Risk Factors Related to Eating Disorders in a Romanian Children Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Procedures
2.2. Measures
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Age and Duration of Symptom Influencing Risk of ARFID
3.3. Eating Patterns
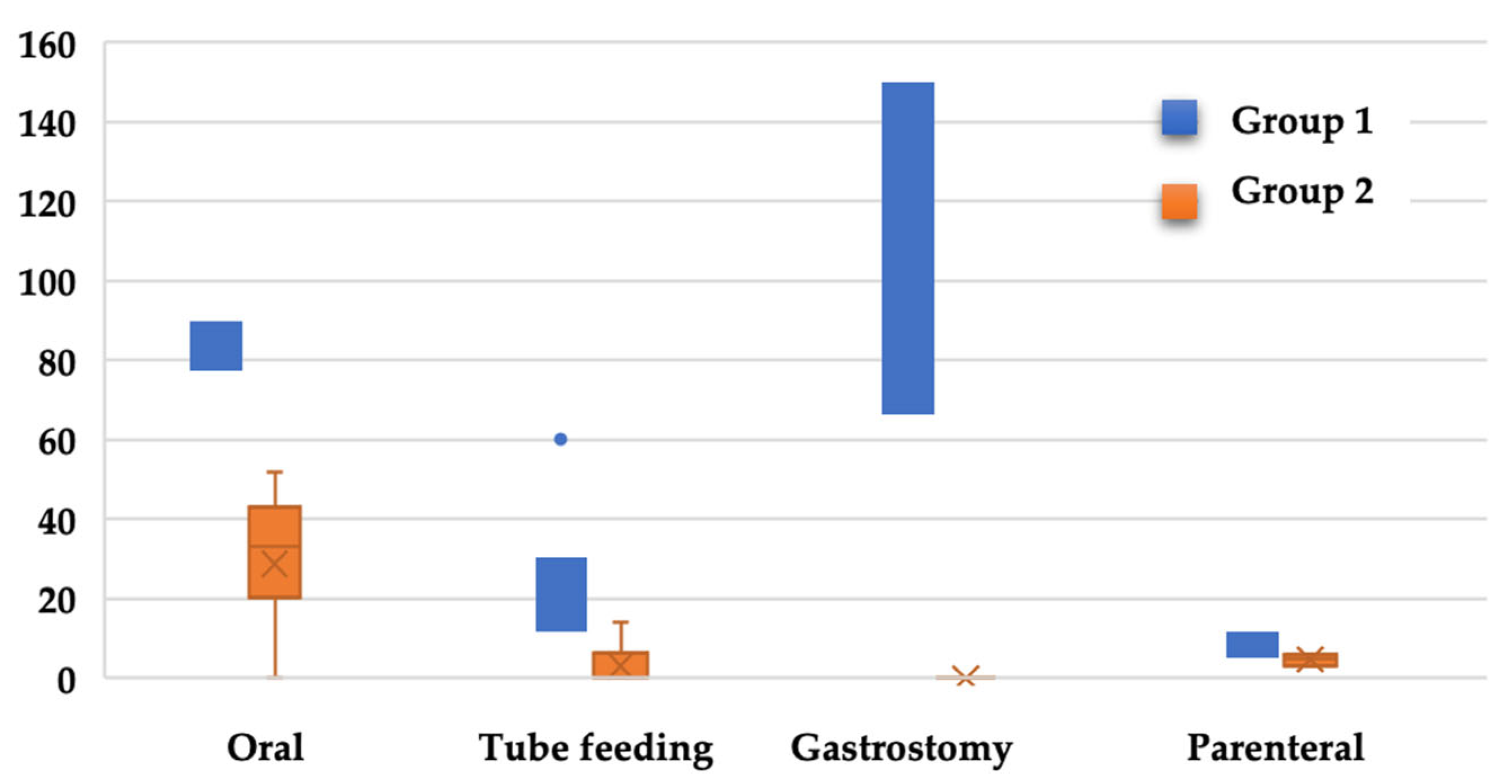
3.4. Nutritional Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grubb, L.K. Avoidant Restrictive Food Intake Disorder-What Are We Missing? What Are We Waiting for? JAMA Pediatr. 2021, 1, e213858. [Google Scholar] [CrossRef] [PubMed]
- Claudino, A.M.; Pike, K.M.; Hay, P.; Keeley, J.W.; Evans, S.C.; Rebello, T.J.; Bryant-Waugh, R.; Dai, Y.; Zhao, M.; Matsumoto, C.; et al. The classification of feeding and eating disorders in the ICD-11: Results of a field study comparing proposed ICD-11 guidelines with existing ICD-10 guidelines. BMC Med. 2019, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Katzman, D.; Stevens, K. Redefining feeding and eating disorders: What is avoidant/restrictive food intake disorder? Paediatr. Child Health 2014, 19, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Dovey, T.M.; Kumari, V.; Blissett, J. Eating behaviour, behavioural problems and sensory pro fi les of children with avoidant/restrictive food intake disorder (ARFID), autistic spectrum disorders or picky eating: Same or different? Eur. Psychiatry 2019, 61, 56–62. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Fisher, M.M.; Rosen, D.S.; Ornstein, R. Characteristics of avoidant/restrictive food intake disorder in children and adolescents: A “new disorder” in DSM-5. J. Adolesc. Health 2014, 55, 49–52. [Google Scholar] [CrossRef]
- Nicely, T.A.; Lane-Loney, S.; Masciulli, E. Prevalence and characteristics of avoidant/restrictive food intake disorder in a cohort of young patients in day treatment for eating disorders. J. Eat. Disord. 2014, 2, 21–25. [Google Scholar] [CrossRef]
- Ellis, J.M.; Galloway, A.T.; Webb, R.M. Recollections of pressure to eat during childhood, but not picky eating, predict young adult eating behavior. Appetite 2016, 97, 58–63. [Google Scholar] [CrossRef]
- Chatoor, I.; Hommel, S.; Sechi, C.; Lucrelli, L. Development of the parent—Child play scale for use in children with feeding disorders. Infant Ment. Health J. 2018, 39, 153–169. [Google Scholar] [CrossRef]
- Bąbik, K.; Rybak, A. Trudności w Karmieniu u Dzieci. (w:) A. Horvath, Szajewska (red.) Żywienie i Leczenie Żywieniowe Dzieci i Młodzieży; PZWL: Kraków, Poland, 2017. [Google Scholar]
- Aviram, I.; Atzaba-Poria, N.; Pike, A.; Meiri, G.; Yerushalmi, B. Mealtime Dynamics in Child Feeding Disorder: The Role of Child Temperament, Parental Sense of Competence, and Paternal Involvement. J. Pediatr. Psychol. 2015, 40, 45–54. [Google Scholar] [CrossRef]
- Lucarelli, L.; Ammaniti, M.; Porreca, A.; Simonelli, A. Infantile Anorexia and Co-parenting: A Pilot Study on Mother–Father–Child Triadic Interactions during Feeding and Play. Front. Psychol. 2017, 8, 259. [Google Scholar] [CrossRef]
- Råstam, M.; Täljemark, J.; Tajnia, A.; Lundström, S.; Gustafsson, P.; Lichtenstein, P.; Gillberg, C.; Anckarsäter, H.; Kerekes, N. Eating problems and overlap with ADHD and autism spectrum disorders in a nationwide twin study of 9- and 12-year-old children. Sci. World J. 2013, 15, 315429. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, S.E.; Zucker, N.L.; Gerke, C.K.; Mitchell, K.S.; Bulik, C.M. Parenting concerns of women with histories of eating disorders. Int. J. Eat. Disord. 2005, 37, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Bryant-Waugh, R.; Micali, N.; Cooke, L.; Lawson, E.A.; Eddy, K.T.; Thomas, J.J. Development of the Pica, ARFID, and Rumination Disorder Interview, a multi-informant, semi-structured interview of feeding disorders across the lifespan: A pilot study for ages 10-22. Int. J. Eat Disord. 2019, 52, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Katzman, D.K.; Norris, M.L.; Zucker, N. Avoidant Restrictive Food Intake Disorder. Psychiatr. Clin. N. Am. 2019, 42, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Zucker, N.L.; LaVia, M.C.; Craske, M.G.; Foukal, M.; Harris, A.A.; Datta, N.; Savereide, E.; Maslow, G.R. Feeling and body investigators (FBI): ARFID division-Anacceptance-based interoceptive exposure treatment for children with ARFID. Int. J. Eat. Disord. 2019, 52, 466–472. [Google Scholar] [CrossRef]
- Pinhas, L.; Morris, A.; Crosby, R.D.; Katzman, D.K. Incidence and age-specific presentation of restrictive eating disorders in children: A Canadian paediatric surveillance program study. Arch. Pediatr. Adolesc. Med. 2011, 165, 895–899. [Google Scholar] [CrossRef]
- Madden, S.; Morris, A.; Zurynski, Y.A.; Kohn, M.; Elliot, E.J. Burden of eating disorders in 5-13-year-old children in Australia. Med. J. Aust. 2009, 190, 410–414. [Google Scholar] [CrossRef]
- Bryant-Waugh, R. Avoidant restrictive food intake disorder: An illustrative case example. Int. J. Eat. Disord. 2013, 46, 420–423. [Google Scholar] [CrossRef]
- Haycraft, E.; Farrow, C.; Meyer, C.; Powell, F.; Blissett, J. Relationships between temperament and eating behaviours in young children. Appetite 2011, 53, 689–692. [Google Scholar] [CrossRef]
- Fichter, M.M.; Quadflieg, N.; Crosby, R.D.; Koch, S. Long-Term Outcome of Anorexia Nervosa: Results from a Large Clinical Longitudinal Study. Int. J. Eat. Disord. 2017, 50, 1018–1030. [Google Scholar] [CrossRef]
- Brown, A.; Lee, M.D. Early influences on child satiety-responsiveness: The role of weaning style. Pediatr. Obes. 2013, 10, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Keery, H.; LeMay-Russell, S.; Barnes, T.L.; Eckhardt, S.; Peterson, C.B.; Lesser, J.; Gorrell, S.; Le Grange, D. Attributes of children and adolescents with avoidant/restrictive food intake disorder. J. Eat. Disord. 2019, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.M.; Barnett, J.A.; Gibson, D.L. A critical analysis of eating disorders and the gut microbiome. J. Eat. Disord. 2022, 10, 154. [Google Scholar] [CrossRef]
- Becheanu, C.A.; Ţincu, I.F.; Smădeanu, R.E.; Coman, O.A.; Coman, L.; Ţincu, R.C.; Păcurar, D. Benefits of oligofructose and inuline in management of functional diarrhoea in children—Interventional study. Farmacia 2019, 67, 511–516. [Google Scholar] [CrossRef]
- Ţincu, I.F.; Păcurar, D.; Ţincu, R.C.; Becheanu, C. Influence of Protein Intake during Complementary Feeding on Body Size and IGF-I Levels in Twelve-month-old Infants. Balk. Med. J. 2019, 37, 54–55. [Google Scholar] [CrossRef]
- Koelman, L.; Markova, M.; Seebeck, N.; Hornemann, S.; Rosenthal, A.; Lange, V.; Pivovarova-Ramich, O.; Aleksandrova, K. Effects of High and Low Protein Diets on Inflammatory Profiles in People with Morbid Obesity: A 3-Week Intervention Study. Nutrients 2020, 12, 3636. [Google Scholar] [CrossRef]
- Pǎcurar, D.; Leşanu, G.; Dijm, I.; Tincu, I.F.; Gherghiceanu, M.; Oraseanu, D. Genetic disorder in carbohydrates metabolism: Hereditary fructose intolerance associated with celiac disease. Rom. J. Morphol. Embryol. 2017, 58, 1109–1113. [Google Scholar]
- Mukkada, V.A.; Haas, A.; Maune, N.C.; Capocelli, K.E.; Henry, M.; Gilman, N.; Petersburg, S.; Moore, W.; Lovell, M.A.; Fleischer, D.M.; et al. Feeding dysfunction in children with eosinophilic gastrointestinal diseases. Pediatrics 2010, 126, e672–e677. [Google Scholar] [CrossRef]
- Thomas, J.J.; Lawson, E.A.; Micali, N.; Misra, M.; Deckersbach, T.; Eddy, K.T. Avoidant/Restrictive Food Intake Disorder: A Three-Dimensional Model of Neurobiology with Implications for Etiology and Treatment. Curr. Psychiatry Rep. 2017, 19, 54. [Google Scholar] [CrossRef]
- Herpertz-Dahlmann, B. Treatment of eating disorders in child and adolescent psychiatry. Curr. Opin. Psychiatry 2017, 30, 438–445. [Google Scholar] [CrossRef]
- Fraker, C.; Fishbein, M.; Walbert, L.; Cox, S. Food Chaining: The Proven 6-Step Plan to Stop Picky Eating, Solve Feeding Problems, and Expand Your Child’s Diet; Da Capo Press: Boston, MA, USA, 2007; Volume 1. [Google Scholar]
- Rosania, K.; Lock, J. Family-Based Treatment for a Preadolescent with Avoidant/Restrictive Food Intake Disorder with Sensory Sensitivity: A Case Report. Front. Psychiatry 2020, 11, 350. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Group 1 | Group 2 | p-Value |
|---|---|---|---|
| Area (U/R), n | 33/16 | 40/9 | 0.105 |
| Sex (M/F), n | 22/27 | 20/29 | 0.683 |
| Age (years) * | 6.91 ± 3.25 | 7.49 ± 2.91 | 0.448 |
| Gestational period (normal/pathologic), n | 37/12 | 41/8 | 0.316 |
| Maternal age (years) * | 29.43 ± 3.81 | 27.03 ± 3.04 | <0.001 |
| Father’s age (years) * | 34.09 ± 5.77 | 31.88 ± 3.94 | 0.004 |
| Mother’s level of education, n (%) | |||
| None/Primary | 8 (16.32) | 6 (12.24) | 0.383 |
| Secondary/College | 14 (28.57) | 14 (28.57) | 0.548 |
| Higher | 27 (55.1) | 29 (59.18) | 0.453 |
| Living standard, n (%) | |||
| Low | 11 (22.44) | 10 (20.4) | 0.562 |
| Medium | 23 (46.93) | 28 (57.14) | 0.312 |
| High | 15 (30.61) | 11 (22.44) | 0.432 |
| Marital status, n (%) | |||
| Married/Cohabiting | 13 (26.53) | 25 (51.02) | 0.001 |
| Single/Divorced | 36 (73.46) | 24 (48.97) | 0.001 |
| Birth weight (g) * | 3095.2 ± 478.77 | 3163.67 ± 360.83 | 0.213 |
| BMI Score Z, n (%) | |||
| −1 to +1 | 29 (59.18) | 47 (95.91) | 0.005 |
| −1 to −2 | 16 (32.65) | 2 (4.08) | 0.001 |
| Less than −2 | 6 (12.24) | 0 (0) | 0.001 |
| Nutrition in the first 6 months (BF/FF/MF), n | 18/25/6 | 22/14/13 | 0.005 |
| Successful weaning, n (%) | 28 (57.14) | 45 (91.83) | 0.001 |
| Regular feeding, n (%) | 20 (40.81) | 33 (67.34) | 0.005 |
| Food allergy, n (%) | 44 (89.79) | 26 (53.06) | <0.001 |
| All N = 49 | Age a < 5 Years N = 23 (46.93%) | Age a > 5 Years N = 25 (51.02%) | pb | c Symptom Onset < 12 Months N = 28 (57.14%) | c Symptom Onset > 12 Months N = 21 (42.85%) | pb |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years | 3.6 ± 2.65 | 10.2 ± 2.60 | <0.001 | 6.25 ± 3.65 | 4.24 ± 2.29 | 0.005 |
| Gender, n (%) | ||||||
| Male | 10 (20.40) | 11 (22.44) | 0.567 | 9 (18.36) | 13 (26.53) | 0.005 |
| Female | 13 (26.53) | 14 (28.57) | 0.645 | 19 (38.77) | 8 (16.32) | 0.005 |
| Allergy history, n (%) | 33 (75) | 11 (25) | <0.001 | 25 (56.81) | 19 (43.18) | 0.253 |
| PARDI a | ||||||
| Sensory profile | 6.2 ± 1.4 | 4.4 ± 1.3 | <0.001 | 6.2 ± 3.1 | 7.4 ± 3.2 | <0.001 |
| Lack of interest profile | 5.4 ± 3.4 | 4.6 ± 1.6 | <0.001 | 5.4 ± 2.1 | 8.4 ± 3.5 | <0.001 |
| Concern profile | 5.8 ± 2.3 | 4.4 ± 1.8 | <0.001 | 5.4 ± 2.3 | 7.2 ± 3.5 | <0.001 |
| ARFID severity scale | 6.4 ± 2.5 | 5.6 ± 2.4 | 0.05 | 4.2 ± 1.2 | 6.8 ± 2.6 | <0.001 |
| Nutritional status a | ||||||
| Kilograms | 15.22 ± 1.16 | 18.16 ± 2.13 | 0.001 | 19.33 ± 2.66 | 16.55 ± 1.22 | 0.005 |
| BMI | 13.11 ± 1.44 | 16.36 ± 2.65 | 0.001 | 15.44 ± 1.22 | 14.33 ± 1.33 | 0.256 |
| BMI Z score | −1.94 ± 0.33 | −1.10 ± 0.88 | 0.05 | −1.22 ± 0.23 | −1.88 ± 0.78 | 0.05 |
| Group 1 | Group 2 | Statistics | p-Value | |
|---|---|---|---|---|
| Number of meals, n (%) | ||||
| 3 | 28 (57.14%) | 6 (12.24%) | χ2 = 22.550 | 0.001 |
| 4 | 4 (8.16%) | 16 (12.24%) | ||
| 5 | 2 (4.08%) | 20 (40.81%) | ||
| At least 3 meals per day | ||||
| Yes | 35 (71.42%) | 42 (85.71%) | χ2 = 2.618 | 0.077 |
| No | 14 (28.57%) | 7 (14.28%) | ||
| Breakfast | ||||
| Yes | 41 (83.67%) | 47 (95.91%) | χ2 = 2.127 | 0.462 |
| No | 8 (16.32%) | 2 (4.08%) | ||
| Special diet restriction | ||||
| Yes | 35 (71.42%) | 40 (81.63%) | χ2 = 38.376 | 0.001 |
| No | 14 (28.57%) | 9 (18.36%) | ||
| Exercise | ||||
| Yes | 22 (44.89%) | 19 (38.77%) | χ2 = 2.176 | 0.377 |
| No | 27 (55.10%) | 30 (61.22%) | ||
| Exercise frequency | ||||
| Everyday | 7 (14.28%) | 5 (10.20%) | χ2 = 2.544 | 0.314 |
| 1–2 times/week | 11 (22.44%) | 12 (24.48%) | ||
| 3 or more times/week | 4 (8.16%) | 2 (4.08%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciurez, B.-T.; Cobilinschi, O.-C.; Luca, A.-R.; Țincu, I.F.; Pleșca, D.A. Risk Factors Related to Eating Disorders in a Romanian Children Population. Nutrients 2023, 15, 2831. https://doi.org/10.3390/nu15132831
Ciurez B-T, Cobilinschi O-C, Luca A-R, Țincu IF, Pleșca DA. Risk Factors Related to Eating Disorders in a Romanian Children Population. Nutrients. 2023; 15(13):2831. https://doi.org/10.3390/nu15132831
Chicago/Turabian StyleCiurez, Bianca-Teodora, Oana-Claudia Cobilinschi, Anamaria-Renata Luca, Iulia Florentina Țincu, and Doina Anca Pleșca. 2023. "Risk Factors Related to Eating Disorders in a Romanian Children Population" Nutrients 15, no. 13: 2831. https://doi.org/10.3390/nu15132831
APA StyleCiurez, B.-T., Cobilinschi, O.-C., Luca, A.-R., Țincu, I. F., & Pleșca, D. A. (2023). Risk Factors Related to Eating Disorders in a Romanian Children Population. Nutrients, 15(13), 2831. https://doi.org/10.3390/nu15132831






