Combination of Panax ginseng and Diospyros kaki Leaf Inhibits White Adipocyte Differentiation and Browning Process through AMP-Activated Protein Kinase (AMPK) Activation In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Panax ginseng and Diospyros kaki Leaf Extract
2.2. HPLC Condition for Analysis
2.3. Animal Experiment
2.4. Serum Assay of Biochemical Parameter
2.5. Tissue Weight and Histological Analysis
2.6. Adipocyte Size Measurement
2.7. Oil Red O Staining
2.8. Western Blotting Analysis
2.9. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.10. Dual-Energy X-ray Absorptiometry (DXA) Scan
2.11. Statistical Analysis
3. Results
3.1. Quantitative Analysis of Ginsenoside Rg1, Rb1, Rg3 and Tannic Acid in Panax ginseng (PG) and Diospyros kaki Leaf (DKL)
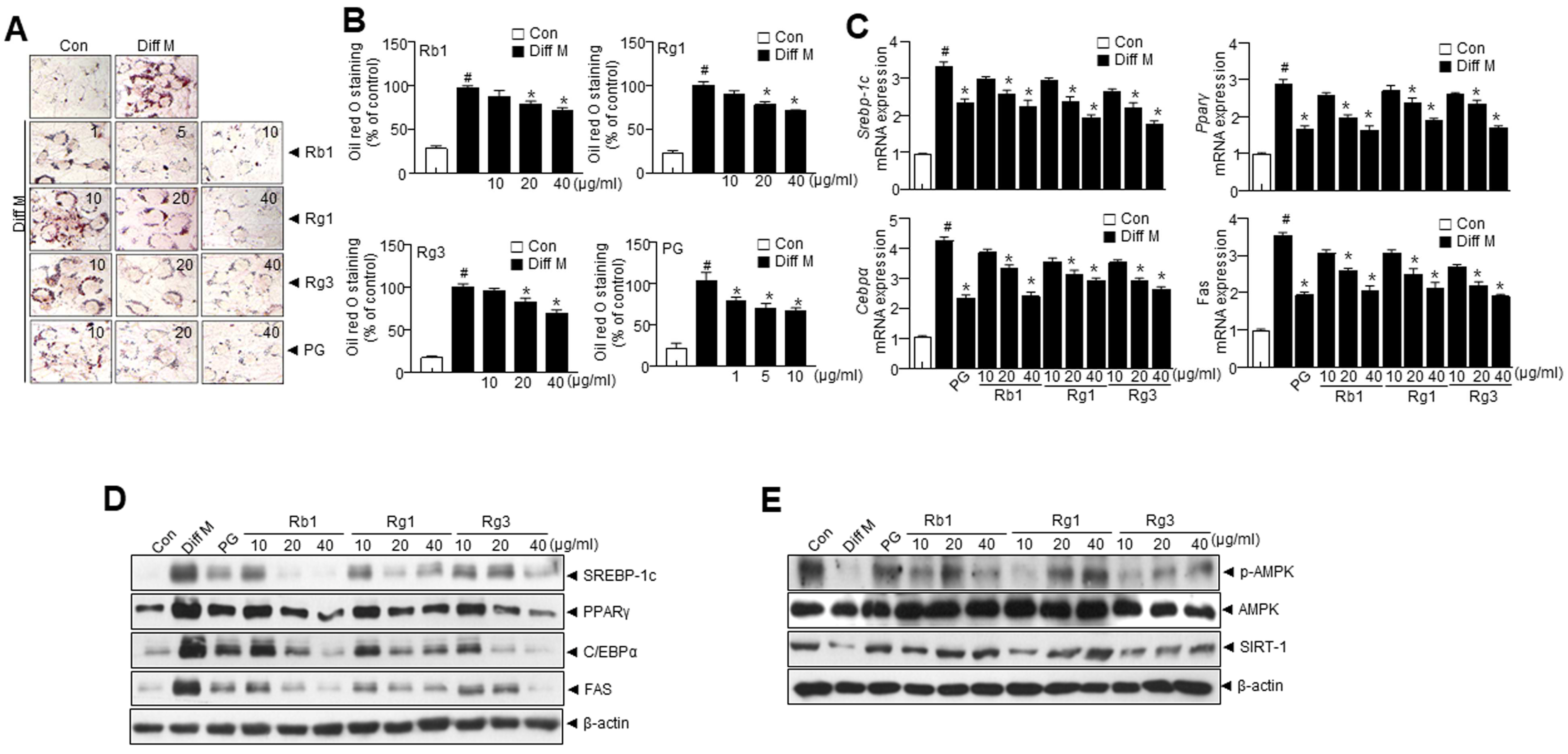
3.2. Panax ginseng (PG) Extract and PG Active Compounds Regulate Adipogenesis in 3T3-L1 Cells
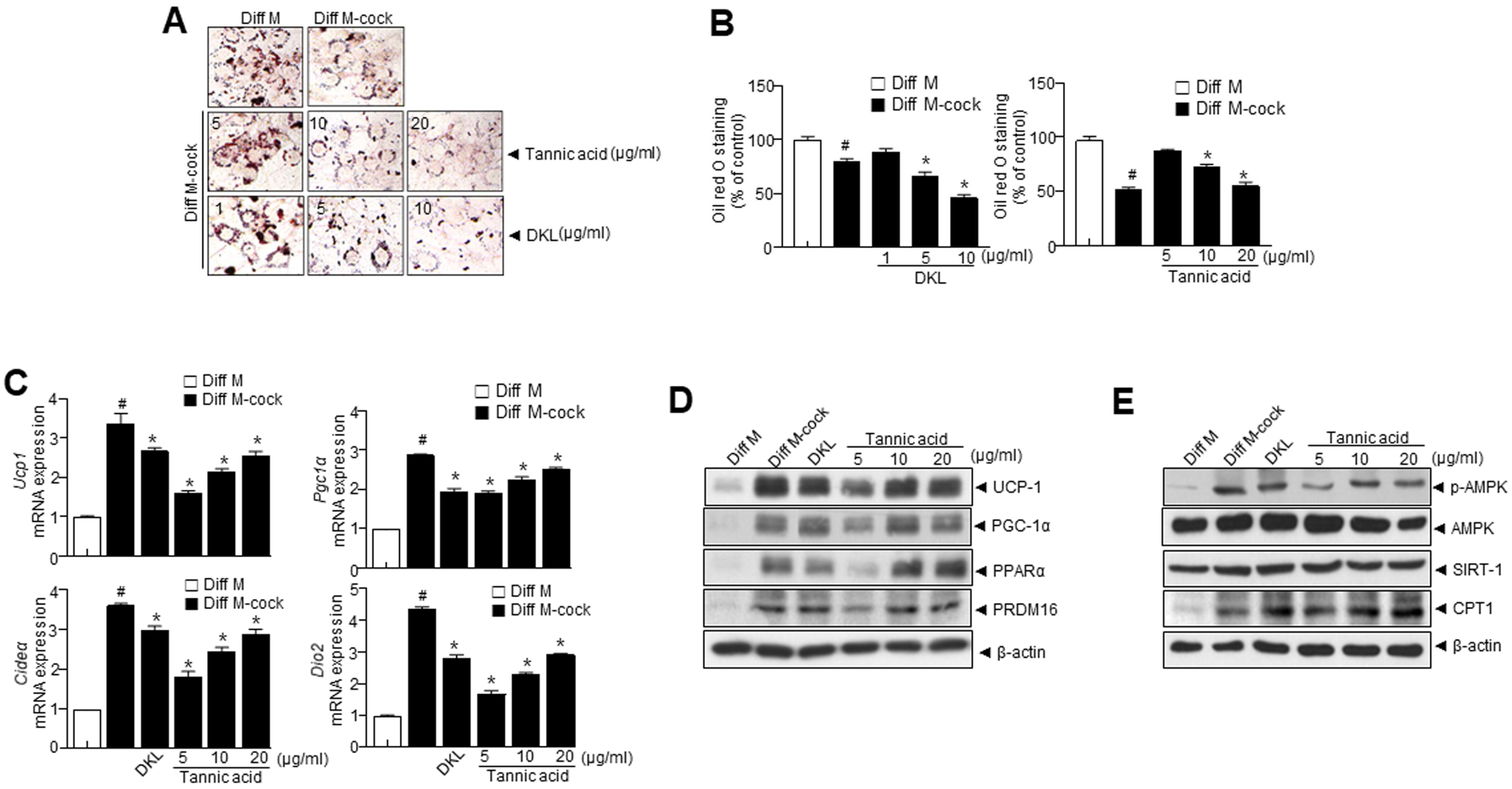
3.3. Diospyros kaki Leaf (DKL) Extract and DKL Active Compounds Induce Browning in 3T3-L1 Adipocytes
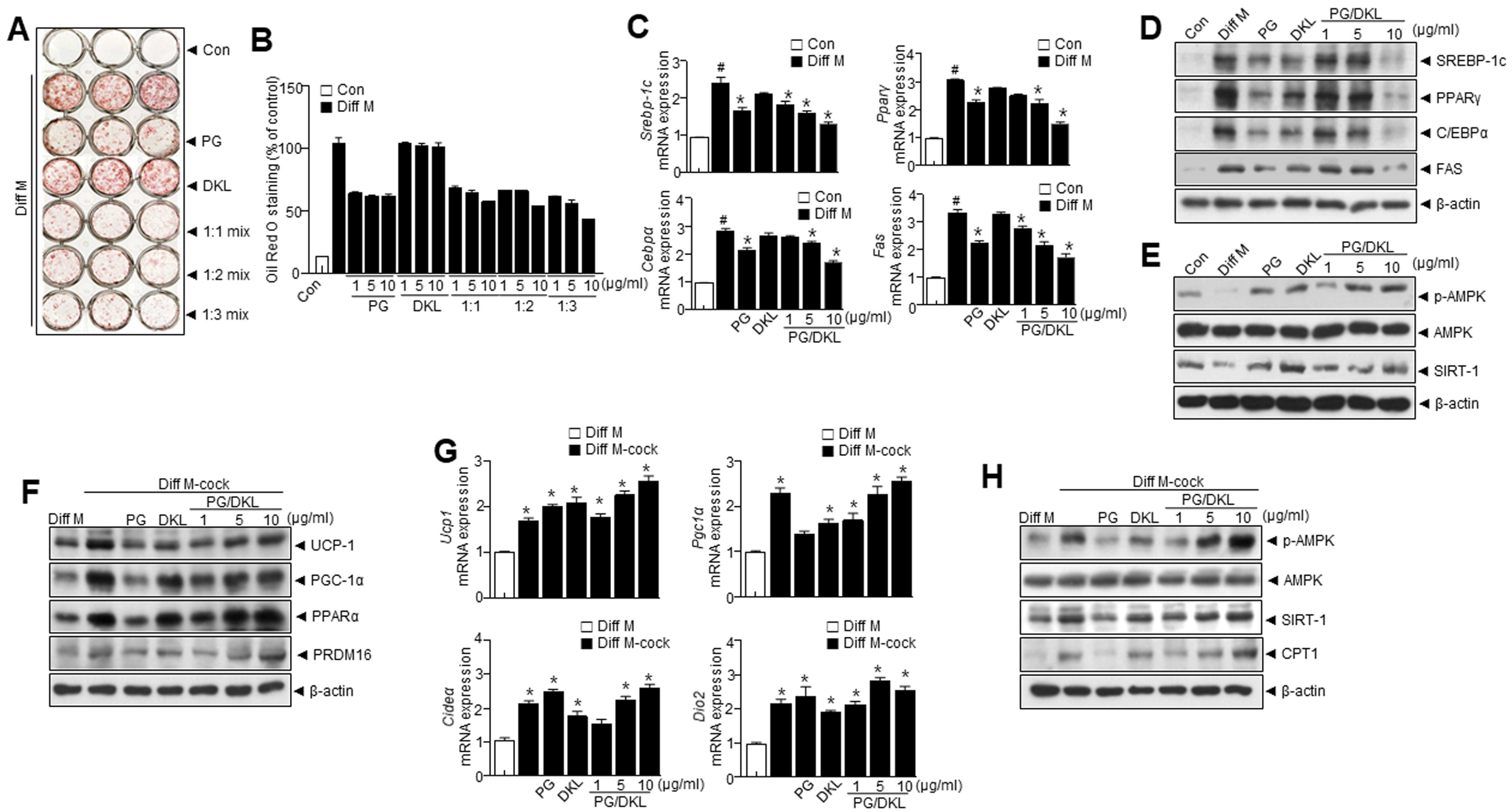
3.4. Synergistic Effect of Panax ginseng (PG) and Diospyros kaki Leaf (DKL) Extracts Regulate Lipid Accumulation and Browning of 3T3-L1 White Adipocytes
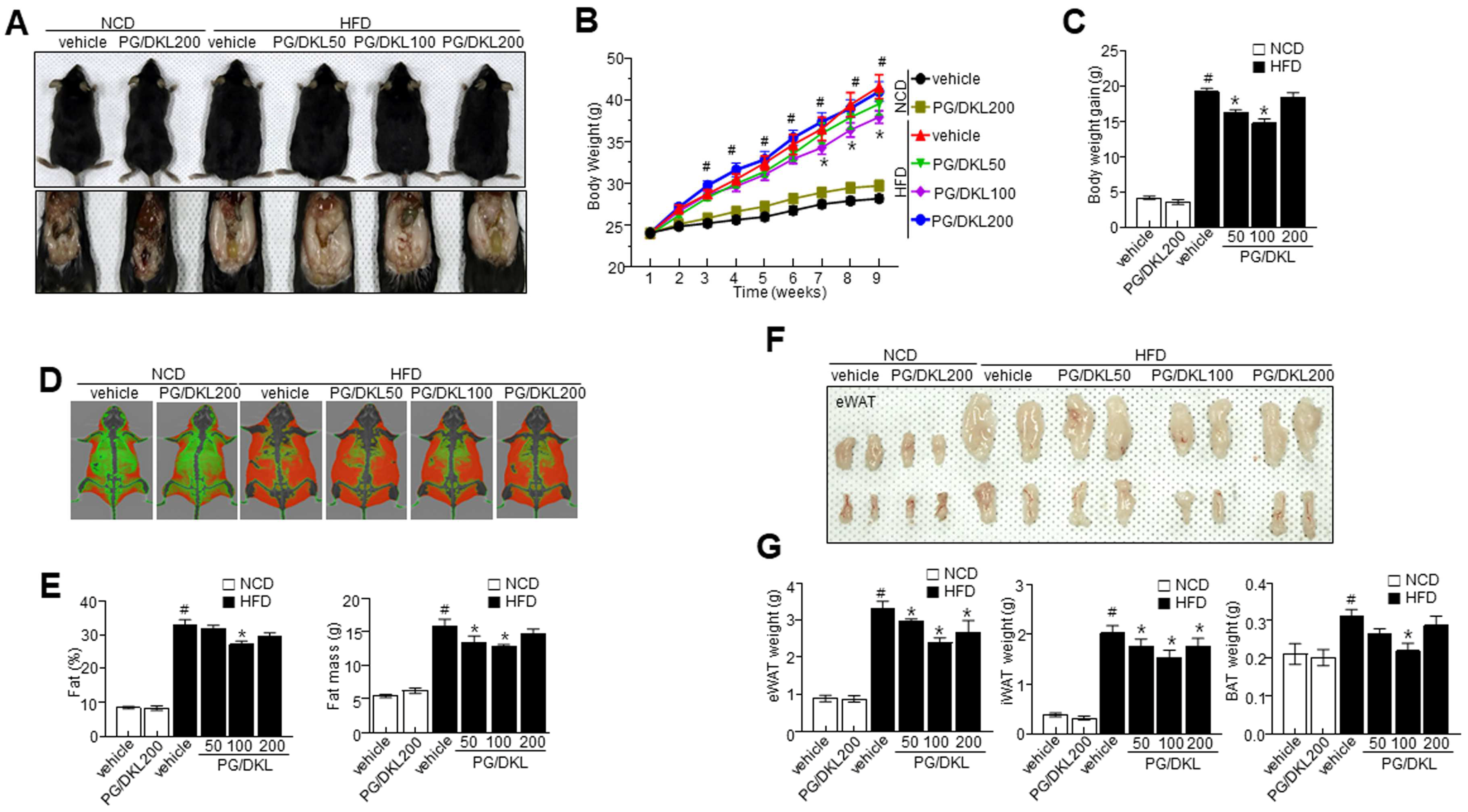
3.5. Panax ginseng (PG) and Diospyros kaki Leaf (DKL) Extracts Synergistically Regulate Body Weight Gain and Their Effect on Metabolic Profile in HFD-Induced Obese Mice
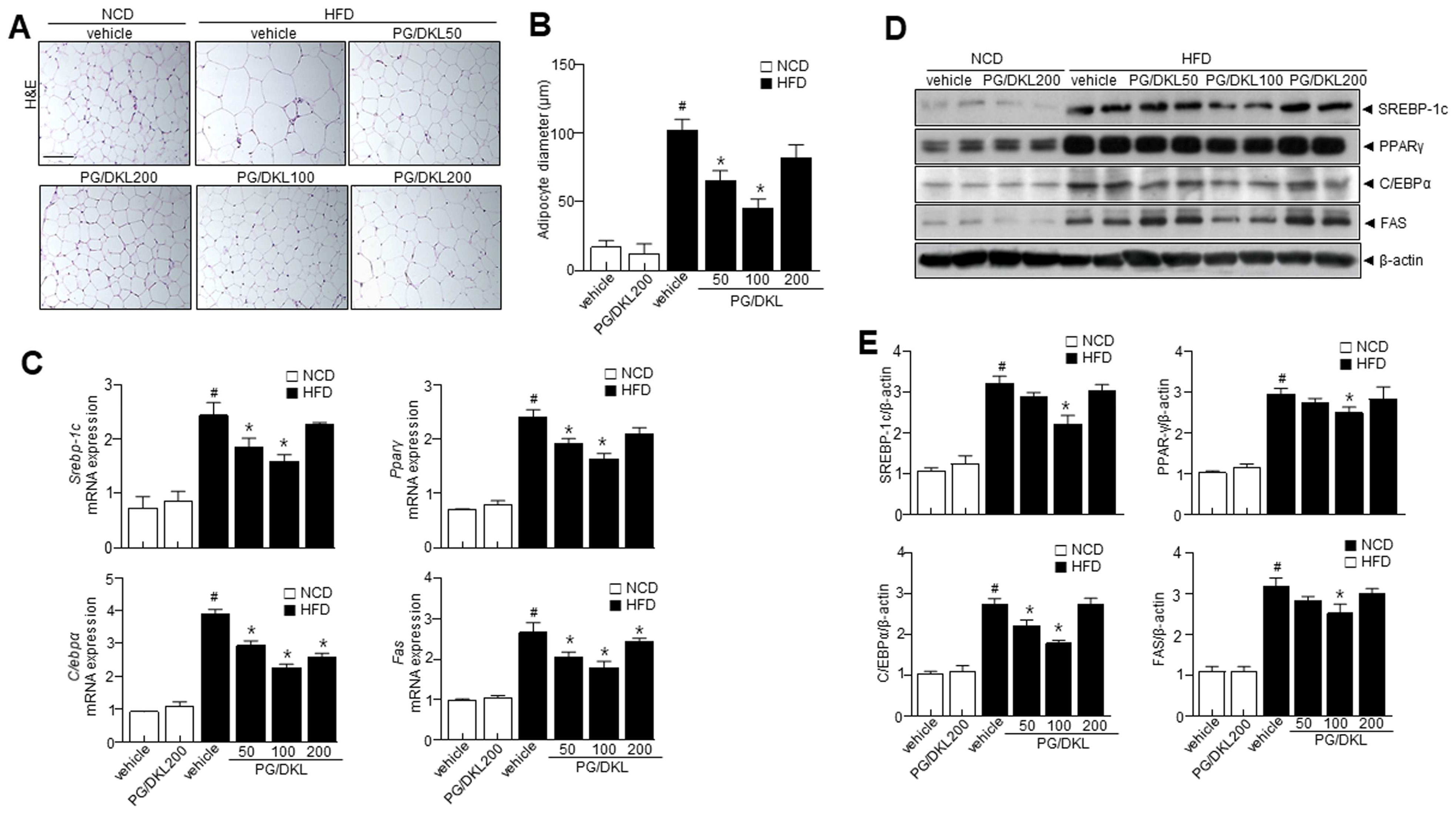
3.6. Panax ginseng (PG) and Diospyros kaki Leaf (DKL) Extracts Synergistically Regulate the Levels of Adipogenesis-Related Proteins in Adipose Tissues of HFD-Induced Obese Mice
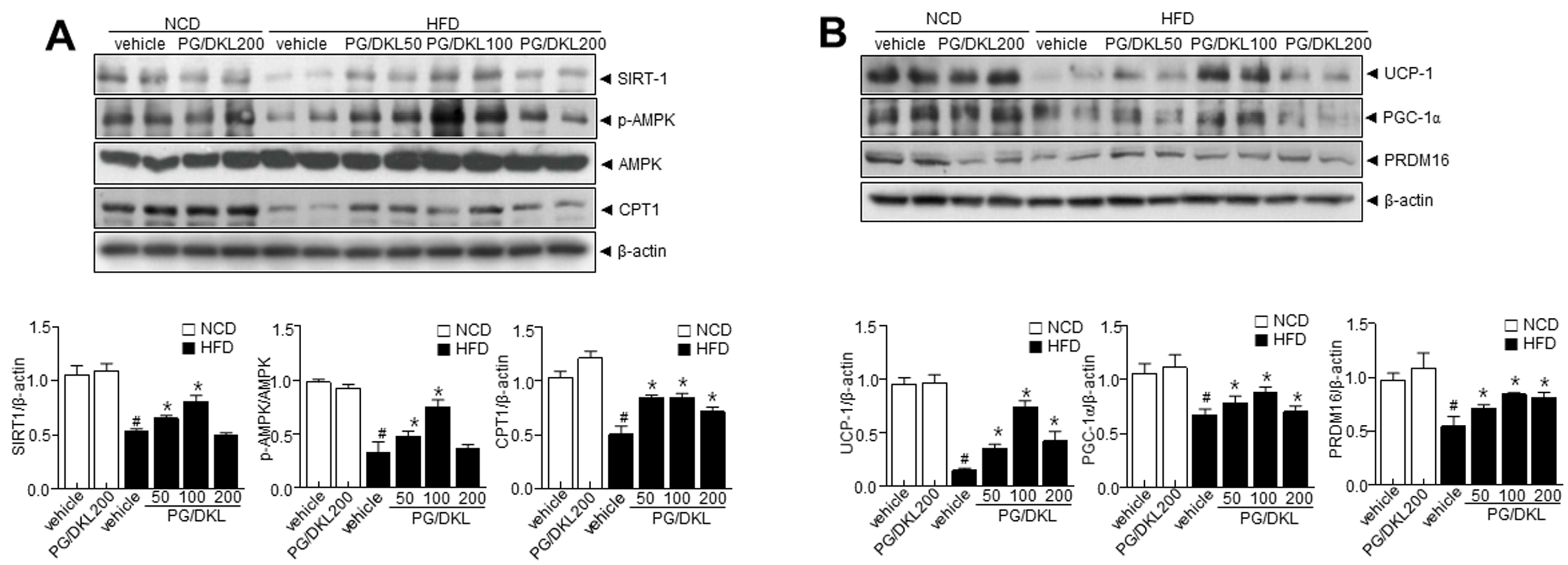
3.7. Combination of Panax ginseng (PG) and Diospyros K kaki Leaf (DKL) Extracts Ameliorate Hepatic Lipid Accumulation in the Liver and Brown Fat-like Phenotype in Adipose Tissue of HFD-Induced Obese Mice
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carbone, S.; Canada, J.M.; Billingsley, H.E.; Siddiqui, M.S.; Elagizi, A.; Lavie, C.J. Obesity paradox in cardiovascular disease: Where do we stand? Vasc. Health Risk Manag. 2019, 15, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Seeley, R.J.; Zeltser, L.M.; Drewnowski, A.; Ravussin, E.; Redman, L.M.; Leibel, R.L. Obesity Pathogenesis: An Endocrine Society Scientific Statement. Endocr. Rev. 2017, 38, 267–296. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.; Tseng, Y.H. Brown adipose tissue: Recent insights into development, metabolic function and therapeutic potential. Adipocyte 2012, 1, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N.; Karpe, F.; Fielding, B.A.; Macdonald, I.A.; Coppack, S.W. Integrative physiology of human adipose tissue. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 875–888. [Google Scholar] [CrossRef]
- Shen, W.; Wang, Y.; Lu, S.F.; Hong, H.; Fu, S.; He, S.; Li, Q.; Yue, J.; Xu, B.; Zhu, B.M. Acupuncture promotes white adipose tissue browning by inducing UCP1 expression on DIO mice. BMC Complement. Altern. Med. 2014, 14, 501. [Google Scholar] [CrossRef]
- Klaus, S. Adipose tissue as a regulator of energy balance. Curr. Drug. Targets 2004, 5, 241–250. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug. Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Review of Ginseng Anti-Diabetic Studies. Molecules 2019, 24, 4501. [Google Scholar] [CrossRef]
- Kim, E.N.; Kim, T.Y.; Park, E.K.; Kim, J.Y.; Jeong, G.S. Panax ginseng Fruit Has Anti-Inflammatory Effect and Induces Osteogenic Differentiation by Regulating Nrf2/HO-1 Signaling Pathway in In Vitro and In Vivo Models of Periodontitis. Antioxidants 2020, 9, 1221. [Google Scholar] [CrossRef]
- Song, M.Y.; Kim, B.S.; Kim, H. Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. J. Ginseng Res. 2014, 38, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: A review for use in cardiovascular diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Huu Tung, N.; Uto, T.; Morinaga, O.; Kim, Y.H.; Shoyama, Y. Pharmacological effects of ginseng on liver functions and diseases: A minireview. Evid. Based Complement. Altern. Med. 2012, 2012, 173297. [Google Scholar] [CrossRef]
- Izuchi, R.; Takahashi, H.; Inada, Y. Preparing a carotenoid polyphenol-enriched extract from the peel of persimmon, Diospyros kaki L.f. Biosci. Biotechnol. Biochem. 2009, 73, 2793–2795. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, M.K.; Ha, T.Y.; Bok, S.H.; Park, H.M.; Jeong, K.S.; Woo, M.N.; Do, G.M.; Yeo, J.Y.; Choi, M.S. Supplementation of whole persimmon leaf improves lipid profiles and suppresses body weight gain in rats fed high-fat diet. Food Chem. Toxicol. 2006, 44, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Kim, D.S.; Kim, S.W.; Lim, S.H.; Kim, D.K.; Shin, T.Y.; Kim, S.H. Inhibitory effects of Diospyros kaki in a model of allergic inflammation: Role of cAMP, calcium and nuclear factor-kappaB. Int. J. Mol. Med. 2013, 32, 945–951. [Google Scholar] [CrossRef]
- Kim, B.M.; Cho, B.O.; Jang, S.I. Anti-obesity effects of Diospyros lotus leaf extract in mice with high-fat diet-induced obesity. Int. J. Mol. Med. 2019, 43, 603–613. [Google Scholar] [CrossRef]
- Nuankaew, W.; Lee, H.K.; Nam, Y.H.; Shim, J.H.; Kim, N.W.; Shin, S.W.; Kim, M.C.; Shin, S.Y.; Hong, B.N.; Dej-Adisai, S.; et al. The Effects of Persimmon (Diospyros kaki L.f.) Oligosaccharides on Features of the Metabolic Syndrome in Zebrafish. Nutrients 2022, 14, 3249. [Google Scholar] [CrossRef]
- Ramirez-Zacarias, J.L.; Castro-Munozledo, F.; Kuri-Harcuch, W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 1992, 97, 493–497. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, G.H.; Hoang, T.H.; Park, S.A.; Lee, J.; Lim, J.; Sa, S.; Kim, G.E.; Han, J.S.; Kim, J.; et al. d-Allulose Ameliorates Hyperglycemia through IRE1alpha Sulfonation-RIDD-Sirt1 Decay Axis in the Skeletal Muscle. Antioxid. Redox Signal. 2022, 37, 229–245. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, G.H.; Hoang, T.H.; Kim, Y.M.; Jang, G.H.; Seok, C.H.; Gwak, Y.G.; Lim, J.; Kim, J.; Chae, H.J. GABA and Fermented Curcuma longa L. Extract Enriched with GABA Ameliorate Obesity through Nox4-IRE1alpha Sulfonation-RIDD-SIRT1 Decay Axis in High-Fat Diet-Induced Obese Mice. Nutrients 2022, 14, 1680. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Moreno, E.; Arias-Rico, J.; Jimenez-Sanchez, R.C.; Estrada-Luna, D.; Jimenez-Osorio, A.S.; Zafra-Rojas, Q.Y.; Ariza-Ortega, J.A.; Flores-Chavez, O.R.; Morales-Castillejos, L.; Sandoval-Gallegos, E.M. Role of Bioactive Compounds in Obesity: Metabolic Mechanism Focused on Inflammation. Foods 2022, 11, 1232. [Google Scholar] [CrossRef]
- Oh, J.; Lee, H.; Park, D.; Ahn, J.; Shin, S.S.; Yoon, M. Ginseng and Its Active Components Ginsenosides Inhibit Adipogenesis in 3T3-L1 Cells by Regulating MMP-2 and MMP-9. Evid. Based Complement. Altern. Med. 2012, 2012, 265023. [Google Scholar] [CrossRef]
- Kim, S.N.; Kim, D.H.; Lee, H.J.; Lim, J.S.; Lee, J.H.; Park, S.Y.; Koh, Y.J. Effects of Ginsenoside Rg3 on Inhibiting Differentiation, Adipogenesis, and ER Stress-Mediated Cell Death in Brown Adipocytes. Evid. Based Complement. Altern. Med. 2021, 2021, 6668665. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.J.; Kim, K.J.; Choi, J.; Jeon, H.J.; Seo, M.J.; Lee, B.Y. Ginsenoside Rg1 suppresses early stage of adipocyte development via activation of C/EBP homologous protein-10 in 3T3-L1 and attenuates fat accumulation in high fat diet-induced obese zebrafish. J. Ginseng Res. 2017, 41, 23–30. [Google Scholar] [CrossRef]
- Fan, W.; Huang, Y.; Zheng, H.; Li, S.; Li, Z.; Yuan, L.; Cheng, X.; He, C.; Sun, J. Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: Pharmacology and mechanisms. Biomed. Pharmacother. 2020, 132, 110915. [Google Scholar] [CrossRef]
- Hwang, J.T.; Lee, M.S.; Kim, H.J.; Sung, M.J.; Kim, H.Y.; Kim, M.S.; Kwon, D.Y. Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-gamma signal pathways. Phytother. Res. 2009, 23, 262–266. [Google Scholar] [CrossRef]
- Mohanan, P.; Subramaniyam, S.; Mathiyalagan, R.; Yang, D.C. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J. Ginseng Res. 2018, 42, 123–132. [Google Scholar] [CrossRef]
- Marlatt, K.L.; Ravussin, E. Brown Adipose Tissue: An Update on Recent Findings. Curr. Obes. Rep. 2017, 6, 389–396. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, G.H.; Yoon, Y.; Hoang, T.H.; Chae, H.J. IBF-R Regulates IRE1alpha Post-Translational Modifications and ER Stress in High-Fat Diet-Induced Obese Mice. Nutrients 2022, 14, 217. [Google Scholar] [CrossRef] [PubMed]
- Khalilpourfarshbafi, M.; Gholami, K.; Murugan, D.D.; Abdul Sattar, M.Z.; Abdullah, N.A. Differential effects of dietary flavonoids on adipogenesis. Eur. J. Nutr. 2019, 58, 5–25. [Google Scholar] [CrossRef] [PubMed]

| Name | Forward (5′–3′) | Reverse (3′–5′) |
|---|---|---|
| Srebp-1c | CCATGGATGCACTTTCGAA | CCAGCATAGGGTGGGTCAA |
| Fas | CGGTACGCGACGGCTGCCTG | GCTGCTCCACGAACTCAAACACCG |
| Pparγ | GCAGCTACTGCATGTGATCAAGA | GTCAGCGGGTGGGACTTTC |
| Cebpα | GACTTGGTGCGTCTAAGATGAG | TAGGCATTGGAGCGGTGAG |
| Ucp-1 | GGCCTCTACGACTCAGTCCA | TAAGCCGGCTGAGATCTTGT |
| Pgc1α | GAAAGGGCCAAACAGAGAGA | GTAAATCACACGGCGCTCTT |
| Cidea | GCCTGCAGGAACTTATCAGC | GCCTGCAGGAACTTATCAGC |
| Dio2 | CTGCGCTGTGTCTGGAAC | GGAATTGGGAGCATCTTCAC |
| β-actin | CCTGGCACCCAGCACAAT | GCCGATCCACACACGGAGTACT |
| Parameter | Group | |||||
|---|---|---|---|---|---|---|
| NCD + Vehicle | NCD + PG/DKL200 | HFD + Vehicle | HFD + PG/DKL50 | HFD + PG/DKL100 | HFD + PG/DKL200 | |
| ALT (U/L) | 24.75 ± 4.47 | 26.25 ± 5.61 | 66.5 ± 7.31 # | 59.13 ± 3.37 *# | 58.25 ± 8.14 *# | 59.00 ± 4.61 *# |
| AST (U/L) | 54.38 ± 6.38 | 58.50 ± 4.27 | 113.00 ± 14.87 # | 98.50 ± 6.12 *# | 88.38 ± 6.14 *# | 86.75 ± 6.16 *# |
| TG (mg/dL) | 41.84 ± 13.47 | 45.04 ± 6.87 | 99.18 ± 15.32 *# | 84.68 ± 13.67 *# | 74.36 ± 14.17 *# | 82.48 ± 11.14 *# |
| T-Chol (mg/dL) | 77.63 ± 9.73 | 73.75 ± 11.40 | 142.63 ± 14.15 # | 135.75 ± 15.28 *# | 122.38 ± 13.58 *# | 125.13 ± 15.49 *# |
| LDL-Chol | 16.40 ± 1.13 | 18.00 ± 3.64 | 131.75 ± 13.08 # | 102.25 ± 11.37 *# | 89.75 ± 6.10 *# | 106.50 ± 13.55 *# |
| Adiponectin (μg/mL) | 10.38 ± 2.45 | 10.25 ± 2.90 | 5.58 ± 1.52 *# | 6.14 ± 1.21 *# | 8.04 ± 1.43 *# | 7.25 ± 1.61 |
| Leptin (ng/mL) | 9.50 ± 3.61 | 7.25 ± 2.49 | 50.75 ± 6.74 # | 45.88 ± 4.91 *# | 35.38 ± 5.10 *# | 40.50 ± 7.86 *# |
| Liver TG(mg/g) | 27.50 ± 6.30 | 23.50 ± 10.10 | 70.88 ± 12.57 # | 64.75 ± 8.94 *# | 61.88 ± 12.54 *# | 62.88 ± 6.57 *# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-Y.; Lee, G.-H.; Kim, H.-J.; Lim, Y.J.; Ko, B.M.; Kim, D.-S.; Kim, T.W.; Kim, H.K.; Kim, T.Y.; Hwang, D.I.; et al. Combination of Panax ginseng and Diospyros kaki Leaf Inhibits White Adipocyte Differentiation and Browning Process through AMP-Activated Protein Kinase (AMPK) Activation In Vitro and In Vivo. Nutrients 2023, 15, 2776. https://doi.org/10.3390/nu15122776
Lee H-Y, Lee G-H, Kim H-J, Lim YJ, Ko BM, Kim D-S, Kim TW, Kim HK, Kim TY, Hwang DI, et al. Combination of Panax ginseng and Diospyros kaki Leaf Inhibits White Adipocyte Differentiation and Browning Process through AMP-Activated Protein Kinase (AMPK) Activation In Vitro and In Vivo. Nutrients. 2023; 15(12):2776. https://doi.org/10.3390/nu15122776
Chicago/Turabian StyleLee, Hwa-Young, Geum-Hwa Lee, Hwa-Jin Kim, Young Jae Lim, Bo Mi Ko, Do-Sung Kim, Tae Won Kim, Hye Kyung Kim, Tae Young Kim, Dae Il Hwang, and et al. 2023. "Combination of Panax ginseng and Diospyros kaki Leaf Inhibits White Adipocyte Differentiation and Browning Process through AMP-Activated Protein Kinase (AMPK) Activation In Vitro and In Vivo" Nutrients 15, no. 12: 2776. https://doi.org/10.3390/nu15122776
APA StyleLee, H.-Y., Lee, G.-H., Kim, H.-J., Lim, Y. J., Ko, B. M., Kim, D.-S., Kim, T. W., Kim, H. K., Kim, T. Y., Hwang, D. I., Choi, H. K., Ju, S. M., Min, K. H., & Chae, H.-J. (2023). Combination of Panax ginseng and Diospyros kaki Leaf Inhibits White Adipocyte Differentiation and Browning Process through AMP-Activated Protein Kinase (AMPK) Activation In Vitro and In Vivo. Nutrients, 15(12), 2776. https://doi.org/10.3390/nu15122776






