Does Self-Perceived Diet Quality Align with Nutrient Intake? A Cross-Sectional Study Using the Food Nutrient Index and Diet Quality Score
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Nutrient Intake Assessment
2.3. Self-Perceived Diet Quality
2.4. The Diet Quality Score
2.5. The Total Nutrient Index and Food Nutrient Index
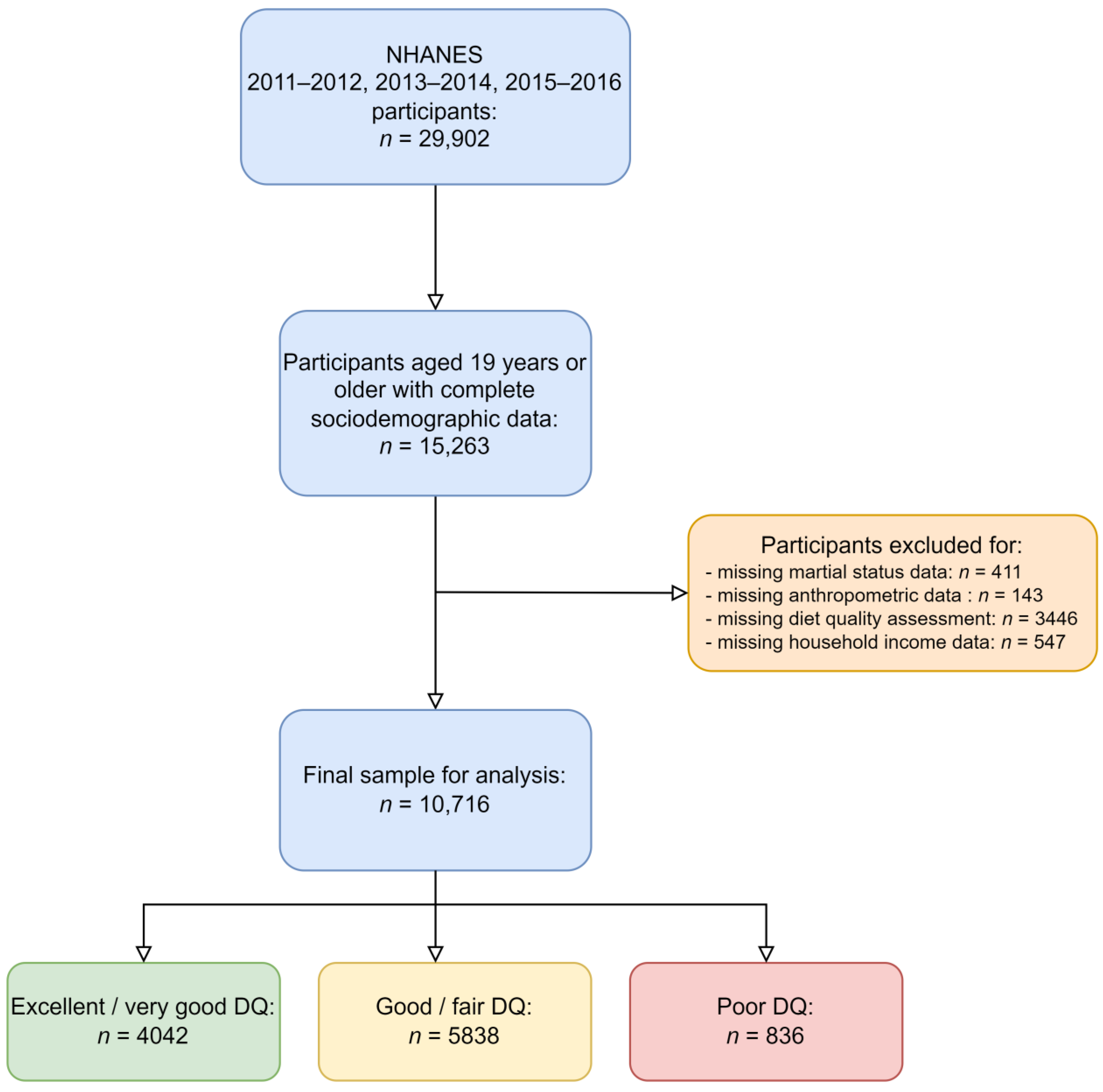
2.6. Inclusion and Exclusion Criteria
2.7. Ethical Approval
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pye, A.; Bash, K.; Joiner, A.; Beenstock, J. Good for the Planet and Good for Our Health: The Evidence for Whole-Food Plant-Based Diets. BJPsych Int. 2022, 19, 90–92. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Huston, P. A Sedentary and Unhealthy Lifestyle Fuels Chronic Disease Progression by Changing Interstitial Cell Behaviour: A Network Analysis. Front. Physiol. 2022, 13, 904107. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Kris-Etherton, P.M. Diet Quality Assessment and the Relationship between Diet Quality and Cardiovascular Disease Risk. Nutrients 2021, 13, 4305. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Kopp, W. How Western Diet and Lifestyle Drive the Pandemic of Obesity and Civilization Diseases. DMSO 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Ford, E.S.; Croft, J.B.; Posner, S.F.; Goodman, R.A.; Giles, W.H. Co-Occurrence of Leading Lifestyle-Related Chronic Conditions Among Adults in the United States, 2002–2009. Prev. Chronic Dis. 2013, 10, E60. [Google Scholar] [CrossRef]
- Health and Economic Costs of Chronic Diseases|CDC. 2023. Available online: https://www.cdc.gov/chronicdisease/about/costs/index.htm (accessed on 16 April 2023).
- Conn, S.; Curtain, S. Health Coaching as a Lifestyle Medicine Process in Primary Care. Aust. J. Gen. Pract. 2019, 48, 677–680. [Google Scholar] [CrossRef]
- Ek, A.; Ekblom, Ö.; Ekström, M.; Börjesson, M.; Kallings, L.V. The Gap between Stated Importance of and Clinical Work in Promoting Healthy Lifestyle Habits by Healthcare Professionals in a Swedish Hospital Setting: A Cross-Sectional Survey. Health Soc. Care Community 2021, 29, 385–394. [Google Scholar] [CrossRef]
- Gordon, N.F.; Salmon, R.D.; Wright, B.S.; Faircloth, G.C.; Reid, K.S.; Gordon, T.L. Clinical Effectiveness of Lifestyle Health Coaching: Case Study of an Evidence-Based Program. Am. J. Lifestyle Med. 2017, 11, 153–166. [Google Scholar] [CrossRef]
- Dalwood, P.; Marshall, S.; Burrows, T.L.; McIntosh, A.; Collins, C.E. Diet Quality Indices and Their Associations with Health-Related Outcomes in Children and Adolescents: An Updated Systematic Review. Nutr. J. 2020, 19, 118. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Smith, T.A.; Wendt, M. How Americans Rate Their Diet Quality: An Increasingly Realistic Perspective. Available online: http://www.ers.usda.gov/publications/pub-details/?pubid=44593 (accessed on 16 April 2023).
- Dietary Guidelines for Americans. Available online: https://www.dietaryguidelines.gov/ (accessed on 8 April 2023).
- Rodrigues, P.R.M.; Gonçalves-Silva, R.M.V.; Ferreira, M.G.; Pereira, R.A. Feasibility of using of a simplified question in assessing diet quality of adolescents. Cien. Saude Colet. 2017, 22, 1565–1578. [Google Scholar] [CrossRef] [PubMed]
- Strachan, S.M.; Brawley, L.R. Healthy-Eater Identity and Self-Efficacy Predict Healthy Eating Behavior: A Prospective View. J. Health Psychol. 2009, 14, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Liu, J.; Cheskin, L.J.; Sheppard, V.B. Discrepancy between Perceived Diet Quality and Actual Diet Quality among US Adult Cancer Survivors. Eur. J. Clin. Nutr. 2020, 74, 1457–1464. [Google Scholar] [CrossRef]
- Fanelli, S.; Heitman, K.; Pisegna, J.; Kelly, O.; Krok-Schoen, J.; Taylor, C. Comparison between Self-Perceived and Actual Diet Quality by Diabetes Status in US Adults. J. Acad. Nutr. Diet. 2020, 120, A76. [Google Scholar] [CrossRef]
- Cowan, A.E.; Bailey, R.L.; Jun, S.; Dodd, K.W.; Gahche, J.J.; Eicher-Miller, H.A.; Guenther, P.M.; Dwyer, J.T.; Potischman, N.; Bhadra, A.; et al. The Total Nutrient Index Is a Useful Measure for Assessing Total Micronutrient Exposures among US Adults. J. Nutr. 2022, 152, 863–871. [Google Scholar] [CrossRef]
- Cowan, A.E.; Jun, S.; Tooze, J.A.; Dodd, K.W.; Gahche, J.J.; Eicher-Miller, H.A.; Guenther, P.M.; Dwyer, J.T.; Potischman, N.; Bhadra, A.; et al. A Narrative Review of Nutrient Based Indexes to Assess Diet Quality and the Proposed Total Nutrient Index That Reflects Total Dietary Exposures. Crit. Rev. Food Sci. Nutr. 2023, 63, 1722–1732. [Google Scholar] [CrossRef]
- Fitzgerald, A.L.; Dewar, R.A.; Veugelers, P.J. Diet Quality and Cancer Incidence in Nova Scotia, Canada. Nutr. Cancer 2002, 43, 127–132. [Google Scholar] [CrossRef]
- NHANES-About the National Health and Nutrition Examination Survey. 2022. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 15 April 2023).
- NHANES-Video-The NHANES Story. 2022. Available online: https://www.cdc.gov/nchs/nhanes/nhanes-story.htm (accessed on 15 April 2023).
- Krok-Schoen, J.L.; Archdeacon Price, A.; Luo, M.; Kelly, O.J.; Taylor, C.A. Low Dietary Protein Intakes and Associated Dietary Patterns and Functional Limitations in an Aging Population: A NHANES Analysis. J. Nutr. Health Aging 2019, 23, 338–347. [Google Scholar] [CrossRef]
- Vaudin, A.M.; Moshfegh, A.J.; Sahyoun, N.R. Measuring Food Insecurity in Older Adults Using Both Physical and Economic Food Access, NHANES 2013–18. J. Nutr. 2022, 152, 1953–1962. [Google Scholar] [CrossRef]
- NHANES–Survey Content Brochure. 2022. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/survey_contents.pdf (accessed on 15 April 2023).
- Li, R.; Chen, Z. Validation and Comparison of Two Dietary Indexes for Predicting Nonalcoholic Fatty Liver Disease in US Adults. J. Nutr. 2022, 152, 2865–2876. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Cowan, A.E.; Bailey, R.L.; Jun, S.; Eicher-Miller, H.A. Usual Nutrient Intake and Dietary Quality of Low-Income U.S. Older Adults. Appl. Econ. Perspect. Policy 2023, 45, 317–335. [Google Scholar] [CrossRef]
- Cowan, A.E.; Tooze, J.A.; Gahche, J.J.; Eicher-Miller, H.A.; Guenther, P.M.; Dwyer, J.T.; Potischman, N.; Bhadra, A.; Carroll, R.J.; Bailey, R.L. Trends in Overall and Micronutrient-Containing Dietary Supplement Use in US Adults and Children, NHANES 2007–2018. J. Nutr. 2022, 152, 2789–2801. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Cowan, A.E.; Dwyer, J.T.; Campbell, W.W.; Thalacker-Mercer, A.E.; Gahche, J.J.; Bailey, R.L. Dietary Protein Intake Is Positively Associated with Appendicular Lean Mass and Handgrip Strength among Middle-Aged US Adults. J. Nutr. 2021, 151, 3755–3763. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Malek, A.M.; Hunt, K.J.; Marriott, B.P. Nutrients in the US Diet: Naturally Occurring or Enriched/Fortified Food and Beverage Sources, Plus Dietary Supplements: NHANES 2009–2012. J. Nutr. 2019, 149, 1404–1412. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Dwyer, J.; Terry, A.; Moshfegh, A.; Johnson, C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy12. Adv. Nutr. 2016, 7, 121–134. [Google Scholar] [CrossRef]
- NHANES-Diet Behavior & Nutrition Section. Available online: https://wwwn.cdc.gov/nchs/nhanes/2013-2014/dbq_h.htm (accessed on 15 April 2023).
- Institute of Medicine (US) Subcommittee on Interpretation and Uses of Dietary Reference Intakes; Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. DRI Dietary Reference Intakes: Applications in Dietary Assessment; National Academies Press (US): Washington, DC, USA, 2000. Available online: http://www.ncbi.nlm.nih.gov/books/NBK222890/ (accessed on 7 April 2023).
- NHANES-NCHS Research Ethics Review Board Approval. 2022. Available online: https://www.cdc.gov/nchs/nhanes/irba98.htm (accessed on 15 April 2023).
- NHANES Tutorials-Weighting Module. Available online: https://wwwn.cdc.gov/nchs/nhanes/tutorials/weighting.aspx (accessed on 15 April 2023).
- Heeringa, S.G.; West, B.T.; Berglund, P.A. Applied Survey Data Analysis Routledge & CRC Press. Available online: https://www.routledge.com/Applied-Survey-Data-Analysis/Heeringa-West-Berglund/p/book/9780367736118 (accessed on 15 April 2023).
- Parker, J.D.; Talih, M.; Malec, D.J.; Beresovsky, V.; Carroll, M.; Gonzalez, J.F.; Hamilton, B.E.; Ingram, D.D.; Kochanek, K.; McCarty, F.; et al. National Center for Health Statistics Data Presentation Standards for Proportions. Vital Health Stat. 2017, 2, 1–22. [Google Scholar]
- Ward, B.W. Kg_nchs: A Command for Korn-Graubard Confidence Intervals and National Center for Health Statistics’ Data Presentation Standards for Proportions. Stata J. 2019, 19, 510–522. [Google Scholar] [CrossRef]
- Long, T.; Zhang, K.; Chen, Y.; Wu, C. Trends in Diet Quality Among Older US Adults from 2001 to 2018. JAMA Netw. Open 2022, 5, e221880. [Google Scholar] [CrossRef]
- Visioli, F.; Marangoni, F.; Poli, A.; Ghiselli, A.; Martini, D. Nutrition and Health or Nutrients and Health? Int. J. Food Sci. Nutr. 2022, 73, 141–148. [Google Scholar] [CrossRef]
- Kostelanetz, S.; Pettapiece-Phillips, M.; Weems, J.; Spalding, T.; Roumie, C.; Wilkins, C.H.; Kripalani, S. Health Care Professionals’ Perspectives on Universal Screening of Social Determinants of Health: A Mixed-Methods Study. Popul. Health Manag. 2022, 25, 367–374. [Google Scholar] [CrossRef]
- Storz, M.A. When the Desire for Lifestyle Medicine Counseling Remains Unfulfilled: A Case Report. J. Patient Exp. 2021, 8, 2374373521996949. [Google Scholar] [CrossRef]
- Storz, M.A. Is There a Lack of Support for Whole-Food, Plant-Based Diets in the Medical Community? Perm. J. 2018, 23, 18–068. [Google Scholar] [CrossRef]
- Glanz, K.; Brug, J.; van Assema, P. Are Awareness of Dietary Fat Intake and Actual Fat Consumption Associated?—A Dutch-American Comparison. Eur. J. Clin. Nutr. 1997, 51, 542–547. [Google Scholar] [CrossRef]
- Variyam, J.N.; Shim, Y.; Blaylock, J. Consumer Misperceptions of Diet Quality. J. Nutr. Educ. 2001, 33, 314–321. [Google Scholar] [CrossRef]
- Brug, J.; van Assema, P.; Kok, G.; Lenderink, T.; Glanz, K. Self-Rated Dietary Fat Intake: Association with Objective Assessment of Fat, Psychosocial Factors, and Intention to Change. J. Nutr. Educ. 1994, 26, 218–223. [Google Scholar] [CrossRef]
- Young, L.M.; Gauci, S.; Scholey, A.; White, D.J.; Lassemillante, A.-C.; Meyer, D.; Pipingas, A. Self-Reported Diet Quality Differentiates Nutrient Intake, Blood Nutrient Status, Mood, and Cognition: Implications for Identifying Nutritional Neurocognitive Risk Factors in Middle Age. Nutrients 2020, 12, 2964. [Google Scholar] [CrossRef]
- Mäkelä, I.; Koivuniemi, E.; Vahlberg, T.; Raats, M.M.; Laitinen, K. Self-Reported Parental Healthy Dietary Behavior Relates to Views on Child Feeding and Health and Diet Quality. Nutrients 2023, 15, 1024. [Google Scholar] [CrossRef]
- Badri, M.A.; Alkhaili, M.; Aldhaheri, H.; Alnahyan, H.; Yang, G.; Albahar, M.; Alrashdi, A. Understanding the Interactions of Happiness, Self-Rated Health, Mental Feelings, Habit of Eating Healthy and Sport/Activities: A Path Model for Abu Dhabi. Nutrients 2022, 14, 55. [Google Scholar] [CrossRef]
- Amaro-Rivera, K.; Carbone, E. Factors Associated with Self-Perceived Diet Quality among Puerto Rican Adults (P04-092-19). Curr. Dev. Nutr. 2019, 3, nzz051.P04-092-19. [Google Scholar] [CrossRef]
- Batis, C.; Castellanos-Gutiérrez, A.; Aburto, T.C.; Jiménez-Aguilar, A.; Rivera, J.A.; Ramírez-Silva, I. Self-Perception of Dietary Quality and Adherence to Food Groups Dietary Recommendations among Mexican Adults. Nutr. J. 2020, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.P.; Vaz, J.D.S.; Mendoza-Sassi, R.A. Positive Self-Perception of Diet: A Population-Based Study in the Far South of Brazil. Epidemiol. Serv. Saude 2019, 28, e2018197. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.E.; Jun, S.; Gahche, J.J.; Tooze, J.A.; Dwyer, J.T.; Eicher-Miller, H.A.; Bhadra, A.; Guenther, P.M.; Potischman, N.; Dodd, K.W.; et al. Dietary Supplement Use Differs by Socioeconomic and Health-Related Characteristics among U.S. Adults, NHANES 2011–2014. Nutrients 2018, 10, 1114. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Stierman, B.; Gahche, J.J.; Potischman, N. Dietary Supplement Use Among Adults: United States, 2017–2018. NCHS Data Brief 2021, 1–8. [Google Scholar]
- Stookey, J.D. Analysis of 2009⁻2012 Nutrition Health and Examination Survey (NHANES) Data to Estimate the Median Water Intake Associated with Meeting Hydration Criteria for Individuals Aged 12⁻80 in the US Population. Nutrients 2019, 11, 657. [Google Scholar] [CrossRef]
- Adams, S.A.; Matthews, C.E.; Ebbeling, C.B.; Moore, C.G.; Cunningham, J.E.; Fulton, J.; Hebert, J.R. The Effect of Social Desirability and Social Approval on Self-Reports of Physical Activity. Am. J. Epidemiol. 2005, 161, 389–398. [Google Scholar] [CrossRef]
- Gibson, R. Principles of Nutritional Assessment, 2nd ed.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Satija, A.; Yu, E.; Willett, W.C.; Hu, F.B. Understanding Nutritional Epidemiology and Its Role in Policy. Adv. Nutr. 2015, 6, 5–18. [Google Scholar] [CrossRef]
- Steinfeldt, L.C.; Martin, C.L.; Clemens, J.C.; Moshfegh, A.J. Comparing Two Days of Dietary Intake in What We Eat in America (WWEIA), NHANES, 2013–2016. Nutrients 2021, 13, 2621. [Google Scholar] [CrossRef]

|
Excellent/Very Good DQ n = 4042 |
Good/Fair DQ n = 5838 |
Poor DQ n = 836 | p -Value | |
|---|---|---|---|---|
| Sex | 0.824 b | |||
| Male | 46.95% (0.84) | 47.76% (0.89) | 46.73% (3.01) | |
| Female | 53.05% (0.84) | 52.24% (0.89) | 53.27% (3.01) | |
| Age (years) | 51.49 (0.53) | 47.11 (0.44) | 43.14 (0.96) | <0.001 c |
| Marital status | <0.001 b | |||
| Married/Living with Partner | 67.37 (1.47) | 62.74% (1.27) | 45.86% (2.64) e | |
| Widowed/Divorced/Separated | 17.34% (0.98) | 18.03% (0.88) | 26.45% (2.21) e | |
| Never married | 15.29% (1.12) | 19.22% (1.33) | 27.69% (2.33) e | |
| Annual household income | <0.001 b | |||
| <$20,000 | 11.42% (0.96) | 14.73% (0.91) | 22.28% (2.19) e | |
| >$20,000 | 88.58% (0.96) | 85.27% (0.91) | 77.72% (2.19) e | |
| Education Level | <0.001 b | |||
| Less than 9th grade | 2.86% (0.42) | 4.39% (0.42) | 6.09% (1.14) e | |
| 9–11th grade | 6.80% (0.68) | 9.17% (0.70) | 17.91% (1.50) e | |
| High school graduate/GED d | 16.47% (1.02) | 21.97% (1.11) | 27.30% (2.64) e | |
| Some college or AA degree | 29.77% (1.28) | 34.13% (1.12) | 37.08% (2.42) e | |
| College graduate or above | 44.10% (1.89) | 30.34% (1.69) | 11.64% (1.75) e | |
| Race/ethnicity | <0.001 b | |||
| Mexican American | 3.63% (0.54) | 7.86% (1.05) | 13.56% (2.26) e | |
| Other Hispanic | 3.95% (0.49) | 6.03% (0.78) | 6.81% (1.28) e | |
| Non-Hispanic White | 73.85% (1.76) | 67.46% (2.16) | 57.02% (3.20) e | |
| Non-Hispanic Black | 8.65% (0.93) | 10.47% (1.15) | 16.33% (1.99) e | |
| Other Race a | 9.92% (0.84) | 8.18% (0.75) | 6.27% (1.06) e | |
| BMI (kg/m2) | 27.24 (0.14) | 28.97 (0.15) | 32.98 (0.47) | <0.001 c |
| Excellent/Very Good DQ n = 4042 | Good/Fair DQ n = 5838 | Poor DQ n = 836 | p-Value | |
|---|---|---|---|---|
| Total energy intake (kcal/d) | 2085.88 (20.46) | 2148.98 (16.65) | 2210.01 (57.80) | 0.038 |
| Carbohydrate intake (g/d) | 244.57 (2.50) | 254.51 (2.31) | 267.53 (8.59) | 0.008 |
| Carbohydrate intake (%tE) | 47.31 (0.26) | 48.00 (0.25) | 48.85 (0.52) | 0.008 |
| Protein intake (g/d) | 84.40 (1.09) | 82.37 (0.61) | 78.06 (2.13) | 0.026 |
| Protein intake (%tE) | 16.60 (0.17) | 15.75 (0.12) | 14.51 (0.29) | 0.026 |
| Fat intake (g/d) | 80.43 (1.04) | 83.57 (0.83) | 85.80 (2.40) | 0.036 |
| Fat intake (%tE) | 34.13 (0.23) | 34.43 (0.21) | 34.34 (0.39) | 0.036 |
| Saturated fat intake (g/d) | 25.31 (0.35) | 27.19 (0.33) | 28.48 (0.91) | <0.001 |
| Saturated fat intake (%tE) | 10.68 (0.09) | 11.16 (0.0844) | 11.31 (0.20) | <0.001 |
| Fiber intake (g/d) | 19.60 (0.32) | 17.03 (0.20) | 13.97 (0.49) | <0.001 |
| Vitamin A intake (mcg RAE/d) | 731.38 (16.52) | 628.68 (22.74) | 539.34 (34.09) | <0.001 |
| Vitamin C intake (mg/d) | 96.87 (2.64) | 77.75 (2.12) | 63.01 (3.66) | <0.001 |
| Vitamin D intake (IE/d) | 212.82 (7.06) | 181.56 (3.07) | 163.24 (12.83) | <0.001 |
| Vitamin E intake (mg/d) | 10.21 (0.21) | 9.03 (0.12) | 7.75 (0.27) | <0.001 |
| Vitamin B1 intake (mg/d) | 1.63 (0.02) | 1.62 (0.01) | 1.50 (0.05) | 0.034 |
| Vitamin B2 intake (mg/d) | 2.26 (0.02) | 2.15 (0.02) | 2.094 (0.09) | 0.003 |
| Vitamin B3 intake (mg/d) | 25.84 (0.27) | 26.01 (0.25) | 26.46 (1.01) | 0.812 |
| Vitamin B6 intake (mg/d) | 2.25 (0.03) | 2.11 (0.02) | 2.10 (0.09) | 0.002 |
| Vitamin B12 intake (mcg/d) | 5.19 (0.11) | 5.07 (0.19) | 5.33 (0.33) | 0.783 |
| Phosphorus intake (mg/d) | 1425.97 (16.45) | 1389.29 (8.91) | 1340.44 (37.49) | 0.047 |
| Magnesium intake (mg/d) | 338.49 (4.23) | 300.62 (2.91) | 267.35 (7.76) | <0.001 |
| Potassium intake (mg/d) | 2913.41 (33.74) | 2639.44 (20.34) | 2369.10 (65.74) | <0.001 |
| Calcium intake (mg/d) | 989.81 (15.07) | 965.25 (10.17) | 930.66 (32.99) | 0.118 |
| Iron intake (mg/d) | 14.99 (0.17) | 14.72 (0.15) | 13.99 (0.60) | 0.189 |
| Zinc intake (mg/d) | 11.41 (0.13) | 11.27 (0.10) | 10.52 (0.39) | 0.075 |
| Choline intake (mg/d) | 291.58 (5.79) | 290.23 (3.54) | 287.99 (9.94) | 0.952 |
| Selenium intake (mcg/d) | 118.31 (1.91) | 114.73 (0.92) | 108.51 (2.60) | 0.111 |
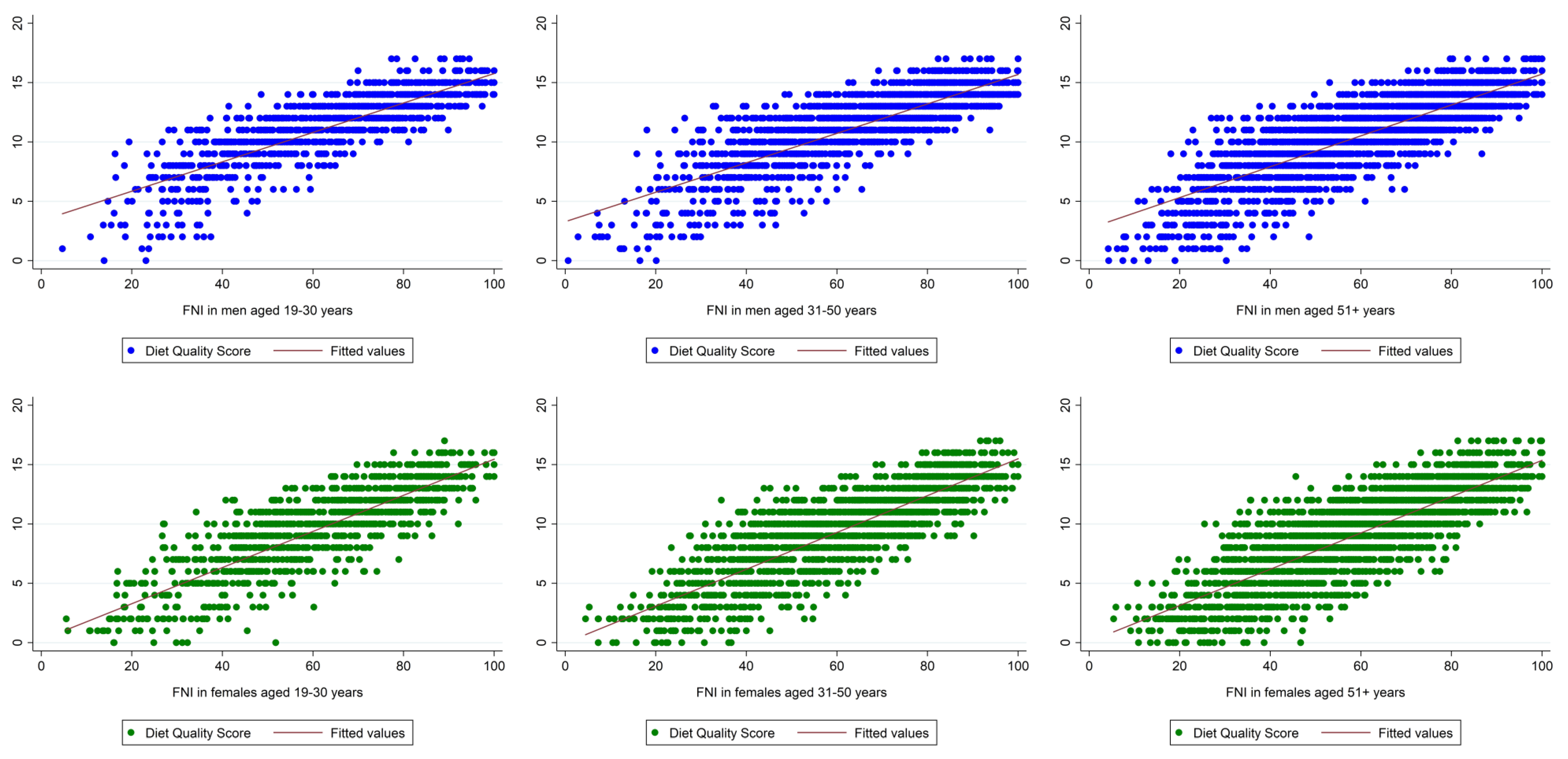
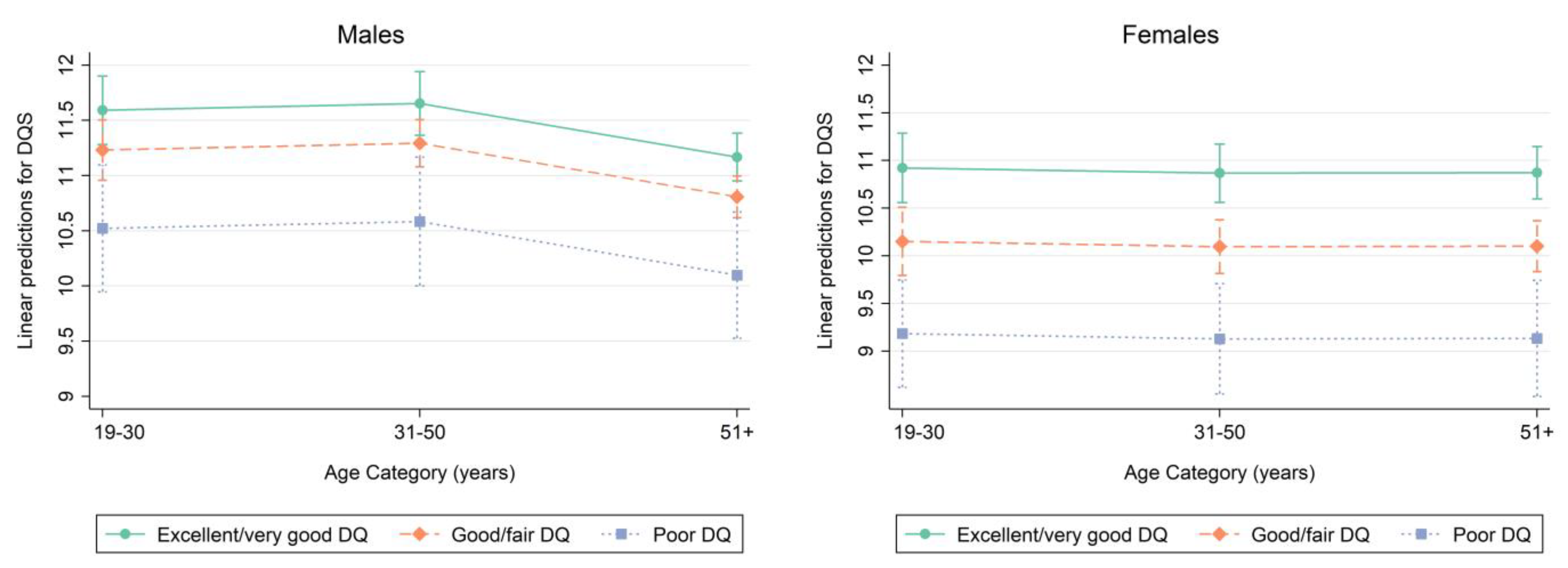
| Participants aged 19–30 years | Excellent/very good DQ n = 319 | Good/fair DQ n = 539 | Poor DQ n = 99 | p-value |
| DQS | 11.77 (0.21) | 11.13 (0.16) | 10.30 (0.40) | 0.004 |
| FNI | 68.93 (1.04) | 62.21 (1.23) | 54.63 (1.94) | <0.001 |
| Participants aged 31–50 years | Excellent/very good DQ n = 566 | Good/fair DQ n = 934 | Poor DQ n = 160 | p-value |
| DQS | 11.86 (0.20) | 11.29 (0.10) | 10.47 (0.35) | <0.001 |
| FNI | 68.35 (1.15) | 64.76 (0.65) | 59.11 (2.04) | <0.001 |
| Participants aged 51+ years | Excellent/very good DQ n = 1143 | Good/fair DQ n = 1300 | Poor DQ n = 110 | p-value |
| DQS | 11.19 (0.14) | 10.86 (0.12) | 9.66 (0.57) | 0.025 |
| FNI | 65.13 (0.91) | 62.47 (0.78) | 56.30 (3.93) | 0.029 |
| Participants aged 19–30 years | Excellent/very good DQ n = 300 | Good/fair DQ n = 639 | Poor DQ n = 103 | p-value |
| DQS | 11.33 (0.22) | 9.90 (0.19) | 9.42 (0.42) | <0.001 |
| FNI | 67.06 (1.34) | 59.32 (1.08) | 57.45 (2.09) | <0.001 |
| Participants aged 31–50 years | Excellent/very good DQ n = 660 | Good/fair DQ n = 1033 | Poor DQ n = 177 | p-value |
| DQS | 11.13 (0.22) | 10.24 (0.17) | 8.79 (0.53) | <0.001 |
| FNI | 67.43 (1.02) | 61.77 (0.88) | 53.61 (2.78) | <0.001 |
| Participants aged 51+ years | Excellent/very good DQ n = 1054 | Good/fair DQ n = 1393 | Poor DQ n = 187 | p-value |
| DQS | 10.84 (0.16) | 9.98 (0.15) | 8.46 (0.30) | <0.001 |
| FNI | 64.98 (0.84) | 59.39 (0.76) | 53.43 (1.75) | <0.001 |
| Independent Variables | β | Linearized SE | p | β | Linearized SE | p |
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| DQ | ||||||
| Excellent/very good | - | - | - | - | - | - |
| Good/Fair | −0.46 | 0.12 | <0.001 | −0.36 | 0.12 | 0.005 |
| Poor | −1.41 | 0.29 | <0.001 | −1.07 | 0.29 | 0.001 |
| Independent Variables | β | Linearized SE | p | β | Linearized SE | p |
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| DQ | ||||||
| Excellent/very good | - | - | - | - | - | - |
| Good/Fair | −0.97 | 0.13 | <0.001 | −0.77 | 0.12 | <0.001 |
| Poor | −2.24 | 0.31 | <0.001 | −1.74 | 0.29 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Storz, M.A. Does Self-Perceived Diet Quality Align with Nutrient Intake? A Cross-Sectional Study Using the Food Nutrient Index and Diet Quality Score. Nutrients 2023, 15, 2720. https://doi.org/10.3390/nu15122720
Storz MA. Does Self-Perceived Diet Quality Align with Nutrient Intake? A Cross-Sectional Study Using the Food Nutrient Index and Diet Quality Score. Nutrients. 2023; 15(12):2720. https://doi.org/10.3390/nu15122720
Chicago/Turabian StyleStorz, Maximilian Andreas. 2023. "Does Self-Perceived Diet Quality Align with Nutrient Intake? A Cross-Sectional Study Using the Food Nutrient Index and Diet Quality Score" Nutrients 15, no. 12: 2720. https://doi.org/10.3390/nu15122720
APA StyleStorz, M. A. (2023). Does Self-Perceived Diet Quality Align with Nutrient Intake? A Cross-Sectional Study Using the Food Nutrient Index and Diet Quality Score. Nutrients, 15(12), 2720. https://doi.org/10.3390/nu15122720






