Therapeutic Effects and Molecular Mechanism of Chlorogenic Acid on Polycystic Ovarian Syndrome: Role of HIF-1alpha
Abstract
1. Introduction
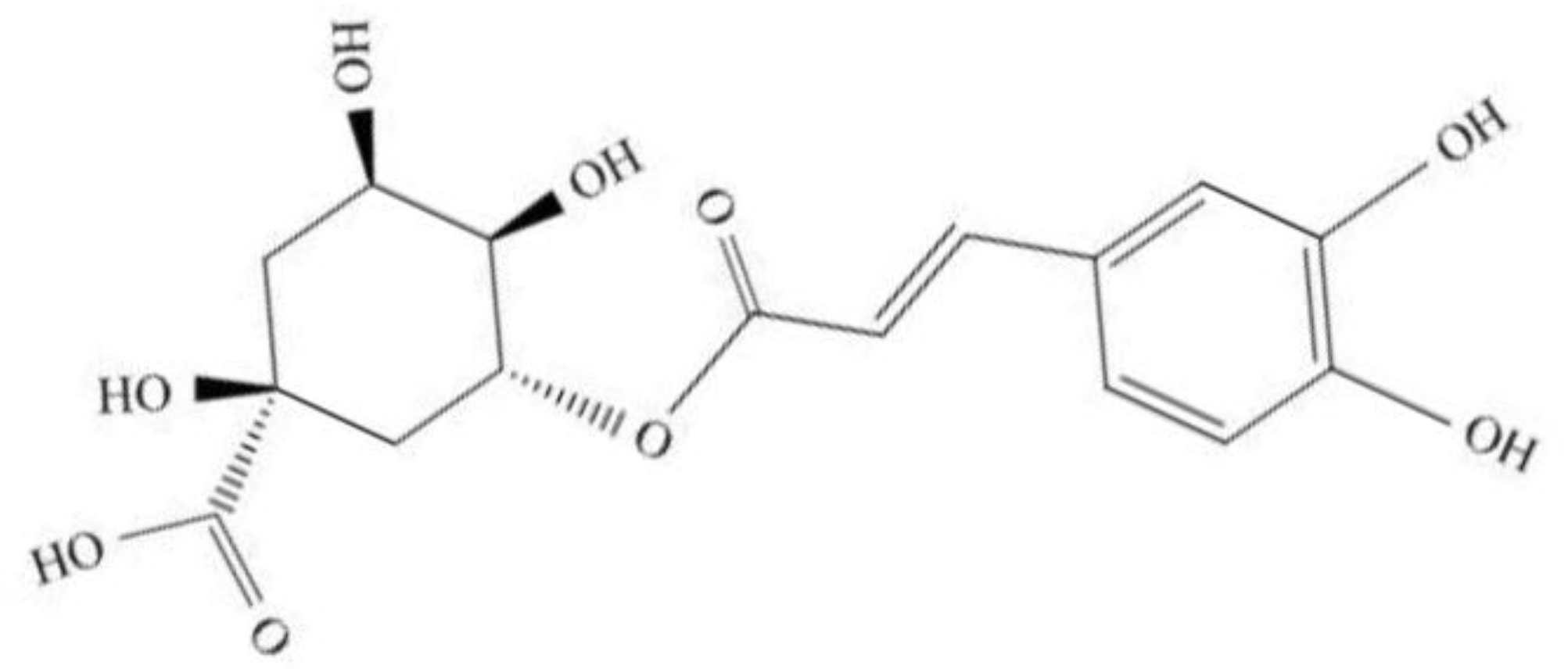
2. Overviews of Chlorogenic Acid (CGA)
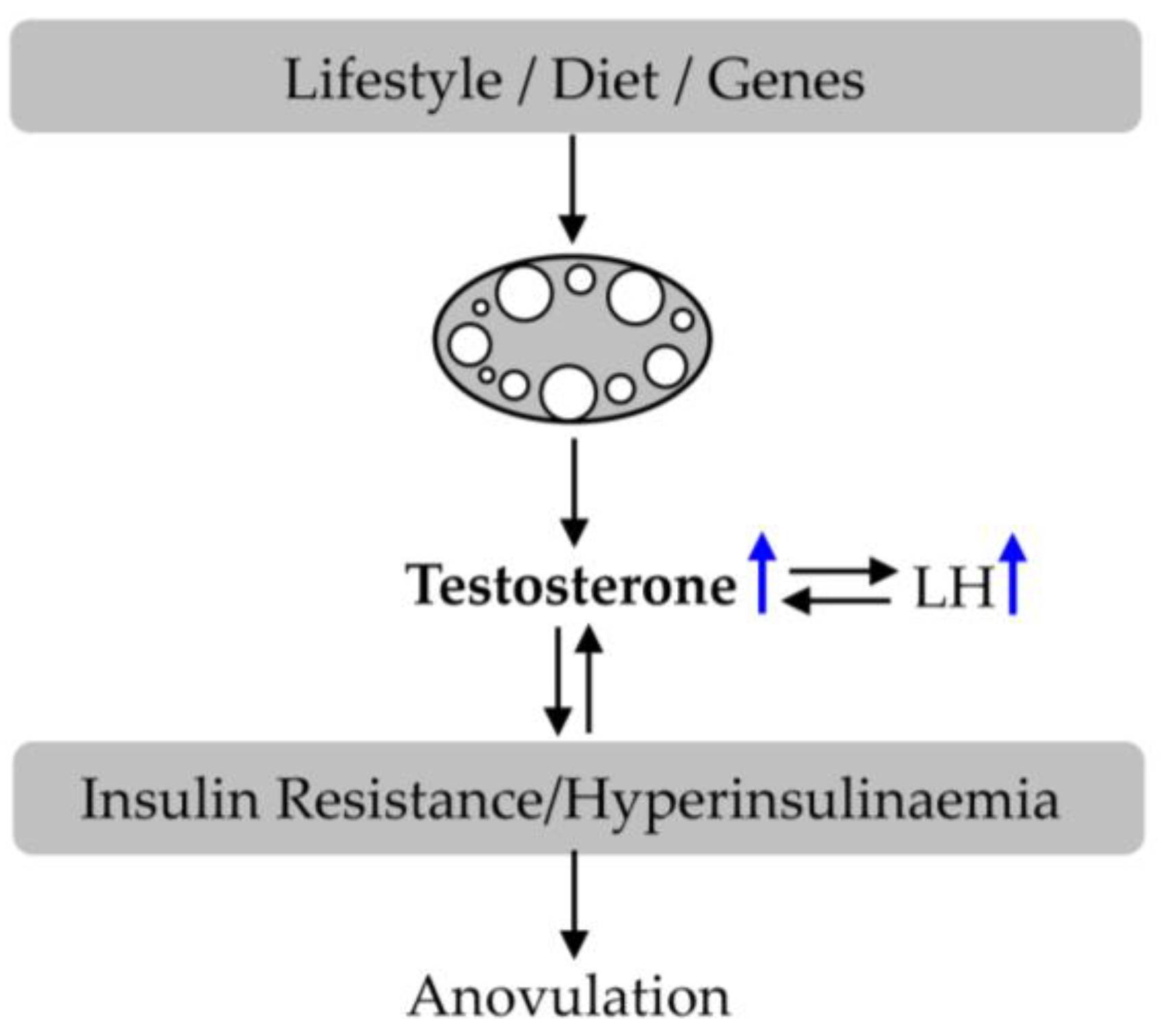
3. Polycystic Ovary Syndrome (PCOS)
4. Hypoxia Inducible Factor-1alpha (HIF-1alpha)
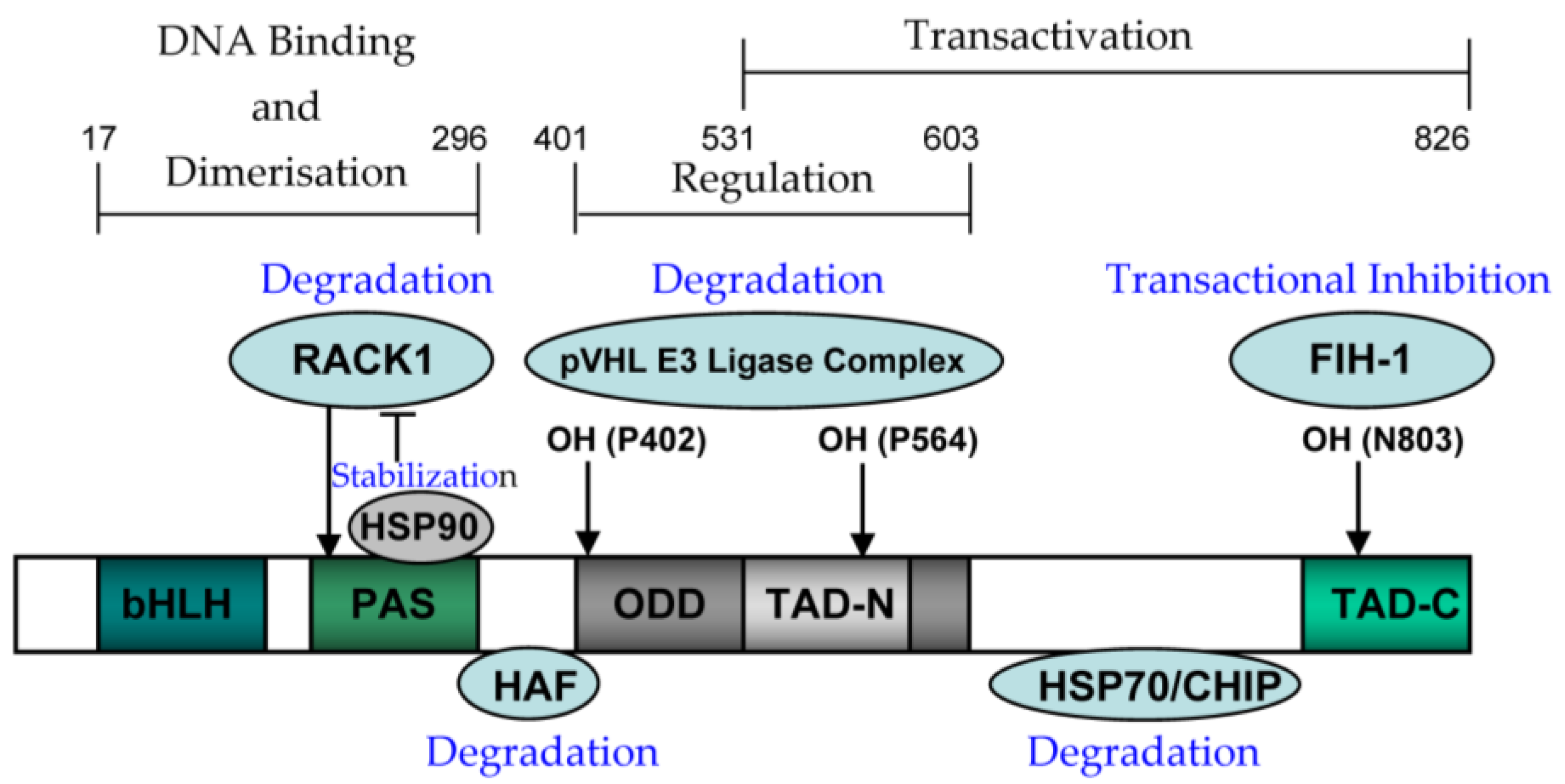
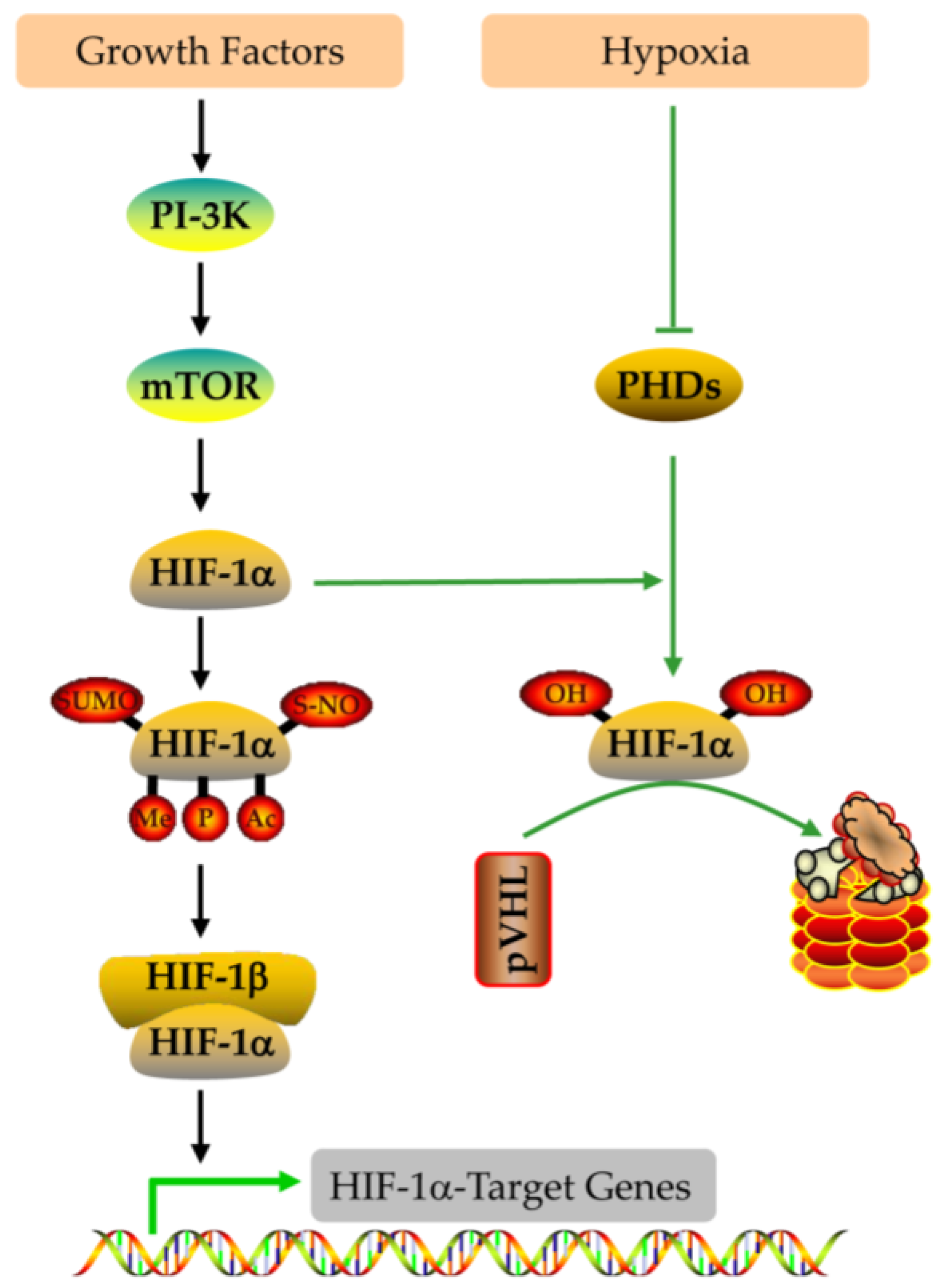
4.1. The Structure and Function of HIF-1alpha
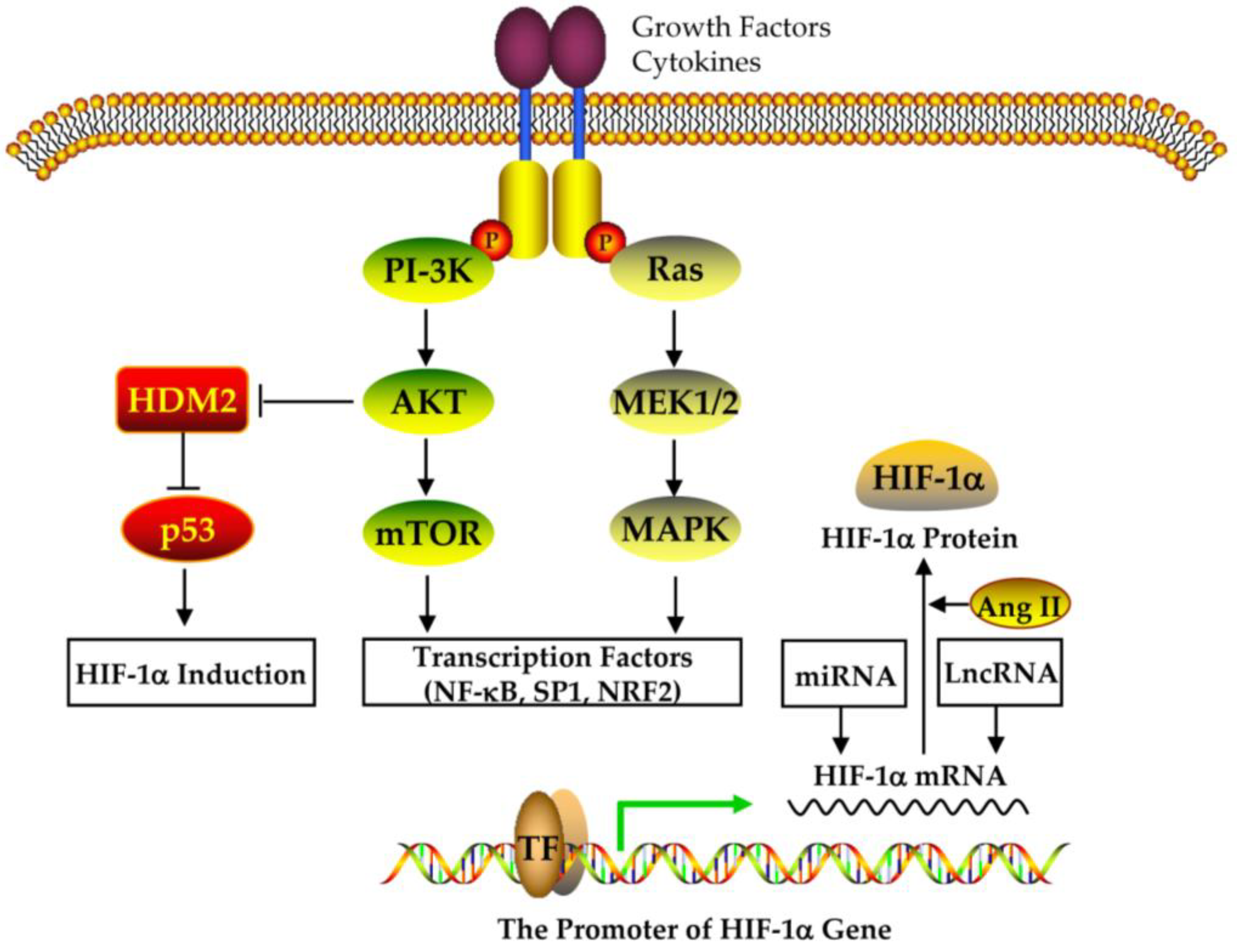
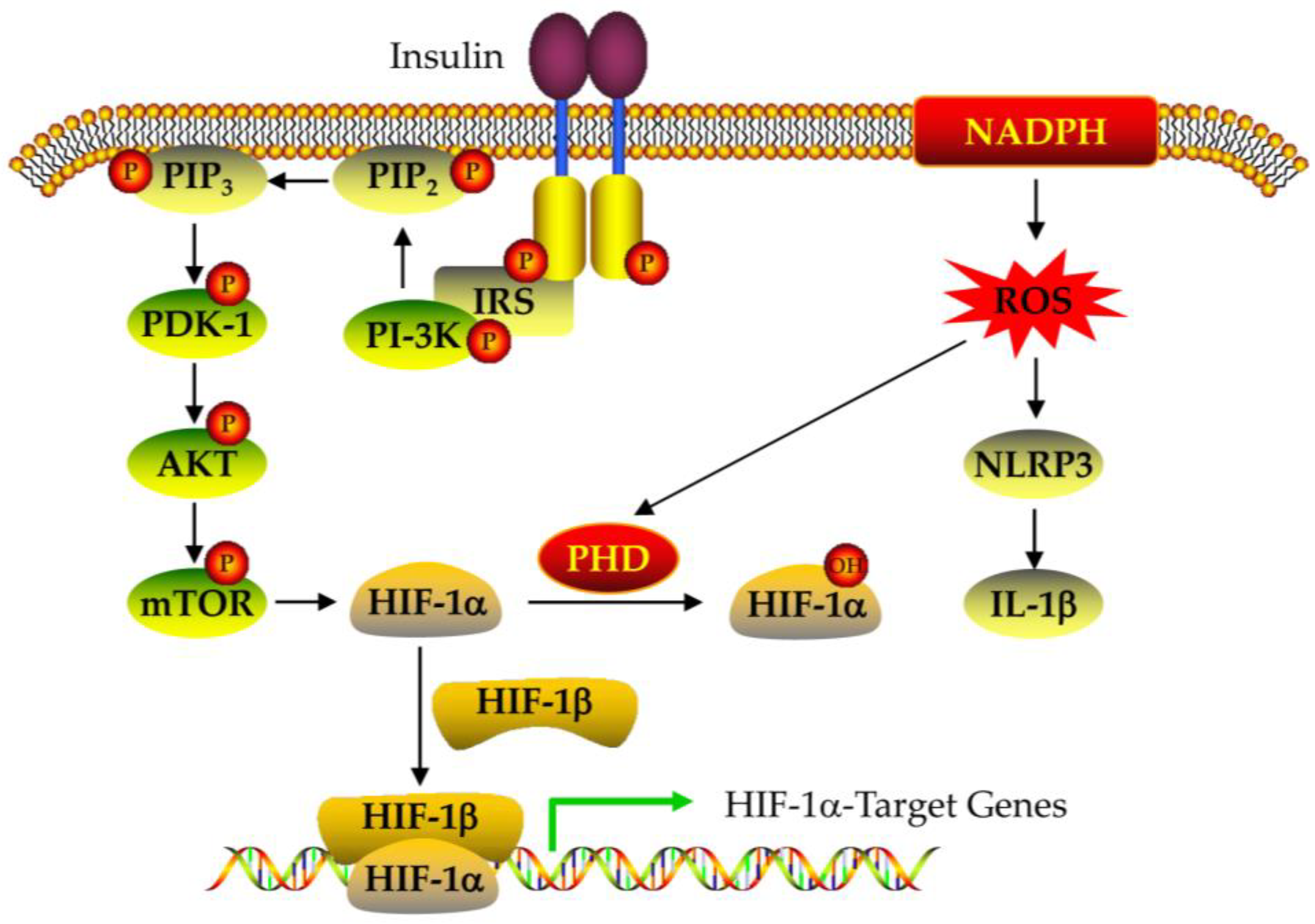
4.2. The Expression and Regulation of HIF-1alpha
5. Therapeutic Effect and Regulation of CGA on PCOS
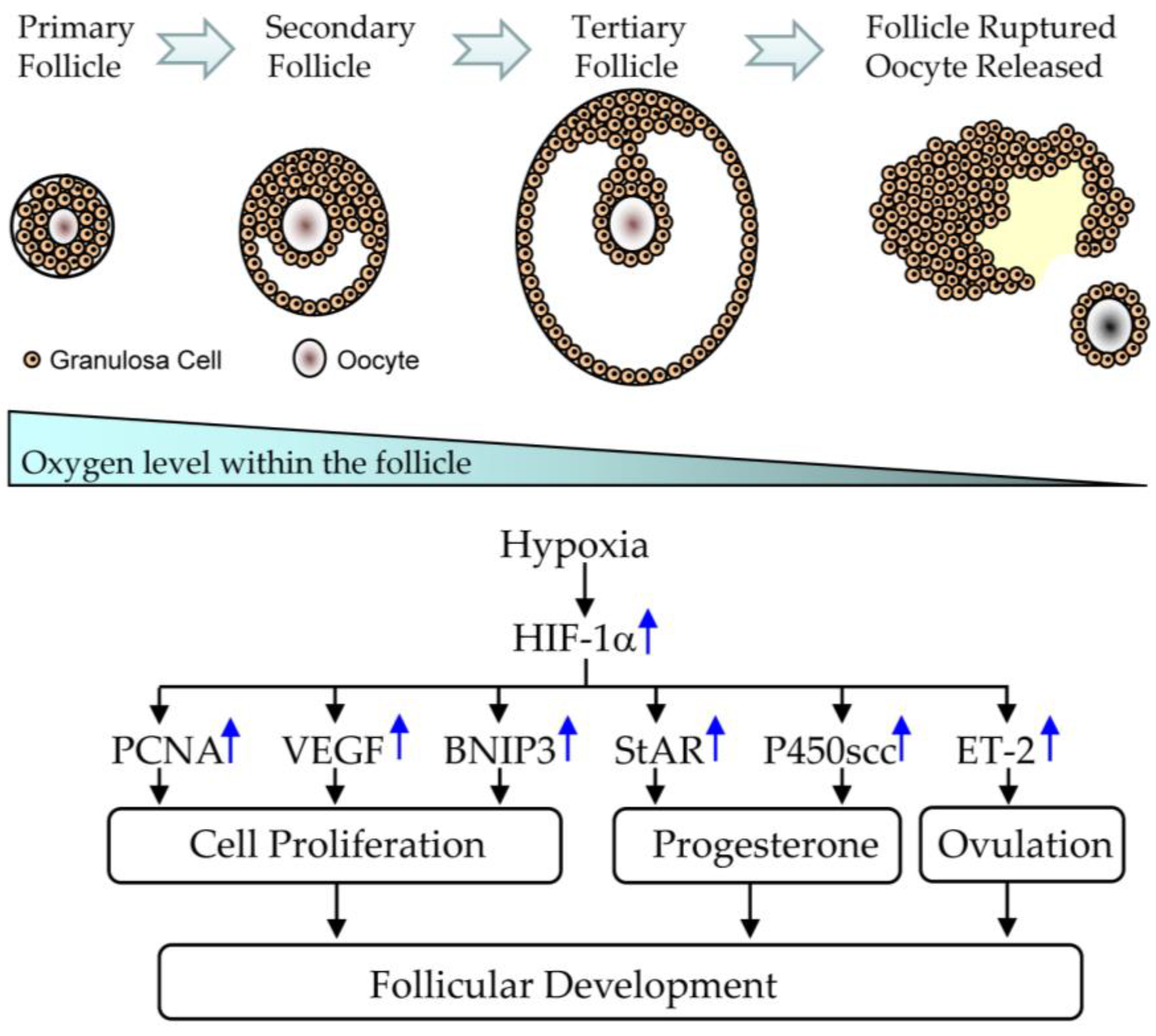
5.1. Effect of CGA on Follicular Development in PCOS
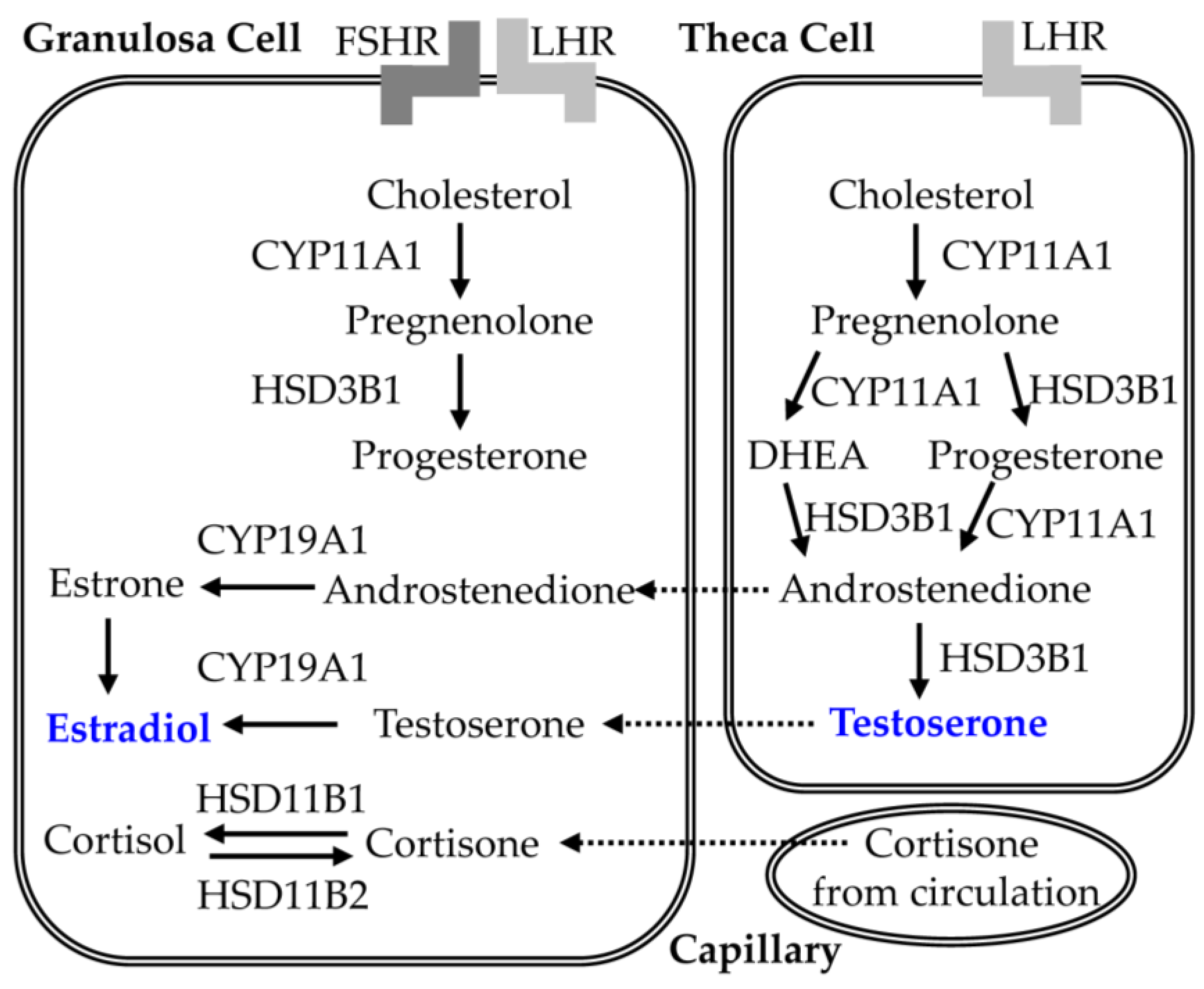
5.2. Effect of CGA on Steroid Synthesis in PCOS
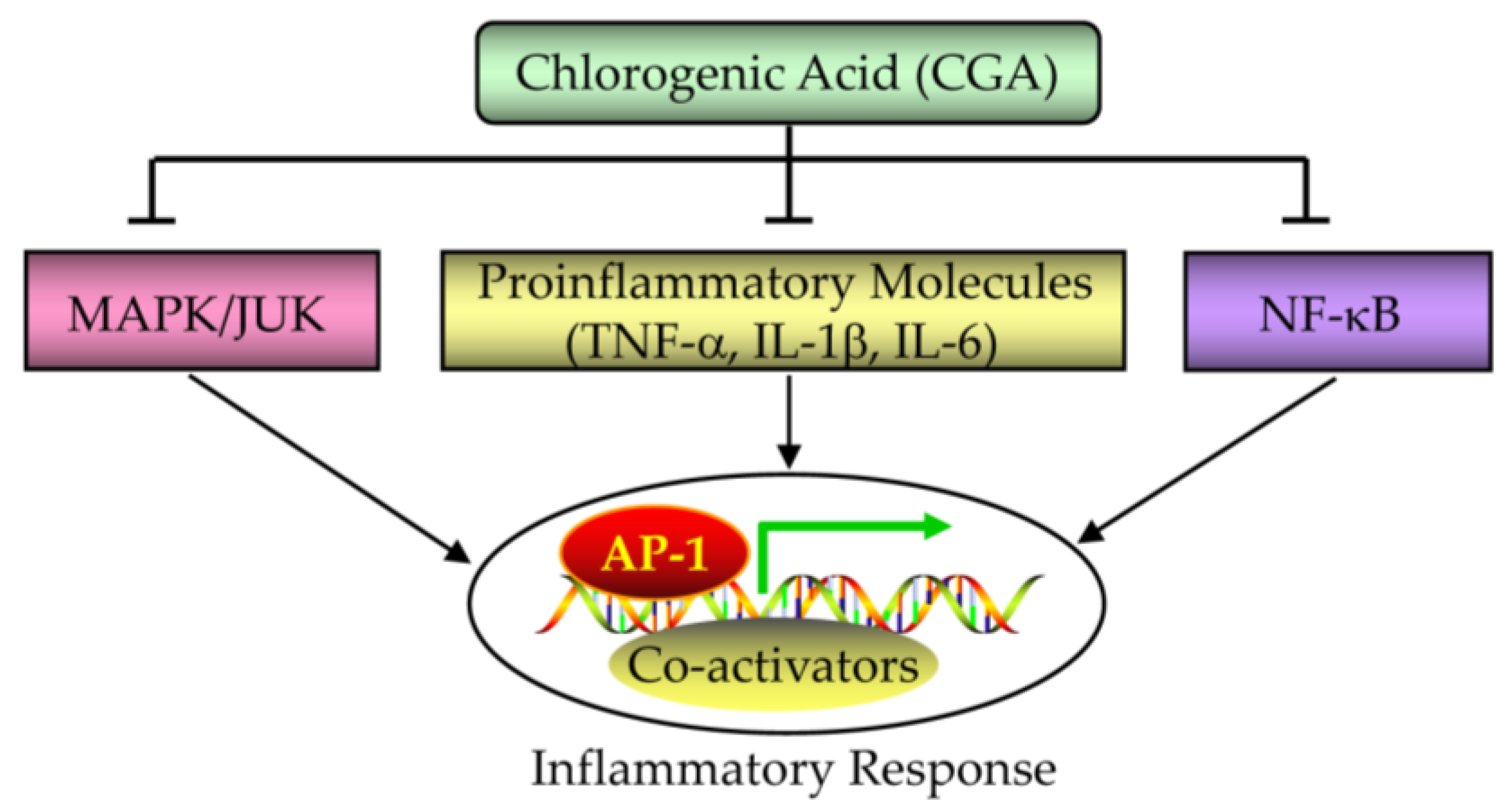
5.3. Effect of CGA on Inflammatory Response in PCOS
5.4. Effect of CGA on Oxidative Stress in PCOS
5.5. Effect of CGA on Insulin Resistance in PCOS
6. Clinical Development of HIF Proline Hydroxylase Inhibitors
7. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shrivastava, S.; Conigliaro, R.L. Polycystic Ovarian Syndrome. Med. Clin. N. Am. 2023, 107, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.Y.; Witchel, S.F. Polycystic ovary syndrome. Pediatr. Ann. 2006, 35, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Shi, Y. Polycystic ovary syndrome. Front. Med. China 2010, 4, 280–284. [Google Scholar] [CrossRef]
- Guzick, D.S. Polycystic ovary syndrome. Obstet. Gynecol. 2004, 103, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef]
- Al Wattar, B.H.; Fisher, M.; Bevington, L.; Talaulikar, V.; Davies, M.; Conway, G.; Yasmin, E. Clinical Practice Guidelines on the Diagnosis and Management of Polycystic Ovary Syndrome: A Systematic Review and Quality Assessment Study. J. Clin. Endocrinol. Metab. 2021, 106, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Presswala, B.; De Souza, L.R. The diagnostic experience of polycystic ovary syndrome: A scoping review of patient perspectives. Patient Educ. Couns. 2023, 113, 107771. [Google Scholar] [CrossRef]
- Bachelot, A. Polycystic ovarian syndrome: Clinical and biological diagnosis. Ann. Biol. Clin. 2016, 74, 661–667. [Google Scholar] [CrossRef]
- Wang, R.; Miao, C.; Chen, Y.; Zhao, Y.; Yang, L.; Cheng, W.; Zhang, Q. Antioxidant supplements relieve insulin resistance but do not improve lipid metabolism in women with polycystic ovary syndrome: A meta-analysis of randomized clinical trials. Gynecol. Endocrinol. 2022, 38, 1047–1059. [Google Scholar] [CrossRef]
- Li, W.; Liu, C.; Yang, Q.; Zhou, Y.; Liu, M.; Shan, H. Oxidative stress and antioxidant imbalance in ovulation disorder in patients with polycystic ovary syndrome. Front. Nutr. 2022, 9, 1018674. [Google Scholar] [CrossRef]
- Papalou, O.; Victor, V.M.; Diamanti-Kandarakis, E. Oxidative Stress in Polycystic Ovary Syndrome. Curr. Pharm. Des. 2016, 22, 2709–2722. [Google Scholar] [CrossRef]
- Haddad-Filho, H.; Tosatti, J.A.G.; Vale, F.M.; Gomes, K.B.; Reis, F.M. Updates in diagnosing polycystic ovary syndrome-related infertility. Expert. Rev. Mol. Diagn. 2023, 23, 123–132. [Google Scholar] [CrossRef]
- Singh, R.; Kaur, S.; Yadav, S.; Bhatia, S. Gonadotropins as pharmacological agents in assisted reproductive technology and polycystic ovary syndrome. Trends Endocrinol. Metab. 2023, 34, 194–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yu, Y.; Duan, T.; Zhou, Q. The role of macrophages in reproductive-related diseases. Heliyon 2022, 8, e11686. [Google Scholar] [CrossRef] [PubMed]
- Glendining, K.A.; Campbell, R.E. Recent advances in emerging PCOS therapies. Curr. Opin. Pharmacol. 2023, 68, 102345. [Google Scholar] [CrossRef]
- Jin, P.; Xie, Y. Treatment strategies for women with polycystic ovary syndrome. Gynecol. Endocrinol. 2018, 34, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Rababa’h, A.M.; Matani, B.R.; Yehya, A. An update of polycystic ovary syndrome: Causes and therapeutics options. Heliyon 2022, 8, e11010. [Google Scholar] [CrossRef] [PubMed]
- Dunaif, A.; Scott, D.; Finegood, D.; Quintana, B.; Whitcomb, R. The insulin-sensitizing agent troglitazone improves metabolic and reproductive abnormalities in the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1996, 81, 3299–3306. [Google Scholar] [CrossRef][Green Version]
- Wang, F.; Han, J.; Wang, X.; Liu, Y.; Zhang, Z. Roles of HIF-1alpha/BNIP3 mediated mitophagy in mitochondrial dysfunction of letrozole-induced PCOS rats. J. Mol. Histol. 2022, 53, 833–842. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Z.; Wang, Z.; Xiao, K.; Wang, Q.; Su, J.; Wang, Z. Expression and clinical significance of the HIF-1a/ET-2 signaling pathway during the development and treatment of polycystic ovary syndrome. J. Mol. Histol. 2015, 46, 173–181. [Google Scholar] [CrossRef]
- Wang, F.; Wang, S.; Zhang, Z.; Lin, Q.; Liu, Y.; Xiao, Y.; Xiao, K.; Wang, Z. Defective insulin signaling and the protective effects of dimethyldiguanide during follicular development in the ovaries of polycystic ovary syndrome. Mol. Med. Rep. 2017, 16, 8164–8170. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Xu, R.; Zhang, Z.; Shi, C.; Zhang, Y.; Yang, H.; Lin, Q.; Liu, Y.; Lin, F.; Geng, B.; et al. HIF-1alpha Protects Granulosa Cells From Hypoxia-Induced Apoptosis During Follicular Development by Inducing Autophagy. Front. Cell. Dev. Biol. 2021, 9, 631016. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, Z.; Lin, Q.; Xu, R.; Chen, J.; Wang, Y.; Zhang, Y.; Tang, Y.; Shi, C.; Liu, Y.; et al. HIF-1alpha/BNIP3-Mediated Autophagy Contributes to the Luteinization of Granulosa Cells During the Formation of Corpus Luteum. Front. Cell. Dev. Biol. 2020, 8, 619924. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Wu, Y.; Chen, L.; Luo, Q.; Zhang, J.; Chen, J.; Luo, Z.; Huang, X.; Cheng, Y. Effects of echinomycin on endothelin-2 expression and ovulation in immature rats primed with gonadotropins. Exp. Mol. Med. 2012, 44, 615–621. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Z.; Han, J.; Zheng, J.; Wang, X.; Wang, Z. Discovery of microglia gonadotropin-releasing hormone receptor and its potential role in polycystic ovarian syndrome. Mol. Med. Rep. 2023, 27, 12964. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Z.H.; Xiao, K.Z.; Wang, Z.C. Roles of Hypothalamic-Pituitary-Adrenal Axis and Hypothalamus-Pituitary-Ovary Axis in the Abnormal Endocrine Functions in Patients with Polycystic Ovary Syndrome. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2017, 39, 699–704. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X. The effects of mitochondrial dysfunction on energy metabolism switch by HIF-1alpha signalling in granulosa cells of polycystic ovary syndrome. Endokrynol. Pol. 2020, 71, 134–145. [Google Scholar] [CrossRef]
- Lebinger, T.G. Metformin and polycystic ovary syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 132–140. [Google Scholar] [CrossRef]
- Naseri, A.; Sanaie, S.; Hamzehzadeh, S.; Seyedi-Sahebari, S.; Hosseini, M.S.; Gholipour-Khalili, E.; Rezazadeh-Gavgani, E.; Majidazar, R.; Seraji, P.; Daneshvar, S.; et al. Metformin: New applications for an old drug. J. Basic. Clin. Physiol. Pharmacol. 2023, 34, 151–160. [Google Scholar] [CrossRef]
- Abedpour, N.; Zirak Javanmard, M.; Karimipour, M.; Pourmajed Liqvan, A. Effect of chlorogenic acid on follicular development, hormonal status and biomarkers of oxidative stress in rats with polycystic ovary syndrome. Vet. Res. Forum 2022, 13, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Wan, S.; Hou, Y.; Cheng, J.; Guo, J.; Wang, S.; Khan, A.; Sun, N.; Li, H. Chlorogenic acid rescues zearalenone induced injury to mouse ovarian granulosa cells. Ecotoxicol. Environ. Saf. 2020, 194, 110401. [Google Scholar] [CrossRef] [PubMed]
- Abedpour, N.; Javanmard, M.Z.; Karimipour, M.; Farjah, G.H. Chlorogenic acid improves functional potential of follicles in mouse whole ovarian tissues in vitro. Mol. Biol. Rep. 2022, 49, 10327–10338. [Google Scholar] [CrossRef]
- Ahmed Nasef, N.; Thota, R.N.; Mutukumira, A.N.; Rutherfurd-Markwick, K.; Dickens, M.; Gopal, P.; Singh, H.; Garg, M.L. Bioactive Yoghurt Containing Curcumin and Chlorogenic Acid Reduces Inflammation in Postmenopausal Women. Nutrients 2022, 14, 4619. [Google Scholar] [CrossRef]
- Shah, M.; Shrivastava, V.K.; Sofi, S.; Jamous, Y.F.; Khan, M.F.; Alkholifi, F.K.; Ahmad, W.; Mir, M.A. Chlorogenic Acid Restores Ovarian Functions in Mice with Letrozole-Induced Polycystic Ovarian Syndrome Via Modulation of Adiponectin Receptor. Biomedicines 2023, 11, 900. [Google Scholar] [CrossRef]
- Hussain, L.; Aamir, N.; Hussain, M.; Asif, M.; Chauhdary, Z.; Manzoor, F.; Siddique, R.; Riaz, M. Therapeutic Investigation of Standardized Aqueous Methanolic Extract of Bitter Melon (Momordica charantia L.) for Its Potential against Polycystic Ovarian Syndrome in Experimental Animals’ Model: In Vitro and In Vivo Studies. Evid. Based Complement. Altern. Med. 2022, 2022, 5143653. [Google Scholar] [CrossRef]
- Meshkani, M.; Saedisomeolia, A.; Yekaninejad, M.; Mousavi, S.A.; Ildarabadi, A.; Vahid-Dastjerdi, M. The Effect of Green Coffee Supplementation on Lipid Profile, Glycemic Indices, Inflammatory Biomarkers and Anthropometric Indices in Iranian Women With Polycystic Ovary Syndrome: A Randomized Clinical Trial. Clin. Nutr. Res. 2022, 11, 241–254. [Google Scholar] [CrossRef]
- Rana, S.; Hussain, L.; Saleem, U.; Asif, M.; Lodhi, A.H.; Barkat, M.Q.; Riaz, M.; Jamil, A. Dose Dependent Effects of Aqueous Extract of Garcinia cambogia Desr. Against Letrozole Induced Polycystic Ovarian Syndrome in Female Adult Rats With Possible Mechanisms Exploration. Dose Response 2023, 21, 15593258231169381. [Google Scholar] [CrossRef]
- Thi Nguyen, N.; Hirata, M.; Tanihara, F.; Hirano, T.; Le, Q.A.; Nii, M.; Otoi, T. Hypothermic storage of porcine zygotes in serum supplemented with chlorogenic acid. Reprod. Domest. Anim. 2019, 54, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Schwab, D.; Herling, A.W.; Hemmerle, H.; Schubert, G.; Hagenbuch, B.; Burger, H.J. Hepatic uptake of synthetic chlorogenic acid derivatives by the organic anion transport proteins. J. Pharmacol. Exp. Ther. 2001, 296, 91–98. [Google Scholar] [PubMed]
- Sitarek, P.; Skala, E.; Wysokinska, H.; Wielanek, M.; Szemraj, J.; Toma, M.; Sliwinski, T. The Effect of Leonurus sibiricus Plant Extracts on Stimulating Repair and Protective Activity against Oxidative DNA Damage in CHO Cells and Content of Phenolic Compounds. Oxid. Med. Cell. Longev. 2016, 2016, 5738193. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Berenbaum, M.R.; Schuler, M.A. Plant allelochemicals differentially regulate Helicoverpa zea cytochrome P450 genes. Insect Mol. Biol. 2002, 11, 343–351. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Di Simone, S.C.; Acquaviva, A.; Libero, M.L.; Campana, C.; Recinella, L.; Leone, S.; Brunetti, L.; Orlando, G.; Vitale, I.; et al. Protective Effects of PollenAid Plus Soft Gel Capsules’ Hydroalcoholic Extract in Isolated Prostates and Ovaries Exposed to Lipopolysaccharide. Molecules 2022, 27, 6279. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules 2020, 25, 4553. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Singh, A.K.; Singla, R.K.; Pandey, A.K. Chlorogenic Acid: A Dietary Phenolic Acid with Promising Pharmacotherapeutic Potential. Curr. Med. Chem. 2023, 30, 3905–3926. [Google Scholar] [CrossRef] [PubMed]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Atanasov, A.G.; Li, Y.; Kumar, N.; Bishayee, A. Chlorogenic acid for cancer prevention and therapy: Current status on efficacy and mechanisms of action. Pharmacol. Res. 2022, 186, 106505. [Google Scholar] [CrossRef]
- Stein, I.F.; Leventhal, M.L. Amenorrhea associated with bilateral polycystic ovaries. Am. J. Obstet. Gynecol. 1935, 29, 181–191. [Google Scholar] [CrossRef]
- Meier, R.K. Polycystic Ovary Syndrome. Nurs. Clin. North. Am. 2018, 53, 407–420. [Google Scholar] [CrossRef]
- Nandi, A.; Chen, Z.; Patel, R.; Poretsky, L. Polycystic ovary syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, D.; Hieronimus, S.; Mirakian, P.; Hugues, J.N. Polycystic ovary syndrome (PCOS). Ann. Endocrinol. 2010, 71, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, N.; Khan, S.Z.; Shaikh, R. Polycystic ovary syndrome (PCOS) and genetic predisposition: A review article. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 3, 100060. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, M.; Christin-Maitre, S.; Kerlan, V. Polycystic ovary syndrome and adipose tissue. Ann. Endocrinol. 2023, 84, 308–315. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Setji, T.L.; Brown, A.J. Polycystic ovary syndrome: Diagnosis and treatment. Am. J. Med. 2007, 120, 128–132. [Google Scholar] [CrossRef]
- Goodarzi, M.O.; Dumesic, D.A.; Chazenbalk, G.; Azziz, R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 2011, 7, 219–231. [Google Scholar] [CrossRef]
- Harada, M. Pathophysiology of polycystic ovary syndrome revisited: Current understanding and perspectives regarding future research. Reprod. Med. Biol. 2022, 21, e12487. [Google Scholar] [CrossRef]
- King, J. Polycystic ovary syndrome. J. Midwifery Womens Health 2006, 51, 415–422. [Google Scholar] [CrossRef]
- Wei, H.; Huo, P.; Liu, S.; Huang, H.; Zhang, S. Posttranslational modifications in pathogenesis of PCOS. Front. Endocrinol. 2022, 13, 1024320. [Google Scholar] [CrossRef]
- Gonzalez, F. Inflammation in Polycystic Ovary Syndrome: Underpinning of insulin resistance and ovarian dysfunction. Steroids 2012, 77, 300–305. [Google Scholar] [CrossRef]
- Herman, R.; Sikonja, J.; Jensterle, M.; Janez, A.; Dolzan, V. Insulin Metabolism in Polycystic Ovary Syndrome: Secretion, Signaling, and Clearance. Int. J. Mol. Sci. 2023, 24, 3140. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Cooney, L.G.; Stanic, A.K. Immune Dysfunction in Polycystic Ovary Syndrome. Immunohorizons 2023, 7, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Psilopatis, I.; Vrettou, K.; Nousiopoulou, E.; Palamaris, K.; Theocharis, S. The Role of Peroxisome Proliferator-Activated Receptors in Polycystic Ovary Syndrome. J. Clin. Med. 2023, 12, 2912. [Google Scholar] [CrossRef]
- Christ, J.P.; Cedars, M.I. Current Guidelines for Diagnosing PCOS. Diagnostics 2023, 13, 1113. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef]
- Belenkaia, L.V.; Lazareva, L.M.; Walker, W.; Lizneva, D.V.; Suturina, L.V. Criteria, phenotypes and prevalence of polycystic ovary syndrome. Minerva Ginecol. 2019, 71, 211–223. [Google Scholar] [CrossRef]
- Pauli, J.M.; Raja-Khan, N.; Wu, X.; Legro, R.S. Current perspectives of insulin resistance and polycystic ovary syndrome. Diabet. Med. 2011, 28, 1445–1454. [Google Scholar] [CrossRef]
- Siddiqui, S.; Mateen, S.; Ahmad, R.; Moin, S. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J. Assist. Reprod. Genet. 2022, 39, 2439–2473. [Google Scholar] [CrossRef]
- Churchill, S.J.; Wang, E.T.; Pisarska, M.D. Metabolic consequences of polycystic ovary syndrome. Minerva Ginecol. 2015, 67, 545–555. [Google Scholar] [PubMed]
- Chang, R.J. The reproductive phenotype in polycystic ovary syndrome. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Qu, Y.; Jiang, H.; Wang, H.; Pan, Y.; Zhang, Y.; Wu, X.; Han, Y.; Zhang, Y. Therapeutic effect and safety of curcumin in women with PCOS: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 1051111. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.Y.; Wang, X.F.; Hua, F.Z. HIF-1alpha in Osteoarthritis: From Pathogenesis to Therapeutic Implications. Front. Pharmacol. 2022, 13, 927126. [Google Scholar] [CrossRef] [PubMed]
- Gunton, J.E. Hypoxia-inducible factors and diabetes. J. Clin. Investig. 2020, 130, 5063–5073. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Takeda, N. The roles of HIF-1alpha signaling in cardiovascular diseases. J. Cardiol. 2023, 81, 202–208. [Google Scholar] [CrossRef]
- Lee, J.W.; Bae, S.H.; Jeong, J.W.; Kim, S.H.; Kim, K.W. Hypoxia-inducible factor (HIF-1)alpha: Its protein stability and biological functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef]
- Metheni, M.; Lombes, A.; Bouillaud, F.; Batteux, F.; Langsley, G. HIF-1alpha induction, proliferation and glycolysis of Theileria-infected leukocytes. Cell. Microbiol. 2015, 17, 467–472. [Google Scholar] [CrossRef]
- Rashid, M.; Zadeh, L.R.; Baradaran, B.; Molavi, O.; Ghesmati, Z.; Sabzichi, M.; Ramezani, F. Up-down regulation of HIF-1alpha in cancer progression. Gene 2021, 798, 145796. [Google Scholar] [CrossRef]
- Peng, J.; He, Z.; Yuan, Y.; Xie, J.; Zhou, Y.; Guo, B.; Guo, J. Docetaxel suppressed cell proliferation through Smad3/HIF-1alpha-mediated glycolysis in prostate cancer cells. Cell. Commun. Signal. 2022, 20, 194. [Google Scholar] [CrossRef]
- Xu, L.; Huan, L.; Guo, T.; Wu, Y.; Liu, Y.; Wang, Q.; Huang, S.; Xu, Y.; Liang, L.; He, X. LncRNA SNHG11 facilitates tumor metastasis by interacting with and stabilizing HIF-1alpha. Oncogene 2020, 39, 7005–7018. [Google Scholar] [CrossRef]
- Luo, J.; Sun, P.; Zhang, X.; Lin, G.; Xin, Q.; Niu, Y.; Chen, Y.; Xu, N.; Zhang, Y.; Xie, W. Canagliflozin Modulates Hypoxia-Induced Metastasis, Angiogenesis and Glycolysis by Decreasing HIF-1alpha Protein Synthesis via AKT/mTOR Pathway. Int. J. Mol. Sci. 2021, 22, 13336. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, B.; Bao, L.; Jin, L.; Yang, M.; Peng, Y.; Kumar, A.; Wang, J.E.; Wang, C.; Zou, X.; et al. ZMYND8 acetylation mediates HIF-dependent breast cancer progression and metastasis. J. Clin. Investig. 2018, 128, 1937–1955. [Google Scholar] [CrossRef]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
- Semba, H.; Takeda, N.; Isagawa, T.; Sugiura, Y.; Honda, K.; Wake, M.; Miyazawa, H.; Yamaguchi, Y.; Miura, M.; Jenkins, D.M.; et al. HIF-1alpha-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat. Commun. 2016, 7, 11635. [Google Scholar] [CrossRef]
- Diaz-Gonzalez, J.A.; Russell, J.; Rouzaut, A.; Gil-Bazo, I.; Montuenga, L. Targeting hypoxia and angiogenesis through HIF-1alpha inhibition. Cancer Biol. Ther. 2005, 4, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Wu, J.L.; Xiao, J.; Fan, L.; Chen, S.H.; Li, W. HIF-1alpha regulates angiogenesis via Notch1/STAT3/ETBR pathway in trophoblastic cells. Cell. Cycle 2019, 18, 3502–3512. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, R.; Wang, Z. Contribution of Oxidative Stress to HIF-1-Mediated Profibrotic Changes during the Kidney Damage. Oxid. Med. Cell. Longev. 2021, 2021, 6114132. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, F.; Yang, H.; Wang, Z. Action Sites and Clinical Application of HIF-1alpha Inhibitors. Molecules 2022, 27, 3426. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, Z.; Xu, R.; Zhang, Y.; Wang, Z. Contribution of HIF-1alpha/BNIP3-mediated autophagy to lipid accumulation during irinotecan-induced liver injury. Sci. Rep. 2023, 13, 6528. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Q.; Li, P.L.; Dhaduk, R.; Zhang, F.; Gehr, T.W.; Li, N. Silencing of hypoxia-inducible factor-1alpha gene attenuates chronic ischemic renal injury in two-kidney, one-clip rats. Am. J. Physiol. Renal Physiol. 2014, 306, F1236–F1242. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, L.; Zhu, Q.; Yi, F.; Zhang, F.; Li, P.L.; Li, N. Hypoxia-inducible factor-1alpha contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney Int. 2011, 79, 300–310. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Q.; Xia, M.; Li, P.L.; Hinton, S.J.; Li, N. Hypoxia-inducible factor prolyl-hydroxylase 2 senses high-salt intake to increase hypoxia inducible factor 1alpha levels in the renal medulla. Hypertension 2010, 55, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Bonello, S.; Zahringer, C.; BelAiba, R.S.; Djordjevic, T.; Hess, J.; Michiels, C.; Kietzmann, T.; Gorlach, A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Minet, E.; Ernest, I.; Michel, G.; Roland, I.; Remacle, J.; Raes, M.; Michiels, C. HIF1A gene transcription is dependent on a core promoter sequence encompassing activating and inhibiting sequences located upstream from the transcription initiation site and cis elements located within the 5’UTR. Biochem. Biophys. Res. Commun. 1999, 261, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Bruning, U.; Cerone, L.; Neufeld, Z.; Fitzpatrick, S.F.; Cheong, A.; Scholz, C.C.; Simpson, D.A.; Leonard, M.O.; Tambuwala, M.M.; Cummins, E.P.; et al. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol. Cell. Biol. 2011, 31, 4087–4096. [Google Scholar] [CrossRef]
- Bertero, T.; Rezzonico, R.; Pottier, N.; Mari, B. Impact of MicroRNAs in the Cellular Response to Hypoxia. Int. Rev. Cell. Mol. Biol. 2017, 333, 91–158. [Google Scholar] [CrossRef]
- Mathew, L.K.; Lee, S.S.; Skuli, N.; Rao, S.; Keith, B.; Nathanson, K.L.; Lal, P.; Simon, M.C. Restricted expression of miR-30c-2-3p and miR-30a-3p in clear cell renal cell carcinomas enhances HIF2alpha activity. Cancer Discov. 2014, 4, 53–60. [Google Scholar] [CrossRef]
- Gee, H.E.; Ivan, C.; Calin, G.A.; Ivan, M. HypoxamiRs and cancer: From biology to targeted therapy. Antioxid. Redox Signal. 2014, 21, 1220–1238. [Google Scholar] [CrossRef] [PubMed]
- Page, E.L.; Robitaille, G.A.; Pouyssegur, J.; Richard, D.E. Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J. Biol. Chem. 2002, 277, 48403–48409. [Google Scholar] [CrossRef]
- Albanese, A.; Daly, L.A.; Mennerich, D.; Kietzmann, T.; See, V. The Role of Hypoxia-Inducible Factor Post-Translational Modifications in Regulating Its Localisation, Stability, and Activity. Int. J. Mol. Sci. 2020, 22, 268. [Google Scholar] [CrossRef] [PubMed]
- Comerford, K.M.; Leonard, M.O.; Karhausen, J.; Carey, R.; Colgan, S.P.; Taylor, C.T. Small ubiquitin-related modifier-1 modification mediates resolution of CREB-dependent responses to hypoxia. Proc. Natl. Acad. Sci. USA 2003, 100, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Zhang, F.P.; Tian, F.; Anders Friberg, P.; Wang, X.; Sjoland, H.; Billig, H. Increase of SUMO-1 expression in response to hypoxia: Direct interaction with HIF-1alpha in adult mouse brain and heart in vivo. FEBS Lett. 2004, 569, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Mennerich, D.; Kubaichuk, K.; Kietzmann, T. DUBs, Hypoxia, and Cancer. Trends Cancer 2019, 5, 632–653. [Google Scholar] [CrossRef] [PubMed]
- Gunter, J.; Ruiz-Serrano, A.; Pickel, C.; Wenger, R.H.; Scholz, C.C. The functional interplay between the HIF pathway and the ubiquitin system—More than a one-way road. Exp. Cell. Res. 2017, 356, 152–159. [Google Scholar] [CrossRef]
- Kindrick, J.D.; Mole, D.R. Hypoxic Regulation of Gene Transcription and Chromatin: Cause and Effect. Int. J. Mol. Sci. 2020, 21, 8320. [Google Scholar] [CrossRef]
- Orlando, I.M.C.; Lafleur, V.N.; Storti, F.; Spielmann, P.; Crowther, L.; Santambrogio, S.; Schodel, J.; Hoogewijs, D.; Mole, D.R.; Wenger, R.H. Distal and proximal hypoxia response elements cooperate to regulate organ-specific erythropoietin gene expression. Haematologica 2020, 105, 2774–2784. [Google Scholar] [CrossRef]
- Schorg, A.; Santambrogio, S.; Platt, J.L.; Schodel, J.; Lindenmeyer, M.T.; Cohen, C.D.; Schrodter, K.; Mole, D.R.; Wenger, R.H.; Hoogewijs, D. Destruction of a distal hypoxia response element abolishes trans-activation of the PAG1 gene mediated by HIF-independent chromatin looping. Nucleic Acids Res. 2015, 43, 5810–5823. [Google Scholar] [CrossRef]
- Lando, D.; Peet, D.J.; Whelan, D.A.; Gorman, J.J.; Whitelaw, M.L. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 2002, 295, 858–861. [Google Scholar] [CrossRef]
- McNeill, L.A.; Hewitson, K.S.; Claridge, T.D.; Seibel, J.F.; Horsfall, L.E.; Schofield, C.J. Hypoxia-inducible factor asparaginyl hydroxylase (FIH-1) catalyses hydroxylation at the beta-carbon of asparagine-803. Biochem. J. 2002, 367, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Volkova, Y.L.; Pickel, C.; Jucht, A.E.; Wenger, R.H.; Scholz, C.C. The Asparagine Hydroxylase FIH: A Unique Oxygen Sensor. Antioxid. Redox Signal. 2022, 37, 913–935. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Park, J.H.; Lee, E.J.; Vo, T.T.; Choi, H.; Jang, J.K.; Wee, H.J.; Ahn, B.J.; Cha, J.H.; Shin, M.W.; et al. Autoacetylation regulates differentially the roles of ARD1 variants in tumorigenesis. Int. J. Oncol. 2015, 46, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.K.; Donato, K.; Dhuli, K.; Stuppia, L.; Bertelli, M. Dietary supplements for polycystic ovary syndrome. J. Prev. Med. Hyg. 2022, 63, E206–E213. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.J.; Cho, Y.M.; Ham, E.; Cacioppo, J.A.; Park, C.J. Endothelin 2: A key player in ovulation and fertility. Reproduction 2022, 163, R71–R80. [Google Scholar] [CrossRef]
- Cacioppo, J.A.; Oh, S.W.; Kim, H.Y.; Cho, J.; Lin, P.C.; Yanagisawa, M.; Ko, C. Loss of function of endothelin-2 leads to reduced ovulation and CL formation. PLoS ONE 2014, 9, e96115. [Google Scholar] [CrossRef]
- Cacioppo, J.A.; Lin, P.P.; Hannon, P.R.; McDougle, D.R.; Gal, A.; Ko, C. Granulosa cell endothelin-2 expression is fundamental for ovulatory follicle rupture. Sci. Rep. 2017, 7, 817. [Google Scholar] [CrossRef]
- Meidan, R.; Klipper, E.; Zalman, Y.; Yalu, R. The role of hypoxia-induced genes in ovarian angiogenesis. Reprod. Fertil. Dev. 2013, 25, 343–350. [Google Scholar] [CrossRef]
- Pagan, Y.L.; Srouji, S.S.; Jimenez, Y.; Emerson, A.; Gill, S.; Hall, J.E. Inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome: Investigation of hypothalamic and pituitary contributions. J. Clin. Endocrinol. Metab. 2006, 91, 1309–1316. [Google Scholar] [CrossRef]
- Duncan, W.C. A guide to understanding polycystic ovary syndrome (PCOS). J. Fam. Plan. Reprod. Health Care 2014, 40, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.J.; Bates, G.W., Jr. Optimal management of subfertility in polycystic ovary syndrome. Int. J. Womens Health 2014, 6, 613–621. [Google Scholar] [CrossRef][Green Version]
- Baddela, V.S.; Sharma, A.; Michaelis, M.; Vanselow, J. HIF1 driven transcriptional activity regulates steroidogenesis and proliferation of bovine granulosa cells. Sci. Rep. 2020, 10, 3906. [Google Scholar] [CrossRef]
- Kowalewski, M.P.; Gram, A.; Boos, A. The role of hypoxia and HIF1alpha in the regulation of STAR-mediated steroidogenesis in granulosa cells. Mol. Cell. Endocrinol. 2015, 401, 35–44. [Google Scholar] [CrossRef]
- Fadhillah; Yoshioka, S.; Nishimura, R.; Yamamoto, Y.; Kimura, K.; Okuda, K. Hypoxia-inducible factor 1 mediates hypoxia-enhanced synthesis of progesterone during luteinization of granulosa cells. J. Reprod. Dev. 2017, 63, 75–85. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Tanihara, F.; Do, L.; Sato, Y.; Taniguchi, M.; Takagi, M.; Van Nguyen, T.; Otoi, T. Chlorogenic acid supplementation during in vitro maturation improves maturation, fertilization and developmental competence of porcine oocytes. Reprod. Domest. Anim. 2017, 52, 969–975. [Google Scholar] [CrossRef]
- Glueck, C.J.; Goldenberg, N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism 2019, 92, 108–120. [Google Scholar] [CrossRef]
- Przybycien, P.; Gasior-Perczak, D.; Placha, W. Cannabinoids and PPAR Ligands: The Future in Treatment of Polycystic Ovary Syndrome Women with Obesity and Reduced Fertility. Cells 2022, 11, 2569. [Google Scholar] [CrossRef]
- Dang, E.V.; Barbi, J.; Yang, H.Y.; Jinasena, D.; Yu, H.; Zheng, Y.; Bordman, Z.; Fu, J.; Kim, Y.; Yen, H.R.; et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 2011, 146, 772–784. [Google Scholar] [CrossRef]
- Kopf, M.; Bachmann, M.F.; Marsland, B.J. Averting inflammation by targeting the cytokine environment. Nat. Rev. Drug. Discov. 2010, 9, 703–718. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Furuta, G.T.; Turner, J.R.; Taylor, C.T.; Hershberg, R.M.; Comerford, K.; Narravula, S.; Podolsky, D.K.; Colgan, S.P. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Exp. Med. 2001, 193, 1027–1034. [Google Scholar] [CrossRef]
- Higgins, D.F.; Kimura, K.; Bernhardt, W.M.; Shrimanker, N.; Akai, Y.; Hohenstein, B.; Saito, Y.; Johnson, R.S.; Kretzler, M.; Cohen, C.D.; et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Investig. 2007, 117, 3810–3820. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Keely, S.; Karhausen, J.; Gerich, M.E.; Furuta, G.T.; Colgan, S.P. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 2008, 134, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Keely, S.; Campbell, E.L.; Baird, A.W.; Hansbro, P.M.; Shalwitz, R.A.; Kotsakis, A.; McNamee, E.N.; Eltzschig, H.K.; Kominsky, D.J.; Colgan, S.P. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol. 2014, 7, 114–123. [Google Scholar] [CrossRef]
- Marks, E.; Goggins, B.J.; Cardona, J.; Cole, S.; Minahan, K.; Mateer, S.; Walker, M.M.; Shalwitz, R.; Keely, S. Oral delivery of prolyl hydroxylase inhibitor: AKB-4924 promotes localized mucosal healing in a mouse model of colitis. Inflamm. Bowel Dis. 2015, 21, 267–275. [Google Scholar] [CrossRef]
- Clark, R.A. Cutaneous tissue repair: Basic biologic considerations. I. J. Am. Acad. Dermatol. 1985, 13, 701–725. [Google Scholar] [CrossRef]
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 1998, 176, 26S–38S. [Google Scholar] [CrossRef]
- Nantel, F.; Denis, D.; Gordon, R.; Northey, A.; Cirino, M.; Metters, K.M.; Chan, C.C. Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation. Br. J. Pharmacol. 1999, 128, 853–859. [Google Scholar] [CrossRef]
- Vitiello, M.; Galdiero, M.; Finamore, E.; Galdiero, S.; Galdiero, M. NF-kappaB as a potential therapeutic target in microbial diseases. Mol. Biosyst. 2012, 8, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Jung, Y.R.; Kim, D.H.; An, H.J.; Kim, M.K.; Kim, N.D.; Chung, H.Y. Caffeic acid regulates LPS-induced NF-kappaB activation through NIK/IKK and c-Src/ERK signaling pathways in endothelial cells. Arch. Pharm. Res. 2014, 37, 539–547. [Google Scholar] [CrossRef]
- Jantan, I.; Ahmad, W.; Bukhari, S.N. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant. Sci. 2015, 6, 655. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Etoz, B.C.; Gul, Z.; Ozyigit, M.O.; Cinkilic, N.; Inan, S.; Buyukcoskun, N.I.; Ozluk, K.; Gurun, M.S. Chlorogenic Acid Enhances Abdominal Skin Flap Survival Based on Epigastric Artery in Nondiabetic and Diabetic Rats. Ann. Plast. Surg. 2016, 77, e21–e25. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Gul, Z.; Meade, J.A.; Cam, B.; Cinkilic, N.; Gurun, M.S. Pharmacologic Overview of Chlorogenic Acid and its Metabolites in Chronic Pain and Inflammation. Curr. Neuropharmacol. 2020, 18, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, D.; Tang, X.; Li, X.; Wo, D.; Yan, H.; Song, R.; Feng, J.; Li, P.; Zhang, J.; et al. Chlorogenic acid prevents isoproterenol-induced hypertrophy in neonatal rat myocytes. Toxicol. Lett. 2014, 226, 257–263. [Google Scholar] [CrossRef]
- Hebeda, C.B.; Bolonheis, S.M.; Nakasato, A.; Belinati, K.; Souza, P.D.; Gouvea, D.R.; Lopes, N.P.; Farsky, S.H. Effects of chlorogenic acid on neutrophil locomotion functions in response to inflammatory stimulus. J. Ethnopharmacol. 2011, 135, 261–269. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Fuentes, E.; Caballero, J.; Alarcon, M.; Rojas, A.; Palomo, I. Chlorogenic acid inhibits human platelet activation and thrombus formation. PLoS ONE 2014, 9, e90699. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic Acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement. Altern. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef]
- Mubarak, A.; Hodgson, J.M.; Considine, M.J.; Croft, K.D.; Matthews, V.B. Supplementation of a high-fat diet with chlorogenic acid is associated with insulin resistance and hepatic lipid accumulation in mice. J. Agric. Food Chem. 2013, 61, 4371–4378. [Google Scholar] [CrossRef]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-kB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef]
- Shi, H.; Shi, A.; Dong, L.; Lu, X.; Wang, Y.; Zhao, J.; Dai, F.; Guo, X. Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 2016, 35, 1366–1373. [Google Scholar] [CrossRef]
- Tsai, K.L.; Hung, C.H.; Chan, S.H.; Hsieh, P.L.; Ou, H.C.; Cheng, Y.H.; Chu, P.M. Chlorogenic Acid Protects Against oxLDL-Induced Oxidative Damage and Mitochondrial Dysfunction by Modulating SIRT1 in Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, e1700928. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Martin, M.A.; Izquierdo-Pulido, M.; Goya, L.; Bravo, L.; Ramos, S. Molecular mechanisms of (-)-epicatechin and chlorogenic acid on the regulation of the apoptotic and survival/proliferation pathways in a human hepatoma cell line. J. Agric. Food Chem. 2007, 55, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A. Reduced glutathione: A radioprotector or a modulator of DNA-repair activity? Nutrients 2013, 5, 525–542. [Google Scholar] [CrossRef]
- Bakuradze, T.; Boehm, N.; Janzowski, C.; Lang, R.; Hofmann, T.; Stockis, J.P.; Albert, F.W.; Stiebitz, H.; Bytof, G.; Lantz, I.; et al. Antioxidant-rich coffee reduces DNA damage, elevates glutathione status and contributes to weight control: Results from an intervention study. Mol. Nutr. Food Res. 2011, 55, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Kotyczka, C.; Boettler, U.; Lang, R.; Stiebitz, H.; Bytof, G.; Lantz, I.; Hofmann, T.; Marko, D.; Somoza, V. Dark roast coffee is more effective than light roast coffee in reducing body weight, and in restoring red blood cell vitamin E and glutathione concentrations in healthy volunteers. Mol. Nutr. Food Res. 2011, 55, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.Y.; Cosma, G.; Gardner, H.; Castranova, V.; Vallyathan, V. Effect of chlorogenic acid on hydroxyl radical. Mol. Cell. Biochem. 2003, 247, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Pan, J.H.; Kim, S.H.; Lee, J.H.; Park, J.W. Chlorogenic acid ameliorates alcohol-induced liver injuries through scavenging reactive oxygen species. Biochimie 2018, 150, 131–138. [Google Scholar] [CrossRef]
- Larki-Harchegani, A.; Fayazbakhsh, F.; Nourian, A.; Nili-Ahmadabadi, A. Chlorogenic acid protective effects on paraquat-induced pulmonary oxidative damage and fibrosis in rats. J. Biochem. Mol. Toxicol. 2023, e23352. [Google Scholar] [CrossRef]
- Moghetti, P. Insulin Resistance and Polycystic Ovary Syndrome. Curr. Pharm. Des. 2016, 22, 5526–5534. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Spritzer, P.M.; Sir-Petermann, T.; Motta, A.B. Insulin resistance and polycystic ovary syndrome through life. Curr. Pharm. Des. 2012, 18, 5569–5576. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, J.; Cheng, X.; Nie, X.; He, B. Insulin resistance in polycystic ovary syndrome across various tissues: An updated review of pathogenesis, evaluation, and treatment. J. Ovarian Res. 2023, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Wu, Y.; Zhang, L.; Yu, Y. Insulin resistance, autophagy and apoptosis in patients with polycystic ovary syndrome: Association with PI3K signaling pathway. Front. Endocrinol. 2022, 13, 1091147. [Google Scholar] [CrossRef] [PubMed]
- Makker, A.; Goel, M.M.; Das, V.; Agarwal, A. PI3K-Akt-mTOR and MAPK signaling pathways in polycystic ovarian syndrome, uterine leiomyomas and endometriosis: An update. Gynecol. Endocrinol. 2012, 28, 175–181. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, C.; Huang, Y.; Yu, Y.; Li, R.; Li, M.; Liu, N.; Liu, P.; Qiao, J. Up-regulated expression of WNT5a increases inflammation and oxidative stress via PI3K/AKT/NF-kappaB signaling in the granulosa cells of PCOS patients. J. Clin. Endocrinol. Metab. 2015, 100, 201–211. [Google Scholar] [CrossRef]
- Venkatesan, A.M.; Dunaif, A.; Corbould, A. Insulin resistance in polycystic ovary syndrome: Progress and paradoxes. Recent. Prog. Horm. Res. 2001, 56, 295–308. [Google Scholar] [CrossRef]
- Azziz, R. Polycystic ovary syndrome, insulin resistance, and molecular defects of insulin signaling. J. Clin. Endocrinol. Metab. 2002, 87, 4085–4087. [Google Scholar] [CrossRef][Green Version]
- Shah, K.N.; Patel, S.S. Phosphatidylinositide-3 kinase: A newer molecular target in metabolic and hormonal pathway of polycystic ovary syndrome. Exp. Clin. Endocrinol. Diabetes 2014, 122, 261–267. [Google Scholar] [CrossRef]
- Siddappa, D.; Kalaiselvanraja, A.; Bordignon, V.; Dupuis, L.; Gasperin, B.G.; Roux, P.P.; Duggavathi, R. Mechanistic target of rapamycin (MTOR) signaling during ovulation in mice. Mol. Reprod. Dev. 2014, 81, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Epstein, A.C.; Gleadle, J.M.; McNeill, L.A.; Hewitson, K.S.; O’Rourke, J.; Mole, D.R.; Mukherji, M.; Metzen, E.; Wilson, M.I.; Dhanda, A.; et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001, 107, 43–54. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. Physiological Effects of Chronic Hypoxia. N. Engl. J. Med. 2017, 376, 1965–1971. [Google Scholar] [CrossRef]
- Schofield, C.J.; Ratcliffe, P.J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell. Biol. 2004, 5, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, S.E.; Abboud, M.I.; Hancock, R.L.; Schofield, C.J. Targeting Protein-Protein Interactions in the HIF System. ChemMedChem 2016, 11, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Semenza, G.L. Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem. Biophys. Res. Commun. 2005, 338, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.A.; Glass, G.A.; Cunningham, J.M.; Bunn, H.F. The regulated expression of erythropoietin by two human hepatoma cell lines. Proc. Natl. Acad. Sci. USA 1987, 84, 7972–7976. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Dunning, S.P.; Bunn, H.F. Regulation of the erythropoietin gene: Evidence that the oxygen sensor is a heme protein. Science 1988, 242, 1412–1415. [Google Scholar] [CrossRef]
- Joharapurkar, A.A.; Pandya, V.B.; Patel, V.J.; Desai, R.C.; Jain, M.R. Prolyl Hydroxylase Inhibitors: A Breakthrough in the Therapy of Anemia Associated with Chronic Diseases. J. Med. Chem. 2018, 61, 6964–6982. [Google Scholar] [CrossRef]
- Yeh, T.L.; Leissing, T.M.; Abboud, M.I.; Thinnes, C.C.; Atasoylu, O.; Holt-Martyn, J.P.; Zhang, D.; Tumber, A.; Lippl, K.; Lohans, C.T.; et al. Molecular and cellular mechanisms of HIF prolyl hydroxylase inhibitors in clinical trials. Chem. Sci. 2017, 8, 7651–7668. [Google Scholar] [CrossRef]


| Items | Specific Descriptions |
|---|---|
| Diagnostic Criteria (Rotterdam 2003) | Oligo or anovulation |
| Hyperandrogenism | |
| Polycystic ovaries | |
| Diagnostic Criteria (AE-PCOS Society 2006) | Biochemical and clinical evidence of hyperandrogenism |
| Dysfunctional ovaries | |
| Polycystic ovary morphology | |
| Clinical Phenotype | Hyperandrogenism + Oligo-Anovulation + Polycystic ovaries |
| Hyperandrogenism + Oligo-Anovulation | |
| Hyperandrogenism + Polycystic ovaries | |
| Oligo-Anovulation + Polycystic ovaries | |
| Treatment Focus | Suppressing and counteracting androgen secretion and action |
| Protecting the endometrium and improving menstrual dysfunction | |
| Improving metabolic status | |
| Improving ovulatory fertility |
| Product | Chemical Name | Molecular Formula | Canonical SMILES | Molecular Weight | Molecular Structure |
|---|---|---|---|---|---|
| Daprodustat | 2-(1,3-dicyclohexyl-2,4,6-trioxohexahydropyrimidine-5-carboxamido)acetic acid | C19H27N3O6 | O=C(O)CNC(C(C(N1C2CCCCC2)=O)C(N(C1=O)C3CCCCC3)=O)=O | 393.43 | 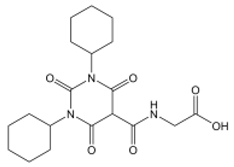 |
| Enarodustat | N-[7-Hydroxy-5-(2-phenylethyl)[1,2,4]triazolo[1,5-a]pyridine-8-carbonyl]glycine | C17H16N4O4 | O=C(C(C1=NC=NN1C(CCC2=CC=CC=C2)=C3)=C3O)NCC(O)=O | 340.33 |  |
| Roxadustat | 2-[(4-hydroxy-1-methyl-7-phenoxyisoquinoline-3-carbonyl)amino]acetic acid | C19H16N2O5 | CC1=NC(=C(C2=C1C=C(C=C2)OC3=CC=CC=C3)O)C(=O)NCC(=O)O | 352.34 | 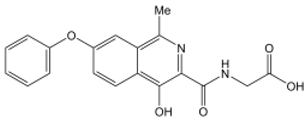 |
| Vadadustat | 2-(5-(3-chlorophenyl)-3-hydroxypicolinamido)acetic acid | C14H11ClN2O4 | OC1=CC(C2=CC=CC(Cl)=C2)=CN=C1C(NCC(O)=O)=O | 306.70 | 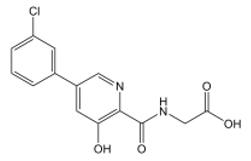 |
| Molidustat | 2-(6-morpholinopyrimidin-4-yl)-4-(1H-1,2,3-triazol-1-yl)-1H-pyrazol-3(2H)-one | C13H14N8O2 | O=C1C(N2C=CN=N2)=CNN1C3=NC=NC(N4CCOCC4)=C3 | 314.30 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Shi, C.; Wang, Z. Therapeutic Effects and Molecular Mechanism of Chlorogenic Acid on Polycystic Ovarian Syndrome: Role of HIF-1alpha. Nutrients 2023, 15, 2833. https://doi.org/10.3390/nu15132833
Zhang Z, Shi C, Wang Z. Therapeutic Effects and Molecular Mechanism of Chlorogenic Acid on Polycystic Ovarian Syndrome: Role of HIF-1alpha. Nutrients. 2023; 15(13):2833. https://doi.org/10.3390/nu15132833
Chicago/Turabian StyleZhang, Zhenghong, Congjian Shi, and Zhengchao Wang. 2023. "Therapeutic Effects and Molecular Mechanism of Chlorogenic Acid on Polycystic Ovarian Syndrome: Role of HIF-1alpha" Nutrients 15, no. 13: 2833. https://doi.org/10.3390/nu15132833
APA StyleZhang, Z., Shi, C., & Wang, Z. (2023). Therapeutic Effects and Molecular Mechanism of Chlorogenic Acid on Polycystic Ovarian Syndrome: Role of HIF-1alpha. Nutrients, 15(13), 2833. https://doi.org/10.3390/nu15132833







