Standard Hypercaloric, Hyperproteic vs. Leucine-Enriched Oral Supplements in Patients with Cancer-Induced Sarcopenia, a Randomized Clinical Trial
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
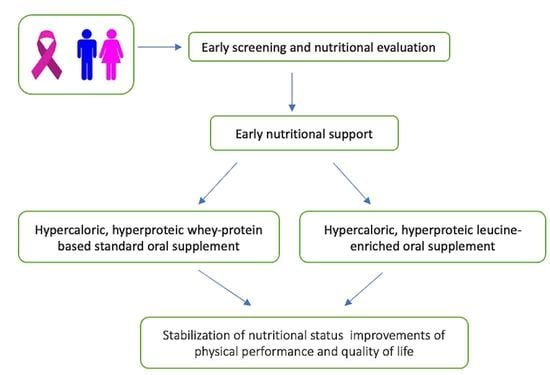
2.2. Study Design
2.3. Outcomes
2.4. Statistical Analysis
3. Results
Primary and Secondary Outcomes

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, A.; Manzoor, M.F.; Ahmad, N.; Aadil, R.M.; Qin, H.; Siddique, R.; Riaz, S.; Ahmad, A.; Korma, S.A.; Khalid, W.; et al. The Burden of Cancer, Government Strategic Policies, and Challenges in Pakistan: A Comprehensive Review. Front. Nutr. 2022, 9, 940514. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.D.A.I. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- World Health Organization. Cancer. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 20 February 2023).
- Beaudry, A.G.; Law, M.L. Leucine Supplementation in Cancer Cachexia: Mechanisms and a Review of the Pre-Clinical Literature. Nutrients 2022, 14, 2824. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J.M.; Stemmler, B.; Lopez-Soriano, F.J.; Busquets, S. Inter-tissue communication in cancer cachexia. Nat. Rev. Endocrinol. 2018, 15, 9–20. [Google Scholar] [CrossRef]
- Tsoli, M.; Robertson, G. Cancer cachexia: Malignant inflammation, tumorkines, and metabolic mayhem. Trends Endocrinol. Metab. 2013, 24, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef]
- Storck, L.J.; Ruehlin, M.; Gaeumann, S.; Gisi, D.; Schmocker, M.; Meffert, P.J.; Imoberdorf, R.; Pless, M.; Ballmer, P.E. Effect of a leucine-rich supplement in combination with nutrition and physical exercise in advanced cancer patients: A randomized controlled intervention trial. Clin. Nutr. 2020, 39, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Garcia Almeida, J.M.; Garcia Garcia, C.; Vegas Aguilar, I.M.; Bellido Castaneda, V.; Bellido Guerrero, D. Morphofunctional assessment of patient s nutritional status: A global approach. Nutr. Hosp. 2021, 38, 592–600. [Google Scholar] [CrossRef]
- Leon-Idougourram, S.; Perez-Gomez, J.M.; Munoz Jimenez, C.; Fernando, L.L.; Manzano Garcia, G.; Molina Puertas, M.J.; Herman-Sanchez, N.; Alonso-Echague, R.; Calanas Continente, A.; Galvez Moreno, M.A.; et al. Morphofunctional and Molecular Assessment of Nutritional Status in Head and Neck Cancer Patients Undergoing Systemic Treatment: Role of Inflammasome in Clinical Nutrition. Cancers 2022, 14, 494. [Google Scholar] [CrossRef]
- Carrillo Lozano, E.; Oses Zarate, V.; Campos Del Portillo, R. Nutritional management of gastric cancer. Endocrinol. Diabetes Nutr. 2021, 68, 428–438. [Google Scholar] [CrossRef]
- Higuera-Pulgar, I.; Ribed, A.; Carrascal-Fabian, M.L.; Romero-Jimenez, R.M.; Velasco-Gimeno, C.; Breton-Lesmes, I.; Camblor-Alvarez, M.; Cuerda-Compes, C.; Garcia-Peris, P. Evolution of nutritional status and survival in patients with cancer on tyrosine kinase inhibitors treatment. Endocrinol. Diabetes Nutr. 2019, 66, 472–479. [Google Scholar] [CrossRef]
- Herrera-Martinez, Y.; Alzas Teomiro, C.; Leon Idougourram, S.; Molina Puertas, M.J.; Calanas Continente, A.; Serrano Blanch, R.; Castano, J.P.; Galvez Moreno, M.A.; Gahete, M.D.; Luque, R.M.; et al. Sarcopenia and Ghrelin System in the Clinical Outcome and Prognosis of Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers 2021, 14, 111. [Google Scholar] [CrossRef]
- Wigmore, S.J.; Plester, C.E.; Ross, J.A.; Fearon, K.C. Contribution of anorexia and hypermetabolism to weight loss in anicteric patients with pancreatic cancer. Br. J. Surg. 1997, 84, 196–197. [Google Scholar] [PubMed]
- Muscaritoli, M.; Corsaro, E.; Molfino, A. Awareness of Cancer-Related Malnutrition and Its Management: Analysis of the Results From a Survey Conducted Among Medical Oncologists. Front. Oncol. 2021, 11, 682999. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8, 1211. [Google Scholar] [CrossRef] [PubMed]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef]
- Johnston, B.C.; Seivenpiper, J.L.; Vernooij, R.W.M.; de Souza, R.J.; Jenkins, D.J.A.; Zeraatkar, D.; Bier, D.M.; Guyatt, G.H. The Philosophy of Evidence-Based Principles and Practice in Nutrition. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 189–199. [Google Scholar] [CrossRef]
- Ocon Breton, M.J.; Tapia Guerrero, M.J.; Ramirez Rodriguez, J.M.; Peteiro Miranda, C.; Ballesteros Pomar, M.D.; Botella Romero, F.; Martinez Olmos, M.A.; Luengo Perez, L.M.; Cancer Minchot, E.; Garcia Malpartida, K.; et al. Multidisciplinary consensus on nutritional and metabolic therapy in enhanced recovery after abdominal surgery programs: NutRICA Project. Endocrinol. Diabetes Nutr. 2022, 69, 98–111. [Google Scholar] [CrossRef]
- Barajas-Galindo, D.E.; Vidal-Casariego, A.; Pintor-de la Maza, B.; Fernandez-Martinez, P.; Ramos-Martinez, T.; Garcia-Arias, S.; Hernandez-Moreno, A.; Urioste-Fondo, A.; Cano-Rodriguez, I.; Ballesteros-Pomar, M.D. Postoperative enteral immunonutrition in head and neck cancer patients: Impact on clinical outcomes. Endocrinol. Diabetes Nutr. 2020, 67, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Dreyer, H.C.; Drummond, M.J.; Glynn, E.L.; Cadenas, J.G.; Yoshizawa, F.; Volpi, E.; Rasmussen, B.B. Nutrient signalling in the regulation of human muscle protein synthesis. J. Physiol. 2007, 582 Pt 2, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Fuchs, C.S.; Niedzwiecki, D.; Zhang, S.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Association of Survival with Adherence to the American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors after Colon Cancer Diagnosis: The CALGB 89803/Alliance Trial. JAMA Oncol. 2018, 4, 783–790. [Google Scholar] [CrossRef]
- Tang, J.E.; Phillips, S.M. Maximizing muscle protein anabolism: The role of protein quality. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 66–71. [Google Scholar] [CrossRef]
- White, P.J.; Newgard, C.B. Branched-chain amino acids in disease. Science 2019, 363, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Safar, A.; Schutzler, S.; Memelink, R.; Ferrando, A.; Spencer, H.; van Helvoort, A.; Wolfe, R.R. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin. Nutr. 2011, 30, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Vashi, P.G.; Trukova, K.; Lis, C.G.; Lammersfeld, C.A. Prevalence of serum vitamin D deficiency and insufficiency in cancer: Review of the epidemiological literature. Exp. Ther. Med. 2011, 2, 181–193. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Muzio, V.; Caini, S.; Raimondi, S.; Martinoli, C.; Chiocca, S.; Miccolo, C.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; et al. Vitamin D Supplementation and Cancer Mortality: Narrative Review of Observational Studies and Clinical Trials. Nutrients 2021, 13, 3285. [Google Scholar] [CrossRef]
- Rondanelli, M.; Klersy, C.; Terracol, G.; Talluri, J.; Maugeri, R.; Guido, D.; Faliva, M.A.; Solerte, B.S.; Fioravanti, M.; Lukaski, H.; et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am. J. Clin. Nutr. 2016, 103, 830–840. [Google Scholar] [CrossRef]
- Rondanelli, M.; Cereda, E.; Klersy, C.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Gasparri, C.; Iannello, G.; Spadaccini, D.; Infantino, V.; et al. Improving rehabilitation in sarcopenia: A randomized-controlled trial utilizing a muscle-targeted food for special medical purposes. J. Cachexia Sarcopenia Muscle 2020, 11, 1535–1547. [Google Scholar] [CrossRef]
- Barichella, M.; Cereda, E.; Pinelli, G.; Iorio, L.; Caroli, D.; Masiero, I.; Ferri, V.; Cassani, E.; Bolliri, C.; Caronni, S.; et al. Muscle-targeted nutritional support for rehabilitation in patients with parkinsonian syndrome. Neurology 2019, 93, e485–e496. [Google Scholar] [CrossRef]
- Bergia, R.E.; Hudson, J.L.; Campbell, W.W. Effect of whey protein supplementation on body composition changes in women: A systematic review and meta-analysis. Nutr. Rev. 2018, 76, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Sepandi, M.; Samadi, M.; Shirvani, H.; Alimohamadi, Y.; Taghdir, M.; Goudarzi, F.; Akbarzadeh, I. Effect of whey protein supplementation on weight and body composition indicators: A meta-analysis of randomized clinical trials. Clin. Nutr. ESPEN 2022, 50, 74–83. [Google Scholar] [CrossRef]
- Guo, Y.; Fu, X.; Hu, Q.; Chen, L.; Zuo, H. The Effect of Leucine Supplementation on Sarcopenia-Related Measures in Older Adults: A Systematic Review and Meta-Analysis of 17 Randomized Controlled Trials. Front. Nutr. 2022, 9, 929891. [Google Scholar] [CrossRef] [PubMed]
- Gómez de Tejada Romero, M.J. Acciones extraóseas de la vitamina D. Rev. Osteoporos. Metab. Miner. 2014, 6, 2173–2345. [Google Scholar] [CrossRef]
- León, S.; Alcántara-Laguna, M.; Molina-Puerta, M.J.; Gálvez-Morenos, M.A.; Herrera-Martínez, A.D. 25-OH-vitamin D and reversal of metabolic comorbidities associated with obesity after bariatric surgery. Rev. Osteoporos. Metab. Miner. 2022, 14, 42–47. [Google Scholar] [CrossRef]
- González-Rozas, M.P.C.J. Regulación endocrina del metabolismo energético a través del hueso. Rev. Osteoporos. Metab. Miner. 2014, 6, 57–62. [Google Scholar] [CrossRef]
- Enko, D.; Moro, T.; Holasek, S.; Baranyi, A.; Schnedl, W.J.; Zelzer, S.; Mangge, H.; Herrmann, M.; Meinitzer, A. Branched-chain amino acids are linked with iron metabolism. Ann. Transl. Med. 2020, 8, 1569. [Google Scholar] [CrossRef]
- Vlachos, A.; Atsidaftos, E.; Lababidi, M.L.; Muir, E.; Rogers, Z.R.; Alhushki, W.; Bernstein, J.; Glader, B.; Gruner, B.; Hartung, H.; et al. L-leucine improves anemia and growth in patients with transfusion-dependent Diamond-Blackfan anemia: Results from a multicenter pilot phase I/II study from the Diamond-Blackfan Anemia Registry. Pediatr. Blood Cancer 2020, 67, e28748. [Google Scholar] [CrossRef]
- Hayaishi, S.; Chung, H.; Kudo, M.; Ishikawa, E.; Takita, M.; Ueda, T.; Kitai, S.; Inoue, T.; Yada, N.; Hagiwara, S.; et al. Oral branched-chain amino acid granules reduce the incidence of hepatocellular carcinoma and improve event-free survival in patients with liver cirrhosis. Dig. Dis. 2011, 29, 326–332. [Google Scholar] [CrossRef]
- Liu, K.A.; Lashinger, L.M.; Rasmussen, A.J.; Hursting, S.D. Leucine supplementation differentially enhances pancreatic cancer growth in lean and overweight mice. Cancer Metab. 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Miura, K.; Nakano, S.; Suzuki, K.; Bannai, M.; Inoue, Y. Leucine-enriched essential amino acids attenuate inflammation in rat muscle and enhance muscle repair after eccentric contraction. Amino Acids 2016, 48, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Nepon, H.; Safran, T.; Reece, E.M.; Murphy, A.M.; Vorstenbosch, J.; Davison, P.G. Radiation-Induced Tissue Damage: Clinical Consequences and Current Treatment Options. Semin. Plast. Surg. 2021, 35, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; Micewicz, E.D.; Ratikan, J.A.; Xie, M.W.; Cheng, G.; McBride, W.H. Radiation and inflammation. Semin. Radiat. Oncol. 2015, 25, 4–10. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Total (n = 46) | Standard OS (n = 25) | Leucine-Enriched OS (n = 21) | p |
|---|---|---|---|---|
| Sex (♂/♀) | 45.7/54.3% (21/25) | 48/52% (12/13) | 42.9/57.1% (9/12) | 0.48 |
| Age at diagnosis (years) | 74.5 (71–78) | 66 (56–74) | 71 (53–78) | 0.7 |
| Tobacco exposure | 0.26 | |||
| No. | 50% (21/42) | 54.2% (13/24) | 44.4% (8/18) | |
| Active | 28.6.% (12/42) | 33.3% (8/24) | 22.2% (4/18) | |
| Previous exposure | 21.4% (9/42) | 12.5% (3/24) | 33.3% (6/18) | |
| Previous other neoplasms | 19.7% (9/46) | 8% (2/25) | 33.3% (7/21) | 0.04 |
| Tumor localization | ||||
| Head/neck | 13% (6/46) | 12 (3/25) | 14.3% (3/21) | |
| Gastrointestinal NET | 8.7% (4/46) | 16 (4/25) | 0% | |
| Gastric cancer | 8.7% (4/46) | 8 (2/25) | 9.5 (2/21) | |
| Colorectal cancer | 19.6% (9/46) | 12 (3/25) | 28.6% (6/21) | |
| Urothelial | 32.6% (15/46) | 24 (7/25) | 9.5% (2/21) | |
| Other | 17.4.% (8/46) | 28 (6/25) | 38.1 (8/21) | |
| Treatment | ||||
| Surgery | 63% (29/46) | 68% (17/25) | 55.1% (12/21) | 0.33 |
| Chemotherapy | 56.5% (26/46) | 52% (13/25) | 61.9% (13/21) | 0.35 |
| Radiotherapy | 19.6% (9/46) | 4% (1/25) | 8% (8/21) | 0.005 |
| Combined treatment | 47.8 (22/46) | 48% (12/25) | 47.6% (10/21) | 0.61 |
| Symptoms | ||||
| Weight loss (3 months) | 84.4% (38/45) | 79.2% (19/24) | 90.5% (19/21) | 0.27 |
| Weight loss in kg (3 months) | 5 (4–6) | 3 (0–19) | 6 (2–6) | 0.5 |
| Weight loss (6 months) | 71.7% (33/46) | 60% (15/25) | 85.7% (18/21) | 0.053 |
| Weight loss in kg (6 months) | 6.5 (6–7) | 3 (0–19) | 6 (4–10) | 0.8 |
| Gastrointestinal symptoms | 43.5% (20/46) | 36% (9/25) | 52.4% (11/21) | 0.21 |
| Abdominal pain | 32.6% (15/46) | 24% (6/25) | 42.9% (9/21) | 0.15 |
| Nausea/vomiting | 22.2% (10/45) | 16.7% (4/25) | 28.6% (6/21) | 0.27 |
| Diarrhea | 15.2% (7/46) | 16% (4/25) | 14.3 (3/21) | 0.60 |
| Dyspnea | 17.4% (8/46) | 20% (5/25) | 14.3% (3/21) | 0.46 |
| Mucositis | 8.7% (4/46) | 4% (1/25) | 14.3% (3/25) | 0.24 |
| Quality of life | ||||
| Any level of dependency | 43.5% (20/46) | 44 (11/25) | 42.9 (9/21) | 0.59 |
| Self-rated health score | 65 (0–80) | 70 (35–84) | 60 (45–75) | 0.3 |
| ECOG | 0.3 | |||
| ECOG 0 | 60.9 (28/46) | 60 (15/25) | 61.9 (13/21) | |
| ECOG 1 | 28.3 (13/46) | 28 (7/21) | 28.6 (6/21) | |
| ECOG 2 | 10.9 (5/46) | 12 (3/25) | 9.5 (2/21) | |
| Mortality | 8.7 (4/46) | 12 (3/25) | 4.8% (1/25) | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Martínez, A.D.; León Idougourram, S.; Muñoz Jiménez, C.; Rodríguez-Alonso, R.; Alonso Echague, R.; Chica Palomino, S.; Sanz Sanz, A.; Manzano García, G.; Gálvez Moreno, M.Á.; Calañas Continente, A.; et al. Standard Hypercaloric, Hyperproteic vs. Leucine-Enriched Oral Supplements in Patients with Cancer-Induced Sarcopenia, a Randomized Clinical Trial. Nutrients 2023, 15, 2726. https://doi.org/10.3390/nu15122726
Herrera-Martínez AD, León Idougourram S, Muñoz Jiménez C, Rodríguez-Alonso R, Alonso Echague R, Chica Palomino S, Sanz Sanz A, Manzano García G, Gálvez Moreno MÁ, Calañas Continente A, et al. Standard Hypercaloric, Hyperproteic vs. Leucine-Enriched Oral Supplements in Patients with Cancer-Induced Sarcopenia, a Randomized Clinical Trial. Nutrients. 2023; 15(12):2726. https://doi.org/10.3390/nu15122726
Chicago/Turabian StyleHerrera-Martínez, Aura D., Soraya León Idougourram, Concepción Muñoz Jiménez, Rosa Rodríguez-Alonso, Rosario Alonso Echague, Sonia Chica Palomino, Ana Sanz Sanz, Gregorio Manzano García, María Ángeles Gálvez Moreno, Alfonso Calañas Continente, and et al. 2023. "Standard Hypercaloric, Hyperproteic vs. Leucine-Enriched Oral Supplements in Patients with Cancer-Induced Sarcopenia, a Randomized Clinical Trial" Nutrients 15, no. 12: 2726. https://doi.org/10.3390/nu15122726
APA StyleHerrera-Martínez, A. D., León Idougourram, S., Muñoz Jiménez, C., Rodríguez-Alonso, R., Alonso Echague, R., Chica Palomino, S., Sanz Sanz, A., Manzano García, G., Gálvez Moreno, M. Á., Calañas Continente, A., & Molina Puertas, M. J. (2023). Standard Hypercaloric, Hyperproteic vs. Leucine-Enriched Oral Supplements in Patients with Cancer-Induced Sarcopenia, a Randomized Clinical Trial. Nutrients, 15(12), 2726. https://doi.org/10.3390/nu15122726