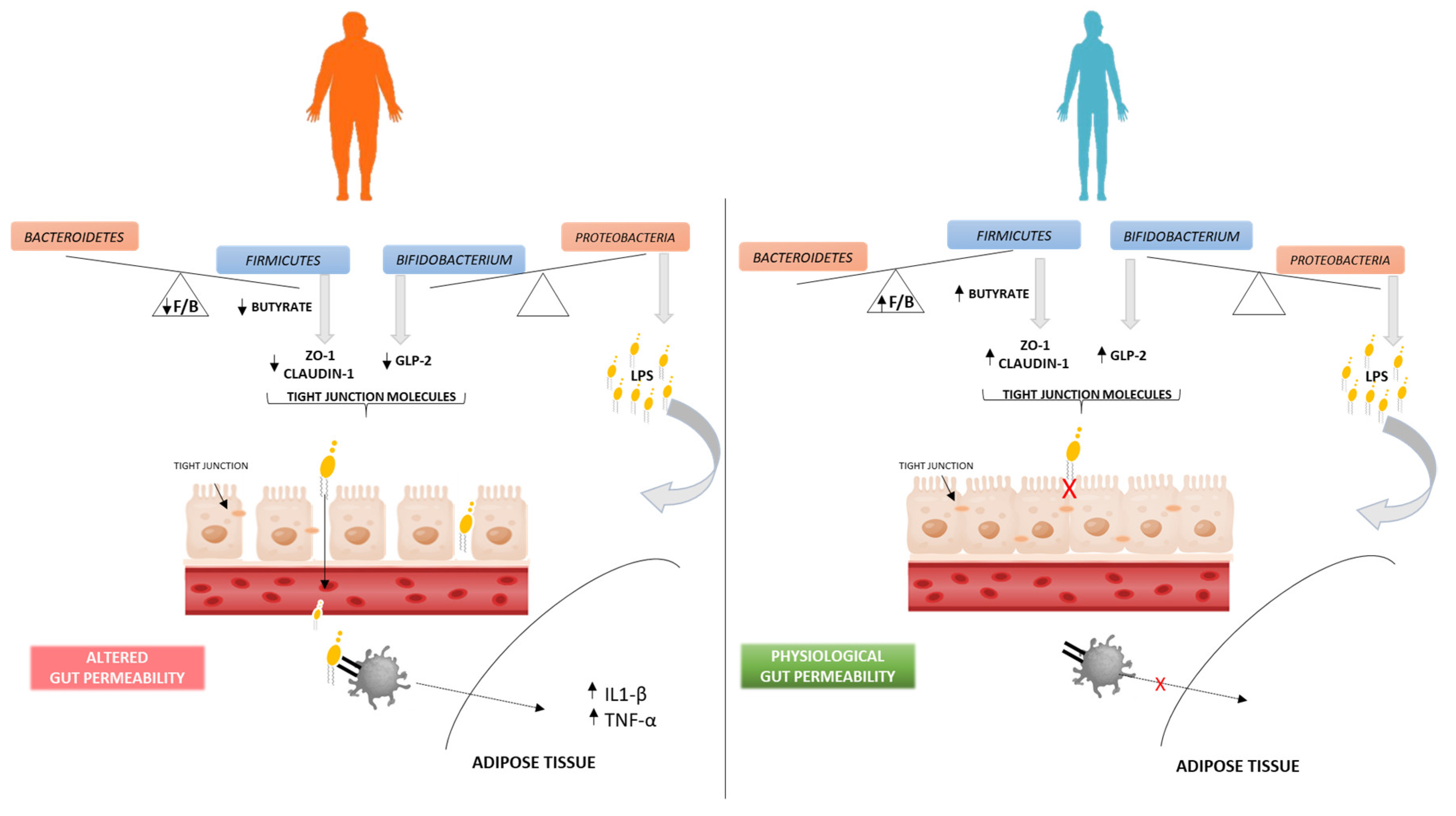
Gut Microbiota–Obesity Association Hypothesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Fraser, C.M.; Ringel, Y.; Sanders, M.E.; Sartor, R.B.; Sherman, P.M.; Versalovic, J.; Young, V.; Finlay, B.B. Defining a healthy human gut microbiome: Current concepts, future directions, and clinical applications. Cell Host Microbe 2012, 12, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Wei, S.; Bahl, M.I.; Baunwall, S.M.D.; Hvas, C.L.; Licht, T.R. Determining Gut Microbial Dysbiosis: A Review of Applied Indexes for Assessment of Intestinal Microbiota Imbalances. Appl. Environ. Microbiol. 2021, 87, e00395-21. [Google Scholar] [CrossRef]
- Sisti, D.; Pazienza, V.; Piccini, F.; Citterio, B.; Baffone, W.; Donati Zeppa, S.; Biavasco, F.; Prospero, E.; De Luca, A.; Artico, M.; et al. A proposal for the reference intervals of the Italian microbiota “scaffold” in healthy adults. Sci. Rep. 2022, 12, 3952. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- Blaut, M. Gut microbiota and energy balance: Role in obesity. Proc. Nutr. Soc. 2015, 74, 227–234. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Tseng, C.H.; Wu, C.Y. The gut microbiome in obesity. J. Formos Med. Assoc. 2019, 118, S3–S9. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, L.; Yang, L.; Chu, H. The critical role of gut microbiota in obesity. Front. Endocrinol. 2022, 13, 1025706. [Google Scholar] [CrossRef]
- Vaiserman, A.; Romanenko, M.; Piven, L.; Moseiko, V.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Koliada, A. Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiol. 2020, 20, 221. [Google Scholar] [CrossRef]
- Mahnic, A.; Rupnik, M. Different host factors are associated with patterns in bacterial and fungal gut microbiota in Slovenian healthy cohort. PLoS ONE 2018, 13, e0209209. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Houtman, T.A.; Eckermann, H.A.; Smidt, H.; de Weerth, C. Gut microbiota and BMI throughout childhood: The role of firmicutes, bacteroidetes, and short-chain fatty acid producers. Sci. Rep. 2022, 12, 3140. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef]
- Grigor’eva, I.N. Gallstone Disease, Obesity and the Firmicutes/Bacteroidetes Ratio as a Possible Biomarker of Gut Dysbiosis. J. Pers. Med. 2020, 11, 13. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 1, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Abdallah Ismail, N.; Ragab, S.H.; Abd Elbaky, A.; Shoeib, A.R.; Alhosary, Y.; Fekry, D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch. Med. Sci. 2011, 7, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Finucane, M.M.; Sharpton, T.J.; Laurent, T.J.; Pollard, K.S. A taxonomic signature of obesity in the microbiome? Getting to the guts of the matter. PLoS ONE 2014, 9, e84689. [Google Scholar] [CrossRef] [PubMed]
- Gallè, F.; Valeriani, F.; Cattaruzza, M.S.; Gianfranceschi, G.; Liguori, R.; Antinozzi, M.; Mederer, B.; Liguori, G.; Romano Spica, V. Mediaterranean Diet, Physical Activity and Gut Microbiome Composition: A Cross-Sectional Study among Healthy Young Italian Adults. Nutrients 2020, 12, 2164. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Fontana, A.; Panebianco, C.; Picchianti-Diamanti, A.; Laganà, B.; Cavalieri, D.; Potenza, A.; Pracella, R.; Binda, E.; Copetti, M.; Pazienza, V. Gut Microbiota Profiles Differ among Individuals Depending on Their Region of Origin: An Italian Pilot Study. Int. J. Environ. Res. Public Health 2019, 16, 4065. [Google Scholar] [CrossRef]
- Bozeman, S.R.; Hoaglin, D.C.; Burton, T.M.; Pashos, C.L.; Ben-Joseph, R.H.; Hollenbeak, C.S. Predicting waist circumference from body mass index. BMC Med. Res. Methodol. 2012, 12, 115. [Google Scholar] [CrossRef]
- Corsaro, L.; Trivellato, L.; Vaccaro, G. 4th Italian Obesity Barometer Report 2022, L’Obesità in Italia: È Tempo di Agire; IBDO Foundation, Istat, CoResearch, BHave: 2022. Available online: https://www.researchgate.net/publication/368825113_ITALIAN_OBESITY_BAROMETER_REPORT_2022 (accessed on 18 June 2023).
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Haro, C.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortés, J.A.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Patil, D.P.; Dhotre, D.P.; Chavan, S.G.; Sultan, A.; Jain, D.S.; Lanjekar, V.B.; Gangawani, J.; Shah, P.S.; Todkar, J.S.; Shah, S.; et al. Molecular analysis of gut microbiota in obesity among Indian individuals. J. Biosci. 2012, 37, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Ignacio, A.; Fernandes, M.R.; Avila-Campos, M.J.; Nakano, V. Enterotoxigenic and non-enterotoxigenic Bacteroides fragilis from fecal microbiota of children. Braz. J. Microbiol. 2015, 46, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Sze, M.A.; Schloss, P.D. Looking for a Signal in the Noise: Revisiting Obesity and the Microbiome. mBio 2016, 7, e01018-16. [Google Scholar] [CrossRef] [PubMed]
- Senghor, B.; Sokhna, C.; Ruimy, R.; Lagier, J.C. Gut microbiota diversity according to dietary habits and geographical provenance. Hum. Microbiome J. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Vetrani, C.; Di Nisio, A.; Paschou, S.A.; Barrea, L.; Muscogiuri, G.; Graziadio, C.; Savastano, S.; Colao, A.; On Behalf Of The Obesity Programs of Nutrition Education Research And Assessment Opera Group. From Gut Microbiota through Low-Grade Inflammation to Obesity: Key Players and Potential Targets. Nutrients 2022, 14, 2103. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.D.; Wang, Y.D. The Relationship Between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages. Front. Microbiol. 2020, 11, 1065. [Google Scholar] [CrossRef]
- Reinold, J.; Farahpour, F.; Fehring, C.; Dolff, S.; Konik, M.; Korth, J.; van Baal, L.; Hoffmann, D.; Buer, J.; Witzke, O.; et al. A Pro-Inflammatory Gut Microbiome Characterizes SARS-CoV-2 Infected Patients and a Reduction in the Connectivity of an Anti-Inflammatory Bacterial Network Associates With Severe COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 747816. [Google Scholar] [CrossRef] [PubMed]
- Fujio-Vejar, S.; Vasquez, Y.; Morales, P.; Magne, F.; Vera-Wolf, P.; Ugalde, J.A.; Navarrete, P.; Gotteland, M. The Gut Microbiota of Healthy Chilean Subjects Reveals a High Abundance of the Phylum Verrucomicrobia. Front. Microbiol. 2017, 8, 1221. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Kassis, A.; Major, G.; Chou, C.J. Is the gut microbiota a new factor contributing to obesity and its metabolic disorders? J. Obes. 2012, 2012, 879151. [Google Scholar] [PubMed]
- Fasano, A. Gut permeability, obesity, and metabolic disorders: Who is the chicken and who is the egg? Am. J. Clin. Nutr. 2017, 105, 3–4. [Google Scholar] [CrossRef]
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids Health Dis. 2016, 15, 108. [Google Scholar] [CrossRef]
- Milano, W.; Carizzone, F.; Foia, M.; Marchese, M.; Milano, M.; Saetta, B.; Capasso, A. Obesity and Its Multiple Clinical Implications between Inflammatory States and Gut Microbiotic Alterations. Diseases 2022, 11, 7. [Google Scholar] [CrossRef]
- Lassenius, M.I.; Pietiläinen, K.H.; Kaartinen, K.; Pussinen, P.J.; Syrjänen, J.; Forsblom, C.; Pörsti, I.; Rissanen, A.; Kaprio, J.; Mustonen, J.; et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 2011, 34, 1809–1815. [Google Scholar] [CrossRef]
- Trøseid, M.; Nestvold, T.K.; Rudi, K.; Thoresen, H.; Nielsen, E.W.; Lappegård, K.T. Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity: Evidence from bariatric surgery. Diabetes Care 2013, 36, 3627–3632. [Google Scholar] [CrossRef]
- Luisi, M.L.E.; Lucarini, L.; Biffi, B.; Rafanelli, E.; Pietramellara, G.; Durante, M.; Vidali, S.; Provensi, G.; Madiai, S.; Gheri, C.F.; et al. Effect of Mediterranean Diet Enriched in High Quality Extra Virgin Olive Oil on Oxidative Stress, Inflammation and Gut Microbiota in Obese and Normal Weight Adult Subjects. Front. Pharm. 2019, 10, 1366. [Google Scholar] [CrossRef]
- Mollica, M.P.; Mattace Raso, G.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A.; et al. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef]
- Gonzalez, A.; Krieg, R.; Massey, H.D.; Carl, D.; Ghosh, S.; Gehr, T.W.B.; Ghosh, S.S. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression. Nephrol. Dial. Transpl. 2019, 34, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Bouzas, C.; Capó, X.; Mateos, D.; Ugarriza, L.; Tur, J.A.; Sureda, A. Peripheral Blood Mononuclear Cells Oxidative Stress and Plasma Inflammatory Biomarkers in Adults with Normal Weight, Overweight and Obesity. Antioxidants 2021, 10, 813. [Google Scholar] [CrossRef] [PubMed]
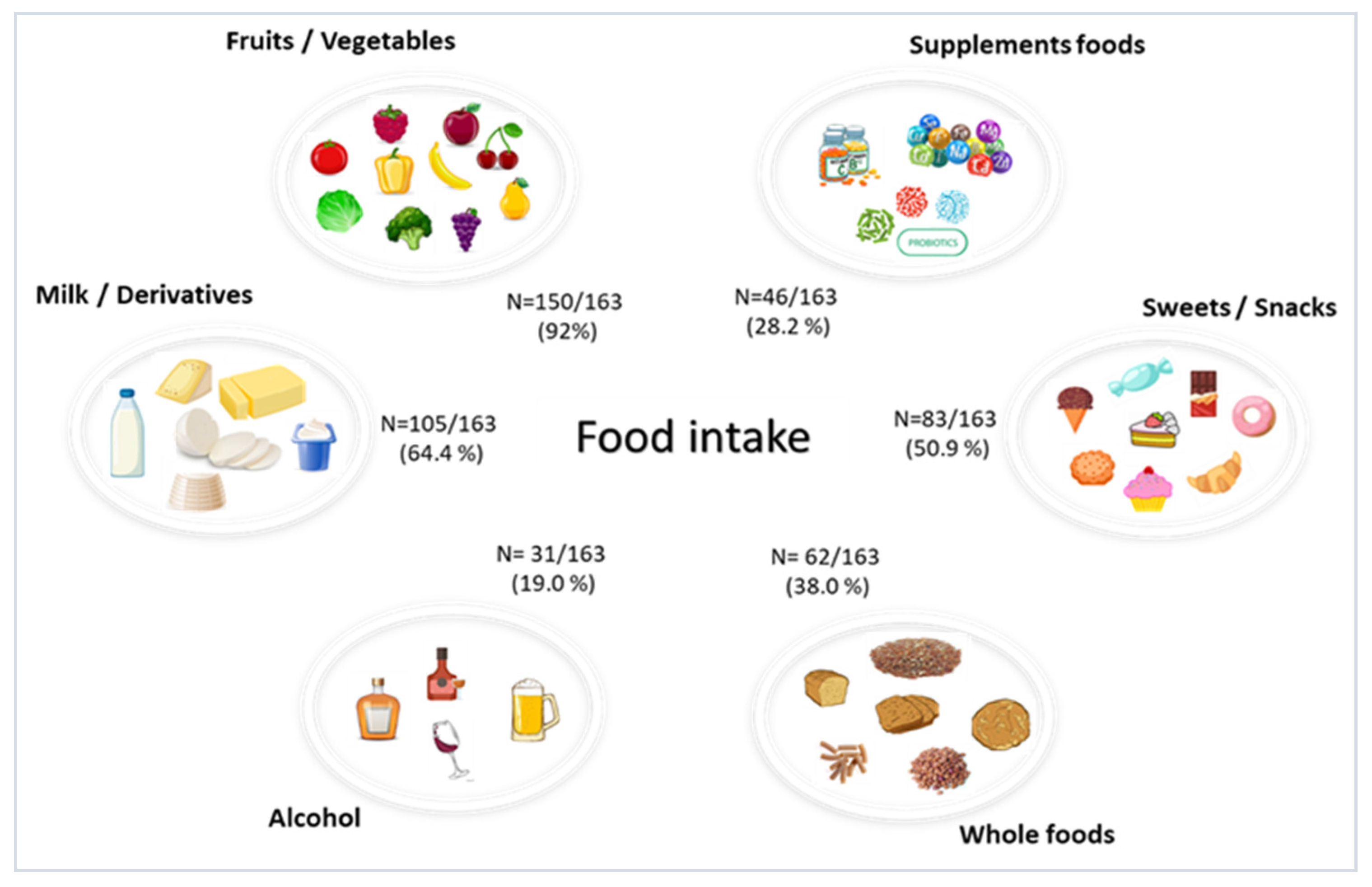

| Demographic and Clinical Characteristics | N = 163 |
|---|---|
| Age, years | 47.5 ± 11.4 |
| Male sex, n (%) | 119 (73.0) |
| Height, cm | 174 (167–178) |
| Weight, kg | 76.3 ± 15.1 |
| BMI, kg/m2 | 25.5 ± 3.70 |
| BMI ≥ 25 kg/m2, n (%) | 88 (54.0) |
| Waist Circumference, cm | 93.2 ± 11 |
| Smoker (y/n), n (%) | 26 (16.0) |
| Physical activity (y/n), n (%) | 92 (56.4) |
| Comorbidities (y/n), n (%) | |
| Cardiovascular diseases | 7 (4.3) |
| Hypertension | 30 (18.4) |
| Hypercholesterolemia | 22 (13.5) |
| Diabetes | 4 (2.5) |
| Thyroid diseases | 16 (9.8) |
| Rheumatic diseases | 8 (4.9) |
| Autoimmune diseases | 4 (2.5) |
| Liver diseases | 1 (0.6) |
| Respiratory diseases | 25 (15.3) |
| Kidney diseases | 5 (3.1) |
| Pharmacological therapies | 40 (24.5) |
| Antibiotic therapies | 10 (6.1) |
| Molecules | N = 163 |
|---|---|
| Vitamin C, µmol/L | 35.5 (23.9–55.2) |
| Vitamin A, µmoli/L | 1.79 ± 0.42 |
| Vitamin E, µmoli/L | 31.1 ± 7.51 |
| Transferrin, gr/L | 2.58 (2.37–2.87) |
| Cortisol, nmol/L | 312 (255–407) |
| Ferritin, ng/mL | 62.6 (19.8–127) |
| Uricemia, mg/dl | 5.10 ± 1.33 |
| Bilirubin, mg/dl | 0.69 (0.55–0.92) |
| 8-iso-PGF2α, pg/mL | 331 ± 136 |
| 3-NT, ng/mL | 54.1 ± 20.7 |
| AGEs, ng/mL | t7.82 (5.19–10.6) |
| MDA, ng/mL | 126 (109–154) |
| CAT, pg/mL | 179 (148–345) |
| OxLDL, ng/mL | 38.4 (15.6–116) |
| GPX1, ng/mL | 22.9 (17.4–30.8) |
| AOPP, ng/mL | 0.49 (0.39–0.59) |
| 8-OHdG, ng/mL | 3.98 ± 0.75 |
| SOD-2, ng/mL | 7.72 (4.48–11.3) |
| 4-HNE, pg/mL | 11987 ± 5719 |
| Dependent Variable: BMI | Model 1/Crude (Beta, p) | Model 2 (Beta, p) | Model 3 (Beta, p) | Model 4 (Beta, p) | Model 5 (Beta, p) | Model 6 (Beta, p) |
|---|---|---|---|---|---|---|
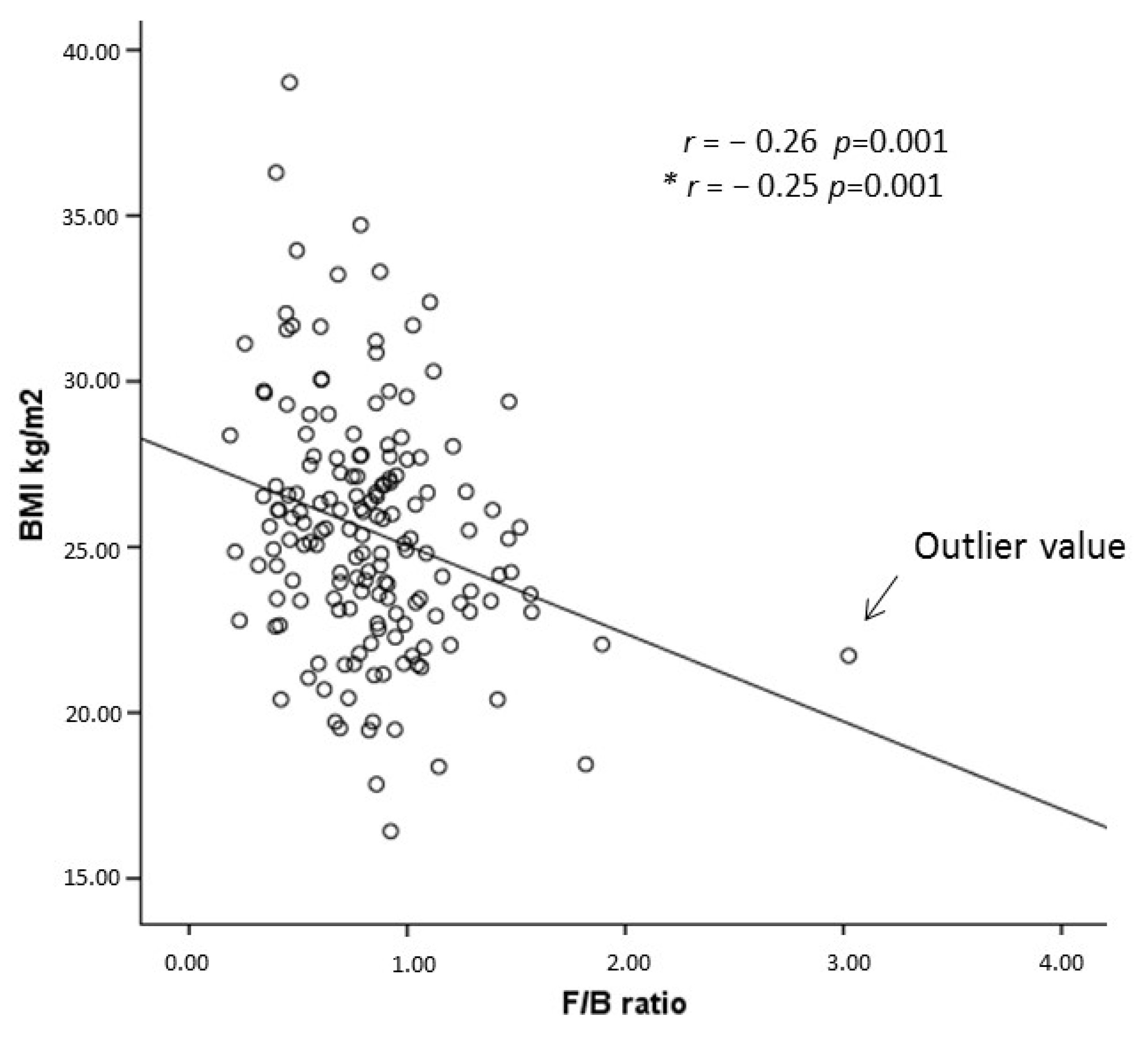
| Firmicutes/Bacteroidetes Ratio | −0.26 (0.001) | −0.26 (<0.001) | −0.19 (0.011) | −0.18 (0.031) | −0.18 (0.035) | −0.12 (0.154) |
| Age, yr | 0.11 (0.106) | 0.12 (0.095) | 0.11 (0.133) | 0.11 (0.128) | 0.14 (0.055) | |
| Male sex, (y/n) | −0.43 (<0.001) | −0.40 (<0.001) | −0.40 (<0.001) | −0.40 (<0.001) | −0.39 (<0.001) | |
| Cardiovascular diseases (y,n) | 0.16 (0.020) | 0.16 (0.022) | 0.17 (0.018) | 0.17 (0.019) | 0.14 (0.043) | |
| Thyroid diseases (y,n) | 0.05 (0.490) | 0.07 (0.315) | 0.07 (0.323) | 0.07 (0.342) | 0.09 (0.202) | |
| Shannon Index | −0.06 (0.443) | −0.05 (0.517) | −0.05 (0.515) | −0.03 (0.664) | ||
| Phylum Actinobacteria | −0.005 (0.946) | 0.03 (0.927) | 0.03 (0.919) | 0.05 (0.879) | ||
| Phylum Proteobacteria | 0.07 (0.340) | 0.04 (0.617) | 0.04 (0.632) | 0.04 (0.629) | ||
| Phylum Verrucomicrobia | −0.13 (0.076) | −0.45 (0.437) | −0.43 (0.470) | −0.23 (0.689) | ||
| Class Actinobacteria | 0.04 (0.898) | 0.04 (0.905) | 0.001 (0.998) | |||
| Class Verrucomicrobia | −0.12 (0.982) | −0.005 (0.999) | 0.14 (0.978) | |||
| Class Betaproteobacteria | 0.03 (0.791) | 0.03 (0.789) | 0.02 (0.838) | |||
| Genus Bifidobacterium | −0.10 (0.387) | −0.10 (0.398) | −0.05 (0.653) | |||
| Genus Akkermansia | 0.45 (0.936) | 0.007 (0.999) | −0.50 (0.928) | |||
| Genus Sutterella | 0.02 (0.862) | 0.02 (0.856) | 0.04 (0.660) | |||
| Species Akkermansia muciniphila | 0.30 (0.741) | 0.44 (0.618) | ||||
| Yogurt intake, (y/n) | −0.08 (0.283) | |||||
| Whole food intake, (y/n) | −0.12 (0.113) | |||||
| Fruit and vegetale intake, (y/n) | −0.16 (0.021) |
| Dependent Variable: 25 < BMI ≥ 25 | Model 1/Crude OR (95%, CI), p | Model 2 OR (95%, CI), p | Model 3 OR (95%, CI), p | Model 4 OR (95%, CI), p | Model 5 OR (95%, CI), p | Model 6 OR (95%, CI), p |
|---|---|---|---|---|---|---|
| Firmicutes/Bacteroidetes Ratio | 0.24 (0.09–0.66), 0.005 | 0.18 (0.06–0.60), 0.005 | 0.25 (0.07–0.87), 0.030 | 0.23 (0.05–0.95), 0.042 | 0.24 (0.06–0.99), 0.049 | 0.39 (0.08–1.92), 0.25 |
| Age, yr | 1.03 (0.99–1.06), 0.076 | 1.03 (0.99–1.07), 0.072 | 1.02 (0.99–1.06), 0.209 | 1.02 (0.99–1.06), 0.203 | 1.03 (0.99–1.07), 0.128 | |
| Male sex, (y/n) | 0.18 (0.07–0.42), <0.001 | 0.18 (0.08–0.44), <0.001 | 0.14 (0.05–0.38), <0.001 | 0.14 (0.05–0.37), 0.135 | 0.09 (0.03–0.29), <0.001 | |
| Cardiovascular diseases, (y/n) | 10.8 (0.69–171), 0.090 | 10.6 (0.69–163), 0.091 | 45.1 (1.24–1634), 0.038 | 45.6 (1.25–1669), 0.037 | 154 (2.31–10,239), 0.019 | |
| Thyroid diseases, (y/n) | 1.07 (0.30–3.77), 0.922 | 1.03 (0.28–3.86), 0.961 | 1.14 (0.28–4.73), 0.857 | 1.11 (0.27–4.62), 0.884 | 1.25 (0.27–5.67), 0.777 | |
| Shannon Index | 0.73 (0.16–3.31), 0.684 | 0.97 (0.20–4.74), 0.967 | 0.95 (0.20–4.66), 0.952 | 1.22 (0.20–7.32), 0.828 | ||
| Phylum Actinobacteria | 1.02 (0.90–1.15), 0.762 | 84.9 (0.79–9145), 0.063 | 92.2 (0.87–9763), 0.057 | 200 (0.87–46,084), 0.056 | ||
| Phylum Proteobacteria | 1.04 (0.95–1.13), 0.404 | 1.00 (0.90–1.11), 0.959 | 1.00 (0.90–1.10), 0.934 | 1.00 (0.90–1.11), 0.947 | ||
| Phylum Verrucomicrobia | 0.98 (0.86–1.11), 0.749 | 0.43 (0.13–1.45), 0.175 | 0.45 (0.13–1.53), 0.202 | 0.45 (0.11–1.77), 0.253 | ||
| Class Actinobacteria | 0.071 (0.0–1.47), 0.072 | 0.01 (0.0–1.33), 0.066 | 0.006 (0.0–1.34), 0.064 | |||
| Class Verrucomicrobia | 0.001 (0.0–288), 0.293 | 0.002 (0.0–363), 0.308 | 0.002 (0.0–1122), 0.350 | |||
| Class Betaproteobacteria | 1.07 (0.85–1.34), 0.568 | 1.07 (0.85–1.35), 0.560 | 1.10 (0.86–1.41), 0.432 | |||
| Genus Bifidobacterium | 0.60 (0.37–0.97), 0.037 | 0.60 (0.38–0.97), 0.038 | 0.58 (0.35–0.98), 0.043 | |||
| Genus Akkermansia | 1693 (0.007–103), 0.243 | 925 (0.003–103), 0.294 | 713 (0.001–103), 0.352 | |||
| Genus Sutterella | 0.95 (0.78–1.16), 0.601 | 0.95 (0.78–1.16), 0.614 | 0.96 (0.78–1.17), 0.675 | |||
| Species Akkermansia muciniphila | 1.54 (0.24–9.76), 0.648 | 2.10 (0.34–13.04), 0.427 | ||||
| Yogurt intake, (y/n) | 0.26 (0.19–0.69), 0.005 | |||||
| Whole food intake, (y/n) | 0.45 (0.18–1.09), 0.076 | |||||
| Fruit and vegetale intake, (y/n) | 0.30 (0.05–1.89), 0.199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Politi, C.; Mobrici, M.; Parlongo, R.M.; Spoto, B.; Tripepi, G.; Pizzini, P.; Cutrupi, S.; Franco, D.; Tino, R.; Farruggio, G.; et al. Role of Gut Microbiota in Overweight Susceptibility in an Adult Population in Italy. Nutrients 2023, 15, 2834. https://doi.org/10.3390/nu15132834
Politi C, Mobrici M, Parlongo RM, Spoto B, Tripepi G, Pizzini P, Cutrupi S, Franco D, Tino R, Farruggio G, et al. Role of Gut Microbiota in Overweight Susceptibility in an Adult Population in Italy. Nutrients. 2023; 15(13):2834. https://doi.org/10.3390/nu15132834
Chicago/Turabian StylePoliti, Cristina, Marco Mobrici, Rosa Maria Parlongo, Belinda Spoto, Giovanni Tripepi, Patrizia Pizzini, Sebastiano Cutrupi, Daniele Franco, Renato Tino, Giuseppe Farruggio, and et al. 2023. "Role of Gut Microbiota in Overweight Susceptibility in an Adult Population in Italy" Nutrients 15, no. 13: 2834. https://doi.org/10.3390/nu15132834
APA StylePoliti, C., Mobrici, M., Parlongo, R. M., Spoto, B., Tripepi, G., Pizzini, P., Cutrupi, S., Franco, D., Tino, R., Farruggio, G., Failla, C., Marino, F., Pioggia, G., & Testa, A. (2023). Role of Gut Microbiota in Overweight Susceptibility in an Adult Population in Italy. Nutrients, 15(13), 2834. https://doi.org/10.3390/nu15132834






