Protective Effect of Citrus Medica limonum Essential Oil against Escherichia coli K99-Induced Intestinal Barrier Injury in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethic Approval
2.2. Chemicals
2.3. Chemical Composition of LEO
2.4. Animal Study
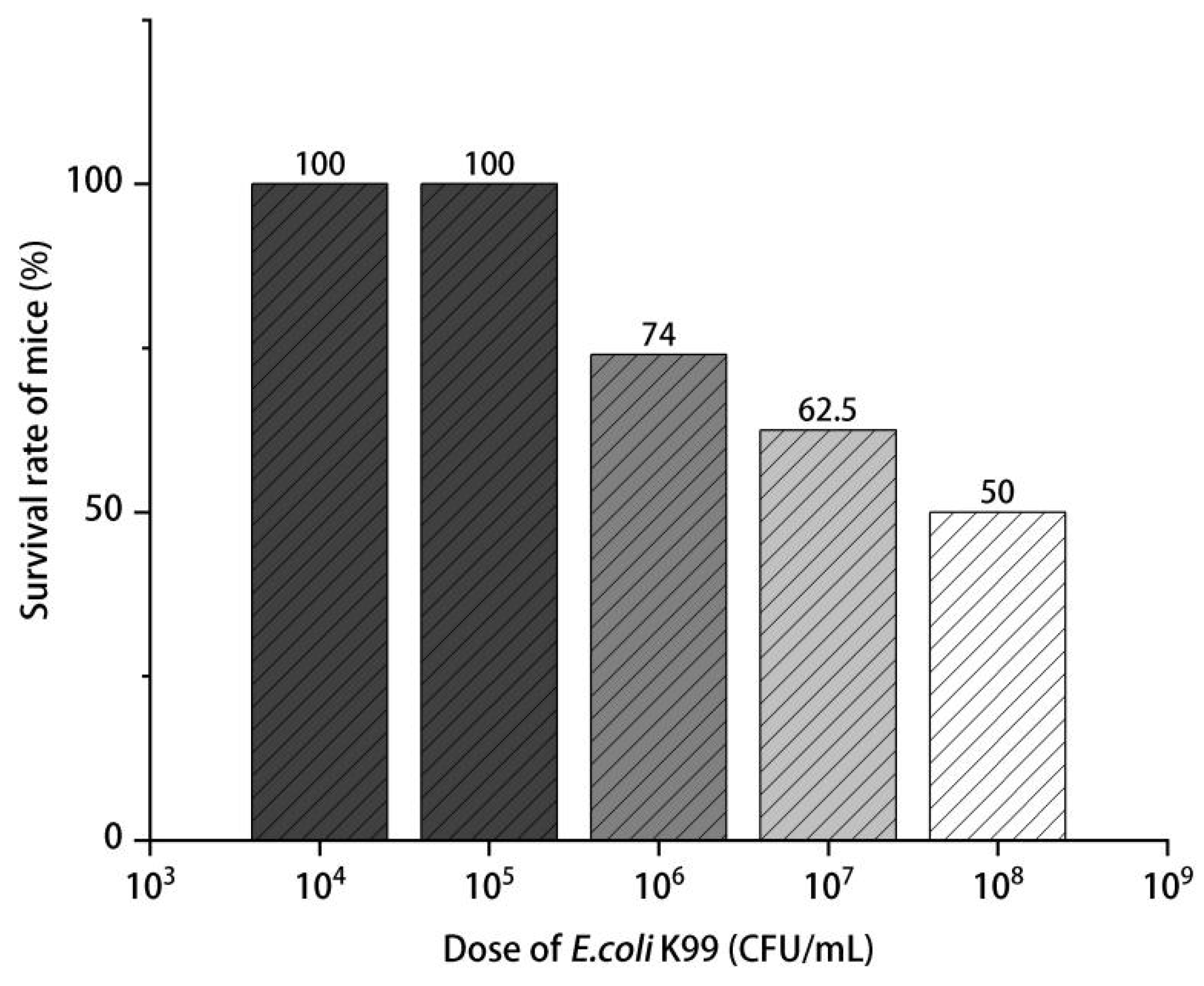
2.4.1. LD50 of E. coli K99
group. i: Logarithm of the ratio of the high dose to the low dose for two
adjacent groups (The difference between the logarithmic doses of two
adjacent groups))
2.4.2. Animals and Experimental Treatment
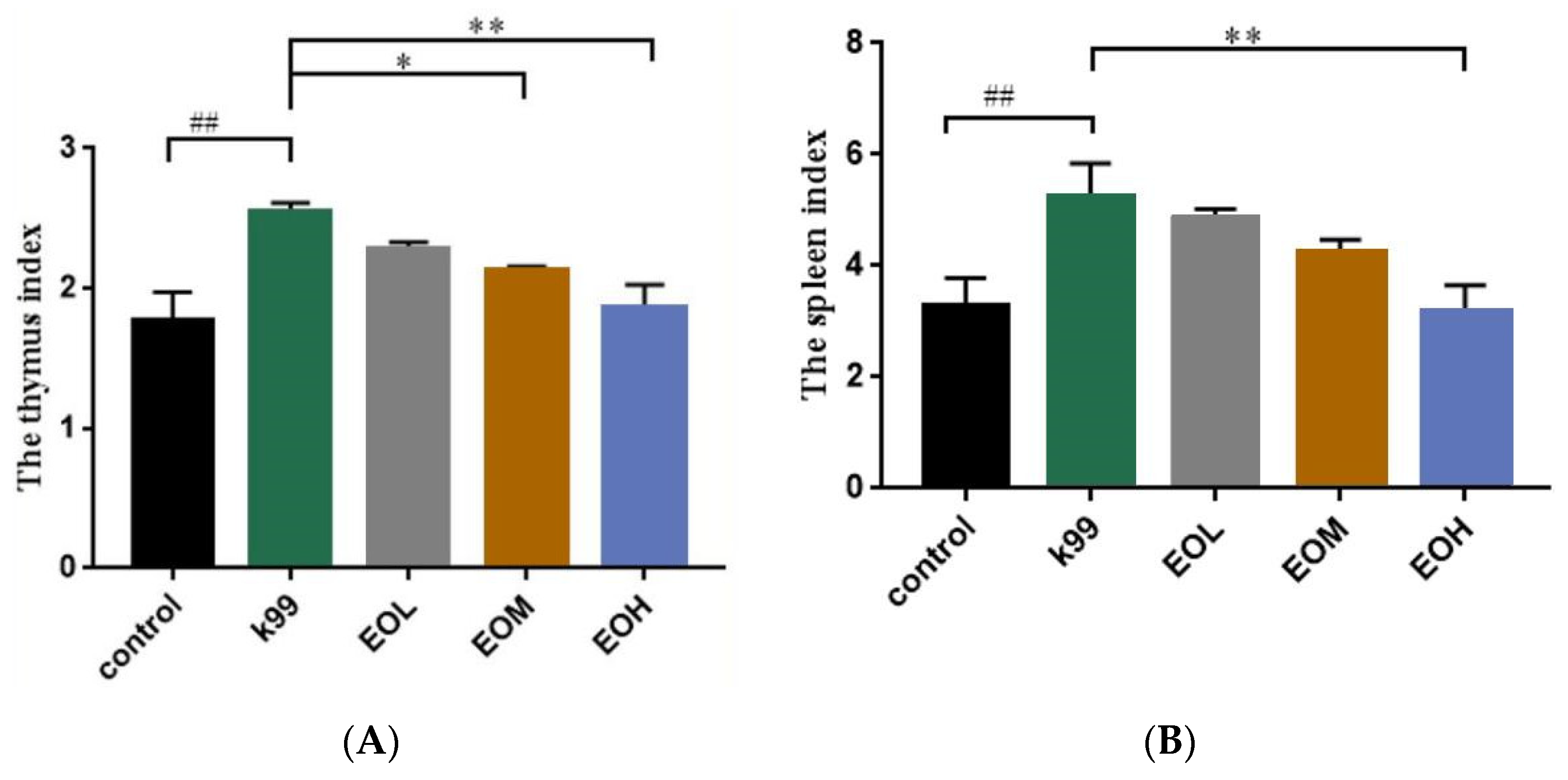
2.4.3. Immune Organs Index in Mice
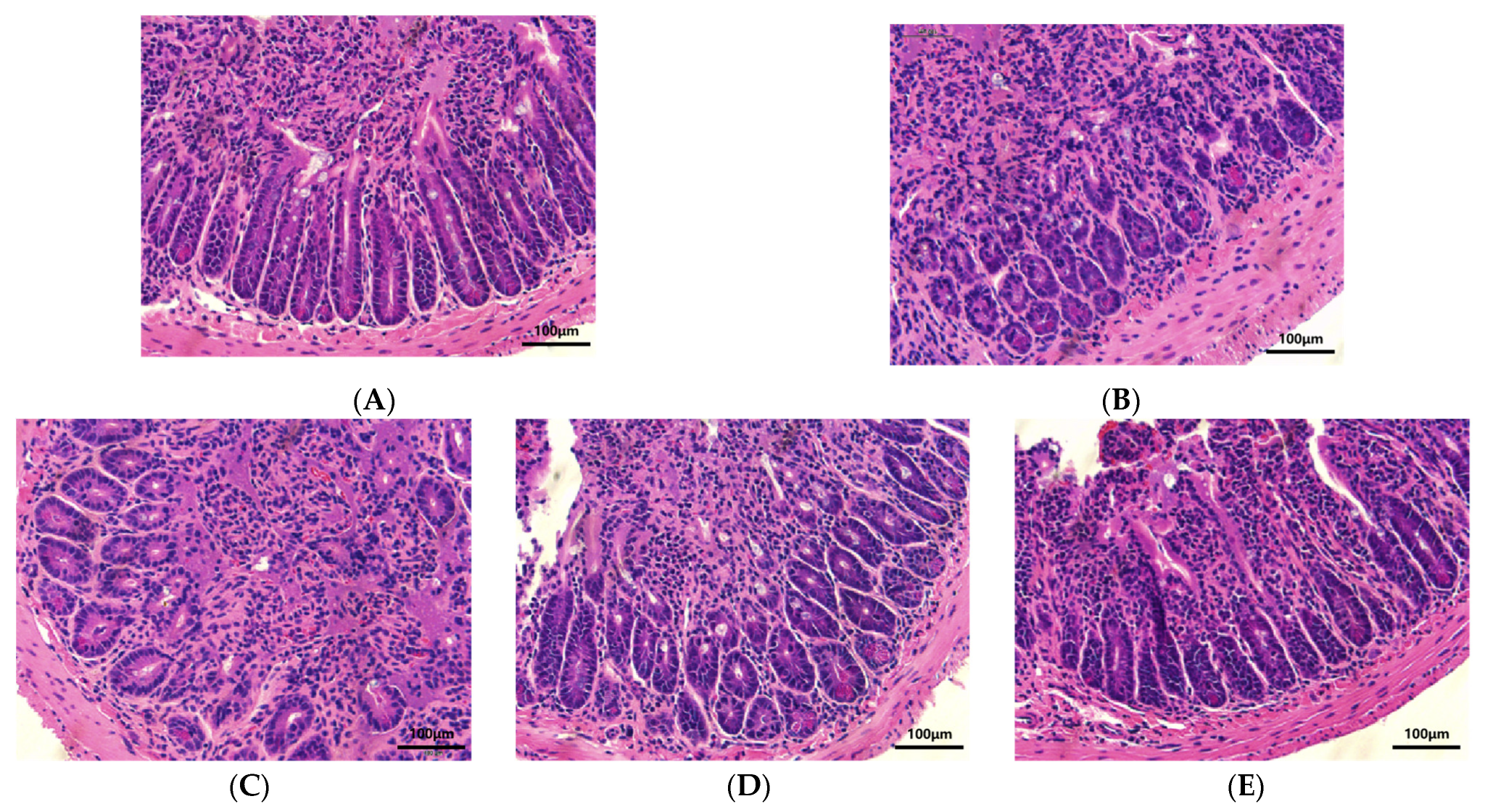
2.4.4. Histopathology
2.4.5. Blood Immune Indexes and Inflammatory Factors in Mice
2.4.6. mRNA Relative Expression of ITF and TGF-β1 in Duodenum
2.5. Statistical Analysis
3. Results
3.1. Chemical Composition of LEO
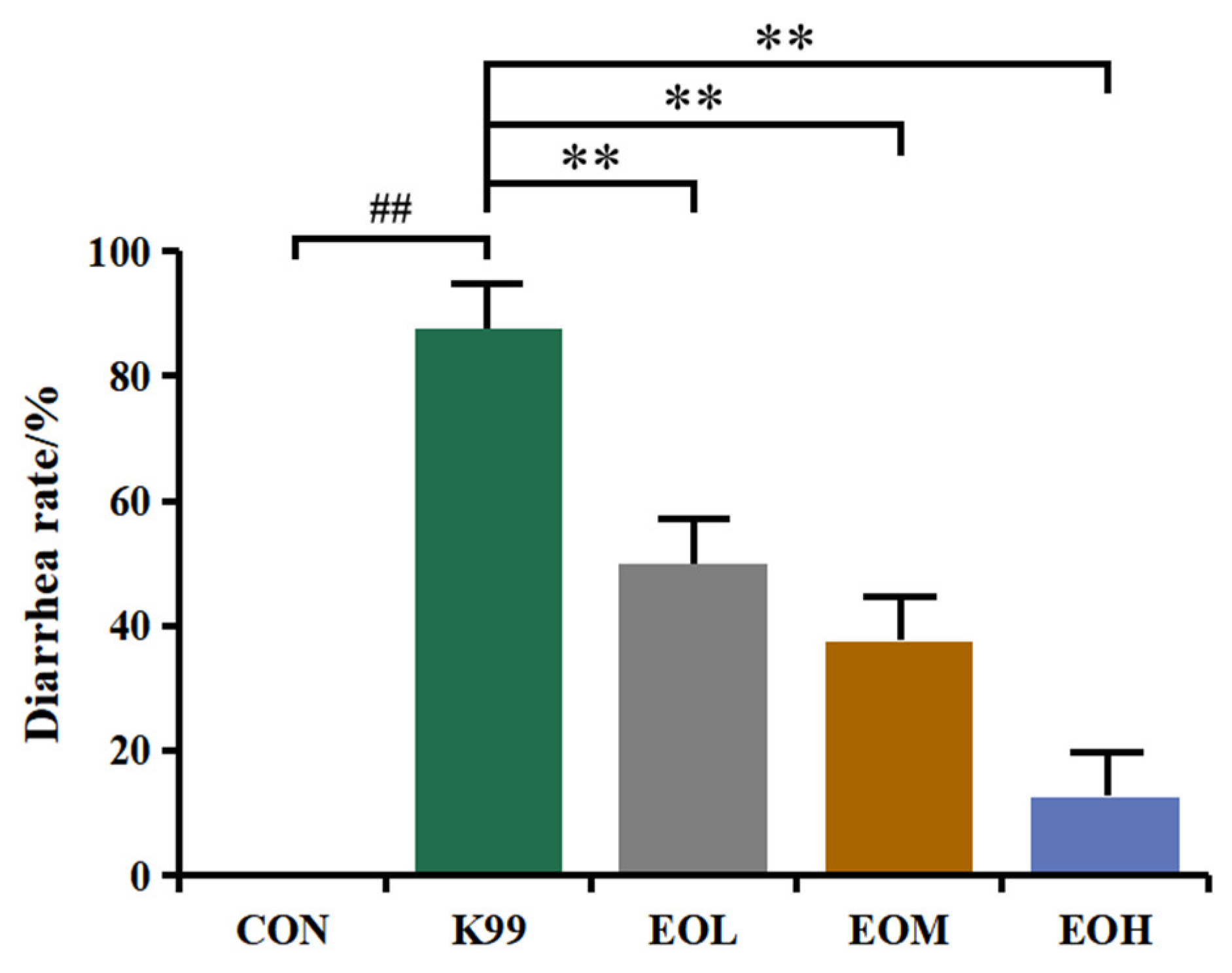
3.2. LD50 of E. coli K99 and Diarrhea Prevention Effect of LEO
3.3. Effects of LEO and E. coli K99 on Organ Indexes in Mice
3.4. Effect of LEO and E. coli K99 on the Morphology of Duodenal Tissue in Mice
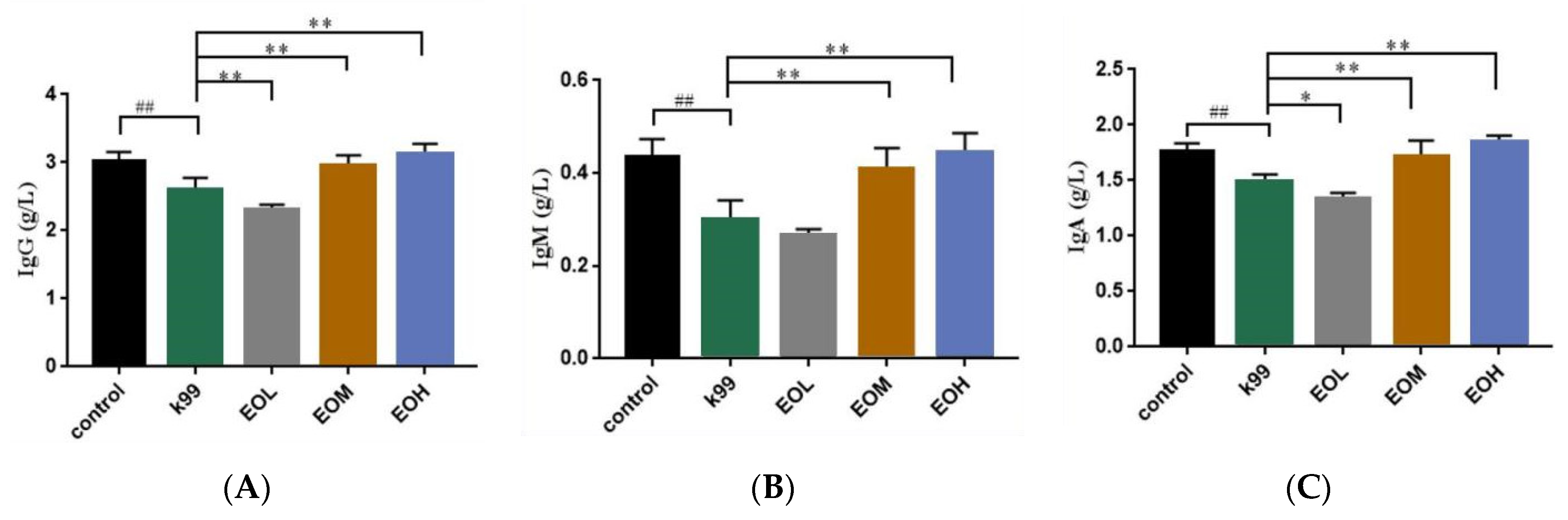
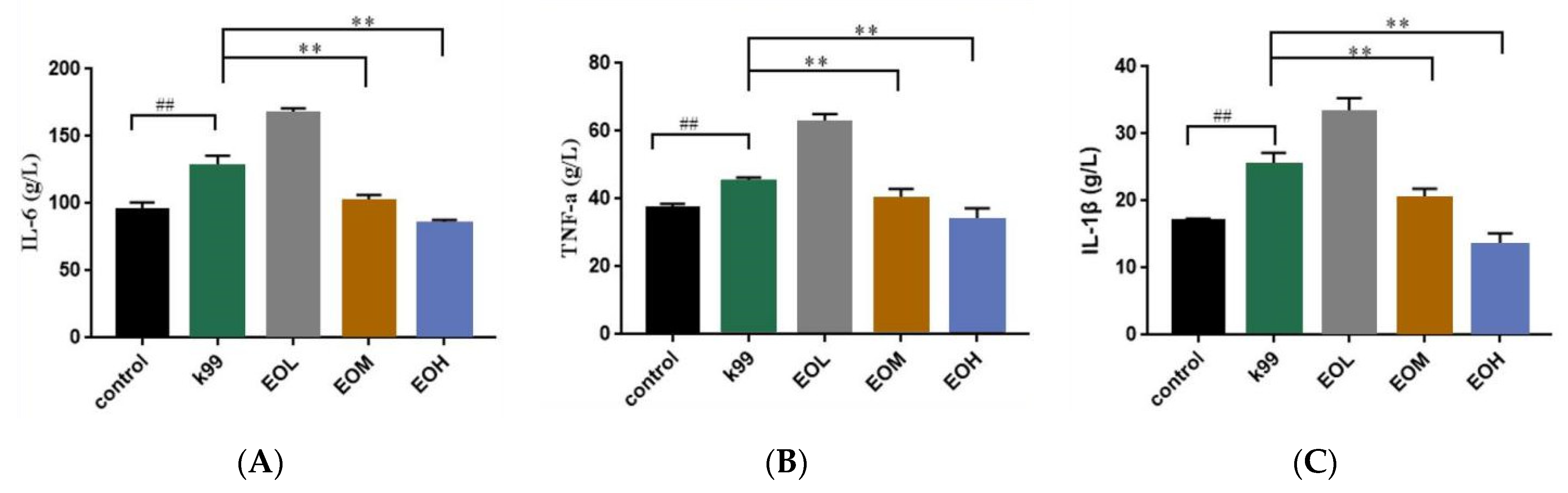
3.5. Effects of LEO and E. coli K99 on Immune and Inflammatory Factors in Blood
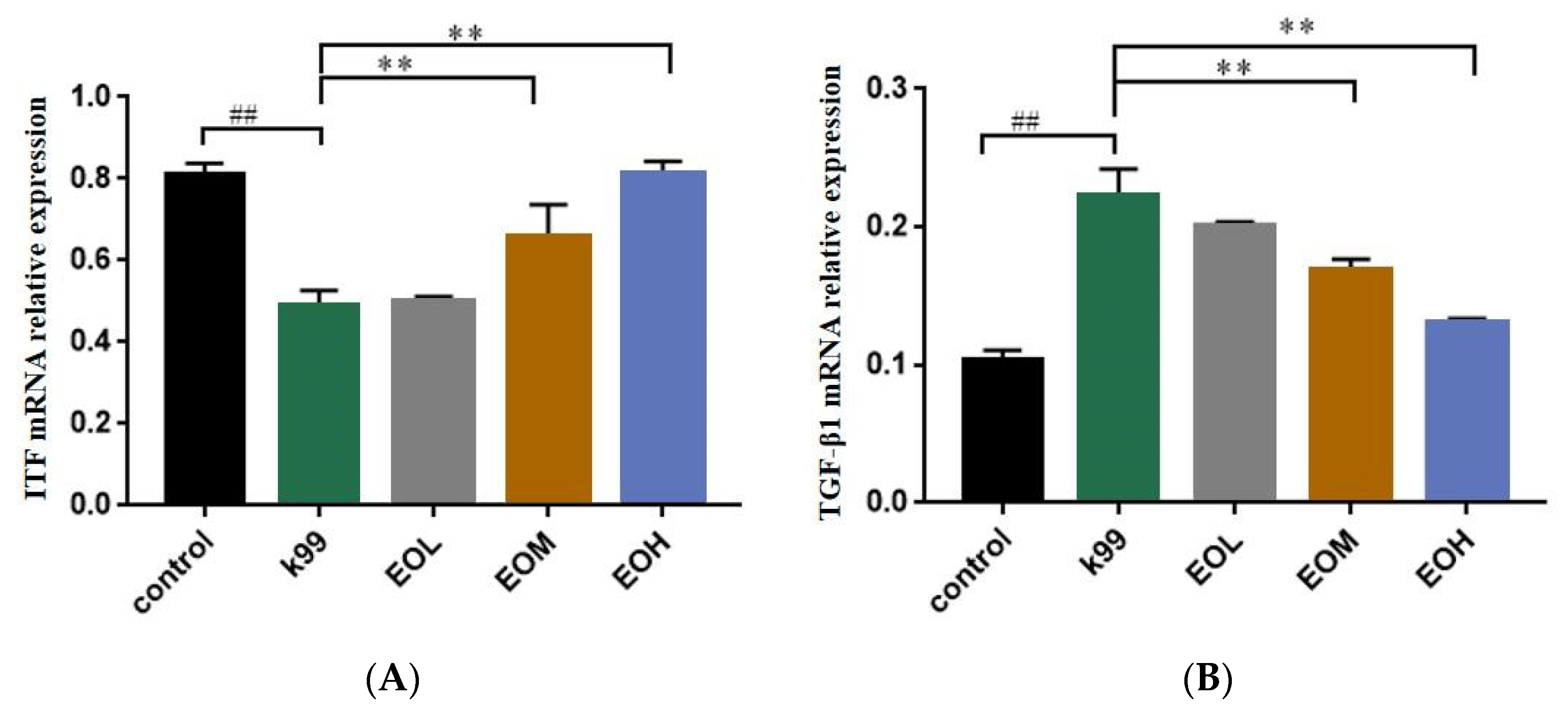
3.6. Effect of LEO and E. coli K99 on mRNA Relative Expression of ITF and TGF-β1 in Duodenum of Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Z.; Ma, Y.; Yang, S.; Zhang, S.; Liu, S.; Xiao, J.; Wang, Y.; Wang, W.; Yang, H.; Li, S.; et al. Gut microbiota-derived ursodeoxycholic acid from neonatal dairy calves improves intestinal homeostasis and colitis to attenuate extended-spectrum β-lactamase-producing enteroaggregative Escherichia coli infection. Microbiome 2022, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Katsoulos, P.D.; Karatzia, M.A.; Dovas, C.I.; Filioussis, G.; Papadopoulos, E.; Kiossis, E.; Arsenopoulos, K.; Papadopoulos, T.; Boscos, C.; Karatzias, H. Evaluation of the in-field efficacy of oregano essential oil administration on the control of neonatal diarrhea syndrome in calves. Res. Vet. Sci. 2017, 115, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Bartels, C.J.M.; Holzhauer, M.; Jorritsma, R.; Swart, W.A.J.M.; Lam, T.J.G.M. Prevalence, prediction and risk factors of enteropathogens in normal and non-normal faeces of young Dutch dairy calves. Prev. Vet. Med. 2010, 93, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.W. Distinguishing Pathovars from Nonpathovars: Escherichia coli. Microbiol. Spectr. 2020, 8, 4. [Google Scholar] [CrossRef]
- Brunauer, M.; Roch, F.; Conrady, B. Prevalence of Worldwide Neonatal Calf Diarrhoea Caused by Bovine Rotavirus in Combination with Bovine Coronavirus, Escherichia coli K99 and Cryptosporidium spp.: A Meta-Analysis. Animals 2021, 11, 1014. [Google Scholar] [CrossRef]
- Orskov, I.; Orskov, F.; Smith, H.W.; Sojka, W.J. The establishment of K99, a thermolabile, transmissible Escherichia coli Kantigen, previously called “Kco”, possessed by calf and lamb enteropathogenic strains. Acta Pathol. Microbiol. Scand. B 1975, 83, 31–36. [Google Scholar] [CrossRef]
- Kim, N.; Gu, M.J.; Kye, Y.C.; Ju, Y.J.; Hong, R.; Ju, D.B.; Pyung, Y.J.; Han, S.H.; Park, B.C.; Yun, C.H. Bacteriophage EK99P-1 alleviates enterotoxigenic Escherichia coli K99-induced barrier dysfunction and inflammation. Sci. Rep. 2022, 12, 941. [Google Scholar] [CrossRef]
- Wang, B.; Yang, C.T.; Diao, Q.Y.; Tu, Y. The influence of mulberry leaf flavonoids and Candida tropicalis on antioxidant function and gastrointestinal development of preweaning calves challenged with Escherichia coli O141:K99. J. Dairy Sci. 2018, 101, 6098–6108. [Google Scholar] [CrossRef]
- Bakkeren, E.; Huisman, J.S.; Fattinger, S.A.; Hausmann, A.; Furter, M.; Egli, A.; Slack, E.; Sellin, M.E.; Bonhoeffer, S.; Regoes, R.R.; et al. Salmonella persisters promote the spread of antibiotic resistance plasmids in the gut. Nature 2019, 573, 276–280. [Google Scholar] [CrossRef]
- Zeissig, S.; Blumberg, R.S. Life at the beginning: Perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat. Immunol. 2014, 15, 307–310. [Google Scholar] [CrossRef]
- Mceachran, A.D.; Blackwell, B.R.; Hanson, J.D.; Wooten, K.J.; Mayer, G.D.; Cox, S.B.; Smith, P.N. Antibiotics, Bacteria, and Antibiotic Resistance Genes: Aerial Transport from Cattle Feed Yards via Particulate Matter. Environ. Health Perspect. 2015, 123, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Salerno, B.; Cornaggia, M.; Sabatino, R.; Di Cesare, A.; Furlan, M.; Barco, L.; Orsini, M.; Cordioli, B.; Mantovani, C.; Bano, L.; et al. Calves as Main Reservoir of Antibiotic Resistance Genes in Dairy Farms. Front. Public Health 2022, 10, 918658. [Google Scholar] [CrossRef] [PubMed]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (Lemon) Phenomenon-A Review of the Chemistry, Pharmacological Properties, Applications in the Modern Pharmaceutical, Food, and Cosmetics Industries, and Biotechnological Studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef]
- NBS. NBS|National Bureau of Statistics. Available online: https://data.stats.gov.cn/easyquery.htm?cn=C01&zb=A0D0K&sj=2021 (accessed on 3 June 2023).
- Luciardi, M.C.; Blázquez, M.A.; Alberto, M.R.; Cartagena, E.; Arena, M.E. Lemon Oils Attenuate the Pathogenicity of Pseudomonas aeruginosa by Quorum Sensing Inhibition. Molecules 2021, 26, 2863. [Google Scholar] [CrossRef] [PubMed]
- Dosoky, N.; Setzer, W. Biological Activities and Safety of Citrus spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef]
- Rozza, A.L.; Moraes, T.D.M.; Kushima, H.; Tanimoto, A.; Marques, M.O.M.; Bauab, T.M.; Hiruma-Lima, C.A.; Pellizzon, C.H. Gastroprotective mechanisms of Citrus lemon (Rutaceae) essential oil and its majority compounds limonene and β-pinene: Involvement of heat-shock protein-70, vasoactive intestinal peptide, glutathione, sulfhydryl compounds, nitric oxide and prostaglandin E2. Chem.-Biol. Interact. 2011, 189, 82–89. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Z.; Nie, D.; Li, Y. Protective Effect of Lemon Essential Oil and Its Major Active Component, D-Limonene, on Intestinal Injury and Inflammation of E. coli-Challenged Mice. Front. Nutr. 2022, 9, 843096. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Zhao, C.; Zhang, Z.; Nie, D.; Tang, W.; Li, Y. The Chemical Composition and Antibacterial and Antioxidant Activities of Five Citrus Essential Oils. Molecules 2022, 27, 7044. [Google Scholar] [CrossRef]
- Xu, X.; Yang, J.; Ning, Z.; Zhang, X. Lentinula edodes-derived polysaccharide rejuvenates mice in terms of immune responses and gut microbiota. Food Funct. 2015, 6, 2653–2663. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Wu, J.; Wang, Q.; Su, A.; Xue, M.; Liu, Q.; Hu, Q. Inhibitory effect of Zanthoxylum bungeanum essential oil (ZBEO) on Escherichia coli and intestinal dysfunction. Food Funct. 2017, 8, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Den Haan, J.M.M.; Kraal, G. Innate Immune Functions of Macrophage Subpopulations in the Spleen. J. Innate Immun. 2012, 4, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Duah, M.; Li, L.; Shen, J.; Lan, Q.; Pan, B.; Xu, K. Thymus Degeneration and Regeneration. Front. Immunol. 2021, 12, 706244. [Google Scholar] [CrossRef]
- Schroeder, H.W.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef]
- Lv, Y.; Zhao, S.; Zhang, J.; Zhang, H.; Xie, Z.; Cai, G.; Jiang, W. Effect of orange peel essential oil on oxidative stress in AOM animals. Int. J. Biol. Macromol. 2012, 50, 1144–1150. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Fan, G.; Ren, J.N.; Zhang, L.L.; Pan, S.Y. Effects of orange essential oil on intestinal microflora in mice. J. Sci. Food Agric. 2019, 99, 4019–4028. [Google Scholar] [CrossRef]
- Terao, R.; Murata, A.; Sugamoto, K.; Watanabe, T.; Nagahama, K.; Nakahara, K.; Kondo, T.; Murakami, N.; Fukui, K.; Hattori, H.; et al. Immunostimulatory effect of kumquat (Fortunella crassifolia) and its constituents, β-cryptoxanthin and R-limonene. Food Funct. 2019, 10, 38–48. [Google Scholar] [CrossRef]
- Feng, T.; Zhang, Q.; Li, Q.; Zhu, T.; Lv, W.; Yu, H.; Qian, B. LUAD transcriptomic profile analysis of D-limonene and potential lncRNA chemopreventive target. Food Funct. 2020, 11, 7255–7265. [Google Scholar] [CrossRef]
- Brenner, D.; Blaser, H.; Mak, T.W. Regulation of tumour necrosis factor signalling: Live or let die. Nat. Rev. Immunol. 2015, 15, 362–374. [Google Scholar] [CrossRef]
- Watson, A.J.M.; Hughes, K.R. TNF-α-induced intestinal epithelial cell shedding: Implications for intestinal barrier function. Ann. N. Y. Acad. Sci. 2012, 1258, 1–8. [Google Scholar] [CrossRef]
- Ye, M.; Joosse, M.E.; Liu, L.; Sun, Y.; Dong, Y.; Cai, C.; Song, Z.; Zhang, J.; Brant, S.R.; Lazarev, M.; et al. Deletion of IL-6 Exacerbates Colitis and Induces Systemic Inflammation in IL-10-Deficient Mice. J. Crohn’s Colitis 2020, 14, 831–840. [Google Scholar] [CrossRef]
- Kaminsky, L.W.; Al-Sadi, R.; Ma, T.Y. IL-1β and the Intestinal Epithelial Tight Junction Barrier. Front. Immunol. 2021, 12, 767456. [Google Scholar] [CrossRef]
- Qi, L.; Mao, H.; Lu, X.; Shi, T.; Wang, J. Cinnamaldehyde Promotes the Intestinal Barrier Functions and Reshapes Gut Microbiome in Early Weaned Rats. Front. Nutr. 2021, 8, 748503. [Google Scholar] [CrossRef]
- Shen, C.; Jiang, J.; Zhu, W.; Ou-Yang, Q. Anti-inflammatory Effect of Essential Oil from Citrus aurantium L. var. amara Engl. J. Agric. Food Chem. 2017, 65, 8586–8594. [Google Scholar] [CrossRef] [PubMed]
- Kummer, R.; Fachini-Queiroz, F.C.; Estevão-Silva, C.F.; Grespan, R.; Silva, E.L.; Bersani-Amado, C.A.; Cuman, R.K.N. Evaluation of Anti-Inflammatory Activity of Citrus latifolia Tanaka Essential Oil and Limonene in Experimental Mouse Models. Evid.-Based Complement. Altern. Med. 2013, 2013, 859083. [Google Scholar] [CrossRef]
- Amorim, J.L.; Simas, D.L.R.; Pinheiro, M.M.G.; Moreno, D.S.A.; Alviano, C.S.; Da Silva, A.J.R.; Dias Fernandes, P. Anti-Inflammatory Properties and Chemical Characterization of the Essential Oils of Four Citrus Species. PLoS ONE 2016, 11, e153643. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhou, R.; Jiang, Q.; Kong, L.; Lei, H. GEO-PGS composite shows synergistic and complementary effect on Escherichia coli and improvement of intestinal dysfunction. Food Chem. Toxicol. 2020, 135, 110936. [Google Scholar] [CrossRef] [PubMed]
- Taupin, D.R.; Kinoshita, K.; Podolsky, D.K. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 799–804. [Google Scholar] [CrossRef]
- Troncone, E.; Marafini, I.; Stolfi, C.; Monteleone, G. Transforming Growth Factor-β1/Smad7 in Intestinal Immunity, Inflammation, and Cancer. Front. Immunol. 2018, 9, 1407. [Google Scholar] [CrossRef] [PubMed]
- Al Khrashi, L.A.; Badr, A.M.; Al Amin, M.A.; Mahran, Y.F. Thymol ameliorates 5-fluorouracil-induced intestinal mucositis: Evidence of down-regulatory effect on TGF-β/MAPK pathways through NF-κB. J. Biochem. Mol. Toxicol. 2022, 36, e22932. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.B.; Rehman, M.U.; Fatima, B.; Ahmad, B.; Hussain, I.; Ahmad, S.P.; Farooq, A.; Muzamil, S.; Razzaq, R.; Rashid, S.M.; et al. Antifibrotic effects of D-limonene (5(1-methyl-4-[1-methylethenyl]) cyclohexane) in CCl4 induced liver toxicity in Wistar rats. Environ. Toxicol. 2018, 33, 361–369. [Google Scholar] [CrossRef]
- Chen, G.; Xie, X.; Peng, F.; Wang, T.; Chen, J.; Li, G.; Liu, J.; Peng, C. Protective effect of the combination of essential oil from patchouli and tangerine peel against gastric ulcer in rats. J. Ethnopharmacol. 2022, 282, 114645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Liu, Z.M.; Zhang, H.F.; Li, Y.S.; Wen, S.H.; Shen, J.T.; Huang, W.Q.; Liu, K.X. TGF-β1 improves mucosal IgA dysfunction and dysbiosis following intestinal ischaemia–reperfusion in mice. J. Cell. Mol. Med. 2016, 20, 1014–1023. [Google Scholar] [CrossRef]
- Walia, B.; Wang, L.; Merlin, D.; Sitaraman, S.V. TGF-β down-regulates IL-6 signaling in intestinal epithelial cells: Critical role of SMAD-2. FASEB J. 2003, 17, 2130–2132. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Cao, S.; Jiao, L.; Song, Z.; Lu, J.; Hu, C. TGF-β1 protects intestinal integrity and influences Smads and MAPK signal pathways in IPEC-J2 after TNF-α challenge. Innate Immun. 2017, 23, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, C.; Mao, Z.; Teng, X.; Ma, L.; Sun, M. Disruption of the F-actin cytoskeleton and monolayer barrier integrity induced by PAF and the protective effect of ITF on intestinal epithelium. Arch. Pharm. Res. 2011, 34, 245–251. [Google Scholar] [CrossRef]
| Gene | Primer Sequence (5′-3′) |
|---|---|
| β-actin | F: CTGGAACGGTGAAGGTGACA R: AAGGGACTTCCTGTAACAATGCA |
| ITF | F: CTGTGCAGTGGTCCTGAAGC R: TTGGAGACAGGCCAACGTAA |
| TGF-β1 | F: CCCCTGCAAGACCATCGAC R: CTGGCGAGCCTTAGTTTGGAC |
| Peak Pegasus | Retention Time RI/min | Chemical Compound | Relative Amount/% |
|---|---|---|---|
| 1 | 6.261 | α-pleuene | 0.24 |
| 2 | 6.395 | α-pinene | 3.4 |
| 3 | 6.677 | Bicyclic [2,2,1] heptane | 0.08 |
| 4 | 7.161 | sabinene | 3.51 |
| 5 | 7.232 | β-pinene | 12.79 |
| 6 | 7.338 | Bicyclic [10,1,0] tridecane | 0.08 |
| 7 | 7.458 | β-myrcene | 3.5 |
| 8 | 7.646 | octanal | 0.26 |
| 9 | 7.715 | α-phellandrene | 0.3 |
| 10 | 7.831 | 3-carene | 0.2 |
| 11 | 7.946 | 4-carene | 0.39 |
| 12 | 8.097 | o-cymene | 1.46 |
| 13 | 8.254 | D-limonene | 47.19 |
| 14 | 8.486 | (Z)-3,7-dimethyl-1,3,6-octadecane triene | 0.14 |
| 15 | 8.715 | γ-terpinene | 11.49 |
| 16 | 8.846 | n-caprylic alcohol | 0.08 |
| 17 | 8.935 | 2-vinyl-2-methyl-5-(1-methylvinyl) tetrahydrofuran | 0.04 |
| 18 | 9.216 | α-terpinolene | 1.16 |
| 19 | 9.365 | linalool | 0.38 |
| 20 | 9.428 | nonanal | 0.17 |
| 21 | 9.975 | 6-methyl-3-(1-methylethyl)-7-oxicycle [4.1.0]-2-heptanone | 0.18 |
| 22 | 10.046 | D-litene oxide | 0.1 |
| 23 | 10.167 | 3-oxacyclic [4.3.0] hept-8-ene-2-ketone | 0.05 |
| 24 | 10.254 | (R)-3,7-dimethyl-6-octenol | 0.04 |
| 25 | 10.717 | 4-terpenenol | 0.05 |
| 26 | 10.917 | α-terpilenol | 0.28 |
| 27 | 11.02 | 3-methylene-1,5,5-trimethylcyclohexene | 0.05 |
| 28 | 11.088 | capraldehyde | 0.18 |
| 29 | 11.351 | 2-cyclohexene-1-ol, 2-methyl-5-(1-methylvinyl)-, mesylate | 0.03 |
| 30 | 11.464 | benzothiazole | 0.05 |
| 31 | 11.536 | 2-cyclohexene 1-alcohol | 0.06 |
| 32 | 11.67 | (Z)-3,7-dimethylocta-2,6-dienal | 2.81 |
| 33 | 11.751 | D-carvone | 0.03 |
| 34 | 12.113 | (E)-3,7-dimethylocta-2,6-dienal | 4.01 |
| 35 | 12.787 | (2E)-1-methoxy-3,7-dimethyl acetamide | 0.49 |
| 36 | 13.116 | 8-chloro-1-octanol | 1.32 |
| 37 | 13.449 | 2,6-octadiene-1-alcohol-3,7-dimethylacetyl | 0.75 |
| 38 | 13.715 | geranyl acetate | 0.57 |
| 39 | 14.449 | caryophyllene | 0.24 |
| 40 | 14.575 | bergapten | 0.45 |
| 41 | 14.923 | humulene | 0.06 |
| 42 | 15.263 | Cyclohexene, 3-(1.5 dimethyl-4-hexene) | 0.04 |
| 43 | 15.426 | 1,4-methylhydroindene | 0.14 |
| 44 | 15.523 | β-bisabolene | 0.67 |
| 45 | 16.531 | 3,5-diethyl-2-propyl pyridine | 0.03 |
| 46 | 17.358 | Cyclooctane siloxane | 0.06 |
| 47 | 19.226 | Octadecymethyl cyclononsiloxane | 0.03 |
| 48 | 20.673 | m-camphorene | 0.23 |
| 49 | 20.73 | N-butyl phthalate | 0.03 |
| 50 | 21.039 | P-camphorene | 0.1 |
| Total | 99.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Zhang, Z.; Nie, D.; Li, Y.; Liu, S.; Li, Y. Protective Effect of Citrus Medica limonum Essential Oil against Escherichia coli K99-Induced Intestinal Barrier Injury in Mice. Nutrients 2023, 15, 2697. https://doi.org/10.3390/nu15122697
Tang W, Zhang Z, Nie D, Li Y, Liu S, Li Y. Protective Effect of Citrus Medica limonum Essential Oil against Escherichia coli K99-Induced Intestinal Barrier Injury in Mice. Nutrients. 2023; 15(12):2697. https://doi.org/10.3390/nu15122697
Chicago/Turabian StyleTang, Weixuan, Zhuo Zhang, Dechao Nie, Yan Li, Shutian Liu, and Yanling Li. 2023. "Protective Effect of Citrus Medica limonum Essential Oil against Escherichia coli K99-Induced Intestinal Barrier Injury in Mice" Nutrients 15, no. 12: 2697. https://doi.org/10.3390/nu15122697
APA StyleTang, W., Zhang, Z., Nie, D., Li, Y., Liu, S., & Li, Y. (2023). Protective Effect of Citrus Medica limonum Essential Oil against Escherichia coli K99-Induced Intestinal Barrier Injury in Mice. Nutrients, 15(12), 2697. https://doi.org/10.3390/nu15122697






