Dietary Supplementation of Ancientino Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composition of the Ancientino
- The ratio of xylan and glucomannan was 80%.
- The fructan and resistant starch ratio was 15%.
- Legume cotyledon fiber vitamin and potato fiber proportions were 5%.
2.3. Animal Model Establishment and Treatment
2.4. Disease Activity Index (DAI) Score
2.5. MPO Activity
2.6. Histological Analysis
2.7. Intestinal Permeability Analysis In Vivo
2.8. Determination of DAO, Endotoxin, and d-Lactate
2.9. Caco-2 Cell Culture and Administration
2.10. Immunofluorescence (IF)
2.11. Measurement of the Levels of Oxidative Stress Biomarkers
2.12. Cytokine Measurement
2.13. Real-Time Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
2.14. Western Blot (WB) Analysis
2.15. Statistical Analysis
3. Results
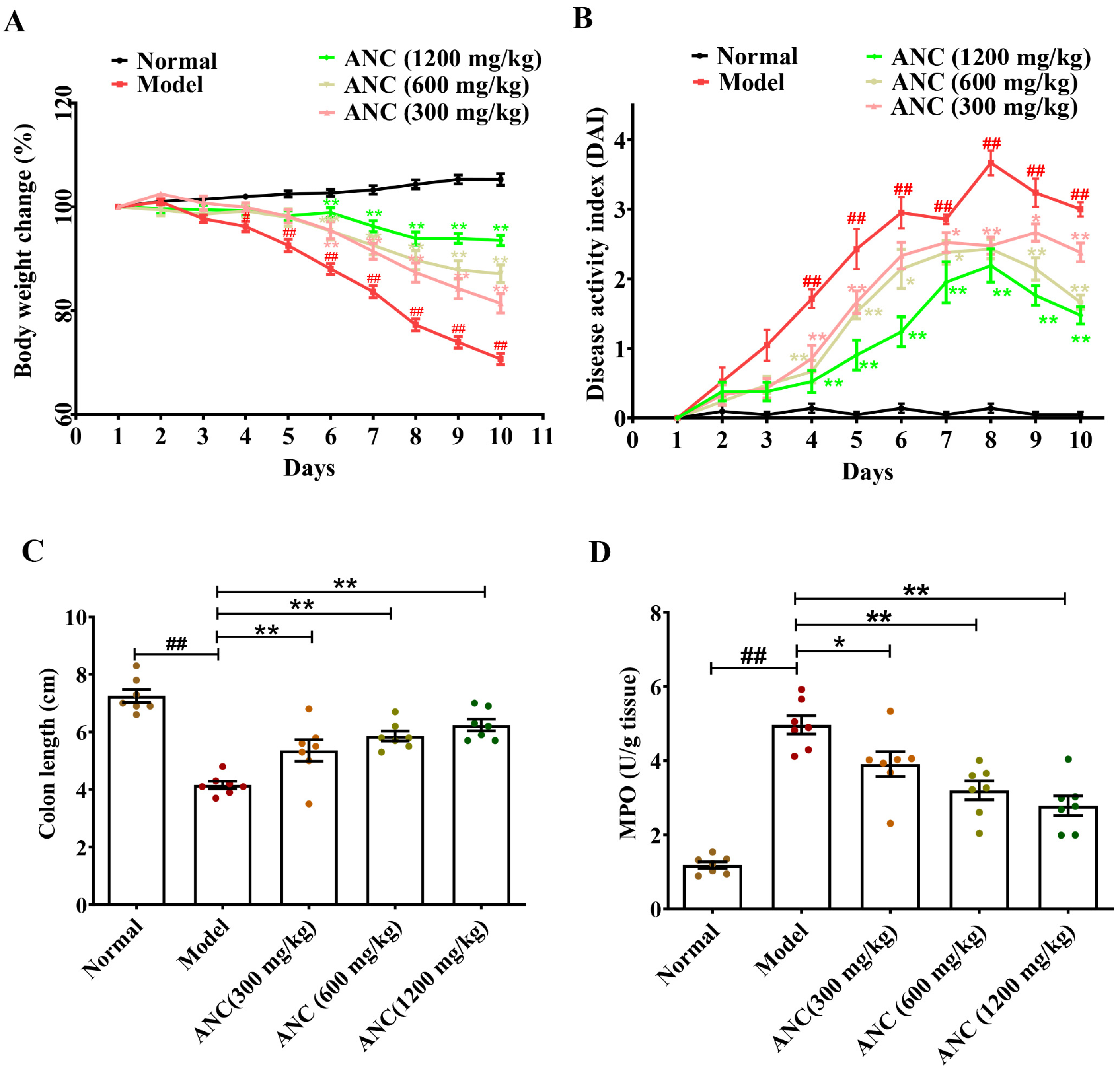
3.1. Ancientino Ameliorated the Symptoms of DSS-Induced Colitis in Mice
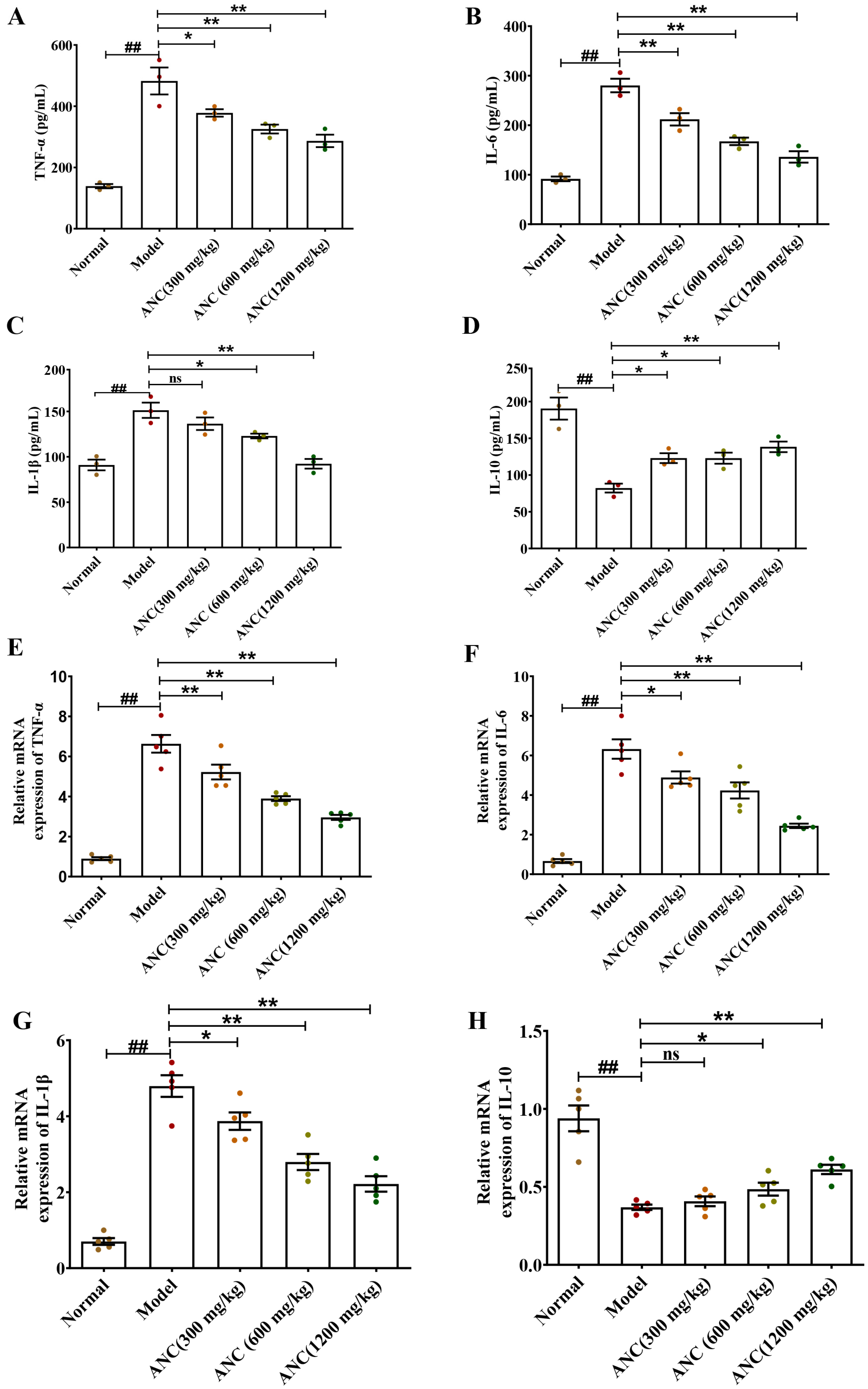
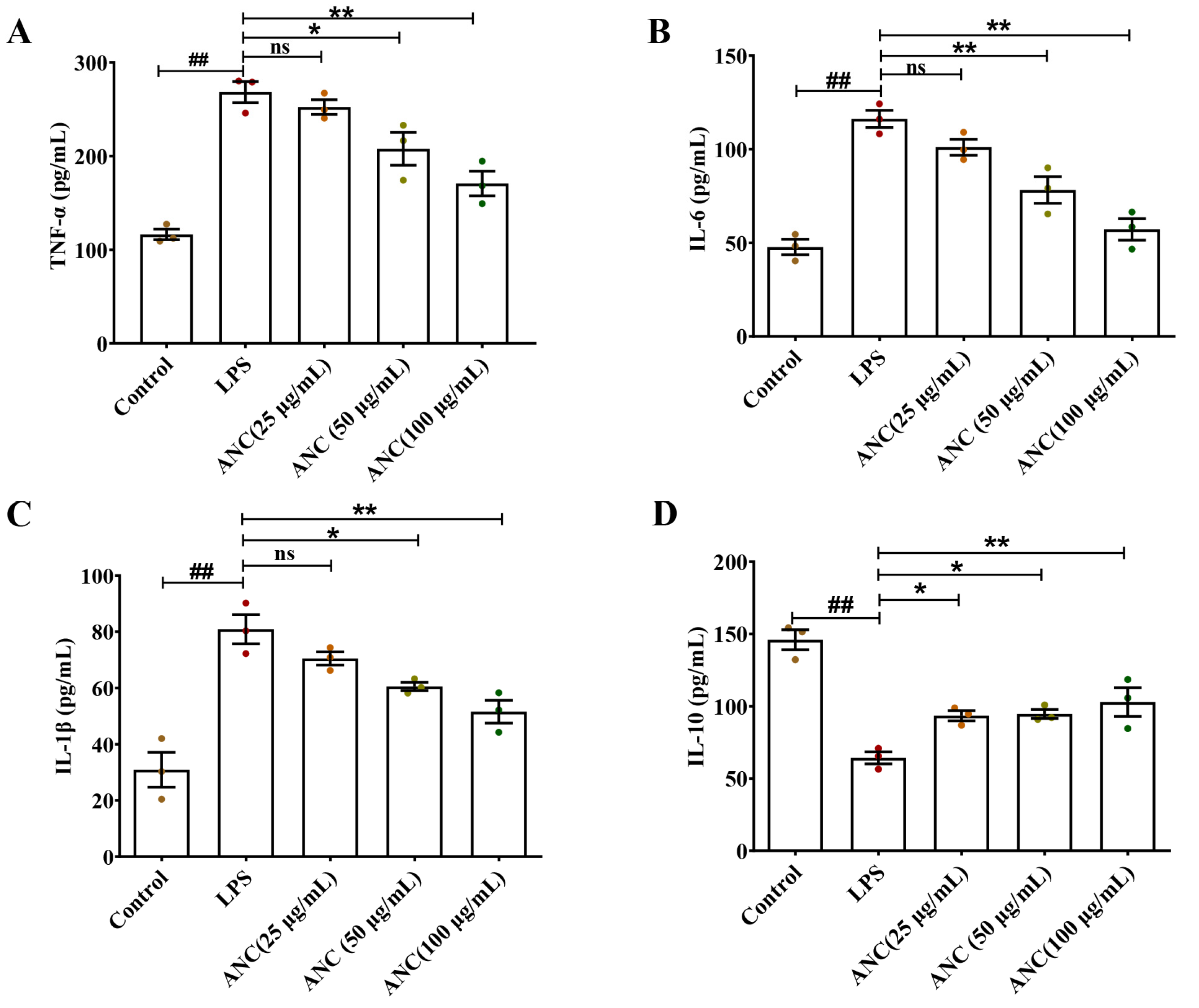
3.2. Ancientino Alleviated Colitis by Suppressing Inflammatory Response In Vivo
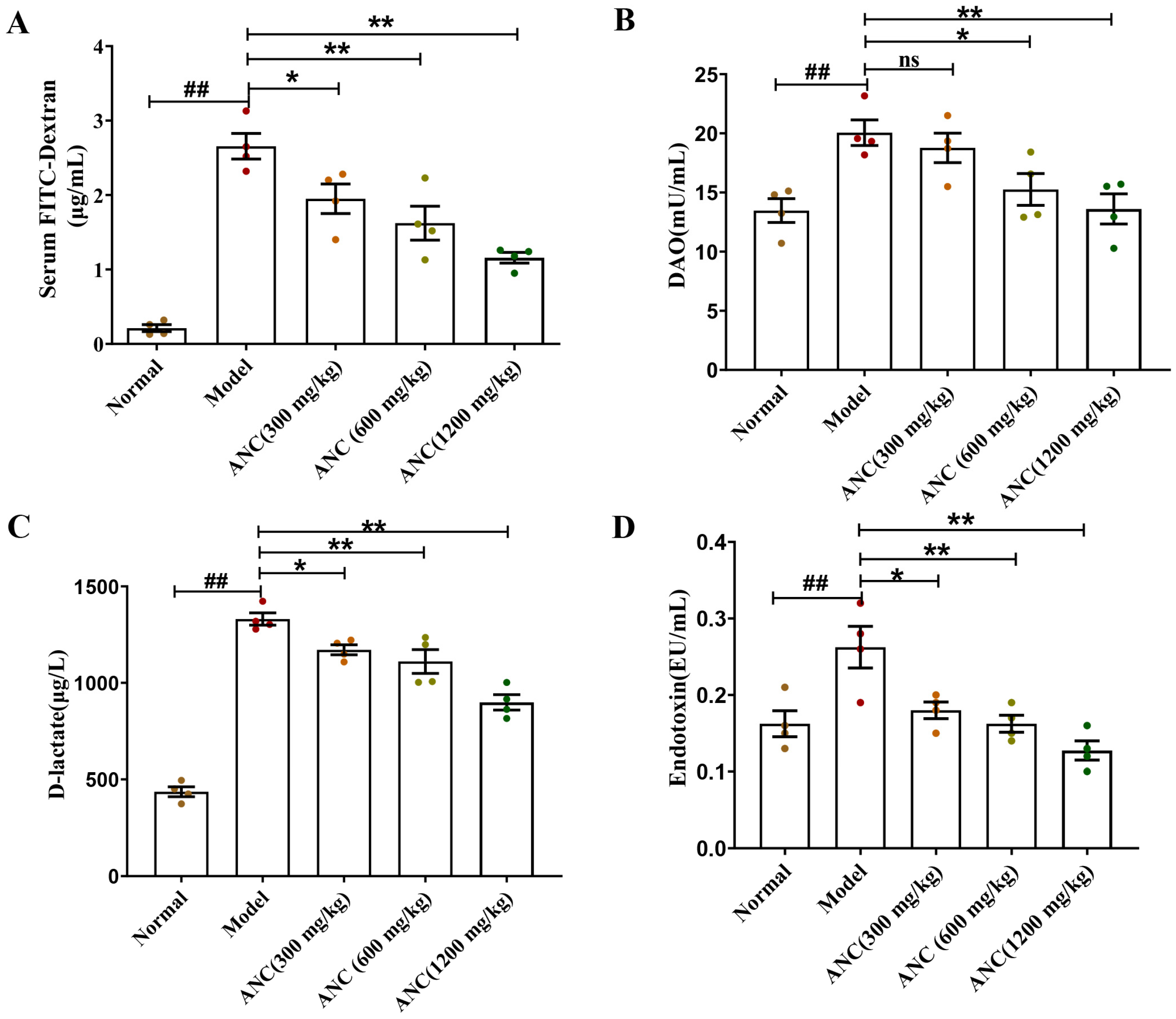
3.3. Ancientino Improved Colitis by Reducing Intestinal Permeability In Vivo
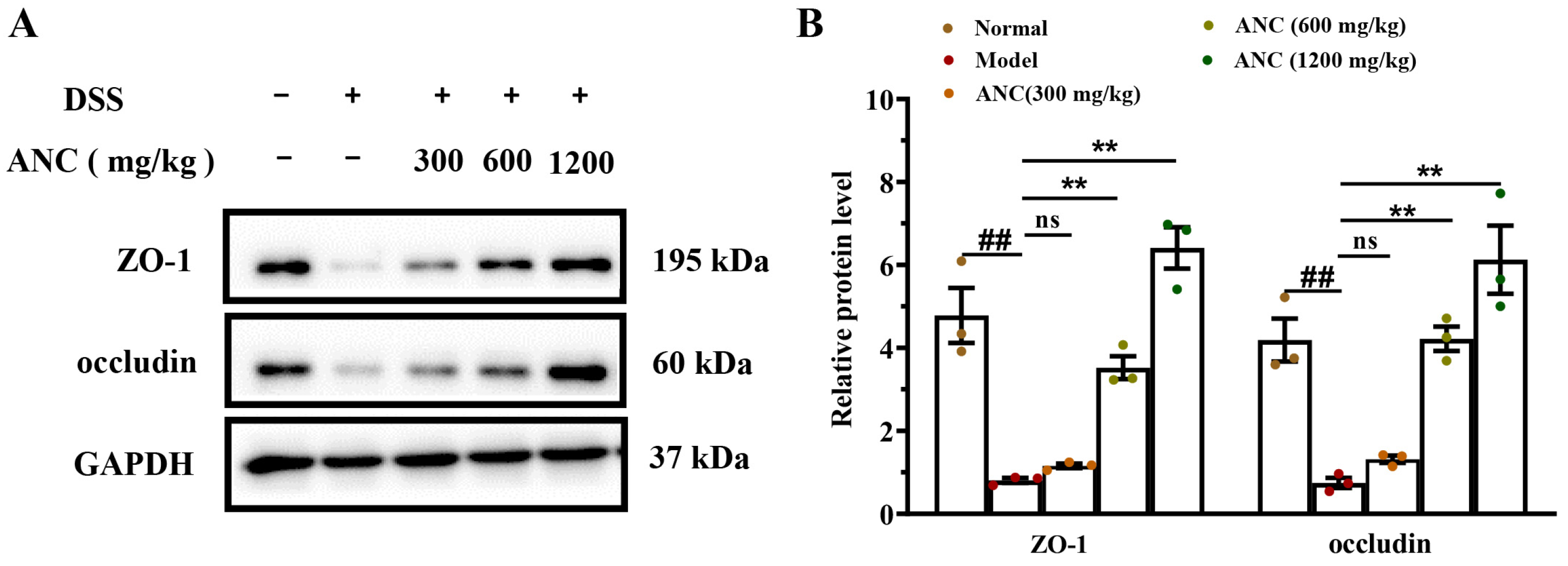
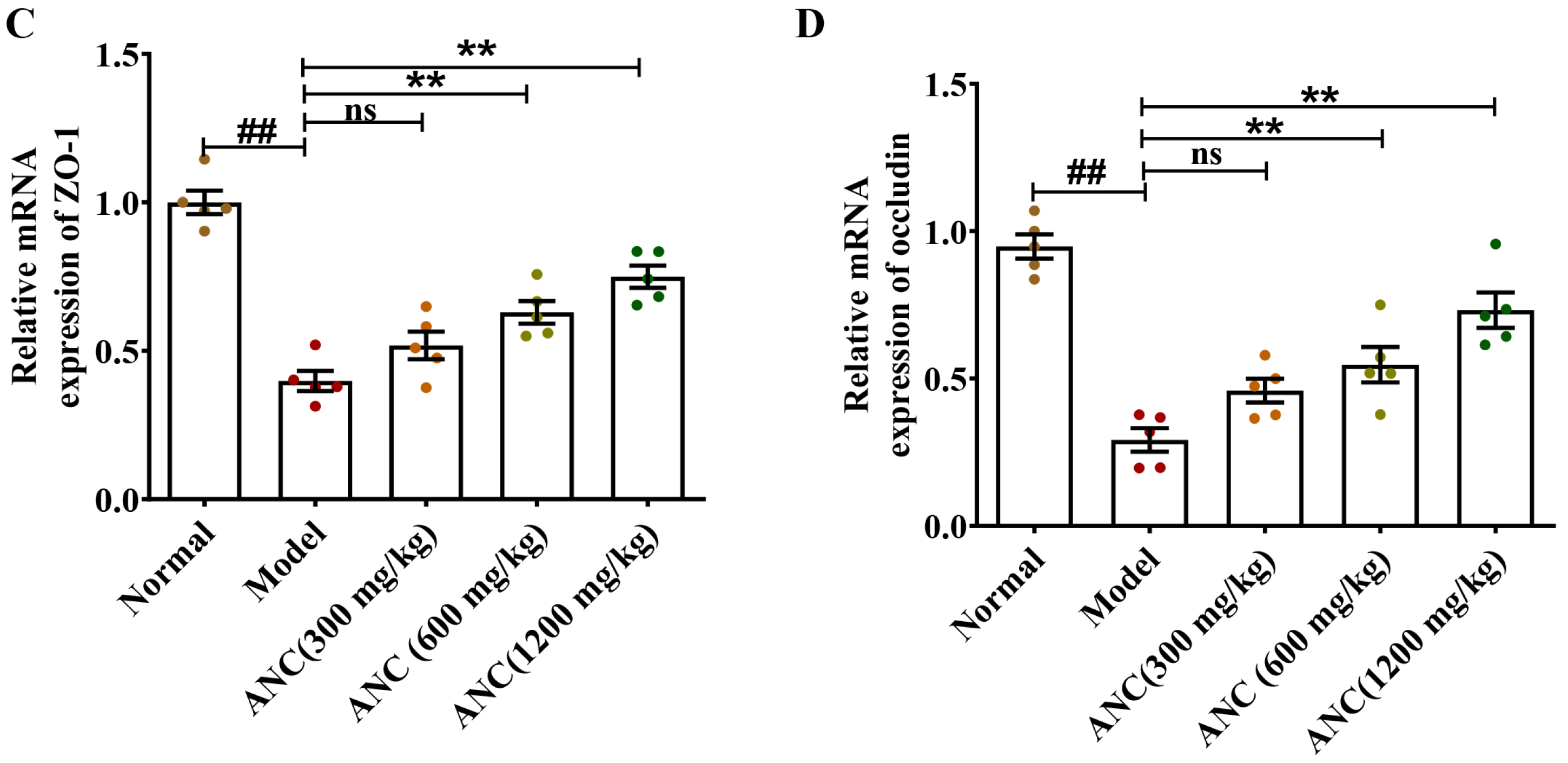
3.4. Ancientino Improved Colitis by Preserving the Expression of Tight Junction (TJ) Proteins In Vivo
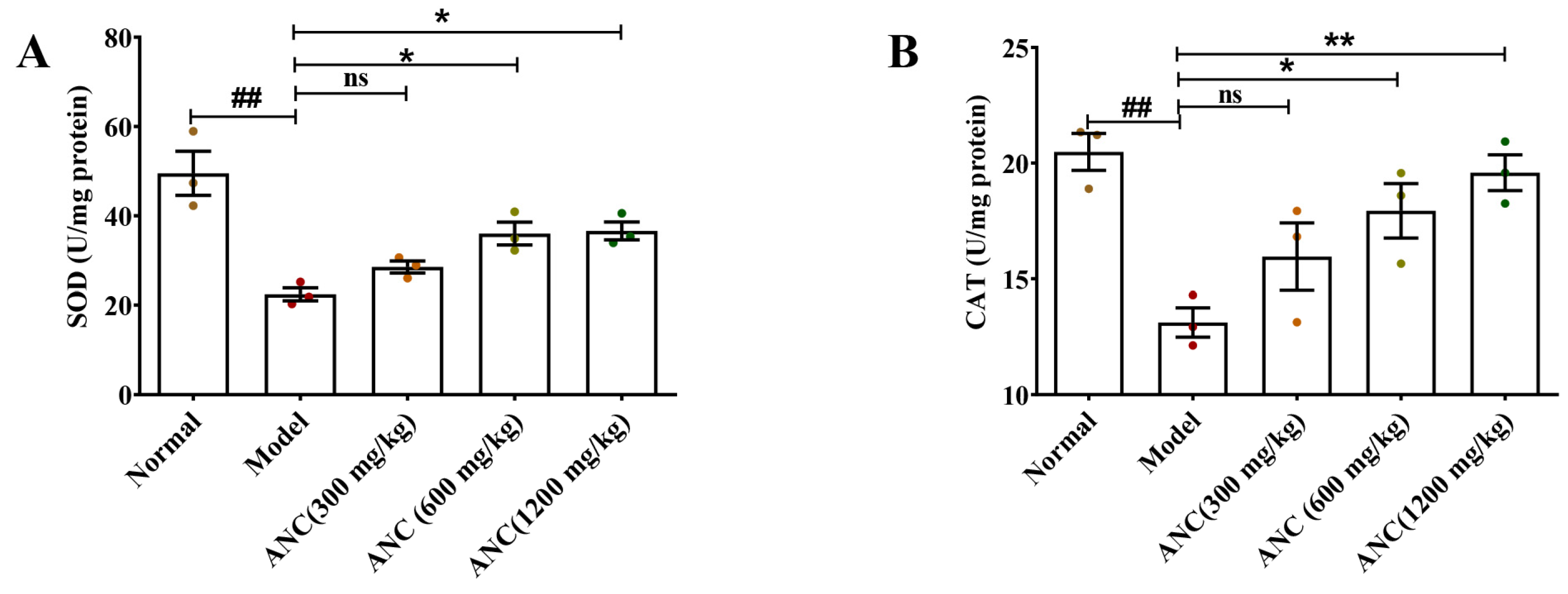
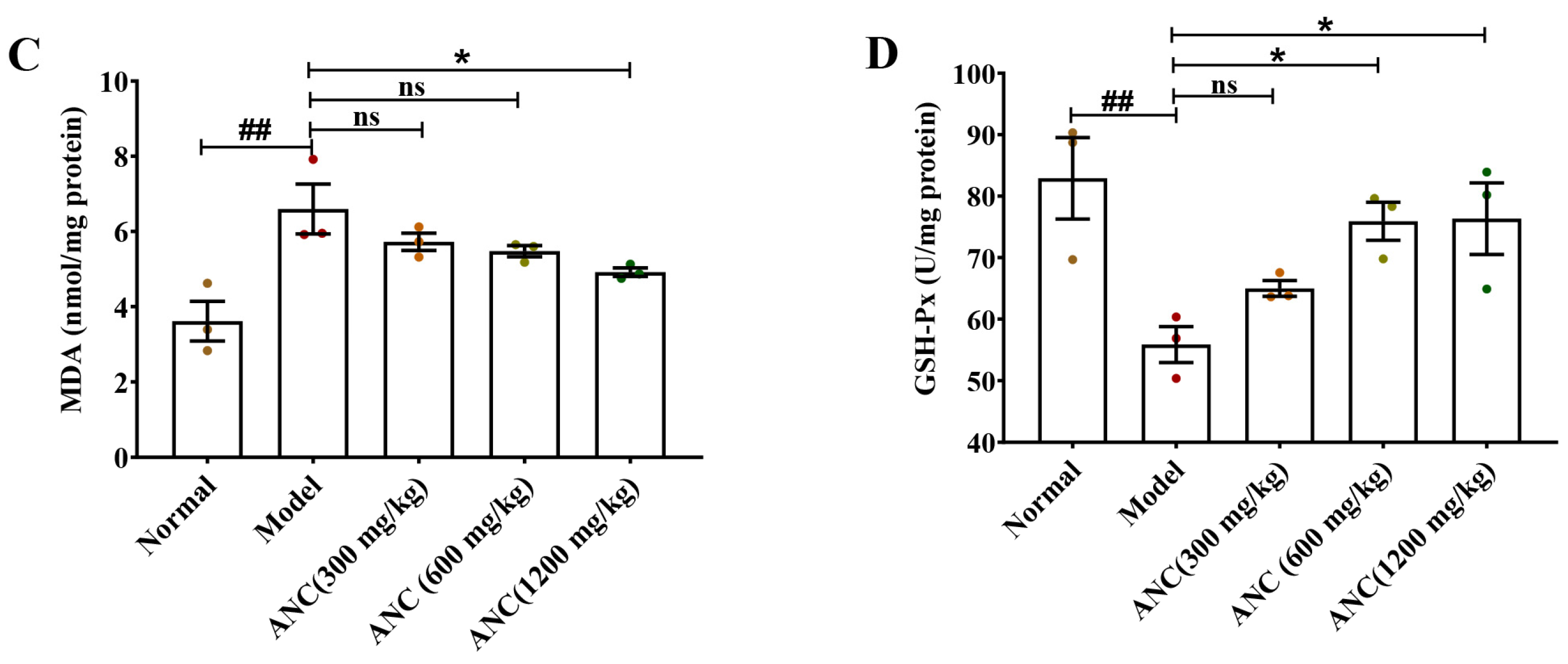
3.5. Ancientino Improved Colitis by Removing the Excessive Oxidative Stress In Vivo
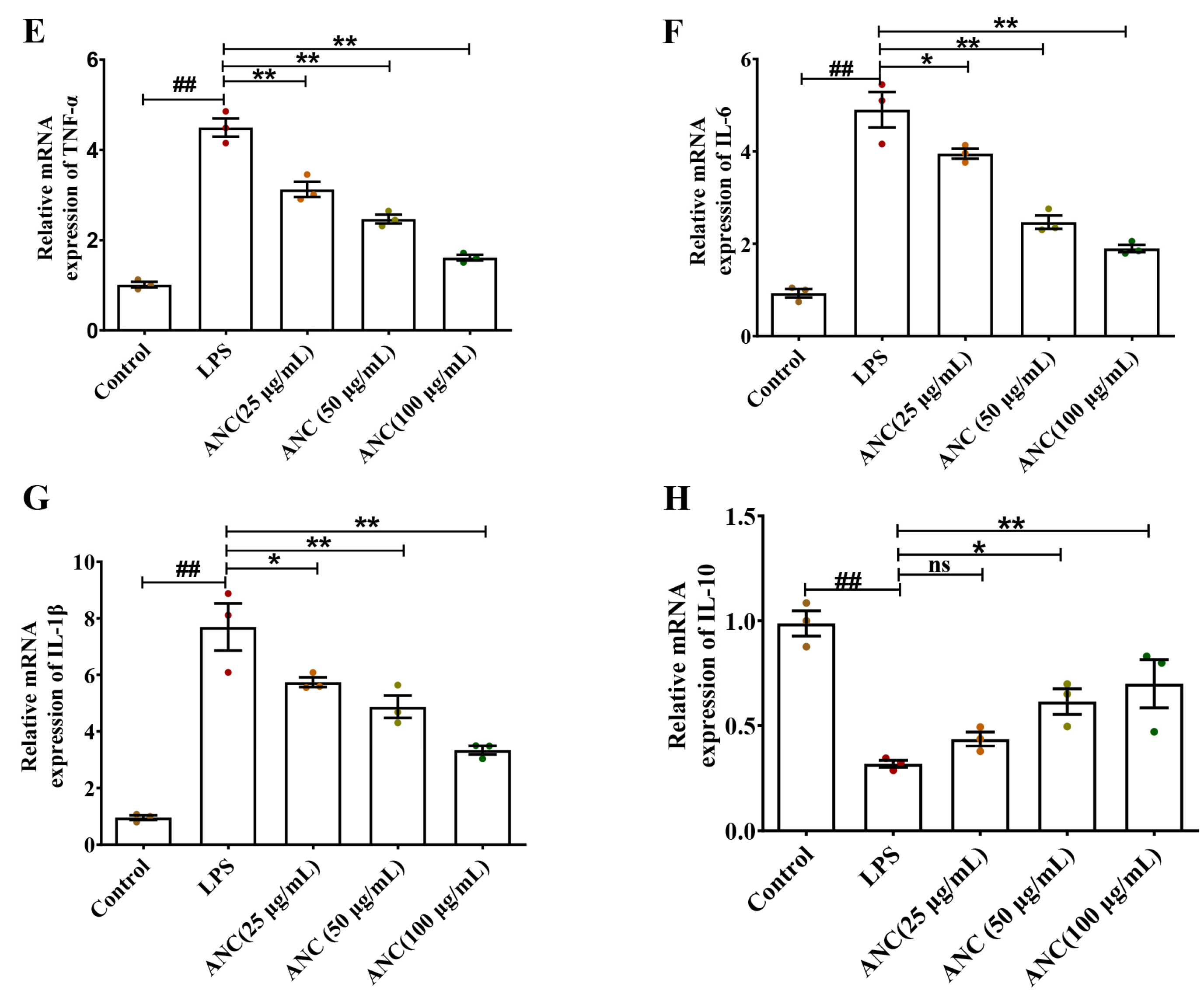
3.6. Ancientino Alleviated Colitis by Suppressing the Inflammatory Response In Vitro
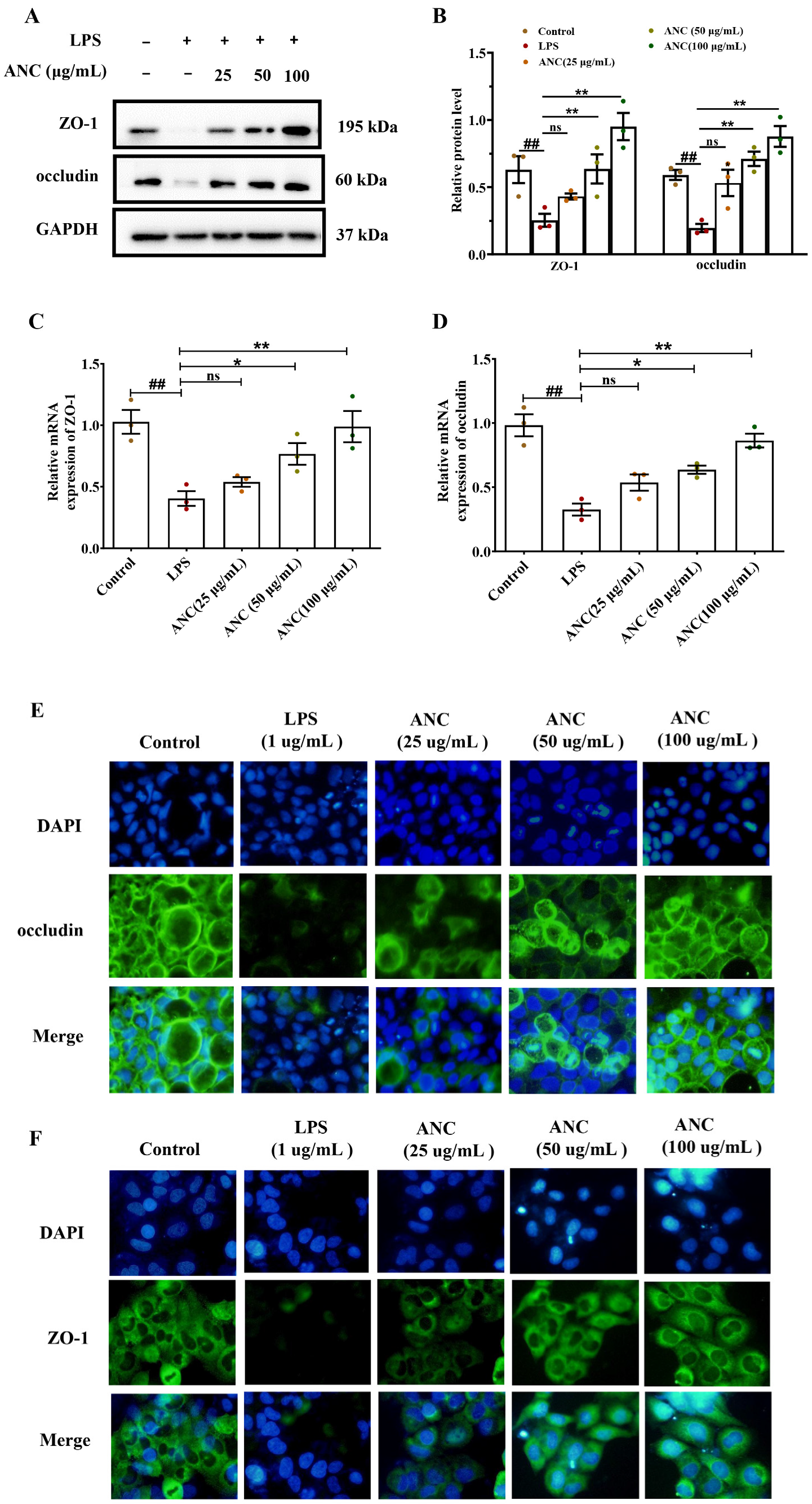
3.7. Ancientino Improved Colitis by Preserving the Expression of TJ Proteins In Vitro
3.8. Ancientino Improved Colitis by Removing the Excessive Oxidative Stress In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Sun, L.; Ouyang, J.; Zeng, F.; Wu, S. An AIEgen-based oral-administration nanosystem for detection and therapy of ulcerative colitis via 3D-MSOT/NIR-II fluorescent imaging and inhibiting NLRP3 inflammasome. Biomaterials 2022, 283, 121468. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ni, J.; Chen, J.; Ma, G.; Zhao, M.; Zhu, S.; Shi, T.; Zhu, J.; Huang, Z.; Zhang, J. Dual-functionalized MSCs that express CX3CR1 and IL-25 exhibit enhanced therapeutic effects on inflammatory bowel disease. Mol. Ther. 2020, 28, 1214–1228. [Google Scholar] [CrossRef]
- Keewan, E.A.; Narasimhulu, C.A.; Rohr, M.; Hamid, S.; Parthasarathy, S. Are fried foods unhealthy? the dietary peroxidized fatty acid, 13-HPODE, induces intestinal inflammation in vitro and in vivo. Antioxidants 2020, 9, 926. [Google Scholar] [CrossRef]
- Krela-Kaźmierczak, I.; Zakerska-Banaszak, O.; Skrzypczak-Zielińska, M.; Łykowska-Szuber, L.; Szymczak-Tomczak, A.; Zawada, A.; Rychter, A.M.; Ratajczak, A.E.; Skoracka, K.; Skrzypczak, D. Where Do We Stand in the Behavioral Pathogenesis of Inflammatory Bowel Disease? The Western Dietary Pattern and Microbiota—A Narrative Review. Nutrients 2022, 14, 2520. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jha, R.; Li, A.; Liu, H.; Zhang, Z.; Zhang, C.; Zhai, Q.; Zhang, J. Probiotics (Lactobacillus plantarum HNU082) supplementation relieves ulcerative colitis by affecting intestinal barrier functions, immunity-related gene expression, gut microbiota, and metabolic pathways in mice. Microbiol. Spectr. 2022, 10, e01651-22. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Yang, L.; Jiang, S.; Qian, D.; Duan, J. Excessive apoptosis in ulcerative colitis: Crosstalk between apoptosis, ROS, ER stress, and intestinal homeostasis. Inflamm. Bowel Dis. 2022, 28, 639–648. [Google Scholar] [CrossRef]
- Zou, Q.; Feng, J.; Li, T.; Cheng, G.; Wang, W.; Rao, G.; He, H.; Li, Y. Antioxidation and anti-inflammatory actions of the extract of Nitraria Tangutorum Bobr. fruits reduce the severity of ulcerative colitis in a dextran sulphate sodium-induced mice model. J. Funct. Foods 2022, 91, 105005. [Google Scholar] [CrossRef]
- Xu, X.; Wei, C.; Yang, Y.; Liu, M.; Luo, A.; Song, H.; Wang, Y.; Duan, X. New discovery of anti-ulcerative colitis active ingredients of Nostoc commune: P-Hydroxy benzaldehyde. J. Funct. Foods 2021, 77, 104327. [Google Scholar] [CrossRef]
- Cao, R.; Wu, X.; Guo, H.; Pan, X.; Huang, R.; Wang, G.; Liu, J. Naringin exhibited therapeutic effects against DSS-induced mice ulcerative colitis in intestinal barrier–dependent manner. Molecules 2021, 26, 6604. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X. Dietary Polyphenols, Gut Microbiota, and Human Health. Front. Pharmacol. 2023, 13, 5595. [Google Scholar] [CrossRef] [PubMed]
- Sałaga, M.; Zatorski, H.; Sobczak, M.; Chen, C.; Fichna, J. Chinese herbal medicines in the treatment of IBD and colorectal cancer: A review. Curr. Treat. Options Oncol. 2014, 15, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-J.; Cha, Y.-S.; Kim, K.-A. Blackcurrant Alleviates Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Foods 2023, 12, 1073. [Google Scholar] [CrossRef]
- Tan, C.; Wang, M.; Kong, Y.; Wan, M.; Deng, H.; Tong, Y.; Lyu, C.; Meng, X. Anti-inflammatory and intestinal microbiota modulation properties of high hydrostatic pressure treated cyanidin-3-glucoside and blueberry pectin complexes on dextran sodium sulfate-induced ulcerative colitis mice. Food Funct. 2022, 13, 4384–4398. [Google Scholar] [CrossRef]
- Xu, X.; Luo, A.; Lu, X.; Liu, M.; Wang, H.; Song, H.; Wei, C.; Wang, Y.; Duan, X. p-Hydroxybenzoic acid alleviates inflammatory responses and intestinal mucosal damage in DSS-induced colitis by activating ERβ signaling. J. Funct. Foods 2021, 87, 104835. [Google Scholar] [CrossRef]
- Tang, S.; Zhong, W.; Li, T.; Li, Y.; Song, G. Isochlorogenic acid A alleviates dextran sulfate sodium-induced ulcerative colitis in mice through STAT3/NF-κB pathway. Int. Immunopharmacol. 2023, 118, 109989. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, P.; Li, C.; Xu, F.; Chen, J. A polysaccharide from Rosa roxburghii Tratt fruit attenuates high-fat diet-induced intestinal barrier dysfunction and inflammation in mice by modulating the gut microbiota. Food Funct. 2022, 13, 530–547. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Lin, X.; Chan, T.F.; Lai, K.P.; Li, R. Uncovering the functions of plasma proteins in ulcerative colitis and identifying biomarkers for BPA-induced severe ulcerative colitis: A plasma proteome analysis. Ecotoxicol. Environ. Saf. 2022, 242, 113897. [Google Scholar] [CrossRef]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L. Multidimensional impact of Mediterranean diet on IBD patients. Inflamm. Bowel Dis. 2021, 27, 1–9. [Google Scholar] [CrossRef]
- Holt, D.; Strauss, B.; Moore, G. Patients with inflammatory bowel disease and their treating clinicians have different views regarding diet. J. Hum. Nutr. Diet. 2017, 30, 66–72. [Google Scholar] [CrossRef]
- Radziszewska, M.; Smarkusz-Zarzecka, J.; Ostrowska, L.; Pogodziński, D. Nutrition and Supplementation in Ulcerative Colitis. Nutrients 2022, 14, 2469. [Google Scholar] [CrossRef]
- Gu, Q.; Xia, C.; Liu, N.; Chen, Z.; Zhou, Q.; Li, P. Lactobacillus plantarum ZJ316 alleviates ulcerative colitis by inhibiting inflammation, regulating short chain fatty acid levels and gut microbiota in a mouse model. Food Funct. 2023, 14, 3982–3993. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Gao, Y.; Kan, R.; Ren, P.; Xue, C.; Kong, B.; Tang, Q. Alginate Oligosaccharides Prevent Dextran-Sulfate-Sodium-Induced Ulcerative Colitis via Enhancing Intestinal Barrier Function and Modulating Gut Microbiota. Foods 2023, 12, 220. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Pettersson, S.; Meyer Zum Büschenfelde, K.-H.; Strober, W. Local administration of antisense phosphorothiate olignucleotides to the p65 subunit of NF–κB abrogates established experimental colitis in mice. Nat. Med. 1996, 2, 998–1004. [Google Scholar] [CrossRef]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Mai, P.; Hao, Y.; Wang, Z.; Wang, J. Quinoa bran soluble dietary fiber ameliorates dextran sodium sulfate induced ulcerative colitis in BALB/c mice by maintaining intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2022, 216, 75–85. [Google Scholar] [CrossRef]
- Mähler, M.; Leiter, E.H. Genetic and environmental context determines the course of colitis developing in IL–10-deficient mice. Inflamm. Bowel Dis. 2002, 8, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Iwańczak, B.M.; Kierkuś, J.; Ryżko, J.; Szczepanik, M.; Więcek, S.; Czaja-Bulsa, G.; Kacperska, M.; Korczowski, B.; Maślana, J.; Iwańczak, F. Induction and maintenance infliximab therapy in children with moderate to severe ulcerative colitis: Retrospective, multicenter study. Adv. Clin. Exp. Med. 2017, 26, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-Y.; Son, J.D.; Hwang, S.-J.; Lee, J.K.; Park, J.Y.; Park, K.I.; Oh, T.W. Fermented Glutinous Rice Extract Mitigates DSS-Induced Ulcerative Colitis by Alleviating Intestinal Barrier Function and Improving Gut Microbiota and Inflammation. Antioxidants 2023, 12, 336. [Google Scholar] [CrossRef]
- Zhu, L.; Gu, P.; Shen, H. Gallic acid improved inflammation via NF-κB pathway in TNBS-induced ulcerative colitis. Int. Immunopharmacol. 2019, 67, 129–137. [Google Scholar] [CrossRef]
- Yuan, Z.; Yang, L.; Zhang, X.; Ji, P.; Hua, Y.; Wei, Y. Huang-lian-jie-du decoction ameliorates acute ulcerative colitis in mice via regulating NF-κB and Nrf2 signaling pathways and enhancing intestinal barrier function. Front. Pharmacol. 2019, 10, 1354. [Google Scholar] [CrossRef]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, N.; Wu, X.; Cao, G.; Qiao, H.; Wang, J.; Hao, R. The preventive effect of Glycyrrhiza polysaccharide on lipopolysaccharide-induced acute colitis in mice by modulating gut microbial communities. Int. J. Biol. Macromol. 2023, 239, 124199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Dai, G.; Wu, X.; Li, L.; Tian, Y.; Tan, L. Protective effects of Fagopyrum dibotrys on oxidized oil-induced oxidative stress, intestinal barrier impairment, and altered cecal microbiota in broiler chickens. Poult. Sci. 2023, 102, 102472. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Chen, M. The Correlation between Endotoxin, D-Lactate and Diamine Oxidase with Endoscopic Activity in Inflammatory Bowel Disease. Dis. Markers 2020, 2022, 9171436. [Google Scholar] [CrossRef]
- Jiang, H.; Shi, G.-F.; Fang, Y.-X.; Liu, Y.-Q.; Wang, Q.; Zheng, X.; Zhang, D.-J.; Zhang, J.; Yin, Z.-Q. Aloin A prevents ulcerative colitis in mice by enhancing the intestinal barrier function via suppressing the Notch signaling pathway. Phytomedicine 2022, 106, 154403. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Q.; Xiong, B.; Chen, H.; Wang, X.; Zhang, D. Discoidin domain receptor 1 (DDR1) promote intestinal barrier disruption in Ulcerative Colitis through tight junction proteins degradation and epithelium apoptosis. Pharmacol. Res. 2022, 183, 106368. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.A.Y.; Söderholm, J.D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 362–381. [Google Scholar] [CrossRef]
- Wei, M.; Ma, Y.; Shen, L.; Xu, Y.; Liu, L.; Bu, X.; Guo, Z.; Qin, H.; Li, Z.; Wang, Z. NDRG2 regulates adherens junction integrity to restrict colitis and tumourigenesis. EBioMedicine 2020, 61, 103068. [Google Scholar] [CrossRef]
- Yang, W.; Ren, D.; Shao, H.; Zhang, X.; Li, T.; Zhang, L.; Liu, L.; Zhao, Y.; Niu, P.; Yang, X. Theabrownin from Fu Brick Tea Improves Ulcerative Colitis by Shaping the Gut Microbiota and Modulating the Tryptophan Metabolism. J. Agric. Food Chem. 2023, 71, 2898–2913. [Google Scholar] [CrossRef]
- Tsopmejio, I.S.N.; Yuan, J.; Diao, Z.; Fan, W.; Wei, J.; Zhao, C.; Li, Y.; Song, H. Auricularia polytricha and Flammulina velutipes reduce liver injury in DSS-induced Inflammatory Bowel Disease by improving inflammation, oxidative stress, and apoptosis through the regulation of TLR4/NF-κB signaling pathways. J. Nutr. Biochem. 2023, 111, 109190. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, X.; Yin, M.; Li, C.; Han, L. Preventive effect of lycopene in dextran sulfate sodium-induced ulcerative colitis mice through the regulation of TLR4/TRIF/NF-κB signaling pathway and tight junctions. J. Agric. Food Chem. 2021, 69, 13500–13509. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Wang, C.; Zeng, Z.; Ren, Y.; Li, D.; Song, J.-L. Ameliorative effect of sinapic acid on dextran sodium sulfate-(DSS-) induced ulcerative colitis in Kunming (KM) mice. Oxidative Med. Cell. Longev. 2020, 2020, 8393504. [Google Scholar] [CrossRef]
- Li, S.; Yuan, R.; Fan, Q.; Zhang, C.; Han, S.; Li, J.; Xu, Z.; Sun, K.; Xu, Q.; Yao, C. Ginsenoside Rb1 exerts therapeutic effects on ulcerative colitis through regulating the Nrf2/PIP2/NLRP3 inflammasome signaling pathway. J. Funct. Foods 2023, 102, 105475. [Google Scholar] [CrossRef]
- Gao, Y.; Zou, J.; Chen, B.; Cao, Y.; Hu, D.; Zhang, Y.; Zhao, X.; Wen, J.; Liu, K.; Wang, K. Hyaluronic acid/serotonin-decorated cerium dioxide nanomedicine for targeted treatment of ulcerative colitis. Biomater. Sci. 2023, 11, 618–629. [Google Scholar] [CrossRef]
- Mandlik, D.S.; Mandlik, S.K.; Patel, S. Protective effect of sarsasapogenin in TNBS induced ulcerative colitis in rats associated with downregulation of pro-inflammatory mediators and oxidative stress. Immunopharmacol. Immunotoxicol. 2021, 43, 571–583. [Google Scholar] [CrossRef]
- Wu, C.; Gao, B.; Gui, Y. Malondialdehyde on postoperative day 1 predicts postoperative cognitive dysfunction in elderly patients after hip fracture surgery. Biosci. Rep. 2019, 39, BSR20190166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, O.; Ma, N.; Yi, J.; Mi, H.; Cai, S. The preventive effect and underlying mechanism of Rhus chinensis Mill. fruits on dextran sulphate sodium-induced ulcerative colitis in mice. Food Funct. 2021, 12, 9965–9978. [Google Scholar] [CrossRef]
- Ren, R.; Zhao, A.Q.; Chen, L.; Wu, S.; Hung, W.L.; Wang, B. Therapeutic effect of Lactobacillus plantarum JS19 on mice with DSS-induced acute and chronic ulcerative colitis. J. Sci. Food Agric. 2022, 103, 4143–4156. [Google Scholar] [CrossRef]
- Lyu, B.; Wang, Y.; Fu, H.; Li, J.; Yang, X.; Shen, Y.; Swallah, M.S.; Yu, Z.; Li, Y.; Wang, H. Intake of high-purity insoluble dietary fiber from Okara for the amelioration of colonic environment disturbance caused by acute ulcerative colitis. Food Funct. 2022, 13, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, K.; Saha, S.; Umar, S. Health benefits of dietary fiber for the management of inflammatory bowel disease. Biomedicines 2022, 10, 1242. [Google Scholar] [CrossRef]
- Woo, J.K.; Choi, S.; Kang, J.-H.; Kim, D.E.; Hurh, B.-S.; Jeon, J.-E.; Kim, S.Y.; Oh, S.H. Fermented barley and soybean (BS) mixture enhances intestinal barrier function in dextran sulfate sodium (DSS)-induced colitis mouse model. BMC Complement. Altern. Med. 2016, 16, 498. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353.e1321. [Google Scholar] [CrossRef]
- Nishiguchi, R.; Basu, S.; Staab, H.A.; Ito, N.; Zhou, X.K.; Wang, H.; Ha, T.; Johncilla, M.; Yantiss, R.K.; Montrose, D.C. Dietary interventions to prevent high-fructose diet–associated worsening of colitis and colitis-associated tumorigenesis in mice. Carcinogenesis 2021, 42, 842–852. [Google Scholar] [CrossRef]
- Faghfoori, Z.; Navai, L.; Shakerhosseini, R.; Somi, M.H.; Nikniaz, Z.; Norouzi, M.F. Effects of an oral supplementation of germinated barley foodstuff on serum tumour necrosis factor-α, interleukin-6 and-8 in patients with ulcerative colitis. Ann. Clin. Biochem. 2011, 48, 233–237. [Google Scholar] [CrossRef]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K. Low-fat, high-fiber diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e1130. [Google Scholar] [CrossRef]
- Di Rosa, C.; Altomare, A.; Imperia, E.; Spiezia, C.; Khazrai, Y.M.; Guarino, M.P.L. The Role of Dietary Fibers in the Management of IBD Symptoms. Nutrients 2022, 14, 4775. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Yoshimura, Y.; Kamada, C.; Tanaka, S.; Horikawa, C.; Okumura, R.; Ito, H.; Ohashi, Y.; Akanuma, Y.; Yamada, N. Intakes of dietary fiber, vegetables, and fruits and incidence of cardiovascular disease in Japanese patients with type 2 diabetes. Diabetes Care 2013, 36, 3916–3922. [Google Scholar] [CrossRef]
- Mihaylova, D.; Popova, A.; Desseva, I.; Manolov, I.; Petkova, N.; Vrancheva, R.; Peltekov, A.; Slavov, A.; Zhivondov, A. Comprehensive evaluation of late season peach varieties (Prunus persica L.): Fruit nutritional quality and phytochemicals. Molecules 2021, 26, 2818. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, C.; Li, Y.; Han, X.; Luo, X.; Chen, L.; Zhang, T.; Wang, N.; Wang, W. Alginate Oligosaccharides Ameliorate DSS-Induced Colitis through Modulation of AMPK/NF-κB Pathway and Intestinal Microbiota. Nutrients 2022, 14, 2864. [Google Scholar] [CrossRef]
- Bhatt, S.; Gupta, M. Dietary fiber from fruit waste as a potential source of metabolites in maintenance of gut milieu during ulcerative colitis: A comprehensive review. Food Res. Int. 2022, 164, 112329. [Google Scholar] [CrossRef] [PubMed]


| DAI Score | Weight Loss (%) | Stool Consistency | Fecal Occult Blood |
|---|---|---|---|
| 0 | 0 | Normal | Normal |
| 1 | 1–5 | Loose stools (mild) | Hemoccult positive |
| 2 | 5–10 | Loose stools (medium) | |
| 3 | 10–20 | Loose stools (high) | |
| 4 | >20 | Diarrhea | Gross bleeding |
| Score | Inflammation Severity | Inflammatory Locations | Crypt Damage |
|---|---|---|---|
| 0 | none | none | none |
| 1 | slight | mucosa | basal third damaged |
| 2 | moderate | mucosa and submucosa | basal two-thirds damaged |
| 3 | severe | transmural | only surface intact |
| 4 | entire crypt and epithelium lost |
| Name | Sequence (5′ to 3′) | GenBank Accession Number |
|---|---|---|
| TNF-α | F-TGTCCCTTTCACTCACTGGC | NM_013693.3 |
| R-TCTTCTGCCAGTTCCACGTC | ||
| IL-1β | F-TCAGCACCTCACAAGCAGAG | NM_008361.4 |
| R-TTCTTGTGACCCTGAGCGAC | ||
| IL-6 | F-TTGGGACTGATGCTGGTGAC | NM_031168.2 |
| R-AGACAGGTCTGTTGGGAGTG | ||
| IL-10 | F-TGAATTCCCTGGGTGAGAAG | M84340.1 |
| R-TTTGTTGGGTGGCTCTAAGG | ||
| occludin | F-TTGACTGGGCTGAACACTCC | NM_008756.2 |
| R-ACATCACAGCTCACACCAGG | ||
| ZO-1 | F-AAACAGCCCTACCAACCTCG | BC138028.1 |
| R-TTCGAGGCAGCTGCTCATAG | ||
| GAPDH | F-CAGCAAGGACACTGAGCAAG | NM_001289726.1 |
| R-GGTCTGGGATGGAAATTGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Wang, Y.; Guan, G.; Lu, X.; Zhu, Y.; Duan, X. Dietary Supplementation of Ancientino Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress. Nutrients 2023, 15, 2798. https://doi.org/10.3390/nu15122798
Liu M, Wang Y, Guan G, Lu X, Zhu Y, Duan X. Dietary Supplementation of Ancientino Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress. Nutrients. 2023; 15(12):2798. https://doi.org/10.3390/nu15122798
Chicago/Turabian StyleLiu, Meng, Yuhui Wang, Guoqiang Guan, Xi Lu, Yizhun Zhu, and Xiaoqun Duan. 2023. "Dietary Supplementation of Ancientino Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress" Nutrients 15, no. 12: 2798. https://doi.org/10.3390/nu15122798
APA StyleLiu, M., Wang, Y., Guan, G., Lu, X., Zhu, Y., & Duan, X. (2023). Dietary Supplementation of Ancientino Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress. Nutrients, 15(12), 2798. https://doi.org/10.3390/nu15122798






