Real-World Management of High Stool Output in Patients with Short Bowel Syndrome: An International Multicenter Survey
Abstract
1. Introduction
2. Materials and Methods
2.1. Questionnaire Survey
2.2. Participating Centers and Data Collection
2.3. Ethical Statements
2.4. Statistical Analysis
3. Results
3.1. Participating Centers
3.2. Dietary Recommendations for Management of High Stool Output
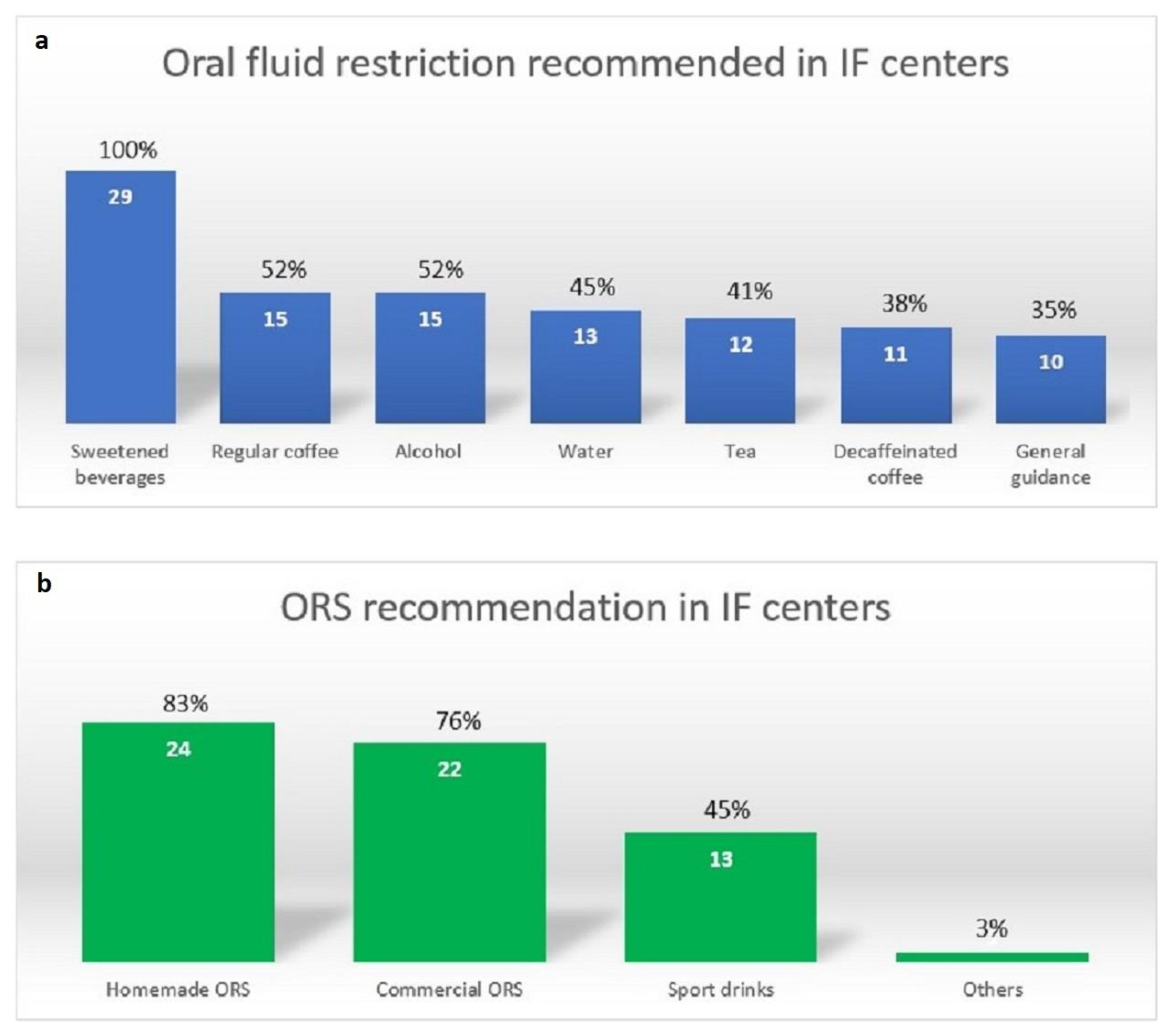
3.3. Fluid Recommendations for Management of High Stool Output
3.4. Medications for Management of High Stool Output
3.4.1. Antimotility Medications
3.4.2. Antisecretory Medications
3.4.3. Other Therapeutic Agents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuerda, C.; Pironi, L.; Arends, J.; Bozzetti, F.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN practical guideline: Clinical nutrition in chronic intestinal failure. Clin. Nutr. 2021, 40, 5196–5220. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Arends, J.; Bozzetti, F.; Cuerda, C.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN guidelines on chronic intestinal failure in adults. Clin. Nutr. 2016, 35, 247–307. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.G.; Tappenden, K.A.; Winkler, M.F. Short bowel syndrome: Highlights of patient management, quality of life, and survival. JPEN J. Parenter. Enteral Nutr. 2014, 38, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Group, E.S.-H.A.N.W.; Van Gossum, A.; Bakker, H.; De Francesco, A.; Ladefoged, K.; Leon-Sanz, M.; Messing, B.; Pironi, L.; Pertkiewicz, M.; Shaffer, J.; et al. Home parenteral nutrition in adults: A multicentre survey in Europe in 1993. Clin. Nutr. 1996, 15, 53–59. [Google Scholar] [CrossRef]
- Baxter, J.P.; Gillanders, L.; Angstmann, K.; Staun, M.; O’Hanlon, C.; Smith, T.; Joly, F.; Thul, P.; Jonkers, C.; Wanten, G.; et al. Home parenteral nutrition: An international benchmarking exercise. e-SPEN J. 2012, 7, e211–e214. [Google Scholar] [CrossRef]
- Nordsten, C.B.; Molsted, S.; Bangsgaard, L.; Fuglsang, K.A.; Brandt, C.F.; Niemann, M.J.; Jeppesen, P.B. High Parenteral Support Volume Is Associated With Reduced Quality of Life Determined by the Short-Bowel Syndrome Quality of Life Scale in Nonmalignant Intestinal Failure Patients. JPEN J. Parenter. Enteral Nutr. 2021, 45, 926–932. [Google Scholar] [CrossRef]
- Pironi, L.; Goulet, O.; Buchman, A.; Messing, B.; Gabe, S.; Candusso, M.; Bond, G.; Gupte, G.; Pertkiewicz, M.; Steiger, E.; et al. Outcome on home parenteral nutrition for benign intestinal failure: A review of the literature and benchmarking with the European prospective survey of ESPEN. Clin. Nutr. 2012, 31, 831–845. [Google Scholar] [CrossRef]
- Siddiqui, M.T.; Al-Yaman, W.; Singh, A.; Kirby, D.F. Short-Bowel Syndrome: Epidemiology, Hospitalization Trends, In-Hospital Mortality, and Healthcare Utilization. JPEN J. Parenter. Enteral Nutr. 2021, 45, 1441–1455. [Google Scholar] [CrossRef]
- Limtrakun, N.; Lakananurak, N. Dietary Strategies for Managing Short Bowel Syndrome. Curr. Treat. Options Gastroenterol. 2022, 20, 376–391. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Shahraz, S.; Hopkins, T.; Worsfold, A.; Genestin, E. Impact of intestinal failure and parenteral support on adult patients with short-bowel syndrome: A multinational, noninterventional, cross-sectional survey. JPEN J. Parenter. Enteral Nutr. 2022, 46, 1650–1659. [Google Scholar] [CrossRef]
- Kumpf, V.J. Pharmacologic management of diarrhea in patients with short bowel syndrome. JPEN J. Parenter. Enteral Nutr. 2014, 38, 38S–44S. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth Wall, H.C.; Lakananurak, N.; Gramlich, L. A Multidisciplinary Team Evaluation of Management Guidelines for Adult Short Bowel Syndrome. Clin. Nutr. ESPEN 2023, in press.
- Lakananurak, N.; Moccia, L.; Wall, E.; Herlitz, J.; Catron, H.; Lozano, E.; Delgado, A.; Vanuytsel, T.; Mercer, D.; Pevny, S.; et al. Characteristics of adult intestinal failure centers: An international multicenter survey. Nutr. Clin. Pract. 2022, 38, 657–663. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Efficacy And Safety Evaluation of Glepaglutide in Treatment of SBS (EASE SBS 1). Available online: https://www.clinicaltrials.gov/ct2/show/NCT03690206 (accessed on 7 March 2023).
- Matarese, L.E. Nutrition and fluid optimization for patients with short bowel syndrome. JPEN J. Parenter. Enteral Nutr. 2013, 37, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Nordgaard, I.; Hansen, B.S.; Mortensen, P.B. Colon as a digestive organ in patients with short bowel. Lancet 1994, 343, 373–376. [Google Scholar] [CrossRef]
- Nordgaard, I.; Hansen, B.S.; Mortensen, P.B. Importance of colonic support for energy absorption as small-bowel failure proceeds. Am. J. Clin. Nutr. 1996, 64, 222–231. [Google Scholar] [CrossRef]
- Hamilton, K.; Crowe, T.; Testro, A. High amylase resistant starch to decrease stool output in people with short bowel syndrome: A pilot trial. Clin. Nutr. ESPEN 2019, 29, 242–244. [Google Scholar] [CrossRef]
- De Vries, F.E.E.; Reeskamp, L.F.; van Ruler, O.; van Arum, I.; Kuin, W.; Dijksta, G.; Haveman, J.W.; Boermeester, M.A.; Serlie, M.J. Systematic review: Pharmacotherapy for high-output enterostomies or enteral fistulas. Aliment. Pharmacol. Ther. 2017, 46, 266–273. [Google Scholar] [CrossRef]
- Tappenden, K.A. Pathophysiology of short bowel syndrome: Considerations of resected and residual anatomy. JPEN J. Parenter. Enteral Nutr. 2014, 38, 14S–22S. [Google Scholar] [CrossRef]
- Nightingale, J.; Woodward, J.M.; Small Bowel and Nutrition Committee of the British Society of Gastroenterology. Guidelines for management of patients with a short bowel. Gut 2006, 55 (Suppl. S4), iv1–iv12. [Google Scholar] [CrossRef]
- Williams, N.S.; Evans, P.; King, R.F. Gastric acid secretion and gastrin production in the short bowel syndrome. Gut 1985, 26, 914–919. [Google Scholar] [CrossRef] [PubMed]
- McLeod, G.M.; Wiggins, H.S. Bile-salts in small intestinal contents after ileal resection and in other malabsorption syndromes. Lancet 1968, 1, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Hardison, W.G.; Rosenberg, I.H. Bile-salt deficiency in the steatorrhea following resection of the ileum and proximal colon. N. Engl. J. Med. 1967, 277, 337–342. [Google Scholar] [CrossRef] [PubMed]

| Dietary Recommendations | Without Colon-in-Continuity Median (Range, %) | With Colon-in-Continuity Median (Range, %) |
|---|---|---|
| A low-simple-sugar diet | 75 (0–100) | 80 (0–100) |
| A low-oxalate diet | 0 (0–100) | 80 (0–100) |
| Separation of fluid from solid food | 90 (0–100) | 75 (0–100) |
| A high-sodium diet | 90 (0–100) | 50 (0–100) |
| A low-fat diet | 10 (0–60) | 35 (0–100) |
| Oral nutritional supplements | 35 (0–100) | 35 (0–100) |
| Addition of soluble fiber | 5 (0–90) | 25 (0–90) |
| Dietary Recommendations | Without Colon-in-Continuity | With Colon-in-Continuity | ||
|---|---|---|---|---|
| US Median (Range, %) | Non-US Median (Range, %) | US Median (Range, %) | Non-US Median (Range, %) | |
| A low-simple-sugar diet | 100 (90–100) | 20 (0–100) | 100 (10–100) | 30 (0–100) |
| A low-oxalate diet | 0 (0–100) | 0 (0–100) | 50 (0–100) | 90 (0–100) |
| Separation of fluid from solid food | 100 (10–100) | 90 (0–100) | 100 (10–100) | 70 (0–100) |
| A high-sodium diet | 100 (0–100) | 80 (0–100) | 60 (0–100) | 40 (0–100) |
| A low-fat diet | 10 (0–60) | 20 (0–60) | 70 (0–100) | 30 (0–100) |
| Oral nutritional supplements | 10 (0–100) | 50 (0–100) | 30 (0–100) | 40 (0–100) |
| Addition of soluble fiber | 20 (0–70) | 0 (0–90) | 40 (0–70) | 20 (0–90) |
| Medications | Usage Median (Range, %) | Initial Dose Median (Range, mg/day) | Maximum Dose Median (Range, mg/day) | Dose Recommendation per Label (mg/day) |
|---|---|---|---|---|
| Loperamide (First-line) | 90 (40–100) | 8 (2–24) | 28 (4–64) | 2–6 mg QID; maximum daily dose, 16 mg |
| Codeine | 20 (0–100) | 60 (30–120) | 240 (60–480) | 15–60 mg QID |
| Tincture of opium | 10 (0–100) | 8 (12–17.5) | 35 (24–50) | 0.3–1 mL QID |
| Diphenoxylate and atropine | 0 (0–100) | 10 (7.5–20) | 20 (10–40) | 2.5–7.5 mg QID; maximum daily dose, 20–25 mg |
| Medication Class | Usage Median (Range, %) | Medications | Initial Dose Median (Range, mg/day) | Maximum Dose Median (Range, mg/day) | Dose Recommendation per Label (mg/day) |
|---|---|---|---|---|---|
| Proton-pump inhibitor (first-line) | 80 (0–100) | Pantoprazole | 40 (20–80) | 80 (40–160) | 20–40 mg BID |
| Omeprazole | 40 (20–80) | 80 | 20–40 mg BID | ||
| Esomeprazole | 80 | 80 | 20–40 mg BID | ||
| Lansoprazole | 60 | 60 | 15–30 BID | ||
| H2RA (oral) | 10 (0–30) | Famotidine | 40 (20–80) | 40 (20–80) | 20–40 mg BID |
| Ranitidine | 300 | 300 | 150–300 mg BID | ||
| Nizatidine | 150 | 300 | 150–300 mg BID | ||
| Somatostatin analogue | 10 (0–50) | Octreotide | 300 (150–600) μg | 600 (300–1500) μg | 50–250 μg SC TID or QID |
| H2RA (added to PS) | 0 (0–90) | Famotidine | 40 (20–80) | 40 (20–80) | 20–40 mg BID |
| Therapeutic Agents | Without Colon-in-Continuity Median (Range, %) | With Colon-in-Continuity Median (Range, %) |
|---|---|---|
| Bile acid binders | 0 (0–100) | 30 (0–100) |
| Fiber (e.g., psyllium) | 10 (0–90) | 20 (0–90) |
| Pancreatic enzymes | 20 (0–80) | 20 (0–80) |
| Probiotics | 0 (0–60) | 10 (0–100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakananurak, N.; Wall, E.; Catron, H.; Delgado, A.; Greif, S.; Herlitz, J.; Moccia, L.; Mercer, D.; Vanuytsel, T.; Kumpf, V.; et al. Real-World Management of High Stool Output in Patients with Short Bowel Syndrome: An International Multicenter Survey. Nutrients 2023, 15, 2763. https://doi.org/10.3390/nu15122763
Lakananurak N, Wall E, Catron H, Delgado A, Greif S, Herlitz J, Moccia L, Mercer D, Vanuytsel T, Kumpf V, et al. Real-World Management of High Stool Output in Patients with Short Bowel Syndrome: An International Multicenter Survey. Nutrients. 2023; 15(12):2763. https://doi.org/10.3390/nu15122763
Chicago/Turabian StyleLakananurak, Narisorn, Elizabeth Wall, Hilary Catron, Adela Delgado, Sophie Greif, Jean Herlitz, Lisa Moccia, David Mercer, Tim Vanuytsel, Vanessa Kumpf, and et al. 2023. "Real-World Management of High Stool Output in Patients with Short Bowel Syndrome: An International Multicenter Survey" Nutrients 15, no. 12: 2763. https://doi.org/10.3390/nu15122763
APA StyleLakananurak, N., Wall, E., Catron, H., Delgado, A., Greif, S., Herlitz, J., Moccia, L., Mercer, D., Vanuytsel, T., Kumpf, V., Berner-Hansen, M., & Gramlich, L. (2023). Real-World Management of High Stool Output in Patients with Short Bowel Syndrome: An International Multicenter Survey. Nutrients, 15(12), 2763. https://doi.org/10.3390/nu15122763






